Parametric and Nonparametric Population Pharmacokinetic Models to Assess Probability of Target Attainment of Imipenem Concentrations in Critically Ill Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population PK Models
2.2. Population Used for Modeling
2.3. Population Used for Validation
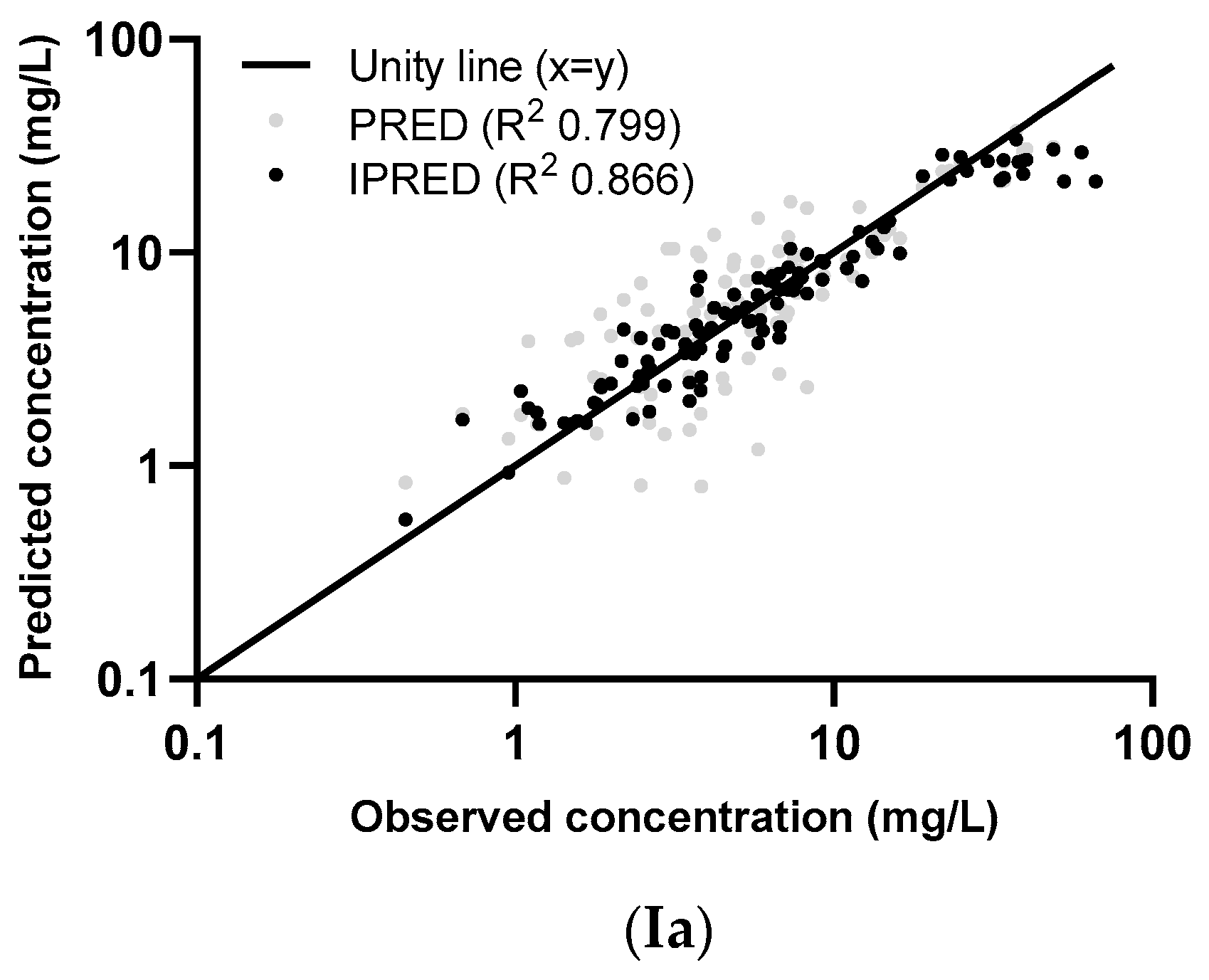
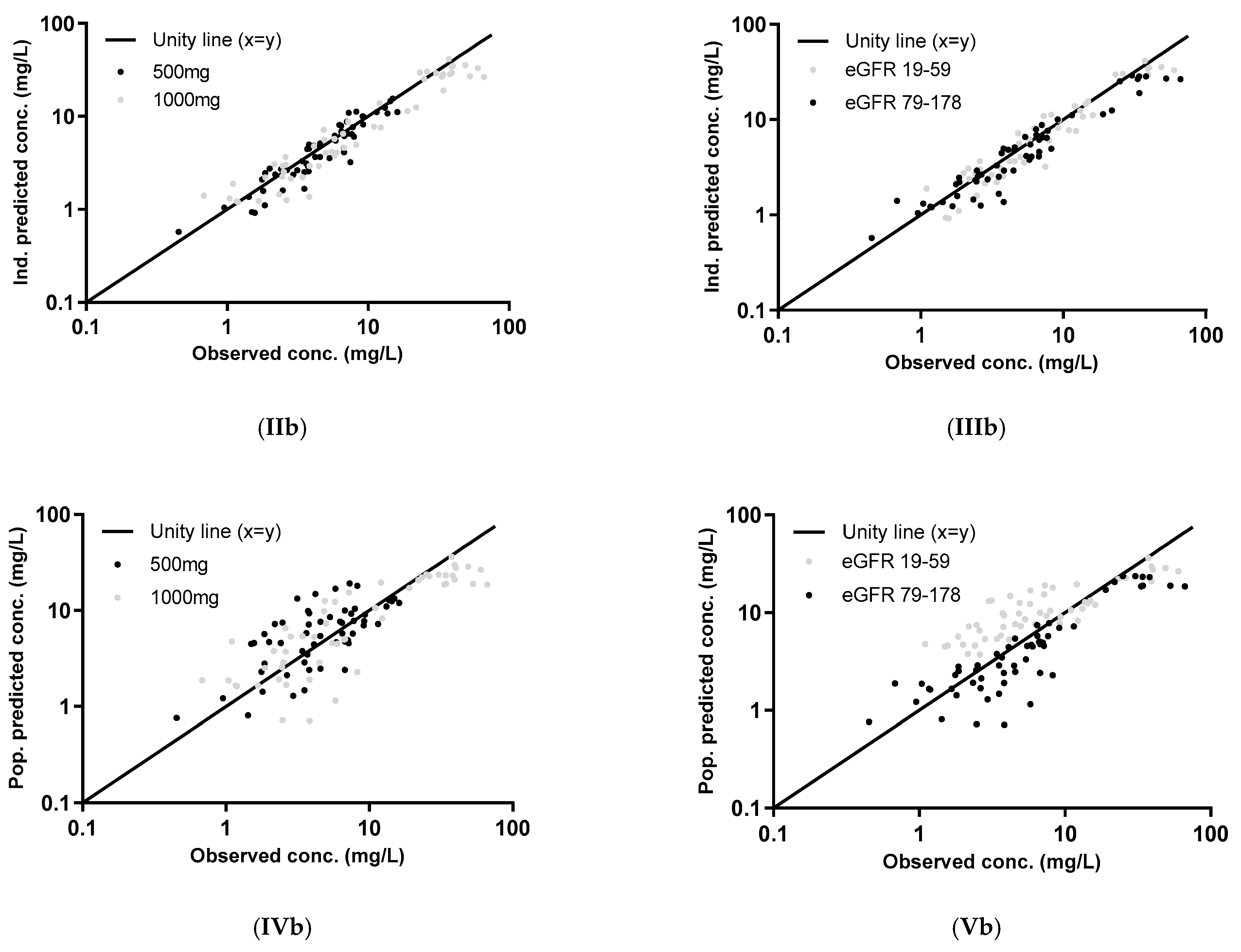
2.4. External Validation
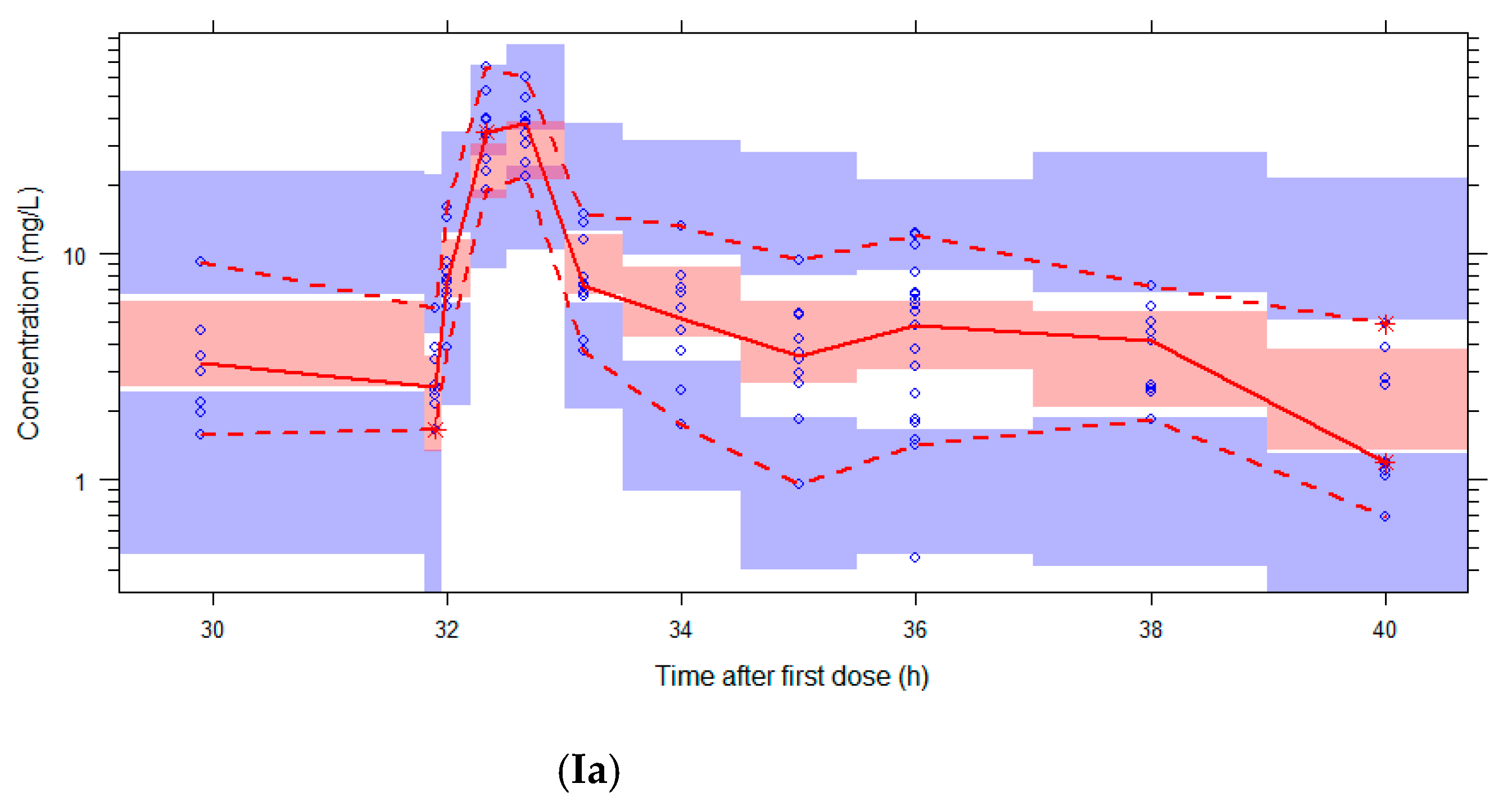
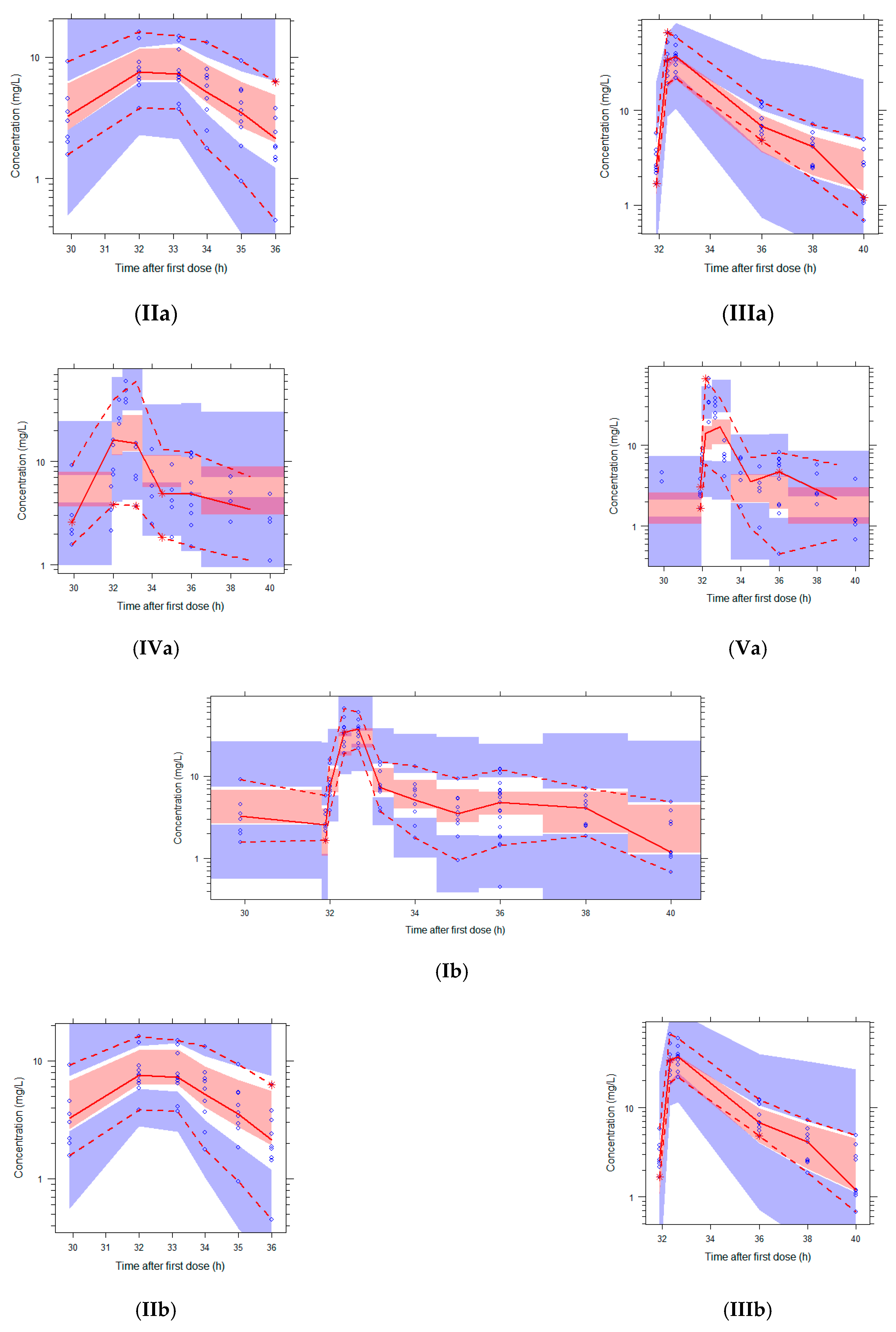
2.5. Simulations
2.6. Software
3. Results
3.1. Population
3.2. External Validation
3.3. Simulations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mouton, J.W.; Ambrose, P.G.; Canton, R.; Drusano, G.L.; Harbarth, S.; MacGowan, A.; Theuretzbacher, U.; Turnidge, J. Conserving antibiotics for the future: New ways to use old and new drugs from a pharmacokinetic and pharmacodynamic perspective. Drug Resist. Updates 2011, 14, 107–117. [Google Scholar] [CrossRef]
- De Velde, F.; Mouton, J.W.; de Winter, B.C.M.; van Gelder, T.; Koch, B.C.P. Clinical applications of population pharmacokinetic models of antibiotics: Challenges and perspectives. Pharmacol. Res. 2018, 134, 280–288. [Google Scholar] [CrossRef]
- Mouton, J.W.; Brown, D.F.; Apfalter, P.; Canton, R.; Giske, C.G.; Ivanova, M.; MacGowan, A.P.; Rodloff, A.; Soussy, C.J.; Steinbakk, M.; et al. The role of pharmacokinetics/pharmacodynamics in setting clinical MIC breakpoints: The EUCAST approach. Clin. Microbiol. Infect. 2012, 18, E37–E45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Racine-Poon, A.; Wakefield, J. Statistical methods for population pharmacokinetic modelling. Stat. Methods Med. Res. 1998, 7, 63–84. [Google Scholar] [CrossRef] [Green Version]
