Combination of Two Kinds of Medicated Microparticles Based on Hyaluronic Acid or Chitosan for a Wound Healing Spray Patch
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Total Polyphenol Content (TPC) Determination in Olive Leaves Water Extract (OLE)
2.3. Bactericidal Assays
2.4. Preparation of Microparticles (MP) Using the Spray-Drying Technique
2.5. MP Characterization
2.5.1. Determination of the OLE Content in Medicated MP
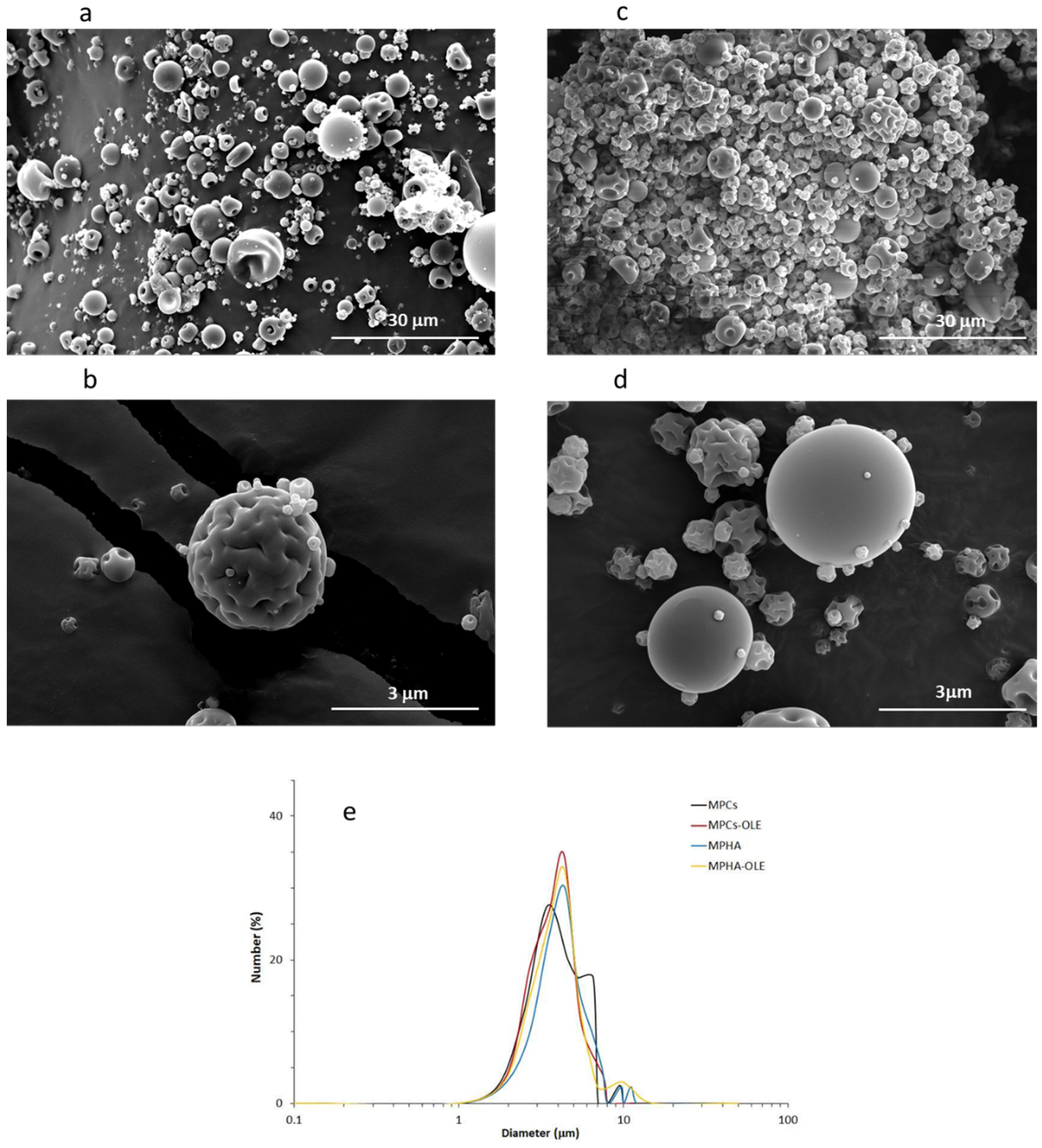
2.5.2. Dimensional and Morphological Analysis: Scanning Electron Microscopy (SEM)
2.5.3. Thermogravimetric Analysis (TGA)
2.5.4. Determination of OLE Stability in MP
2.5.5. Determination of the MP Swelling Degree
2.5.6. Study of the Release Profiles from Medicated MP
2.6. Cell Culture Techniques
2.6.1. Cell Viability and Proliferation Test by WST-1 Assay
2.6.2. In Vitro Scratch Wound Healing Assay
3. Results
3.1. TPC Determination in OLE
3.2. OLE Antibacterial Activity
3.3. MP Characterization
3.4. TGA Analysis
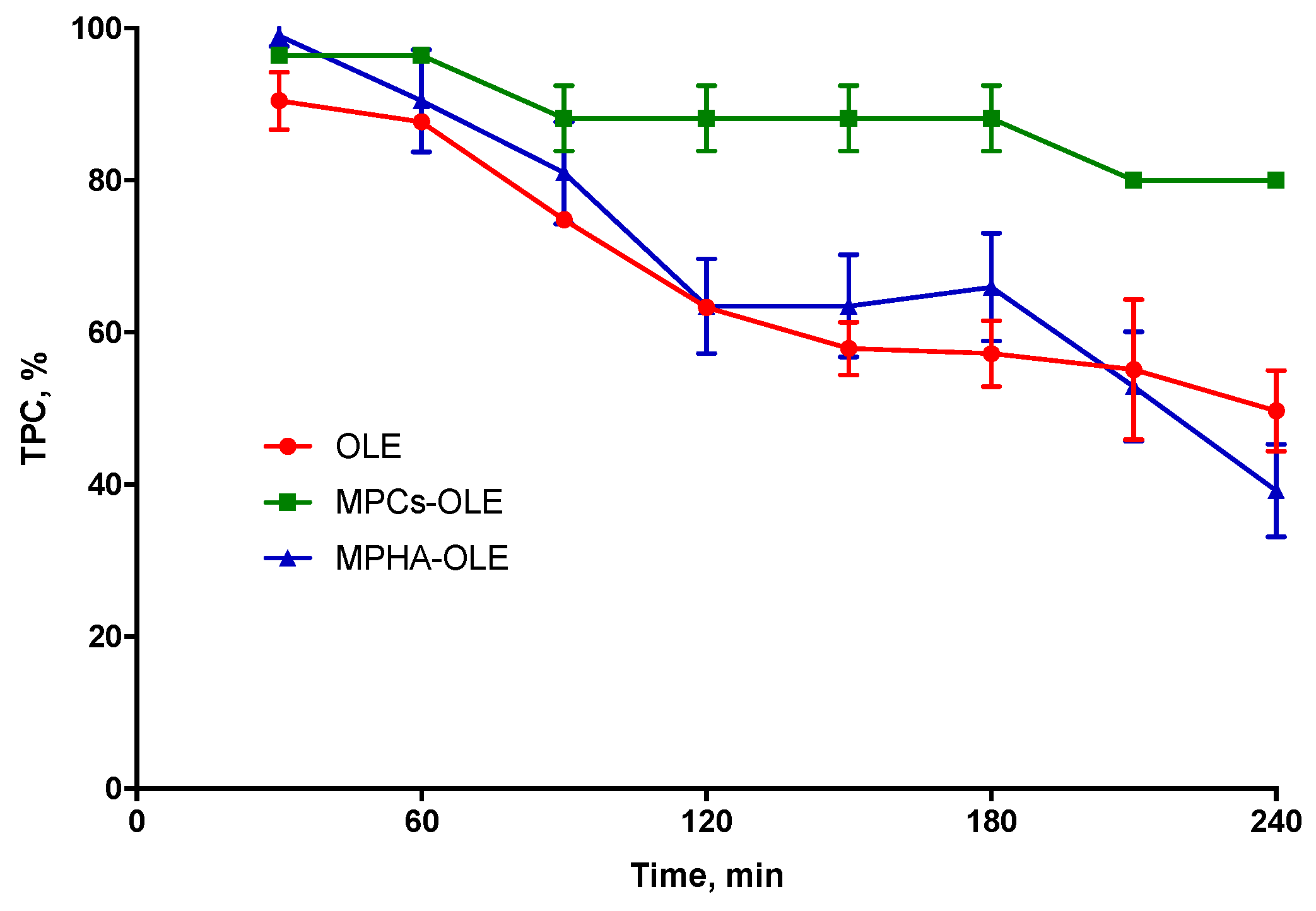
3.5. Determination of OLE Stability in MP
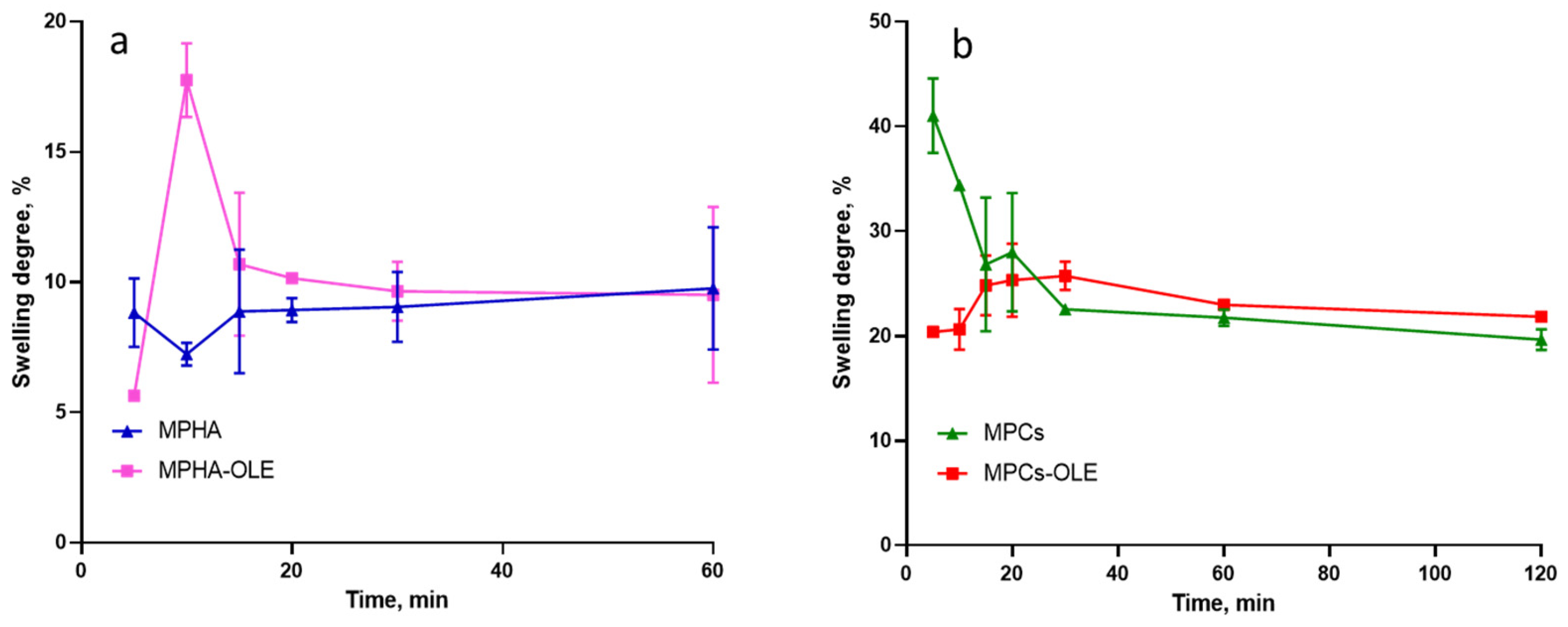
3.6. Determination the MP Swelling Degree
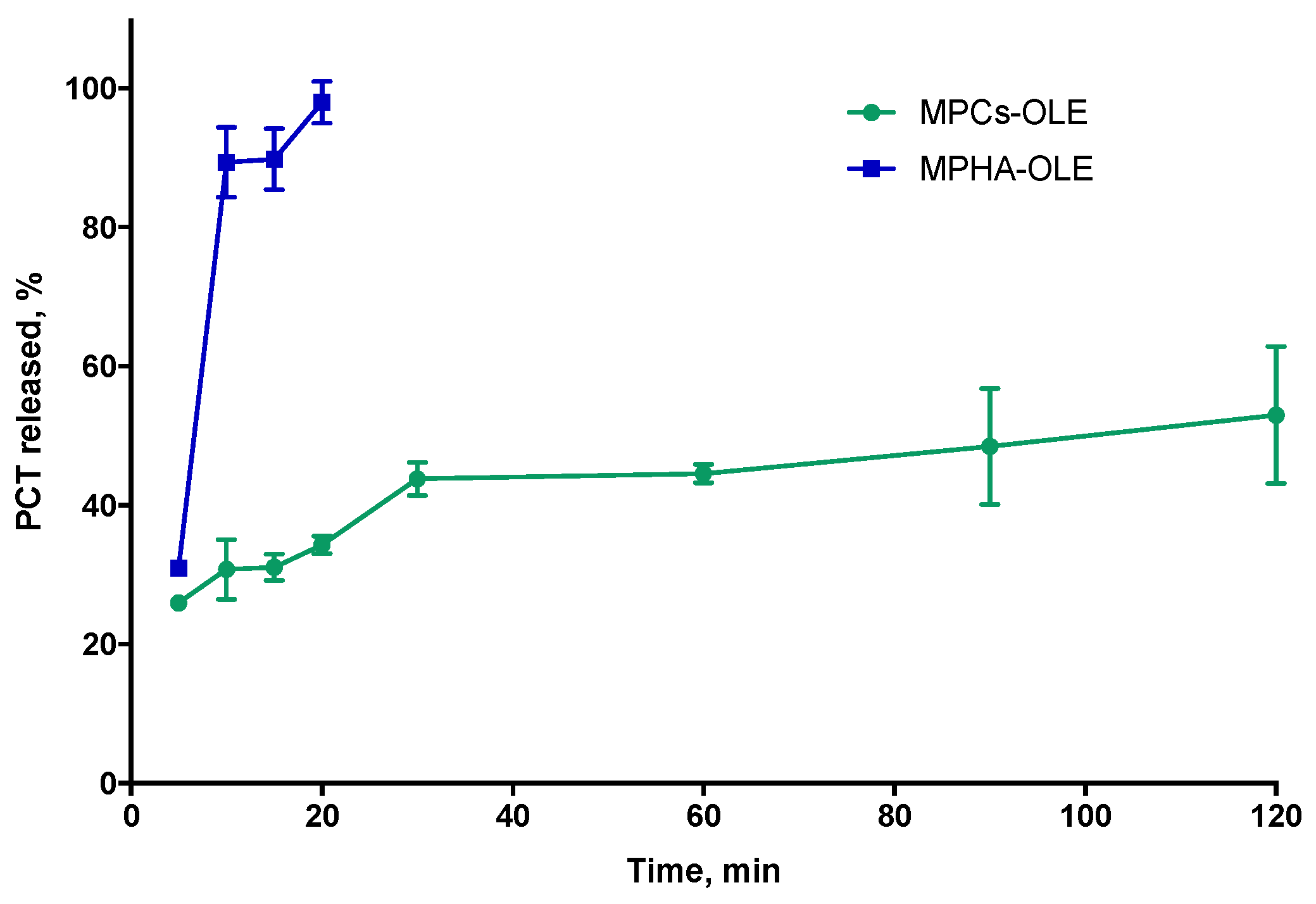
3.7. Study of Release Profile from Medicated MP
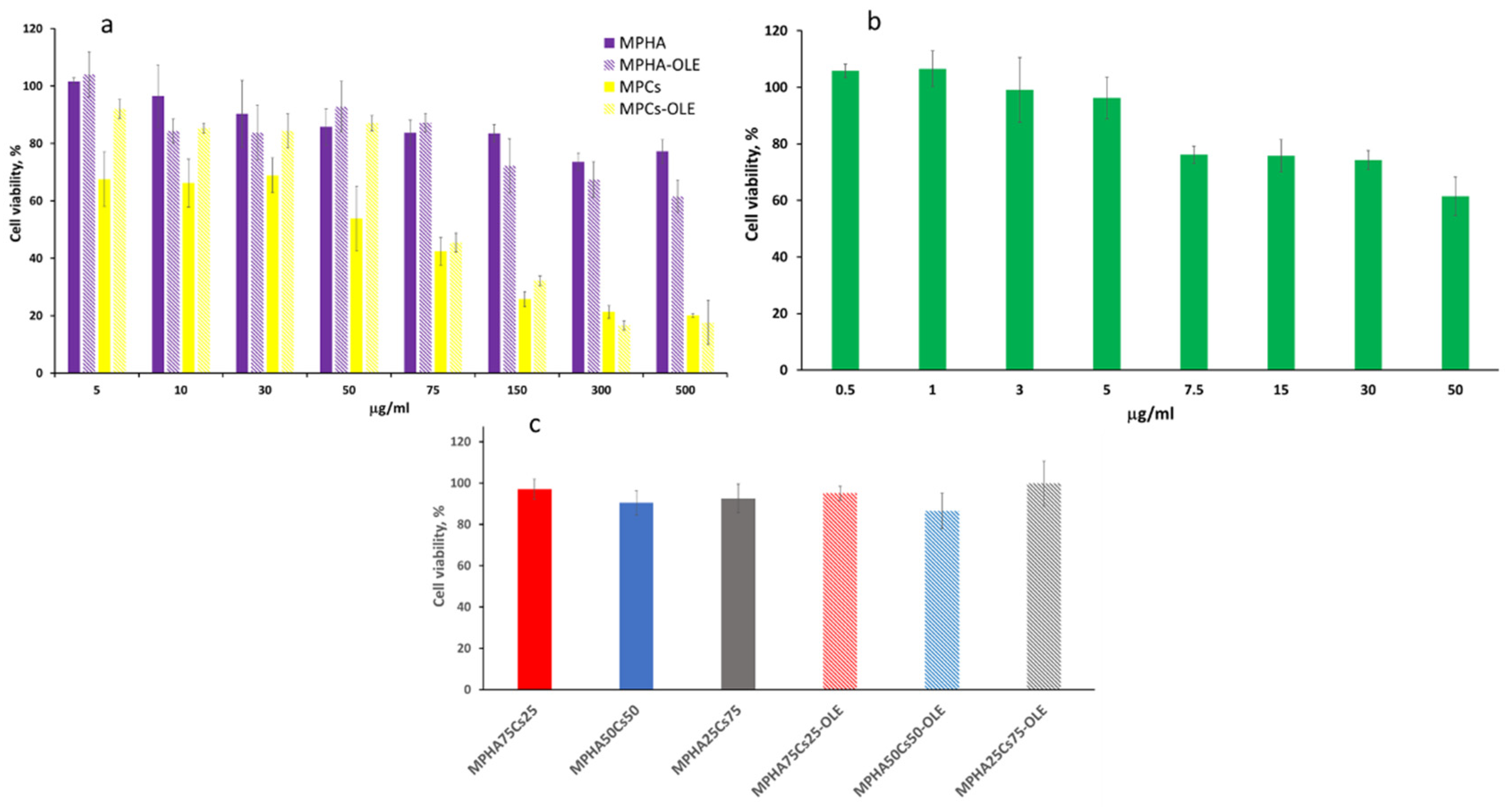
3.8. Cell Viability and Proliferation Test by WST-1 Essay
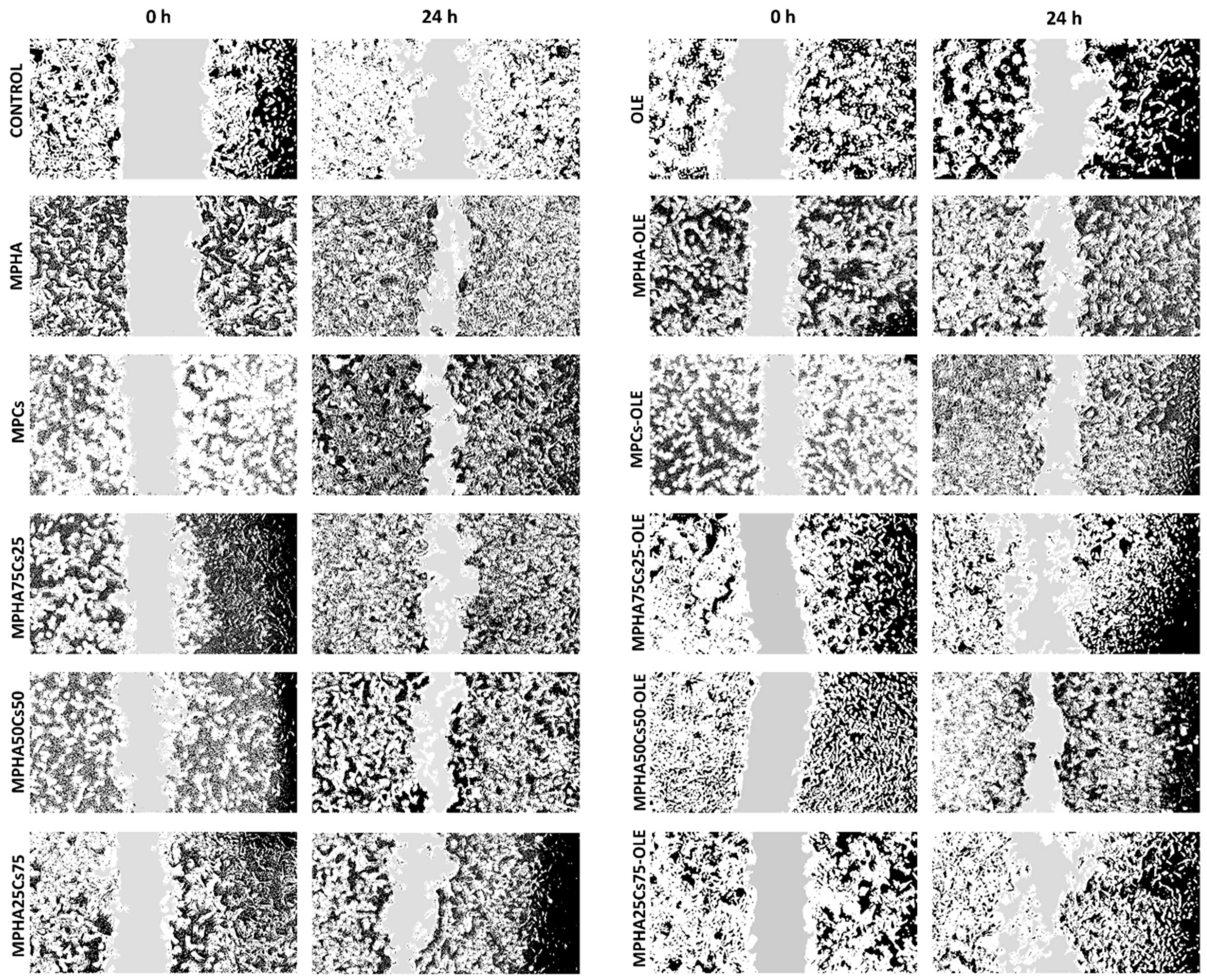
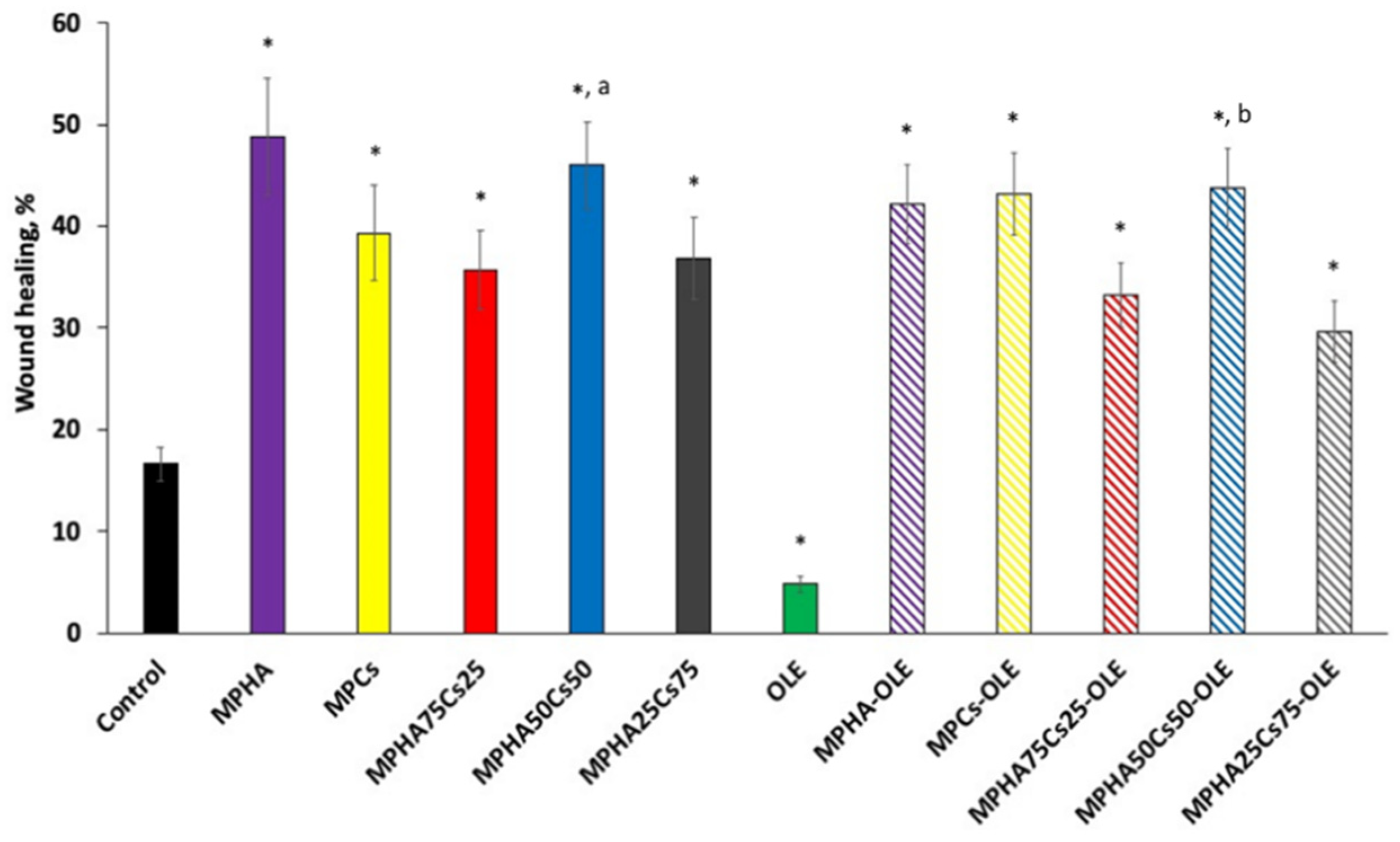
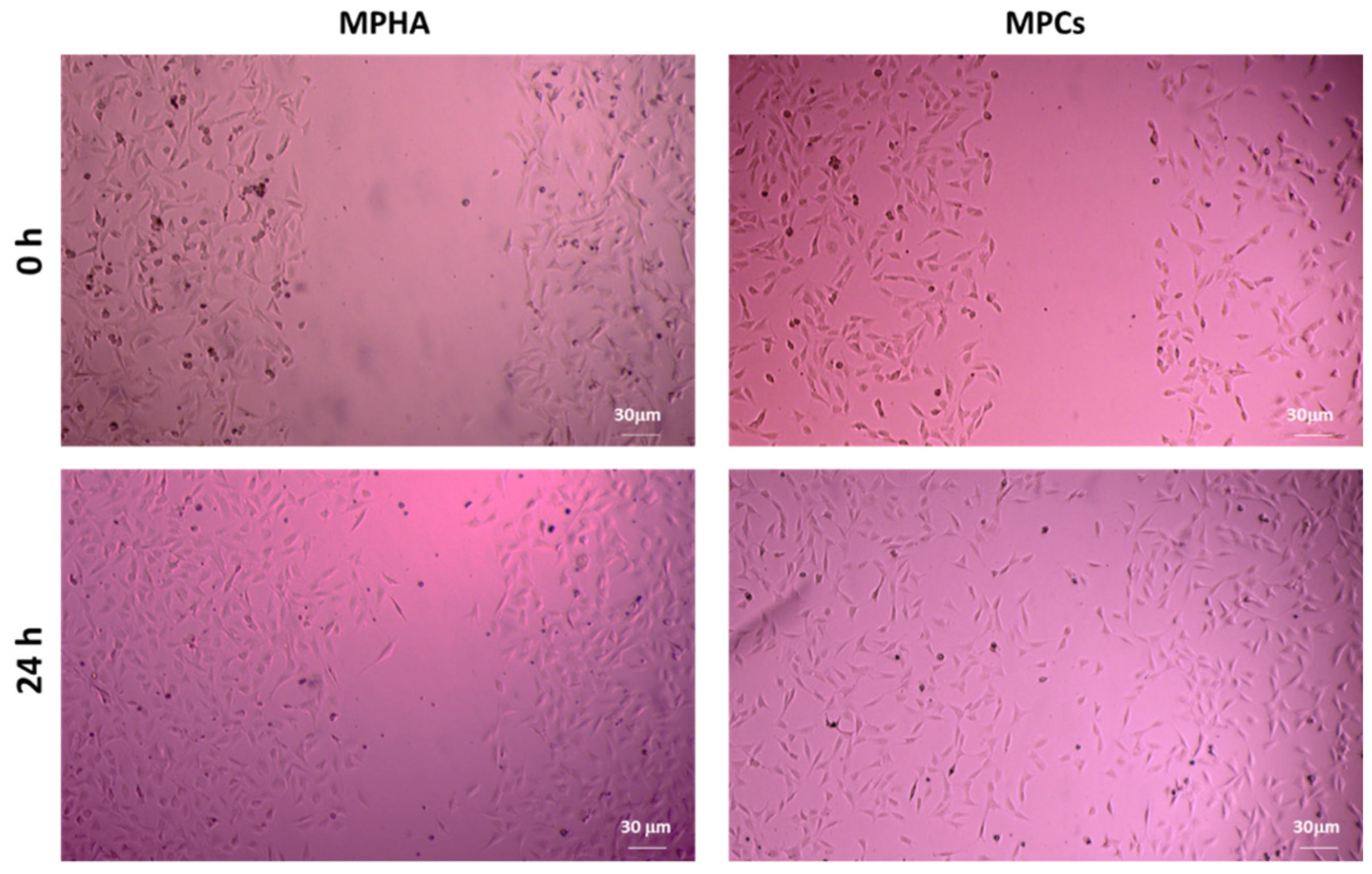
3.9. In Vitro Scratch Wound Healing Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salomone, R.; Giuseppe, I. Environmental impacts of olive oil production: A Life Cycle Assessment case study in the province of Messina (Sicily). J. Clean. Prod. 2012, 28, 88–100. [Google Scholar] [CrossRef]
