Polymeric Materials for Hemostatic Wound Healing
Abstract
1. Introduction
2. Polymer-Based Hemostatic Materials
2.1. Natural Hemostatic Polymers
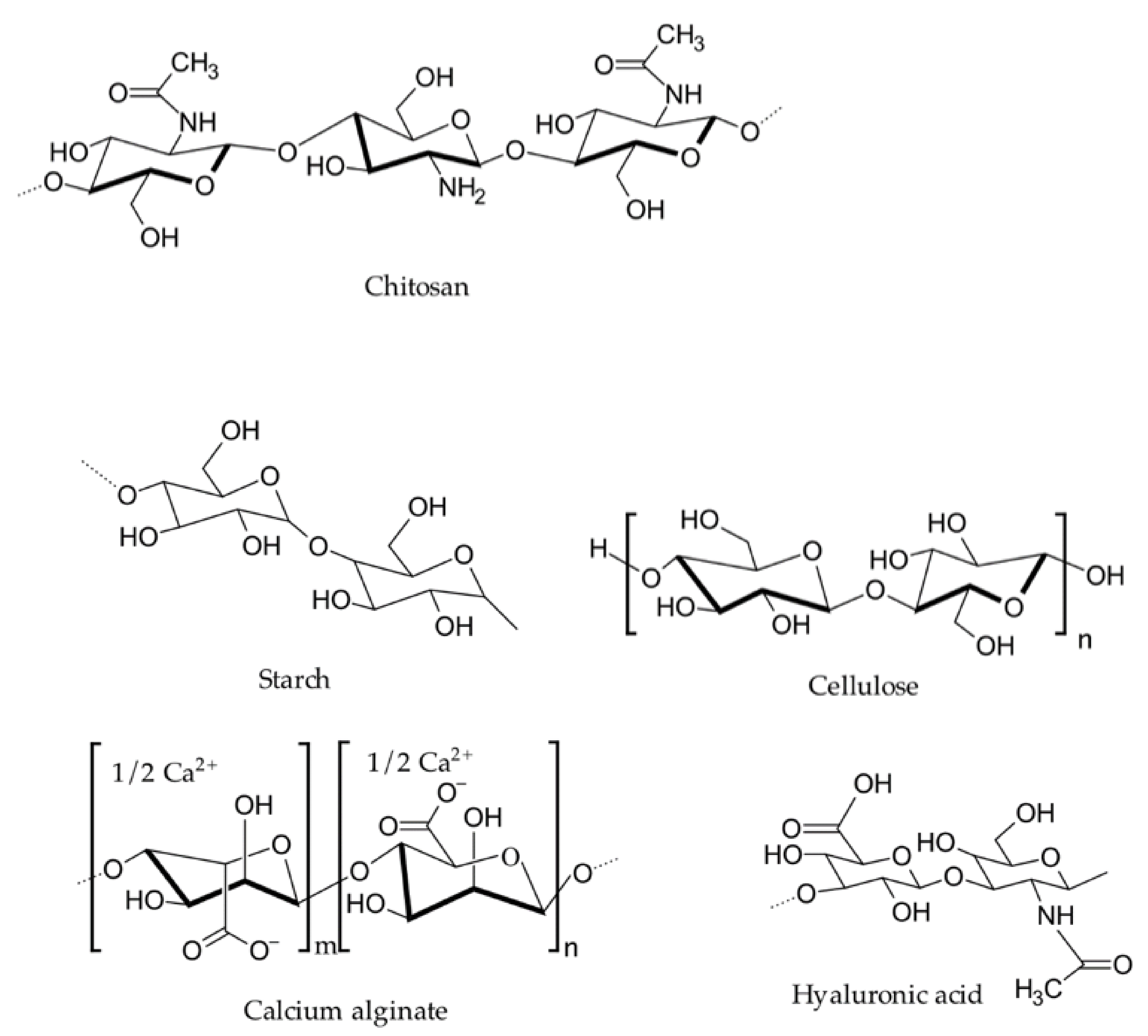
2.1.1. Chitosan
2.1.2. Collagen/Gelatin
2.1.3. Alginate
2.1.4. Oxidized Cellulose
2.1.5. Dextran
2.1.6. Hyaluronic Acid
2.1.7. Starch
2.2. Synthetic Hemostatic Polymers
2.2.1. Polyester
2.2.2. Polyethylene Glycol (PEG)
2.2.3. Polycyanoacrylate
2.2.4. Polyurethane (PU)
2.2.5. PolySTAT
2.2.6. Other Synthetic Hemostatic Polymers
3. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, M.A.; Mujahid, M. A review on recent advances in chitosan based composite for hemostatic dressings. Int. J. Biol. Macromol. 2019, 124, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Neuffer, M.C.; McDivitt, J.; Rose, D.; King, K.; Cloonan, C.C.; Vayer, J.S. Hemostatic dressings for the first responder: A Review. Mil. Med. 2004, 169, 716–720. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arnaud, F.; Teranishi, K.; Okada, T.; Parreño-Sacdalan, D.; Hupalo, D.; McNamee, G.; Carr, W.; Burris, D.; McCarron, R. Comparison of Combat Gauze and TraumaStat in two severe groin injury models. J. Surg. Res. 2011, 169, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.B.; Reynolds, B.Z.; Shiver, S.A.; Lerner, E.B.; Greenfield, E.M.; Solis, R.A.; Kimpel, N.A.; Coule, P.L.; McManus, J.G. Comparison of two packable hemostatic Gauze dressings in a porcine hemorrhage model. Prehosp. Emerg. Care 2011, 15, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.H.; Goodnough, L.T. How I use fibrinogen replacement therapy in acquired bleeding. Blood 2015, 125, 1387–1393. [Google Scholar] [CrossRef] [PubMed]
- Leaper, D.J.; Durani, P. Topical antimicrobial therapy of chronic wounds healing by secondary intention using iodine products. Int. Wound J. 2008, 5, 361–368. [Google Scholar] [CrossRef]
- Galante, J.M.; Smith, C.A.; Sena, M.J.; Scherer, L.A.; Tharratt, R.S. Identification of barriers to adaptation of battlefield technologies into civilian trauma in California. Mil. Med. 2013, 178, 1227–1230. [Google Scholar] [CrossRef][Green Version]
- Gao, Y.; Xiang, H.-F.; Wang, X.-X.; Yan, K.; Liu, Q.; Li, X.; Liu, R.-Q.; Yu, M.; Long, Y.-Z. A portable solution blow spinning device for minimally invasive surgery hemostasis. Chem. Eng. J. 2020, 387, 124052. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.-T.; Hu, P.-Y.; Liu, J.-J.; Liu, X.-F.; Hu, M.; Cui, Z.; Wang, N.; Niu, Z.; Xiang, H.-F.; et al. Laparoscopic electrospinning for in situ hemostasis in minimally invasive operation. Chem. Eng. J. 2020, 395, 125089. [Google Scholar] [CrossRef]
- Aisina, R.; Mukhametova, L.; Ivanova, E. Influence cationic and anionic PAMAM dendrimers of low generation on selected hemostatic parameters in vitro. Mater. Sci. Eng. C 2020, 109, 110605. [Google Scholar] [CrossRef] [PubMed]
- Khoshmohabat, H.; Paydar, S.; Kazemi, H.M.; Dalfardi, B. Overview of agents used for emergency hemostasis. Trauma Mon. 2016, 21, e26023. [Google Scholar] [CrossRef] [PubMed]
- Biranje, S.S.; Madiwale, P.V.; Patankar, K.C.; Chhabra, R.; Bangde, P.; Dandekar, P.; Adivarekar, R.V. Cytotoxicity and hemostatic activity of chitosan/carrageenan composite wound healing dressing for traumatic hemorrhage. Carbohydr. Polym. 2020, 239, 116106. [Google Scholar] [CrossRef] [PubMed]
- Tackett, S.M.; Sugarman, R.; Kreuwel, H.T.; Alvarez, P.; Nasso, G. Hospital economic impact from hemostatic matrix usage in cardiac surgery. J. Med. Econ. 2014, 17, 670–676. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Joshi, M.R.; Latham, J.; Okorogheye, G. Use of a flowable haemostat versus an oxidised regenerated cellulose agent in primary elective cardiac surgery: Economic impact from a UK healthcare perspective. J. Cardiothorac. Surg. 2017, 12, 107. [Google Scholar] [CrossRef] [PubMed]
- Rivard, G.E.; Brummel-Ziedins, K.E.; Mann, K.G.; Fan, L.; Hofer, A.; Cohen, E. Evaluation of the profile of thrombin generation during the process of whole blood clotting as assessed by thrombelastography. J. Thromb. Haemost. 2005, 3, 2039–2043. [Google Scholar] [CrossRef] [PubMed]
- Hickman, D.A.; Pawlowski, C.L.; Sekhon, U.D.S.; Marks, J.; Gupta, A.S. Biomaterials and Advanced Technologies for Hemostatic Management of Bleeding. Adv. Mater. 2018, 30, 1700859. [Google Scholar] [CrossRef] [PubMed]
- Achneck, H.E.; Sileshi, B.; Jamiolkowski, R.M.; Albala, D.M.; Shapiro, M.L.; Lawson, J.H. A Comprehensive Review of Topical Hemostatic Agents: Efficacy and Recommendations for Use. Ann Surg. 2010, 251, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Zeng, Q.; Pimpi, S.; Wu, W.; Han, K.; Dong, K.; Lu, T. Research status and development potential of composite hemostatic materials. J. Mater. Chem. B 2020, 8, 5395–5410. [Google Scholar] [CrossRef]
- Kate Hopper, S.B. An updated view of hemostasis: Mechanism of hemostatic dysfunction associated with sepsis. J. Vet. Emerg. Crit. Care 2005, 15, 83–91. [Google Scholar] [CrossRef]
- Behrens, A.M.; Sikorski, M.J.; Kofinas, P. Hemostatic strategies for traumatic and surgical bleeding. J. Biomed. Mater. Res. A 2014, 102, 4182–4194. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.T.; Shek, P.N. Novel wound sealants: Biomaterials and applications. Expert. Rev. Med. Devices 2010, 7, 639–659. [Google Scholar] [CrossRef]
- De la Torre, R.A.; Bachman, S.L.; Wheeler, A.A.; Bartow, K.N.; Scott, J.S. Hemostasis and hemostatic agents in minimally invasive surgery. Surgery 2007, 142, S39–S45. [Google Scholar] [CrossRef]
- Te Grotenhuis, R.; Van Grunsven, P.M.; Heutz, W.M.; Tan, E.C. Prehospital use of hemostatic dressings in emergency medical services in the Netherlands: A prospective study of 66 cases. Injury 2016, 47, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, W.; Li, N.; Wang, M.; Liang, B.; Ullah, I.; Neve, A.L.; Feng, Y.; Chen, H.; Shi, C. Design and development of polysaccharide hemostatic materials and their hemostatic mechanism. Biomater. Sci. 2017, 12, 2357–2368. [Google Scholar] [CrossRef] [PubMed]