- Tatarinova, T.; Neely, M.; Bartroff, J.; van Guilder, M.; Yamada, W.; Bayard, D.; Jelliffe, R.; Leary, R.; Chubatiuk, A.; Schumitzky, A. Two general methods for population pharmacokinetic modeling: Non-parametric adaptive grid and non-parametric Bayesian. J. Pharmacokinet. Pharmacodyn. 2013, 40, 189–199. [Google Scholar] [CrossRef] [Green Version]
- De Velde, F.; de Winter, B.C.M.; Neely, M.N.; Yamada, W.M.; Koch, B.C.P.; Harbarth, S.; von Dach, E.; van Gelder, T.; Huttner, A.; Mouton, J.W.; et al. Population Pharmacokinetics of Imipenem in Critically Ill Patients: A Parametric and Nonparametric Model Converge on CKD-EPI Estimated Glomerular Filtration Rate as an Impactful Covariate. Clin. Pharmacokinet. 2020, 59, 885–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Ambrose, P.G.; Bhavnani, S.M.; Rubino, C.M.; Louie, A.; Gumbo, T.; Forrest, A.; Drusano, G.L. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: It’s not just for mice anymore. Clin. Infect. Dis. 2007, 44, 79–86. [Google Scholar] [CrossRef]
- Crandon, J.L.; Luyt, C.E.; Aubry, A.; Chastre, J.; Nicolau, D.P. Pharmacodynamics of carbapenems for the treatment of Pseudomonas aeruginosa ventilator-associated pneumonia: Associations with clinical outcome and recurrence. J. Antimicrob. Chemother. 2016, 71, 2534–2537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ariano, R.E.; Nyhlen, A.; Donnelly, J.P.; Sitar, D.S.; Harding, G.K.; Zelenitsky, S.A. Pharmacokinetics and pharmacodynamics of meropenem in febrile neutropenic patients with bacteremia. Ann. Pharmacother. 