- Flamini, G.; Cioni, P.L.; Morelli, I. Volatiles from leaves, fruits, and virgin oil from Olea europaea Cv. Olivastra Seggianese from Italy. J. Agric. Food Chem. 2003, 51, 1382–1386. [Google Scholar] [CrossRef] [PubMed]
- Milanesi, C.; Sorbi, A.; Paolucci, E.; Antonucci, F.; Menesatti, P.; Costa, C.; Pallottino, F.; Vignani, R.; Cimato, A.; Ciacci Mauro, A.; et al. Pomology observations, morphometric analysis, ultrastructural study and allelic profiles of “olivastra Seggianese” endocarps from ancient olive trees (Olea europaea L.). C. R. Biol. 2011, 334, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Şahin, S.; Bilgin, M. Olive tree (Olea europaea L.) leaf as a waste by-product of table olive and olive oil industry: A review. J. Sci. Food and Agric. 2018, 98, 1271–1279. [Google Scholar] [CrossRef]
- Lee, O.H.; Lee, B.Y. Antioxidant and antimicrobial activities of individual and combined phenolics in Olea europaea leaf extract. Bioresour. Technol. 2010, 101, 3751–3754. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.; Toral, M.; Gómez-Guzmán, M.; Jiménez, R.; Galindo, P.; Sánchez, M.; Olivares, M.; Gálvez, J.; Duarte, J. Antihypertensive effects of oleuropein-enriched olive leaf extract in spontaneously hypertensive rats. Food Funct. 2016, 7, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Susalit, E.; Agus, N.; Effendi, I.; Tjandrawinata, R.R.; Nofiarny, D.; Perrinjaquet-Moccetti, T.; Verbruggen, M. Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: Comparison with Captopril. Phytomedicine 2011, 18, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Lockyer, S.; Rowland, I.; Spencer, J.P.E.; Yaqoob, P.; Stonehouse, W. Impact of phenolic-rich olive leaf extract on blood pressure, plasma lipids and inflammatory markers: A randomised controlled trial. Eur. J. Nutr. 2017, 56, 1421–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crespo, M.C.; Tome-Carneiro, J.; Davalos, A.; Visioli, F. Pharma-nutritional properties of olive oil phenols. Transfer of new findings to human nutrition. Foods 2018, 7, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.J. Functions of hyaluronan in wound repair. Hyaluronan 2002, 2, 147–156. [Google Scholar]
- Nebbioso, G.; Petrella, F.; Caprarella, E. Role of hyaluronic acid in chronic non-responder skin lesions. Acta Vulnologica 2010, 8, 15–19. [Google Scholar]
- Singla, A.K.; Chawla, M. Chitosan: Some pharmaceutical and biological aspects-an update. J. Pharm. Pharmac. 2001, 53, 1047–1067. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Takashi, M.; Toru, F. Topical formulations and wound healing applications of chitosan. Adv. Drug Deliv. Rev. 2001, 52, 105–115. [Google Scholar] [CrossRef]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a wound dressing starting material: Antimicrobial properties and mode of action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayakumar, R.; Prabaharan, M.; Kumar, P.S.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Pagano, C.; Perioli, L.; Baiocchi, C.; Bartoccini, A.; Beccari, T.; Blasi, F.; Calarco, P.; Ceccarini, M.R.; Cossignani, L.; di Michele, A.; et al. Preparation and characterization of polymeric microparticles loaded with Moringa oleifera leaf extract for exuding wound treatment. Int. J. Pharm. 2020, 587, 119700. [Google Scholar] [CrossRef] [PubMed]