- Satterly, S.; Nelson, D.; Zwintscher, N.; Oguntoye, M.; Causey, W.; Theis, B.; Huang, R.; Haque, M.; Martin, M.; Bickett, G.; et al. Hemostasis in a noncompressible hemorrhage model: An end-user evaluation of hemostatic agents in a proximal arterial injury. J. Surg. Educ. 2013, 70, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Palm, M.D.; Altman, J.S. Topical hemostatic agents: A review. Dermatol. Surg. 2008, 34, 431–445. [Google Scholar]
- Schuhmacher, C.; Pratschke, J.; Weiss, S.; Schneeberger, S.; Mihaljevic, A.L.; Schirren, R.; Winkler, M.; Emmanouilidis, N. Safety and effectiveness of a synthetic hemostatic patch for intraoperative soft tissue bleeding. Med. Devices 2015, 8, 167–174. [Google Scholar]
- Hino, M.; Ishiko, O.; Honda, K.-I.; Yamane, T.; Ohta, K.; Takubo, T.; Tatsumi, N. Transmission of symptomatic parvovirus B19 infection by fibrin sealant used during surgery. Br. J. Haematol. 2000, 108, 194–195. [Google Scholar] [CrossRef] [PubMed]
- Dogan, S.; Kocaeli, H.; Doygun, M. Oxidised regenerated cellulose as a cause of paraplegia after thoracotomy: Case report and review of the literature. Spinal Cord 2005, 43, 445–447. [Google Scholar] [CrossRef][Green Version]
- U.S. Food and Drug Administration. 24 Hour Summary General and Plastic Surgery Devices Advisory Committee Meeting; FDA: Gaithersburg, MD, USA, 2019. [Google Scholar]
- Guo, B.; Dong, R.; Liang, Y.; Li, M. Haemostatic materials for wound healing applications. Nat. Rev. Chem. 2021, 5, 773–791. [Google Scholar] [CrossRef]
- Hong, Y.; Zhou, F.; Hua, Y.; Zhang, X.; Ni, C.; Pan, D.; Zhang, Y.; Jiang, D.; Yang, L.; Lin, Q.; et al. A strongly adhesive hemostatic hydrogel for the repair of arterial and heart bleeds. Nat. Commun. 2019, 10, 2060. [Google Scholar] [CrossRef] [PubMed]
- Burkoth, A.K.; Anseth, K.S. A review of photocrosslinked polyanhydrides: In situ forming degradable networks. Biomaterials 2000, 21, 2395–2404. [Google Scholar] [CrossRef]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. Biomedicine 2015, 5, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Altun, I. An Experimental Study of Histopathologic Effects of hemostatic agents used in spinal surgery. World Neurosurg. 2016, 90, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xin, Y.; Yin, B.; Ye, G.L.; Wang, J.X.; Shen, J.F.; Li, L.; Yang, Q.H. Synthesis and properties of crosslinked carboxymethyl chitosan and its hemostatic and wound healing effects on liver injury of rats. J. Biomater. Appl. 2019, 34, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.M.; Atlee, H.D.; Mannone, A.J.; Dwyer, J.; Lin, L.; Goppelt, A.; Redl, H. Comparison of two gelatin and thrombin combination hemostats in a porcine liver abrasion model. J. Investig. Surg. 2013, 26, 141–148. [Google Scholar] [CrossRef]
- Gustafson, S.B.; Fulkerson, P.; Bildfell, R.; Aguilera, L.; Hazzard, T.M. Chitosan dressing provides hemostasis in swine femoral arterial injury model. Prehosp. Emerg. Care 2007, 11, 172–178. [Google Scholar] [CrossRef]
- Wang, T.; Zhong, X.; Wang, S.; Lv, F.; Zhao, X. Molecular mechanisms of RADA16-1 peptide on fast stop bleeding in rat models. Int. J. Mol. Sci. 2012, 13, 15279–15290. [Google Scholar] [CrossRef] [PubMed]
- Spinella, P.C.; Dunne, J.; Beilman, G.J.; O’Connell, R.J.; Borgman, M.A.; Cap, A.P.; Rentas, F. Constant challenges and evolution of US military transfusion medicine and blood operations in combat. Transfusion 2012, 52, 1146–1153. [Google Scholar] [CrossRef]
- Sundaram, C.P.; Keenan, A.C. Evolution of hemostatic agents in surgical practice. Indian J. Urol. 2010, 26, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Alam, H.B.; Burris, D.; DaCarta, J.A.; Rhee, P. Hemorrhage control in the battlefield: Role of new hemostatic agents. Mil. Med. 2005, 170, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.C.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An update on potential biomedical and pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef] [PubMed]
- Di Lena, F. Hemostatic polymers: The concept, state of the art and perspectives. J. Mater. Chem. B 2014, 2, 3567–3577. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical applications of chitosan and its derivative nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef] [PubMed]
- Pourshahrestani, S.; Zeimaran, E.; Kadri, N.A.; Mutlu, N.; Boccaccini, A.R. Polymeric hydrogel systems as emerging biomaterial platforms to enable hemostasis and wound healing. Adv. Healthc. Mater. 2020, 9, e2000905. [Google Scholar] [CrossRef]
- Malette, W.G.; Quigley, H.J.; Gaines, R.D.; Johnson, N.D.; Rainer, W.G. Chitosan: A new hemostatic. Ann. Thorac. Surg. 1983, 36, 55–58. [Google Scholar] [CrossRef]
- Chou, T.-C.; Fu, E.; Wu, C.-J.; Yeh, J.-H. Chitosan enhances platelet adhesion and aggregation. Biochem. Biophys. Res. Commun. 2003, 302, 480–483. [Google Scholar] [CrossRef]
- Li, D.; Chen, J.; Wang, X.; Zhang, M.; Li, C.; Zhou, J. Recent advances on synthetic and polysaccharide adhesives for biological hemostatic applications. Front. Bioeng. Biotechnol. 2020, 8, 926. [Google Scholar] [CrossRef]
- Nie, W.; Yuan, X.; Zhao, J.; Zhou, Y.; Bao, H. Rapidly in situ forming chitosan/epsilon-polylysine hydrogels for adhesive sealants and hemostatic materials. Carbohydr. Polym. 2013, 96, 342–348. [Google Scholar] [CrossRef]
- Sundaram, M.N.; Amirthalingam, S.; Mony, U.; Varma, P.K.; Jayakumar, R. Injectable chitosan-nano bioglass composite hemostatic hydrogel for effective bleeding control. Int. J. Biol. Macromol. 2019, 129, 936–943. [Google Scholar] [CrossRef]
- Satitsri, S.; Muanprasat, C. Chitin and chitosan derivatives as biomaterial resources for biological and biomedical applications. Molecules 2020, 25, 5961. [Google Scholar] [CrossRef] [PubMed]
- Erosy, G.; Kayank, M.F.; Yilmaz, O.; Rodoplu, U.; Gokeman, N. Hemostatic effects of microporous polysaccharide Hemosphere® in a rat model with severe femoral artery bleeding. Adv. Ther. 2007, 24, 485–492. [Google Scholar] [CrossRef] [PubMed]