2005, 39, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Abdul-Aziz, M.H.; Lipman, J.; Mouton, J.W.; Vinks, A.A.; Felton, T.W.; Hope, W.W.; Farkas, A.; Neely, M.N.; Schentag, J.J.; et al. Individualised antibiotic dosing for patients who are critically ill: Challenges and potential solutions. Lancet Infect. Dis. 2014, 14, 498–509. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Du, X.; Kuti, J.L.; Nicolau, D.P. Clinical pharmacodynamics of meropenem in patients with lower respiratory tract infections. Antimicrob. Agents Chemother. 2007, 51, 1725–1730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller, A.E.; Punt, N.; Mouton, J.W. Optimal exposures of ceftazidime predict the probability of microbiological and clinical outcome in the treatment of nosocomial pneumonia. J. Antimicrob. Chemother. 2013, 68, 900–906. [Google Scholar] [CrossRef] [Green Version]
- Craig, W.A. Pharmacokinetic/pharmacodynamic parameters: Rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 1998, 26, 1–10. [Google Scholar] [CrossRef]
- Couffignal, C.; Pajot, O.; Laouenan, C.; Burdet, C.; Foucrier, A.; Wolff, M.; Armand-Lefevre, L.; Mentre, F.; Massias, L. Population pharmacokinetics of imipenem in critically ill patients with suspected ventilator-associated pneumonia and evaluation of dosage regimens. Br. J. Clin. Pharmacol. 