- Valachová, K.; Šoltés, L. Self-Associating Polymers Chitosan and Hyaluronan for Constructing Composite Membranes as Skin-Wound Dressings Carrying Therapeutics. Molecules 2021, 26, 2535. [Google Scholar] [CrossRef] [PubMed]
- Fabiano, A.; Beconcini, D.; Migone, C.; Piras, A.M.; Zambito, Y. Quaternary ammonium chitosans: The importance of the positive fixed charge of the drug delivery systems. Int. J. Mol. Sci. 2020, 21, 6617. [Google Scholar] [CrossRef] [PubMed]
- De la Ossa, J.G.; Fusco, A.; Azimi, B.; Esposito Salsano, J.; Digiacomo, M.; Coltelli, M.B.; De Clerck, K.; Roy, I.; Macchia, M.; Lazzeri, A.; et al. Immunomodulatory Activity of Electrospun Polyhydroxyalkanoate Fiber Scaffolds Incorporating Olive Leaf Extract. Appl. Sci. 2021, 11, 40. [Google Scholar] [CrossRef]
- Zambito, Y.; Zaino, C.; Uccello-Barretta, G.; Balzano, F.; Di Colo, G. Improved synthesis of quaternary ammonium-chitosan conjugates (N+-Ch) for enhanced intestinal drug permeation. Eur. J. Pharm. Sci. 2008, 33, 343–350. [Google Scholar] [CrossRef]
- Beconcini, D.; Fabiano, A.; Zambito, Y.; Berni, R.; Santoni, T.; Piras, A.M.; Di Stefano, R. Chitosan-based nanoparticles containing cherry extract from Prunus avium L. to improve the resistance of endothelial cells to oxidative stress. Nutrients 2018, 10, 1598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, B.; Pal, L. Development of sterculia gum based wound dressings for use in drug delivery. Eur. Polym. J. 2008, 44, 3222–3230. [Google Scholar] [CrossRef]