- King, D.R.; Chen, S.M.; Proctor, K.G. Modified rapid deployment hemostat bandage terminates bleeding in coagulopathic patients with severe visceral injuries. J. Trauma 2004, 57, 756–759. [Google Scholar] [CrossRef] [PubMed]
- Manon-Jensen, T.; Kjeld, N.G.; Karsdal, M.A. Collagen-mediated hemostasis. J. Thromb. Haemost. 2016, 14, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Guajardo, S.; Figueroa, T.; Borges, J.; Aguayo, C.; Fernández, K. Graphene oxide-gelatin aerogels as wound dressings with improved hemostatic properties. Mater. Today Chem. 2021, 20, 438–448. [Google Scholar] [CrossRef]
- Chen, K.; Pan, H.; Yan, Z.; Li, Y.; Ji, D.; Yun, K.; Su, Y.; Liu, D.; Pan, W. A novel alginate/gelatin sponge combined with curcumin-loaded electrospun fibers for postoperative rapid hemostasis and prevention of tumor recurrence. Int. J. Biol. Macromol. 2021, 182, 1339–1350. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.L.; Tripathi, G.; Bae, S.H.; Lee, B.T. Physico-mechanical and biological evaluation of an injectable m-TG cross-linked thrombin loaded amended gelatin hemostat to heal liver trauma. Int. J. Biol. Macromol. 2021, 181, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Sarode, A.; Kokoroskos, N.; Ukidve, A.; Zhao, Z.; Guo, S.; Flaumenhaft, R.; Gupta, A.S.; Saillant, N.; Mitragotri, S. A polymer-based systemic hemostatic agent. Sci. Adv. 2020, 6, eaba0588. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, X.; Li, Z.; Wang, B. Efficacy, safety, and physicochemical properties of a flowable hemostatic agent made from absorbable gelatin sponge via vacuum pressure steam sterilization. J. Biomater. Appl. 2021, 35, 776–789. [Google Scholar] [CrossRef]
- Sneha Letha, S.; Shukla, S.K.; Haridas, N.; Smitha, R.P.; Sidharth Mohan, M.; Archana, V.; Rosemary, M.J. In vitro and In vivo biocompatibility evaluation of freeze dried gelatin haemostat. Fibers Polym. 2021, 22, 621–628. [Google Scholar] [CrossRef]
- Segal, H.C.; Hunt, B.J. The effects of alginate and non-alginate wound dressings on blood coagulation and platelet activation. J. Biomater. Appl. 1998, 12, 249–257. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.; Mooney, D.J. Hydrogels of tissue engineering. Chem. Rev. 2001, 101, 1869–1879. [Google Scholar]
- Asadi, L.; Mokhtari, J.; Abbasi, M. An alginate-PHMB-AgNPs based wound dressing polyamide nanocomposite with improved antibacterial and hemostatic properties. J. Mater. Sci. Mater. Med. 2021, 32, 7. [Google Scholar] [CrossRef] [PubMed]
- Hama, C.; Umeda, T.; Musha, Y.; Koda, S.; Itatani, K. novel hemostatic material containing spherical porous hydroxyapatite/alginate granules. J. Ceram. Soc. Japan 2010, 118, 446–450. [Google Scholar] [CrossRef]
- Chen, Z.; Song, J.; Xia, Y.; Jiang, Y.; Murillo, L.L.; Tsigkou, O.; Wang, T.; Li, Y. High strength and strain alginate fibers by a novel wheel spinning technique for knitting stretchable and biocompatible wound-care materials. Mater. Sci. Eng. C 2021, 127, 100418. [Google Scholar] [CrossRef] [PubMed]
- Hattori, H.; Amano, Y.; Nogami, Y.; Takase, B.; Ishihara, M. Hemostasis for severe hemorrhage with photocrosslinkable chitosan hydrogel and calcium alginate. Ann. Biomed. Eng. 2010, 38, 3724–3732. [Google Scholar] [CrossRef] [PubMed]
- Knill, C.J.; Kennedy, J.F.; Mistry, J.; Miraftab, M.; Smart, G.; Groocock, M.R.; Williams, H.J. Alginate fibres modified with unhydrolysed and hydrolysed chitosans for wound dressings. Carbohydr. Polym. 2004, 55, 65–76. [Google Scholar] [CrossRef]
- Wei, X.; Ding, S.; Liu, S.; Yang, K.; Cai, J.; Li, F.; Wang, C.; Lin, S.; Tian, F. Polysaccharides-modified chitosan as improved and rapid hemostasis foam sponges. Carbohydr. Polym. 2021, 264, 118028. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, Z.; Liang, Y.; He, J.; Guo, B. Multifunctional tissue-adhesive cryogel wound dressing for rapid nonpressing surface hemorrhage and wound repair. ACS Appl. Mater. Interfaces 2020, 12, 35856–35872. [Google Scholar] [CrossRef] [PubMed]
- Dragu, A.; Unglaub, F.; Schwarz, S.; Beier, J.P.; Kneser, U.; Bach, A.D.; Horch, R.E. Foreign body reaction after usage of tissue adhesives for skin closure: A case report and review of the literature. Arch. Orthop. Trauma. Surg. 2009, 129, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Seyednejad, H.; Imani, M.; Jamieson, T.; Seifalian, A.M. Topical haemostatic agents. Br. J. Surg. 2008, 95, 1197–1225. [Google Scholar] [CrossRef] [PubMed]
- Amit, M.; Binenbaum, Y.; Cohen, J.T.; Gil, Z. Effectiveness of an oxidized cellulose patch hemostatic agent in thyroid surgery: A prospective, randomized, controlled study. J. Am. Coll. Surg. 2013, 217, 221–225. [Google Scholar] [CrossRef]
- Kobylkevich, B.M.; Raihan, M.J.; Uprety, T.; Kaushik, R.S.; Shore, J.S.; Sohn, J.J.; Messerli, M.A. Linear polysaccharides reduce production of inflammatory cytokines by LPS-stimulated bovine fibroblasts. Vet. Immunol. Immunopathol. 2021, 234, 110220. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.M.; Spazierer, D.; Urban, M.D.; Lin, L.; Redl, H.; Goppelt, A. Comparison of regenerated and non-regenerated oxidized cellulose hemostatic agents. Eur. Surg. 2013, 45, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Sigurjonsson, J.; Hedman, D.; Bansch, P.; Schott, U. Comparison of dextran and albumin on blood coagulation in patients undergoing major gynaecological surgery. Perioper. Med. 2018, 7, 21. [Google Scholar] [CrossRef]
- Liu, C.; Liu, X.; Liu, C.; Wang, N.; Chen, H.; Yao, W.; Sun, G.; Song, Q.; Qiao, W. A highly efficient, in situ wet-adhesive dextran derivative sponge for rapid hemostasis. Biomaterials 2019, 205, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Nie, J.; Yang, D. Dextran and gelatin based photocrosslinkable tissue adhesive. Carbohydr Polym 2012, 90, 1428–1436. [Google Scholar] [CrossRef] [PubMed]
- Stecco, C.; Stern, R.; Porzionato, A.; Macchi, V.; Masiero, S.; Stecco, A.; De Caro, R. Hyaluronan within fascia in the etiology of myofascial pain. Surg. Radiol. Anat. 2011, 33, 891–896. [Google Scholar] [CrossRef]
- Geng, H.; Dai, Q.; Sun, H.; Zhuang, L.; Song, A.; Caruso, F.; Hao, J.; Cui, J. Injectable and sprayable polyphenol-based hydrogels for controlling hemostasis. ACS Appl. Biomater. 2020, 3, 1258–1266. [Google Scholar] [CrossRef]