2014, 78, 1022–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakka, S.G.; Glauner, A.K.; Bulitta, J.B.; Kinzig-Schippers, M.; Pfister, W.; Drusano, G.L.; Sorgel, F. Population pharmacokinetics and pharmacodynamics of continuous versus short-term infusion of imipenem-cilastatin in critically ill patients in a randomized, controlled trial. Antimicrob. Agents Chemother. 2007, 51, 3304–3310. [Google Scholar] [CrossRef] [Green Version]
- Suchankova, H.; Lips, M.; Urbanek, K.; Neely, M.N.; Strojil, J. Is continuous infusion of imipenem always the best choice? Int. J. Antimicrob. Agents 2017, 49, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Paul, S.K.; Akova, M.; Bassetti, M.; De Waele, J.J.; Dimopoulos, G.; Kaukonen, K.M.; Koulenti, D.; Martin, C.; Montravers, P.; et al. DALI: Defining antibiotic levels in intensive care unit patients: Are current beta-lactam antibiotic doses sufficient for critically ill patients? Clin. Infect. Dis. 2014, 58, 1072–1083. [Google Scholar] [CrossRef]
- Bricheux, A.; Lenggenhager, L.; Hughes, S.; Karmime, A.; Lescuyer, P.; Huttner, A. Therapeutic drug monitoring of imipenem and the incidence of toxicity and failure in hospitalized patients: A retrospective cohort study. Clin. Microbiol. Infect. 2019, 25, 383.e1–383.e4. [Google Scholar] [CrossRef] [Green Version]
- Huttner, A.; Von Dach, E.; Renzoni, A.; Huttner, B.D.; Affaticati, M.; Pagani, L.; Daali, Y.; Pugin, J.; Karmime, A.; Fathi, M.; et al. Augmented renal clearance, low beta-lactam concentrations and clinical outcomes in the critically ill: An observational prospective cohort study. Int. J. Antimicrob. Agents 2015, 45, 385–392. [Google Scholar] [CrossRef]