- Sadeghi, D.; Solouk, A.; Samadikuchaksaraei, A.; Seifalian, A.M. Preparation of internally-crosslinked alginate microspheres: Optimization of process parameters and study of pH-responsive behaviors. Carbohydr. Polym. 2021, 255, 117336. [Google Scholar] [CrossRef] [PubMed]
- Felice, F.; Zambito, Y.; Belardinelli, E.; Fabiano, A.; Santoni, T.; Di Stefano, R. Effect of different chitosan derivatives on in vitro scratch wound assay: A comparative study. Int. J. Biol. Macromol. 2015, 76, 236–241. [Google Scholar] [CrossRef] [PubMed]
- De la Ossa, J.G.; Felice, F.; Azimi, B.; Esposito Salsano, J.; Digiacomo, M.; Macchia, M.; Danti, S.; Di Stefano, R. Waste autochthonous tuscan olive leaves (Olea europaea var. Olivastra seggianese) as antioxidant source for biomedicine. Int. J. Mol. Sci. 2019, 20, 5918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De la Ossa, J.G.; El Kadri, H.; Gutierrez-Merino, J.; Wantock, T.; Harle, T.; Seggiani, M.; Danti, S.; Di Stefano, R.; Velliou, E. Combined antimicrobial effect of bio-waste olive leaf extract and remote cold atmospheric plasma effluent. Molecules 2021, 26, 1890. [Google Scholar] [CrossRef]
- Hashmi, M.A.; Khan, A.; Hanif, M.; Farooq, U.; Perveen, S. Traditional uses, phyto-chemistry, and pharmacology of Olea europaea (Olive). Evid.-Based Complement. Altern. Med. 2015, 2015, 541591. [Google Scholar] [CrossRef] [Green Version]