- Luo, J.W.; Liu, C.; Wu, J.H.; Lin, L.X.; Fan, H.M.; Zhao, D.H.; Zhuang, Y.Q.; Sun, Y.L. In situ injectable hyaluronic acid/gelatin hydrogel for hemorrhage control. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 628–634. [Google Scholar] [CrossRef]
- Landoulsi, J.; Genet, M.J.; Fleith, S.; Touré, Y.; Liascukiene, I.; Méthivier, C.; Rouxhet, P.G. Organic adlayer on inorganic materials: XPS analysis selectivity to cope with adventitious contamination. Appl. Surf. Sci. 2016, 383, 71–83. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Hu, T.; Chen, B.; Yin, Z.; Ma, P.X.; Guo, B. Adhesive hemostatic conducting injectable composite hydrogels with sustained drug release and photothermal antibacterial activity to promote full-thickness skin regeneration during wound healing. Small 2019, 15, e1900046. [Google Scholar] [CrossRef]
- Huang, A.P.; Lai, D.M.; Hsu, Y.H.; Tsai, H.H.; Su, C.Y.; Hsu, S.H. An anti-inflammatory gelatin hemostatic agent with biodegradable polyurethane nanoparticles for vulnerable brain tissue. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 121, 111799. [Google Scholar] [CrossRef]
- Sakoda, M.; Kaneko, M.; Ohta, S.; Qi, P.; Ichimura, S.; Yatomi, Y.; Ito, T. Injectable hemostat composed of a polyphosphate-conjugated hyaluronan hydrogel. Biomacromolecules 2018, 19, 3280–3290. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef]
- Hou, S.; Liu, Y.; Feng, F.; Zhou, J.; Feng, X.; Fan, Y. Polysaccharide-peptide cryogels for multidrug-resistant-bacteria infected wound healing and hemostasis. Adv. Healthc. Mater. 2020, 9, e1901041. [Google Scholar] [CrossRef] [PubMed]
- Bjorses, K.; Faxalv, L.; Montan, C.; Wildt-Persson, K.; Fyhr, P.; Holst, J.; Lindahl, T.L. In vitro and in vivo evaluation of chemically modified degradable starch microspheres for topical haemostasis. Acta. Biomater. 2011, 7, 2558–2565. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.; Chen, F.; Zhao, Y.; Huang, W.; Liu, C. A novel injectable starch-based tissue adhesive for hemostasis. J. Mater. Chem. B 2020, 8, 8282–8293. [Google Scholar] [CrossRef] [PubMed]
- Panwar, V.; Sharma, A.; Thomas, J.; Chopra, V.; Kaushik, S.; Kumar, A.; Ghosh, D. In-vitro and In-vivo evaluation of biocompatible and biodegradable calcium-modified carboxymethyl starch as a topical hemostat. Materialia 2019, 7, 100373. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, G.; Wu, L.; Qu, H.; Song, D.; Huang, H.; Wu, C.; Xu, M. Rational design of porous starch/hyaluronic acid composites for hemostasis. Int. J. Biol. Macromol. 2020, 20, 1319–1329. [Google Scholar] [CrossRef]
- Tavakoli, S.; Kharaziha, M.; Nemati, S.; Kalateh, A. Nanocomposite hydrogel based on carrageenan-coated starch/cellulose nanofibers as a hemorrhage control material. Carbohydr. Polym. 2021, 251, 117013. [Google Scholar] [CrossRef]
- Yang, X.; Liu, W.; Shi, Y.; Xi, G.; Wang, M.; Liang, B.; Feng, Y.; Ren, X.; Shi, C. Peptide-immobilized starch/PEG sponge with rapid shape recovery and dual-function for both uncontrolled and noncompressible hemorrhage. Acta. Biomater. 2019, 99, 220–235. [Google Scholar] [CrossRef]
- Leonhardt, E.E.; Kang, N.; Hamad, M.A.; Wooley, K.L.; Elsabahy, M. Absorbable hemostatic hydrogels comprising composites of sacrificial templates and honeycomb-like nanofibrous mats of chitosan. Nat. Commun. 2019, 10, 2307. [Google Scholar] [CrossRef]
- Yin, M.; Wang, Y.; Zhang, Y.; Ren, X.; Qiu, Y.; Huang, T.S. Novel quaternarized N-halamine chitosan and polyvinyl alcohol nanofibrous membranes as hemostatic materials with excellent antibacterial properties. Carbohydr. Polym. 2020, 232, 115823. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, J.H.; Kim, J.S.; Choe, J.H. Evaluation of a Novel collagen hemostatic matrix: Comparison of two hemostatic matrices in a rabbits jejunal artery injury model. J. Surg. Res. 2019, 243, 553–559. [Google Scholar] [CrossRef]
- Leixnering, M.; Reichetseder, J.; Schultz, A.; Figl, M.; Wassermann, E.; Thurnher, M.; Redl, H. Gelatin thrombin granules for hemostasis in a severe traumatic liver and spleen rupture model in swine. J. Trauma 2008, 64, 456–461. [Google Scholar] [CrossRef]
- Yang, Q.; Lei, S. Alginate dressing application in hemostasis after using seldinger peripherally inserted central venous catheter in tumor patients. Indian J. Hematol. Blood Transfus. 2015, 31, 434–438. [Google Scholar] [CrossRef]
- Tong, Z.; Yang, J.; Lin, L.; Wang, R.; Cheng, B.; Chen, Y.; Tang, L.; Chen, J.; Ma, X. In situ synthesis of poly (gamma- glutamic acid)/alginate/AgNP composite microspheres with antibacterial and hemostatic properties. Carbohydr. Polym. 2019, 221, 21–28. [Google Scholar] [CrossRef]
- Karahaliloglu, Z.; Demirbilek, M.; Ulusoy, I.; Gumuskaya, B.; Denkbas, E.B. Active nano/microbilayer hemostatic agents for diabetic rat bleeding model. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1573–1585. [Google Scholar] [CrossRef]
- Sagar, P.; Prasad, K.; Lalitha, R.M.; Ranganath, K. Cyanoacrylate for intraoral wound closure: A possibility? Int. J. Biomater. 2015, 2015, 165428. [Google Scholar] [CrossRef]
- Zhu, J.; Sun, Y.; Sun, W.; Meng, Z.; Shi, Q.; Zhu, X.; Gan, H.; Gu, R.; Wu, Z.; Dou, G. Calcium ion-exchange cross-linked porous starch microparticles with improved hemostatic properties. Int. J. Biol. Macromol. 2019, 134, 435–444. [Google Scholar] [CrossRef]
- Qian, J.; Chen, Y.; Yang, H.; Zhao, C.; Zhao, X.; Guo, H. Preparation and characterization of crosslinked porous starch hemostatic. Int. J. Biol. Macromol. 2020, 160, 429–436. [Google Scholar] [CrossRef]
- Ferreira, P.; Coelho, J.F.; Gil, M.H. Development of a new photocrosslinkable biodegradable bioadhesive. Int. J. Pharm. 2008, 352, 172–181. [Google Scholar] [CrossRef]
- Della Puppa, A.; Rossetto, M.; Scienza, R. Use of a new absorbable sealing film for preventing postoperative cerebrospinal fluid leaks: Remarks on a new approach. Br. J. Neurosurg. 2010, 24, 609–611. [Google Scholar] [CrossRef]