- Cockcroft, D.W.; Gault, M.H. Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef]
- Merck Sharp & Dohme BV. Summary of Product Characteristics Tienam 500/500mg Powder for Solution for Infusion. Haarlem, The Netherlands, 2020. Available online: https://www.geneesmiddeleninformatiebank.nl/smpc/h11089_smpc.pdf (accessed on 8 November 2021).
- Legrand, T.; Chhun, S.; Rey, E.; Blanchet, B.; Zahar, J.R.; Lanternier, F.; Pons, G.; Jullien, V. Simultaneous determination of three carbapenem antibiotics in plasma by HPLC with ultraviolet detection. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2008, 875, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Byon, W.; Smith, M.K.; Chan, P.; Tortorici, M.A.; Riley, S.; Dai, H.; Dong, J.; Ruiz-Garcia, A.; Sweeney, K.; Cronenberger, C. Establishing best practices and guidance in population modeling: An experience with an internal population pharmacokinetic analysis guidance. CPT Pharmacomet. Syst. Pharmacol. 2013, 2, e51. [Google Scholar] [CrossRef]
- Lips, M.; Siller, M.; Strojil, J.; Urbanek, K.; Balik, M.; Suchankova, H. Pharmacokinetics of imipenem in critically ill patients during empirical treatment of nosocomial pneumonia: A comparison of 0.5-h and 3-h infusions. Int. J. Antimicrob. Agents 2014, 44, 358–362. [Google Scholar] [CrossRef]
- Merck Sharp & Dohme Corp. Prescribing Information Primaxin (Imipenem and Cilastatin) for Injection, for Intravenous Use. USA, NJ, Whitehouse Station. 2018. Available online: https://www.merck.com/product/usa/pi_circulars/p/primaxin/primaxin_iv_pi.pdf (accessed on 8 November 2021).