- Cesare, M.M.; Felice, F.; Conti, V.; Cerri, L.; Zambito, Y.; Romi, M.; Cai, G.; Cantini, C.; Di Stefano, R. Impact of Peels Extracts from an Italian Ancient Tomato Variety Grown under Drought Stress Conditions on Vascular Related Dysfunction. Molecules 2021, 26, 4289. [Google Scholar] [CrossRef] [PubMed]
- Bessa, L.J.; Fazii, P.; Di Giulio, M.; Cellini, L. Bacterial isolates from infected wounds and their antibiotic susceptibility pattern: Some remarks about wound infection. Int. Wound J. 2015, 12, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Warnke, P.H.; Lott, A.J.; Sherry, E.; Wiltfang, J.; Podschun, R. The ongoing battle against multi-resistant strains: In-vitro inhibition of hospital-acquired MRSA, VRE, Pseudomonas, ESBL E. coli and Klebsiella species in the presence of plant-derived antiseptic oils. J. Cranio-Maxillofac. Surg. 2013, 41, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Garcia, L.G.S.; da Rocha, M.G.; Lima, L.R.; Cunha, A.P.; de Oliveira, J.S.; de Andrade, A.R.C.; Ricardo, N.M.P.; Pereira-Neto, W.A.; Sidrim, J.J.C.; Rocha, M.F.G.; et al. Essential oils encapsulated in chitosan microparticles against Candida albicans biofilms. Int. J. Biol. Macromol. 2021, 166, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Sahiner, N.; Suner, S.S.; Ayyala, R.S. Mesoporous, degradable hyaluronic acid microparticles for sustainable drug delivery application. Colloids Surf. B. 2019, 177, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Mezzetta, A.; Guazzelli, L.; Chiappe, C. Access to cross-linked chitosans by exploiting CO2 and the double solvent-catalytic effect of ionic liquids. Green Chem. 2017, 19, 1235–1239. [Google Scholar] [CrossRef]