- Von der Brelie, C.; Soehle, M.; Clusmann, H.R. Intraoperative sealing of dura mater defects with a novel, synthetic, self adhesive patch: Application experience in 25 patients. Br. J. Neurosurg. 2012, 26, 231–235. [Google Scholar] [CrossRef]
- Yuan, W.; Liu, Z. Surgical wound healing using hemostatic gauze scaffold loaded with nanoparticles containing sustained-release granulocyte colony-stimulating factor. Int. J. Nanomed. 2011, 6, 3139–3149. [Google Scholar] [CrossRef][Green Version]
- Fornaguera, C.; Caldero, G.; Mitjans, M.; Vinardell, M.P.; Solans, C.; Vauthier, C. Interactions of PLGA nanoparticles with blood components: Protein adsorption, coagulation, activation of the complement system and hemolysis studies. Nanoscale 2015, 7, 6045–6058. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Chhabra, H.; Narwane, S.; Rege, N.; Bellare, J.R. Hemostatic efficacy of nanofibrous matrix in rat liver injury model. Surg. Innov. 2017, 24, 23–28. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, Y.; Zhang, N.; Shi, J.; Zhang, X.; Qi, C.; Midgley, A.C.; Wang, S. Potentials of sandwich-like chitosan/polycaprolactone/gelatin scaffolds for guided tissue regeneration membrane. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 109, 110618. [Google Scholar] [CrossRef]
- Giri Dev, V.R.; Hemamalini, T. Porous electrospun starch rich polycaprolactone blend nanofibers for severe hemorrhage. Int. J. Biol. Macromol. 2018, 118, 1276–1283. [Google Scholar] [CrossRef]
- Park, J.-Y.; Kyung, K.-H.; Tsukada, K.; Kim, S.-H.; Shiratori, S. Biodegradable polycaprolactone nanofibres with β-chitosan and calcium carbonate produce a hemostatic effect. Polymer 2017, 123, 194–202. [Google Scholar] [CrossRef]
- Zhu, J.; Li, F.; Wang, X.; Yu, J.; Wu, D. Hyaluronic acid and polyethylene glycol hybrid hydrogel encapsulating nanogel with hemostasis and sustainable antibacterial property for wound healing. ACS Appl. Mater. Interfaces 2018, 10, 13304–13316. [Google Scholar] [CrossRef]
- Orgill, D.P.; Ehret, F.W.; Regan, J.F.; Glowacki, J.; Mulliken, J.B. Polyethylene glycol/microfibrillar collagen composite as a new resorbable hemostatic bone wax. J. Biomed. Mater. Res. 1998, 39, 358–363. [Google Scholar] [CrossRef]
- Lewis, K.M.; Spazierer, D.; Slezak, P.; Baumgartner, B.; Regenbogen, J.; Gulle, H. Swelling, sealing, and hemostatic ability of a novel biomaterial: A polyethylene glycol-coated collagen pad. J. Biomater. Appl. 2014, 29, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Lih, E.; Lee, J.S.; Park, K.M.; Park, K.D. Rapidly curable chitosan-PEG hydrogels as tissue adhesives for hemostasis and wound healing. Acta. Biomater. 2012, 8, 3261–3269. [Google Scholar] [CrossRef]
- Pérez, M.; Fernández, I.; Márquez, D.; Bretaña, R.M. Use of N-Butyl-2-Cyanoacrylate in oral surgery: Biological and clinical evaluation. Artif. Organs 2000, 24, 241–243. [Google Scholar] [CrossRef] [PubMed]
- Park, W.T.; Son, I.; Park, H.W.; Chung, K.B.; Xu, Y.; Lee, T.; Noh, Y.Y. Facile Routes to improve performance of solution-processed amorphous metal oxide thin film transistors by water vapor annealing. ACS Appl. Mater. Interfaces 2015, 7, 13289–13294. [Google Scholar] [CrossRef] [PubMed]
- Montanaro, L.; Arciola, C.R.; Cenni, E.; Ciapetti, G.; Savioli, F.; Filippini, F.; Barsanti, L.A. Cytotoxicity, blood compatibility and antimicrobial activity of two cyanoacrylate glues for surgical use. Biomaterials 2001, 22, 59–66. [Google Scholar] [CrossRef]
- Aghajani, B.; Hosseini, B. Hydroxyapatite-Hardystonite nanocomposite scaffolds prepared by the replacing the polyurethane polymeric sponge technique for tissue engineering applications. Nanomed. J. 2017, 4, 254–262. [Google Scholar]
- Zumbardo-Bacelis, G.A.; Meza-Villegas, L.A.; Pérez-Aranda, C.A.; Vargas-Coronado, R.; Castillo-Cruz, O.; Montaño-Machado, V.; Mantovani, D.; Cauich-Rodríguez, J.V. On arginine-based polyurethane-blends specific to vascular prostheses. J. Appl. Polym. Sci. 2021, 38, e51247. [Google Scholar] [CrossRef]
- Gabriel, L.P.; Rodrigues, A.A.; Macedo, M.; Jardini, A.L.; Maciel Filho, R. Electrospun polyurethane membranes for Tissue Engineering applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 72, 113–117. [Google Scholar] [CrossRef]
- Zahedi, P.; Rezaeian, I.; Ranaei-Siadat, S.-O.; Jafari, S.-H.; Supaphol, P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym. Adv. Tech. 2010, 21, 77–95. [Google Scholar] [CrossRef]
- Ohlinger, R.; Gieron, L.; Rutkowski, R.; Kohlmann, T.; Zygmunt, M.; Unger, J. The use of tissuGlu(R) surgical adhesive for mastectomy with or without lymphonodectomy. In Vivo 2018, 32, 625–631. [Google Scholar]
- Burnett, L.R.; Richter, J.G.; Rahmany, M.B.; Soler, R.; Steen, J.A.; Orlando, G.; Abouswareb, T.; Van Dyke, M.E. Novel keratin (KeraStat) and polyurethane (Nanosan(R)-Sorb) biomaterials are hemostatic in a porcine lethal extremity hemorrhage model. J. Biomater. Appl. 2014, 28, 869–879. [Google Scholar] [CrossRef]
- Landsman, T.L.; Touchet, T.; Hasan, S.M.; Smith, C.; Russell, B.; Rivera, J.; Maitland, D.J.; Cosgriff-Hernandez, E. A shape memory foam composite with enhanced fluid uptake and bactericidal properties as a hemostatic agent. Acta. Biomater. 2017, 47, 91–99. [Google Scholar] [CrossRef]
- Chang, J.C.; Holloway, B.C.; Zamisch, M.; Hepburn, M.J.; Ling, G.S. ResQFoam for the treatment of non-compressible hemorrhage on the front line. Mil. Med. 2015, 180, 932–933. [Google Scholar] [CrossRef]
- Duggan, M.; Rago, A.; Sharma, U.; Zugates, G.; Freyman, T.; Busold, R.; Caulkins, J.; Pham, Q.; Chang, Y.; Mejaddam, A.; et al. Self-expanding polyurethane polymer improves survival in a model of noncompressible massive abdominal hemorrhage. J. Trauma Acute Care Surg. 2013, 74, 1462–1467. [Google Scholar] [CrossRef]
- Klein, M.K.; Tsihlis, N.D.; Pritts, I.A.; Kibbe, M.R. Emerging therapies for prehospital control of hemorrhage. J. Surg. Res. 2019, 248, 182–190. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Wei, H.; Pozzo, L. A synthetic fibrin-crosslinking polymer for modulating clot properties and inducing hemostasis. Sci. Transl. Med. 2015, 7, 277ra29. [Google Scholar]