- Keizer, R.J.; Karlsson, M.O.; Hooker, A. Modeling and Simulation Workbench for NONMEM: Tutorial on Pirana, PsN, and Xpose. CPT Pharmacomet. Syst. Pharmacol. 2013, 2, e50. [Google Scholar] [CrossRef] [PubMed]
- Neely, M.N.; van Guilder, M.G.; Yamada, W.M.; Schumitzky, A.; Jelliffe, R.W. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther. Drug Monit. 2012, 34, 467–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baptista, J.P.; Neves, M.; Rodrigues, L.; Teixeira, L.; Pinho, J.; Pimentel, J. Accuracy of the estimation of glomerular filtration rate within a population of critically ill patients. J. Nephrol. 2014, 27, 403–410. [Google Scholar] [CrossRef]
- Hobbs, A.L.; Shea, K.M.; Roberts, K.M.; Daley, M.J. Implications of Augmented Renal Clearance on Drug Dosing in Critically Ill Patients: A Focus on Antibiotics. Pharmacotherapy 2015, 35, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Belzberg, H.; Zhu, J.; Cornwell, E.E., 3rd; Murray, J.A.; Sava, J.; Salim, A.; Velmahos, G.C.; Gill, M.A. Imipenem levels are not predictable in the critically ill patient. J. Trauma 2004, 56, 111–117. [Google Scholar] [CrossRef]
- Roberts, J.A.; Lipman, J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit. Care Med. 2009, 37, 840–851, quiz 859. [Google Scholar] [CrossRef] [Green Version]
- Jaruratanasirikul, S.; Sudsai, T. Comparison of the pharmacodynamics of imipenem in patients with ventilator-associated pneumonia following administration by 2 or 0.5 h infusion. J. Antimicrob. Chemother. 2009, 63, 560–563. [Google Scholar] [CrossRef] [Green Version]
- Novelli, A.; Adembri, C.; Livi, P.; Fallani, S.; Mazzei, T.; De Gaudio, A.R. Pharmacokinetic evaluation of meropenem and imipenem in critically ill patients with sepsis. Clin. Pharmacokinet. 2005, 44, 539–549. [Google Scholar] [CrossRef]
- Abhilash, B.; Tripathi, C.D.; Gogia, A.R.; Meshram, G.G.; Kumar, M.; Suraj, B. Pharmacokinetic/pharmacodynamic profiling of imipenem in patients admitted to an intensive care unit in India: A nonrandomized, cross-sectional, analytical, open-labeled study. Indian J. Crit. Care Med. 2015, 19, 587–592. [Google Scholar] [CrossRef]
- Brendel, K.; Dartois, C.; Comets, E.; Lemenuel-Diot, A.; Laveille, C.; Tranchand, B.; Girard, P.; Laffont, C.M.; Mentre, F. Are population pharmacokinetic and/or pharmacodynamic models adequately evaluated? A survey of the literature from 2002 to 2004. Clin. Pharmacokinet. 2007, 46, 221–234. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 11.0. 2021. Available online: www.eucast.org (accessed on 8 November 2021).
- Launay-Iliadis, M.C.; Bruno, R.; Cosson, V.; Vergniol, J.C.; Oulid-Aissa, D.; Marty, M.; Clavel, M.; Aapro, M.; Le Bail, N.; Iliadis, A. Population pharmacokinetics of docetaxel during phase I studies using nonlinear mixed-effect modeling and nonparametric maximum-likelihood estimation. Cancer Chemother. Pharmacol. 1995, 37, 47–54. [Google Scholar] [CrossRef]
- Vermes, A.; Mathot, R.A.; van der Sijs, I.H.; Dankert, J.; Guchelaar, H.J. Population pharmacokinetics of flucytosine: Comparison and validation of three models using STS, NPEM, and NONMEM. Ther. Drug Monit. 2000, 22, 676–687. [Google Scholar] [CrossRef]
- Patoux, A.; Bleyzac, N.; Boddy, A.V.; Doz, F.; Rubie, H.; Bastian, G.; Maire, P.; Canal, P.; Chatelut, E. Comparison of nonlinear mixed-effect and non-parametric expectation maximisation modelling for Bayesian estimation of carboplatin clearance in children. Eur. J. Clin. Pharmacol. 2001, 57, 297–303. [Google Scholar] [CrossRef]