- Talón, E.; Trifkovic, K.T.; Vargas, M.; Chiralt, A.; González-Martínez, C. Release of polyphenols from starch-chitosan based films containing thyme extract. Carbohydr. Polym. 2017, 175, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grada, A.; Otero-Vinas, M.; Prieto-Castrillo, F.; Obagi, Z.; Falanga, V. Research techniques made simple: Analysis of collective cell migration using the wound healing assay. J. Investig. Dermatol. 2017, 137, e11–e16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elnahas, R.A.; Elwakil, B.H.; Elshewemi, S.S.; Olama, Z.A. Egyptian Olea europaea leaves bioactive extract: Antibacterial and wound healing activity in normal and diabetic rats. J. Trad. Complement. Med. 2021, 11, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Koca, U.; Suntar, I.; Akkol, E.K.; Yilmazer, D.; Alper, M. Wound repair potential of Olea europaea L. leaf extracts revealed by in vivo experimental models and comparative evaluation of the extracts’ antioxidant activity. J. Med. Food 2011, 14, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Patrulea, V.; Sublet, E.; Borchard, G.; Iyoda, T.; Kageyama, R.; Morita, A.; Seino, S.; Yoshida, H.; Jordan, O.; et al. Wound Healing Promotion by Hyaluronic Acid: Effect of Molecular Weight on Gene Expression and In Vivo Wound Closure. Pharmaceuticals 2021, 14, 301. [Google Scholar] [CrossRef] [PubMed]
- Patrulea, V.; Laurent-Applegate, L.A.; Ostafe, V.; Borchard, G.; Jordan, O. Polyelectrolyte nanocomplexes based on chitosan derivatives for wound healing application. Eur. J. Pharm. Biopharm. 