- Chan, L.W.; White, N.J.; Pun, S.H. A fibrin cross-linking polymer enhances clot formation similar to factor concentrates and tranexamic acid in an in vitro model of coagulopathy. ACS Biomater. Sci. Eng. 2016, 2, 403–408. [Google Scholar] [CrossRef]
- Lamm, R.J.; Lim, E.B.; Weigandt, K.M.; Pozzo, L.D.; White, N.J.; Pun, S.H. Peptide valency plays an important role in the activity of a synthetic fibrin-crosslinking polymer. Biomaterials 2017, 132, 96–104. [Google Scholar] [CrossRef]
- Lamm, R.J.; Pichon, T.J.; Huyan, F.; Wang, X.; Prossnitz, A.N.; Manner, K.T.; White, N.J.; Pun, S.H. Optimizing the polymer chemistry and synthesis method of PolySTAT, an injectable hemostat. ACS Biomater. Sci. Eng. 2020, 6, 7011–7020. [Google Scholar] [CrossRef]
- Chan, L.W.; Kim, C.H.; Wang, X.; Pun, S.H.; White, N.J.; Kim, T.H. PolySTAT-modified chitosan gauzes for improved hemostasis in external hemorrhage. Acta. Biomater. 2016, 31, 178–185. [Google Scholar] [CrossRef]
- Mukhopadhyay, K.; Kasthuri, R.; Sudarshan, T.S. Siloxane-based artificial blockage to control bleeding. U.S. Patent No. 009707251B2, 18 July 2017. [Google Scholar]
- Barba, B.J.D.; Aranilla, C.T.; Relleve, L.S.; Cruz, V.R.C.; Vista, J.R.; Abad, L.V. Hemostatic granules and dressing prepared from formulations of carboxymethyl cellulose, kappa-carrageenan and polyethylene oxide crosslinked by gamma radiation. Rad. Phys. Chem. 2018, 144, 180–188. [Google Scholar] [CrossRef]
- Wang, D.; Li, W.; Wang, Y.; Yin, H.; Ding, Y.; Ji, J.; Wang, B.; Hao, S. Fabrication of an expandable keratin sponge for improved hemostasis in a penetrating trauma. Colloids Surf. B Biointerfaces 2019, 182, 110367. [Google Scholar] [CrossRef]
- Li, Z.; Milionis, A.; Zheng, Y.; Yee, M.; Codispoti, L.; Tan, F.; Poulikakos, D.; Yap, C.H. Superhydrophobic hemostatic nanofiber composites for fast clotting and minimal adhesion. Nat. Commun. 2019, 10, 5562. [Google Scholar] [CrossRef]
- Bu, Y.; Zhang, L.; Liu, J.; Zhang, L.; Li, T.; Shen, H.; Wang, X.; Yang, F.; Tang, P.; Wu, D. Synthesis and properties of hemostatic and bacteria-responsive in situ hydrogels for emergency treatment in critical situations. ACS Appl. Mater. Interfaces 2016, 8, 12674–12683. [Google Scholar] [CrossRef]
- Broekema, F.; Oeveren, W.; Bos, R. Analysis of the hemostatic efficacy of polyurethane foam using a novel method to compare topical hemostatic agents in a rat tail-tip model. Int. Surg. J. 2016, 1551–1556. [Google Scholar] [CrossRef]
- Morani, A.C.; Platt, J.F.; Thomas, A.J.; Kaza, R.K.; Al-Hawary, M.M.; Cohan, R.H.; Francis, I.R.; Elsayes, K.M. Hemostatic agents and tissue sealants: Potential mimics of abdominal abnormalities. AJR Am. J. Roentgenol. 2018, 211, 760–766. [Google Scholar] [CrossRef]
- Kolar, M.; Primc, G. Haemostatic response of polyethylene terephthalate treated by oxygen and nitrogen plasma afterglows. Int. J. Polym. Sci. 2016, 2016, 1749285. [Google Scholar] [CrossRef]
- Boerman, M.A.; Roozen, E.; Sanchez-Fernandez, M.J.; Keereweer, A.R.; Felix Lanao, R.P.; Bender, J.; Hoogenboom, R.; Leeuwenburgh, S.C.; Jansen, J.A.; Van Goor, H.; et al. Next generation hemostatic materials based on NHS-Ester functionalized Poly(2-oxazoline)s. Biomacromolecules 2017, 18, 2529–2538. [Google Scholar] [CrossRef]
- Matteri, K.V.; Doddi, N. Synthetic absorbale hemostatic composition. U.S. Patent No. 4,440,789, 3 April 1984. [Google Scholar]
- Marin, S.; Albu Kaya, M.G.; Ghica, M.V.; Dinu-Pirvu, C.; Popa, L.; Udeanu, D.I.; Mihai, G.; Enachescu, M. Collagen-polyvinyl alcohol-indomethacin biohybrid matrices as wound dressings. Pharmaceutics 2018, 10, 224. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, X.; Zhang, Z.; Liang, Y.; Yin, Z.; Chen, B.; Bai, L.; Han, Y.; Guo, B. Degradable gelatin-based IPN cryogel hemostat for rapidly stopping deep noncompressible hemorrhage and simultaneously improving wound healing. Chem. Mater. 2020, 32, 6595–6610. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, B.; Wu, H.; Liang, Y.; Ma, P.X. Injectable antibacterial conductive nanocomposite cryogels with rapid shape recovery for noncompressible hemorrhage and wound healing. Nat. Commun. 2018, 9, 2784. [Google Scholar] [CrossRef]
- Shi, Z.; Lan, G.; Hu, E.; Lu, F.; Qian, P.; Liu, J.; Dai, F.; Xie, R. Targeted delivery of hemostats to complex bleeding wounds with magnetic guidance for instant hemostasis. Chem. Eng. J. 2022, 427, 130916. [Google Scholar] [CrossRef]
- Ziv, O.; Lublin-Tennenbaum, T.; Margel, S. Synthesis and characterization of thrombin conjugated g-Fe2O3 magnetic nanoparticles for nemostasis. Adv. Eng. Mater. 2009, 11, B251–B260. [Google Scholar] [CrossRef]
- Davies, G.L.; Govan, J.; Tekoriute, R.; Serrano-Garcia, R.; Nolan, H.; Farrell, D.; Hajatpour, O.; Gun’ko, Y.K. Magnetically activated adhesives: Towards on-demand magnetic triggering of selected polymerisation reactions. Chem. Sci. 2017, 8, 7758–7764. [Google Scholar] [CrossRef]
- Luo, W.L.; Zhang, J.; Qiu, X.; Chen, L.J.; Fu, J.; Hu, P.Y.; Li, X.; Hu, R.J.; Long, Y.Z. Electric-field-modified in situ precise deposition of electrospun medical glue fibers on the liver for rapid hemostasis. Nanoscale. Res. Lett. 2018, 13, 278. [Google Scholar] [CrossRef]
- Jia, N.; Yang, J.; Liu, J.; Zhang, J. Electric field: A key signal in wound healing. Chin. J. Plast. Reconst. Surg. 2021, 3, 95–102. [Google Scholar] [CrossRef]
- Vyas, K.S.; Saha, S.P. Comparison of hemostatic agents used in vascular surgery. Expert. Opin. Biol. Ther. 2013, 13, 1663–1672. [Google Scholar] [CrossRef]
- Nooh, N.; Abdullah, W.A.; Grawish Mel, A.; Ramalingam, S.; Javed, F.; Al-Hezaimi, K. The effects of surgicel and bone wax hemostatic agents on bone healing: An experimental study. Indian. J. Orthop. 2014, 48, 319–325. [Google Scholar] [CrossRef]
- Zhang, J. Traumastem® versus Surgicel® for the Secondary Treatment of Local Bleeding in Patients Undergoing Hepatic Resection (TSFHR); ClinicalTrials.gov. Identifier: NCT03489070; U.S. National Laboratory of Medicine: Bethesda, MD, USA, 2018; pp. 1–4.