- de Hoog, M.; Schoemaker, R.C.; van den Anker, J.N.; Vinks, A.A. NONMEM and NPEM2 population modeling: A comparison using tobramycin data in neonates. Ther. Drug Monit. 2002, 24, 359–365. [Google Scholar] [CrossRef]
- Woillard, J.B.; Debord, J.; Benz-de-Bretagne, I.; Saint-Marcoux, F.; Turlure, P.; Girault, S.; Abraham, J.; Choquet, S.; Marquet, P.; Barin-Le Guellec, C. A Time-Dependent Model Describes Methotrexate Elimination and Supports Dynamic Modification of MRP2/ABCC2 Activity. Ther. Drug Monit. 2017, 39, 145–156. [Google Scholar] [CrossRef]
- Woillard, J.B.; Lebreton, V.; Neely, M.; Turlure, P.; Girault, S.; Debord, J.; Marquet, P.; Saint-Marcoux, F. Pharmacokinetic tools for the dose adjustment of ciclosporin in haematopoietic stem cell transplant patients. Br. J. Clin. Pharmacol. 2014, 78, 836–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Premaud, A.; Weber, L.T.; Tonshoff, B.; Armstrong, V.W.; Oellerich, M.; Urien, S.; Marquet, P.; Rousseau, A. Population pharmacokinetics of mycophenolic acid in pediatric renal transplant patients using parametric and nonparametric approaches. Pharmacol. Res. 2011, 63, 216–224. [Google Scholar] [CrossRef] [Green Version]
- Bustad, A.; Terziivanov, D.; Leary, R.; Port, R.; Schumitzky, A.; Jelliffe, R. Parametric and nonparametric population methods: Their comparative performance in analysing a clinical dataset and two Monte Carlo simulation studies. Clin. Pharmacokinet. 2006, 45, 365–383. [Google Scholar] [CrossRef]
- Baverel, P.G.; Savic, R.M.; Wilkins, J.J.; Karlsson, M.O. Evaluation of the nonparametric estimation method in NONMEM VI: Application to real data. J. Pharmacokinet. Pharmacodyn. 2009, 36, 297–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlsson, K.C.; van de Schootbrugge, M.; Eriksen, H.O.; Moberg, E.R.; Karlsson, M.O.; Hoem, N.O. A population pharmacokinetic model of gabapentin developed in nonparametric adaptive grid and nonlinear mixed effects modeling. Ther. Drug Monit. 2009, 31, 86–94. [Google Scholar] [CrossRef]
- Bulitta, J.B.; Landersdorfer, C.B.; Kinzig, M.; Holzgrabe, U.; Sorgel, F. New semiphysiological absorption model to assess the pharmacodynamic profile of cefuroxime axetil using nonparametric and parametric population pharmacokinetics. Antimicrob. Agents Chemother. 2009, 53, 3462–3471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulitta, J.B.; Landersdorfer, C.B.; Huttner, S.J.; Drusano, G.L.; Kinzig, M.; Holzgrabe, U.; Stephan, U.; Sorgel, F. Population pharmacokinetic comparison and pharmacodynamic breakpoints of ceftazidime in cystic fibrosis patients and healthy volunteers. Antimicrob. Agents Chemother. 2010, 54, 1275–1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Parameter | Modeling Population | Validation Population |
|---|---|---|
| Male, n (%) | 18 (69) | 14 (74) |
| APACHE II score, median (range) | 22 (7–35) | 26 (13–42) |
| Age (years), median (range) | 51 (25–59) | 64 (26–90) |
| Creatinine at inclusion (μmol/L), median (range) | 59 (28–108) | 98 (44–235) |
| eGFR CKD-EPI at inclusion (ml/min/1.73 m2), median (range) | 116 (50–143) | 73 (20–145) |
| eGFR absolute CKD-EPI at inclusion, unadjusted for BSA (ml/min), median (range) | 119 (51–172) | 79 (19–178) |
| Height (cm), median (range) | 175 (155–190) | 170 (150–190) |
| Total bodyweight (kg), median (range) | 75 (50–107) | 78 (45–110) |
| BMI (kg/m2), median (range) | 25 (18–35) | 28 (18–34) |
| BSA (m2), median (range) | 1.89 (1.51–2.23) | 1.92 (1.40–2.29) |
| Presumed infection, n (%) | ||
| Respiratory tract infection | 16 (62) | 19 (100) |
| Intra-abdominal infection | 4 (15) | - |
| Bloodstream infection | 3 (12) | - |
| Surgical site infection | 1 (4) | - |
| Meningitis | 1 (4) | - |