2019, 140, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Sudjana, A.N.; D’Orazio, C.; Ryan, V.; Rasool, N.; Ng, J.; Islam, N.; Riley, T.V.; Hammer, K.A. Antimicrobial activity of commercial Olea europaea (olive) leaf extract. Int. J. Antimicrob. Agents 2009, 33, 461–463. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabiano, A.; Migone, C.; Cerri, L.; Piras, A.M.; Mezzetta, A.; Maisetta, G.; Esin, S.; Batoni, G.; Di Stefano, R.; Zambito, Y. Combination of Two Kinds of Medicated Microparticles Based on Hyaluronic Acid or Chitosan for a Wound Healing Spray Patch. Pharmaceutics 2021, 13, 2195. https://doi.org/10.3390/pharmaceutics13122195
Fabiano A, Migone C, Cerri L, Piras AM, Mezzetta A, Maisetta G, Esin S, Batoni G, Di Stefano R, Zambito Y. Combination of Two Kinds of Medicated Microparticles Based on Hyaluronic Acid or Chitosan for a Wound Healing Spray Patch. Pharmaceutics. 2021; 13(12):2195. https://doi.org/10.3390/pharmaceutics13122195
Chicago/Turabian StyleFabiano, Angela, Chiara Migone, Luca Cerri, Anna Maria Piras, Andrea Mezzetta, Giuseppantonio Maisetta, Semih Esin, Giovanna Batoni, Rossella Di Stefano, and Ylenia Zambito. 2021. "Combination of Two Kinds of Medicated Microparticles Based on Hyaluronic Acid or Chitosan for a Wound Healing Spray Patch" Pharmaceutics 13, no. 12: 2195. https://doi.org/10.3390/pharmaceutics13122195
APA StyleFabiano, A., Migone, C., Cerri, L., Piras, A. M., Mezzetta, A., Maisetta, G., Esin, S., Batoni, G., Di Stefano, R., & Zambito, Y. (2021). Combination of Two Kinds of Medicated Microparticles Based on Hyaluronic Acid or Chitosan for a Wound Healing Spray Patch. Pharmaceutics, 13(12), 2195. https://doi.org/10.3390/pharmaceutics13122195