- Pereira, B.M.; Bortoto, J.B.; Fraga, G.P. Topical hemostatic agents in surgery: Review and prospects. Rev. Col. Bras. Cir. 2018, 45, e1900. [Google Scholar]
- Echave, M.; Oyagüez, I.; Casado, M.A. Use of Floseal®, a human gelatine-thrombin matrix sealant, in surgery: A systematic review. BMC Surgery 2014, 14, 111. [Google Scholar] [CrossRef]
- Sanders, L.; Nagatomi, J. Clinical applications of surgical adhesives and sealants. Crit. Rev. Biomed. Eng. 2014, 42, 271–292. [Google Scholar] [CrossRef]
- Peng, H.T. Hemostatic agents for prehospital hemorrhage control: A narrative review. Mil. Med. Res. 2020, 7, 13. [Google Scholar] [CrossRef]
- Xie, T.; Ding, J.; Han, X.; Jia, H.; Yang, Y.; Liang, S.; Wang, W.; Liu, W.; Wang, W. Wound dressing change facilitated by spraying zinc ions. Mater. Horiz. 2020, 7, 605–614. [Google Scholar] [CrossRef]
- Wang, L.; Zhong, Y.; Qian, C.; Yang, D.; Nie, J.; Ma, G. A natural polymer-based porous sponge with capillary-mimicking microchannels for rapid hemostasis. Acta. Biomater. 2020, 114, 193–205. [Google Scholar] [CrossRef]
- Walker, R.M.; Gillespie, B.M.; Thalib, L.; Higgins, N.S.; Whitty, J.A. Foam dressings for treating pressure ulcers. Cochrane Database Syst. Rev. 2017, 10, CD011332. [Google Scholar] [CrossRef]
- Chiara, O.; Cimbanassi, S.; Bellanova, G.; Chiarugi, M.; Mingoli, A.; Olivero, G.; Ribaldi, S.; Tugnoli, G.; Basilicò, S.; Bindi, F.; et al. A systematic review on the use of topical hemostats in trauma and emergency surgery. BMC Surg. 2018, 18, 68. [Google Scholar] [CrossRef]
- Transparency Market Research. Flowable Hemostats. 2020. Available online: https://www.transparencymarketresearch.com/flowable-hemostats-market.html (accessed on 9 May 2021).




| Name [Ref.] | Polymeric System | Mechanism of Action | Case Study Model | Findings |
|---|---|---|---|---|
| Chitosan [50,51,81,95,96] | Chitosan/ε-polylysine hydrogel Chitosan-nano-bioglass composite CMCS-TA-BDBA hydrogel PVA/CSENDHM nanofibrous membrane. | Employed for hemostasis, in the form of powder, films, sponges, hydrogels, particles, fibers. Powders: Manual compression, as well as the interaction with erythrocytes, provide rapid coagulation. Hydrogels: Facilitates barrier formation and prevents blood flow from the cavity. | In vivo study of acute liver puncher models in rats, rabbits, and pigs. | Anti-microbial and anti-bacterial activities; excellent adhesion ability; rapid blood coagulation; high water absorption; biocompatible. |
| Collagen [97] | CollaStat®® (Collagen and thrombin) and Floseal®® (Gelatin and thrombin) | Provides a site for platelet adherence, activation, and aggregation. The activated platelets agglomerate around the wound and stop the blood flow. | The hemostatic efficacy of CollaStat®® and FloSeal®® have been compared in a rabbit jejunal artery injury model. | The mean hemostasis time for CollaStat®® was found to be significantly shorter compared to Floseal®® (64.0 ± 0.5 s vs. 84.0 ± 7.8 s). |
| Dextran | Aldehyde dextran sponge | Accelerates coagulation by rapid wound closure, cells’ initiation, and aggregation, as well as coagulation factors’ aggregation on the wound site. | In vivo study of the femoral artery and liver injuries model in rabbits. | Low cytotoxicity; remarkable blood loss; quick blood absorption and strong tissue adhesion. |
| Gelatin [56,57,58,98] | FloSeal®® (Gelatin-thrombin granules). Graphene oxide-gelatin aerogels. Curcumin-alginate-gelatin sponge. m-TG-thrombin-gelatin | Gelatin granules swell when they come in contact with the blood. These swollen granules stop hemorrhage by blocking the bleeding site. | In vivo (liver and spleen rupture model in swine, rat liver trauma model, liver abrasion model in rabbit). In vitro (platelets adhesion, whole blood-clotting time, total blood absorption). | Excellent clot integration into the surrounding tissues; safe to implant in the body; reduced blood-clotting time; potential for preventing tumor recurrence. |
| Alginate [65,67,99,100] | PGA/alginate/AgNP Alginate fibers Alginate-PHMB-AgNP polyamide nanocomposites Hydroxy apatite/alginate granules. | Calcium ions are released in exchange for sodium ions when calcium alginate comes in contact with blood. These released calcium ions promote prothrombin activation in the clotting cascade which leads to rapid hemostasis. Alginate granules swell enough to block the bleeding site. | The alginate-based hemostatic dressing efficacy was characterized by blood-clotting time. Biocompatibility of the dressings was studied using degradation weight change. | pH-sensitive swelling properties; excellent hemostatic performance; anti-bacterial properties. |
| Cellulose [49,70,74,101] | Cellulose-modified chitosan foam sponge. Oxidized cellulose patch (Surgicel®®). | Facilitates hemostasis by quickly absorbing liquid, entrapping platelets and erythrocytes, and increasing blood coagulating factors. | Rabbit femoral artery injury model. Mouse tail amputation model. | Excellent water-absorbing ability, improved mechanical strength; low hemolysis rate, benign cytotoxicity; good resilience ability; superior hemostasis; good candidate for chronic wound treatment. |
| Hyaluronic acid [32,59,82,94,102] | GelMA-HA-NB hydrogel HA/gelatin hydrogel HAPPI HA-Serotonin hydrogel | HA-based hydrogels act as tissue sealants for hemorrhage control. | In vivo (rat femoral artery bleeding model, liver bleeding rat model, mouse tail bleeding model, mouse abdominal wall abrasion model).In vitro (shear test, adhesion test, compression test, total blood-clotting time test) | Shorter gelation time (< 2 min), good stability; strong burst strength; excellent sealant strength; improved hemostatic capability; potential application as a trauma wound sealant. |
| Starch [90,93,94,103,104] | kCA-coated starch/cellulose nanofibers St-Dopa hydrogel SPS-STMP hydrogel TRAP-Starch-PEG sponge | When Ca2+ CPSMs are applied to the bleeding sites, it provides sites for RBCs and platelets’ adhesion, and forms gel-like matrices which block the irregular bleeding. Starch-based sponge provides pressure to the wound and promotes hemostasis. | In vivo mouse tail amputation method; rat tail bleeding model; rat liver laceration model. | Hydrogels: rapid sol-gel transition; good swelling ratio; excellent cyto/hemocompatibility. Sponge: high resilience; good mechanical strength; high expandable; useful as a topical hemostatic agent for uncontrolled and non-compressible hemorrhage. |
| Synthetic Polymer | Synthetic Polymeric System [Ref.] | Mode of Application | Case Study Model | Time to Achieve Hemostasis | Findings |