| Gynecological infection | 1 (4) | - |
| KERRYPNX | External Database | Simulations | Simulations (Selection) | Simulations (Selection) | ||||
|---|---|---|---|---|---|---|---|---|
| 111 Concentrations | 1000 × 111 Concentrations | 1000 × 17 trough eGFR19-59 | 1000 × 18 trough eGFR79-178 | |||||
| Parametric | PE (mg/L) | RPE (%) | PE (mg/L) | RPE (%) | PE (mg/L) | RPE (%) | PE (mg/L) | RPE (%) |
| 97.5% | 3.83 | 105 | 8.97 | 252 | 9.74 | 360 | 2.03 | 225 |
| 75% | 0.61 | 19 | 1.97 | 56 | 3.92 | 167 | 0.38 | 31 |
| 50% | −0.02 | −1 | −0.04 | −1 | 2.13 | 83 | −0.50 | −24 |
| 25% | −1.52 | −20 | −2.20 | −31 | 0.72 | 23 | −1.64 | −53 |
| 2.5% | −30.55 | −52 | −28.63 | −74 | −3.16 | −41 | −3.15 | −82 |
| Nonparametric | PE (mg/L) | RPE (%) | PE (mg/L) | RPE (%) | PE (mg/L) | RPE (%) | PE (mg/L) | RPE (%) |
| 97.5% | 3.89 | 54 | 30.68 | 594 | 28.96 | 996 | 7.08 | 564 |
| 75% | 0.51 | 15 | 3.22 | 83 | 5.66 | 221 | 0.80 | 58 |
| 50% | −0.43 | −9 | 0.02 | 0.5 | 2.24 | 88 | −0.33 | −19 |
| 25% | −1.74 | −29 | −2.47 | −39 | 0.32 | 11 | −1.56 | −56 |
| 2.5% | −25.99 | −58 | −24.77 | −79 | −4.15 | −63 | −3.36 | −91 |
| eGFR (ml/min) | Dose Regimen | Target fT > MIC | Highest MIC (mg/L) with PTA > 97.5% | |
|---|---|---|---|---|
| Parametric | Nonparametric | |||
| 150 | 500 mg q6h | 100% | 0.125 | 0.06 |
| 1000 mg q8h | 100% | 0.125 | 0.03 | |
| 1000 mg q6h | 100% | 0.25 | 0.125 | |
| 500 mg q6h | 50% | 0.5 | 0.25 | |
| 1000 mg q8h | 50% | 0.5 | 0.5 | |
| 1000 mg q6h | 50% | 1 | 1 | |
| 120 | 500 mg q6h | 100% | 0.125 | 0.125 |
| 1000 mg q8h | 100% | 0.25 | 0.06 | |
| 1000 mg q6h | 100% | 0.25 | 0.25 | |
| 500 mg q6h | 50% | 0.5 | 0.5 | |
| 1000 mg q8h | 50% | 1 | 0.5 | |
| 1000 mg q6h | 50% | 2 | 1 | |
| 90 | 500 mg q6h | 100% | 0.25 | 0.25 |
| 1000 mg q8h | 100% | 0.25 | 0.25 | |
| 1000 mg q6h | 100% | 0.5 | 0.5 | |
| 500 mg q6h | 50% | 1 | 0.5 | |
| 1000 mg q8h | 50% | 1 | 1 | |
| 1000 mg q6h | 50% | 2 | 1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Velde, F.; de Winter, B.C.M.; Neely, M.N.; Strojil, J.; Yamada, W.M.; Harbarth, S.; Huttner, A.; van Gelder, T.; Koch, B.C.P.; Muller, A.E.; et al. Parametric and Nonparametric Population Pharmacokinetic Models to Assess Probability of Target Attainment of Imipenem Concentrations in Critically Ill Patients. Pharmaceutics 2021, 13, 2170. https://doi.org/10.3390/pharmaceutics13122170
de Velde F, de Winter BCM, Neely MN, Strojil J, Yamada WM, Harbarth S, Huttner A, van Gelder T, Koch BCP, Muller AE, et al. Parametric and Nonparametric Population Pharmacokinetic Models to Assess Probability of Target Attainment of Imipenem Concentrations in Critically Ill Patients. Pharmaceutics. 2021; 13(12):2170. https://doi.org/10.3390/pharmaceutics13122170
Chicago/Turabian Stylede Velde, Femke, Brenda C. M. de Winter, Michael N. Neely, Jan Strojil, Walter M. Yamada, Stephan Harbarth, Angela Huttner, Teun van Gelder, Birgit C. P. Koch, Anouk E. Muller, and et al. 2021. "Parametric and Nonparametric Population Pharmacokinetic Models to Assess Probability of Target Attainment of Imipenem Concentrations in Critically Ill Patients" Pharmaceutics 13, no. 12: 2170. https://doi.org/10.3390/pharmaceutics13122170
APA Stylede Velde, F., de Winter, B. C. M., Neely, M. N., Strojil, J., Yamada, W. M., Harbarth, S., Huttner, A., van Gelder, T., Koch, B. C. P., Muller, A. E., & on behalf of the COMBACTE-NET Consortium. (2021). Parametric and Nonparametric Population Pharmacokinetic Models to Assess Probability of Target Attainment of Imipenem Concentrations in Critically Ill Patients. Pharmaceutics, 13(12), 2170. https://doi.org/10.3390/pharmaceutics13122170