|---|---|---|---|---|---|
| Polylactic co-glycolic acid (PLGA) | TissuePatchDuralTM [107] G-CSF-dextran nanoparticle-PLGA [108] | Adhesive patch Gauze | Patients underwent an intradural neurosurgical procedure. Femoral artery model in rats. | 1 min - | Excellent for postoperative cerebrospinal fluid (CSF) leakage; no foreign body reaction. Provides hemostasis; increases neutrophil activity. |
| Polycaprolactone (PCL) | Gelatin/PCL [110] Chitosan/PCL/Gelatin [111] PCL/Starch [112] | Nanofibrous matrix sheet Composite scaffold Mat | In vivo rat liver injury model. In vitro whole blood clotting. Blood-clotting time was determined using the modified Lee and White method. | - - - | Safe and effective hemostat; helps in liver regeneration. Strong blood coagulation ability; prevent cell infiltration; biocompatible. Good hemostatic potential with a faster blood-clotting rate. |
| PolySTAT | PolySTAT [131] PolySTAT/Chitosan [135] | Gauze Injectable polymer | Rat femoral artery injury model. Trauma and fluid resuscitation model in rat. | - - | Rapid blood adsorption; withstand arterial pressure. Stabilizes fibrin clot, assists in the formation of a strong clot, mimics the function of transglutaminase factor XIII. |
| Siloxane | Siloxane-based mixtures [136] | Semi-solid gel | Porcine model | - | Semisolid matrix forms an artificial blockage to control bleeding. |
| Polyethylene oxide (PEO) | CMC-PEO-KC [137] | Granules | Femoral artery model in rats. | 90 s | CMC-PEO-KC hydrogels are capable of clotting whole blood, adhering to platelets, and accelerating clotting time. |
| Polyacrylamide (PAM) | Keratin-PAM [138] | Sponge | Rat penetrating liver trauma model | 8 mm wound—48 s 11 mm wound—57 s. | Highly expandable upon blood adsorption; useful in trauma application. |
| Polyethylene glycol (PEG) | HA-PEG [114] Chitosan-PEG [117] PEG-NHS [140] | Hydrogel Hydrogel Hydrogel | Laceration model in rabbit liver and pig skin. Liver penetration model in rat. Hemorrhaging liver mouse model; rat skin incision model. | 30 s - 5 s | Rapid hemorrhage control; prevent from infection; good candidate for first-aid treatment of critical wound. Rapid hemostasis along with accelerated wound healing; excellent swelling and mechanical properties; low cytotoxicity. Excellent bioadhesive hydrogel; excellent hemostatic ability; possible alternative for sutures. |
| Polyurethane [106] | PU-chitosan [141] | Foam | Rat tail tip model | PU—23.9 min PU-chitosan—21.5 min | Possible alternative for topical hemostatic agents, chitosan added to PU decreases the bleeding time. |
| Cyanoacrylate | Octyl-cyanoacrylate [142] | Hydrogel | Porcine epistaxis model in pigs. | 259 s | Cost-effective. No follow-up required. |
| Polyethylene terephthalate | Oxygen- and nitrogen-treated PET coated with heparin [143] | - | In vitro study characterized by platelet adhesion in whole human blood using optical imaging techniques. | - | Oxygen-functionalized PET shows better hemostasis compared to nitrogen-functionalized PET. |
| Poly-2-oxazoline (POx) | POx-NHS [144] | Powder | Liver and spleen injury model of profuse bleedings in heparinized pigs. | 20–25 s | NHS-ester and hydrophilic groups required for better hemostatic application. |
| Polydioxanone (PDS) | PDS + Sesame oil/Castor oil/Almond oil/Carbowax 400 [145] | Putty | Rat penetrating liver model | - | Can be used as a bone sealant; effective to osseous hemorrhage; no irritation. |
| Name [Ref.] | Polymeric System | Form of Application | Hemostatic Efficacy (Based on Clinical Trials) | Reported Drawbacks |
|---|---|---|---|---|
| GelFoam®® [41] | Gelatin | Compressed sponge | Capable of absorbing up to 45 times its weight of whole blood. Hemostatic success in 10 min. | Abscess formation, breathing difficulties, fluid encapsulation. |
| Tachosil®® [154] | Equine collagen | Two-layer patch/sponge material with equine collagen on one side and fibrinogen-thrombin on the other side. | Hemostasis achieved in 3 min. | Hypertension, increased transaminases. |
| Surgicel®® [154,155] | Cellulose (ORC) | Loose knit absorbable powder | Hemostatic success in 5 min. | Foreign body reactions, tissue necrosis, nerve damage. |
| Traumastem®® [156] | Cellulose (ONRC) | Fibrous re-absorbable dressing | Hemostatic success in 10 min. | No adverse reactions reported. |
| InStat®®, HeliStat®® [154,157] | Bovine collagen | Dry absorbent hemostatic agent in microfibrillar form. | Hemostatic success in 5 min. Left in place, reabsorbed within 8 to 10 weeks. | Swelling and allergic reactions. |
| FloSeal®® [154,158] | Gelatin | Adjunct hemostat–collagen granules dispersed in human thrombin (syringe application). | Hemostatic success within 10 min. Forms mechanically stable clot. Reabsorbed within 8 to 10 weeks. | Rare reports of inflammatory responses. |
| Celox Rapid®® [11] Duraseal®® [154] TissuGlu®® [159] Tegaderm®® [160] | Polyurethane Chitosan Polyurethane PEG | Adhesive mesh. Hemostatic gauze. Adhesive sterile dressing. Absorbable hydrogel delivered by a dual-syringe applicator. | Adhesive crosslinking takes place in 30 to 40 min, allowing surgeons to reapproximate skin layer before the adhesive sets in. Can eliminate the need of post-surgical drains. Stops bleeding within one minute of compression. Stops bleeding within one minute of compression. Crosslinks immediately and creates watertight closure within 5 min. | Seroma formation, hematoma, immunological reactions, wound separation. Embolism, pain. Embolism. Pain. Renal compromise, inflammatory reactions, delayed healing. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghimire, S.; Sarkar, P.; Rigby, K.; Maan, A.; Mukherjee, S.; Crawford, K.E.; Mukhopadhyay, K. Polymeric Materials for Hemostatic Wound Healing. Pharmaceutics 2021, 13, 2127. https://doi.org/10.3390/pharmaceutics13122127
Ghimire S, Sarkar P, Rigby K, Maan A, Mukherjee S, Crawford KE, Mukhopadhyay K. Polymeric Materials for Hemostatic Wound Healing. Pharmaceutics. 2021; 13(12):2127. https://doi.org/10.3390/pharmaceutics13122127
Chicago/Turabian StyleGhimire, Suvash, Pritha Sarkar, Kasey Rigby, Aditya Maan, Santanu Mukherjee, Kaitlyn E. Crawford, and Kausik Mukhopadhyay. 2021. "Polymeric Materials for Hemostatic Wound Healing" Pharmaceutics 13, no. 12: 2127. https://doi.org/10.3390/pharmaceutics13122127
APA StyleGhimire, S., Sarkar, P., Rigby, K., Maan, A., Mukherjee, S., Crawford, K. E., & Mukhopadhyay, K. (2021). Polymeric Materials for Hemostatic Wound Healing. Pharmaceutics, 13(12), 2127. https://doi.org/10.3390/pharmaceutics13122127








