Comparison of Synthetic Membranes to Heat-Separated Human Epidermis in Skin Permeation Studies In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Sample Preparations
2.2.2. Preparation of Heat-Separated Epidermis
2.2.3. Franz Diffusion Cell Method
2.2.4. Skin PAMPA Method
2.2.5. Permeation Analysis
2.2.6. Statistical Analysis
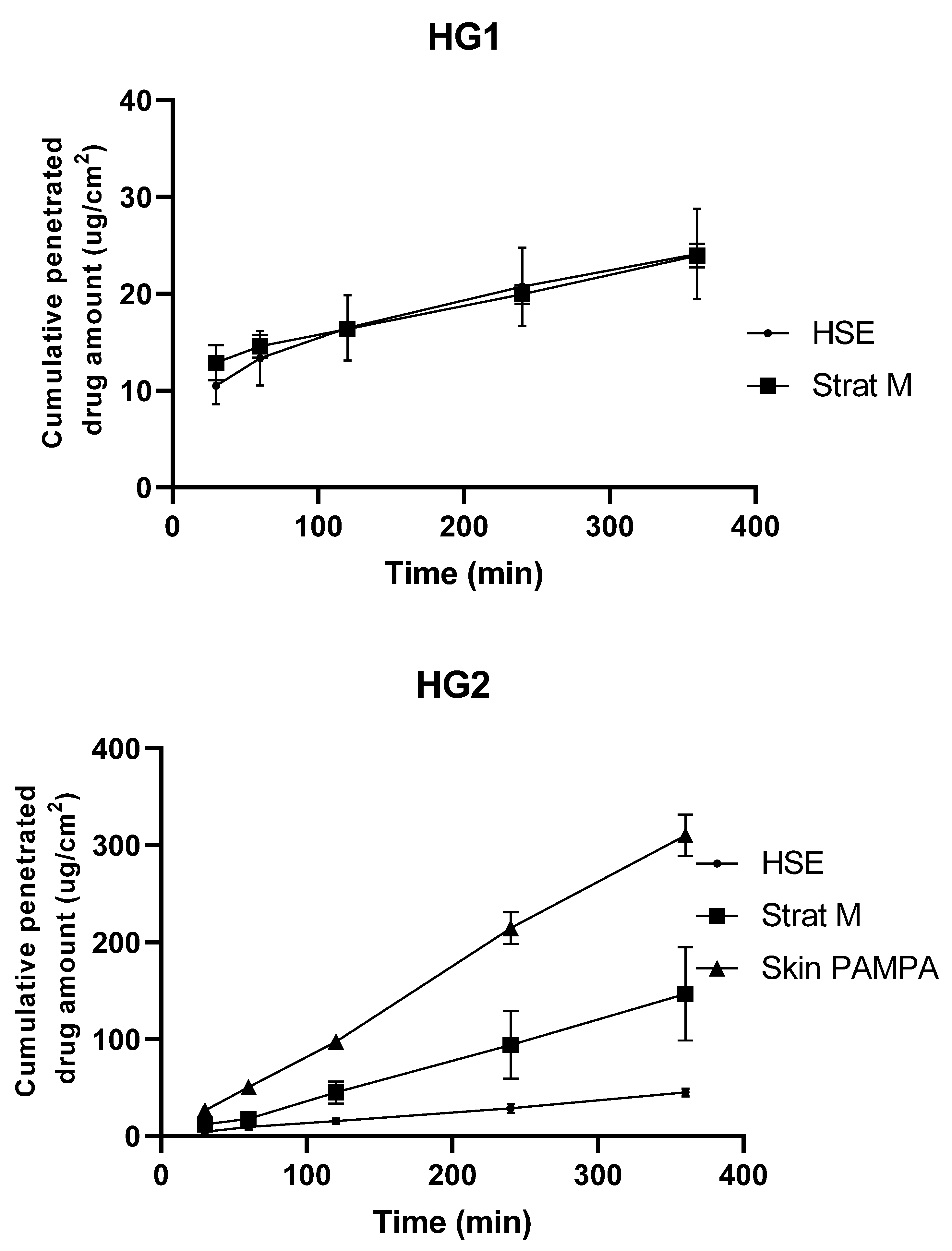
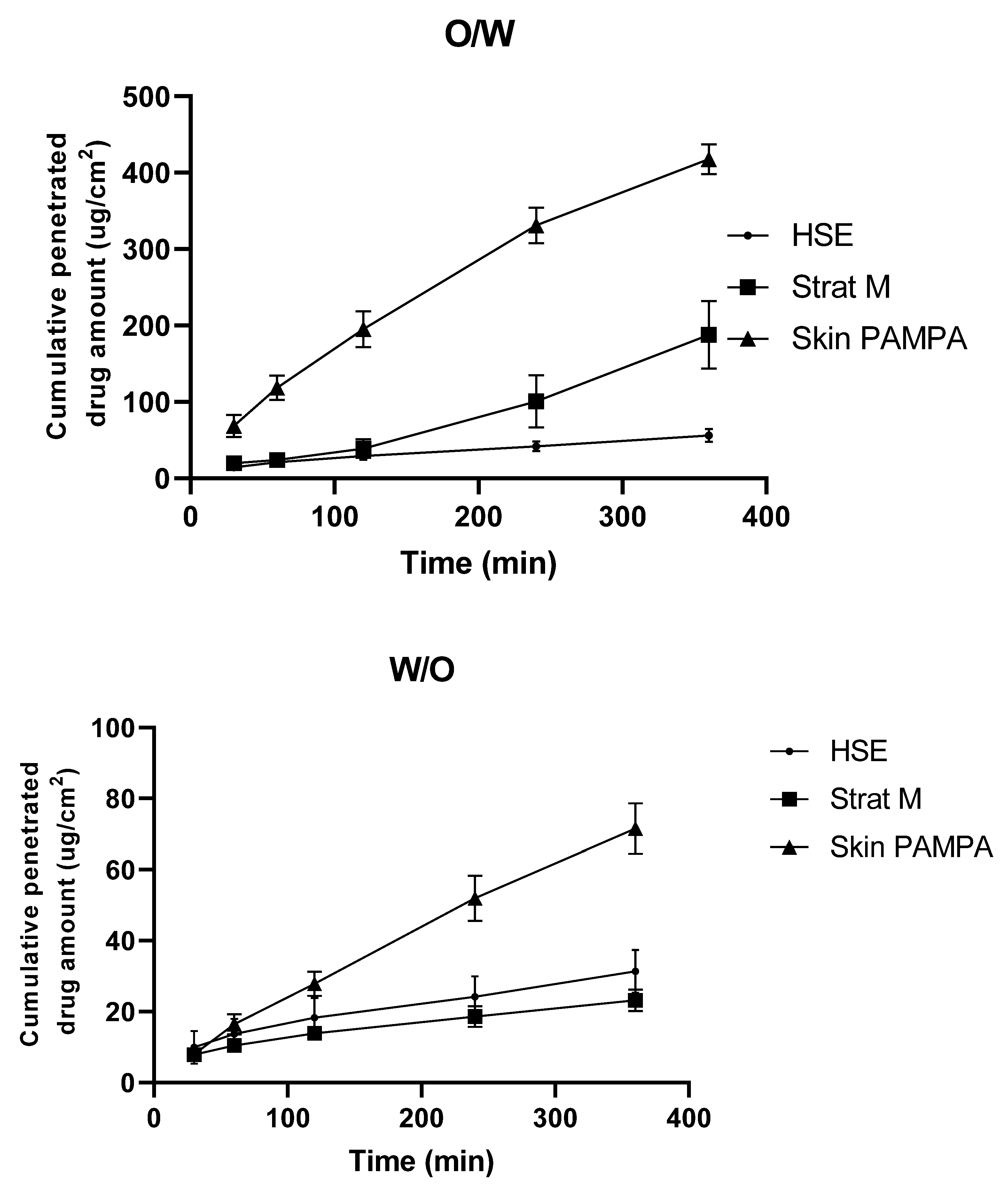
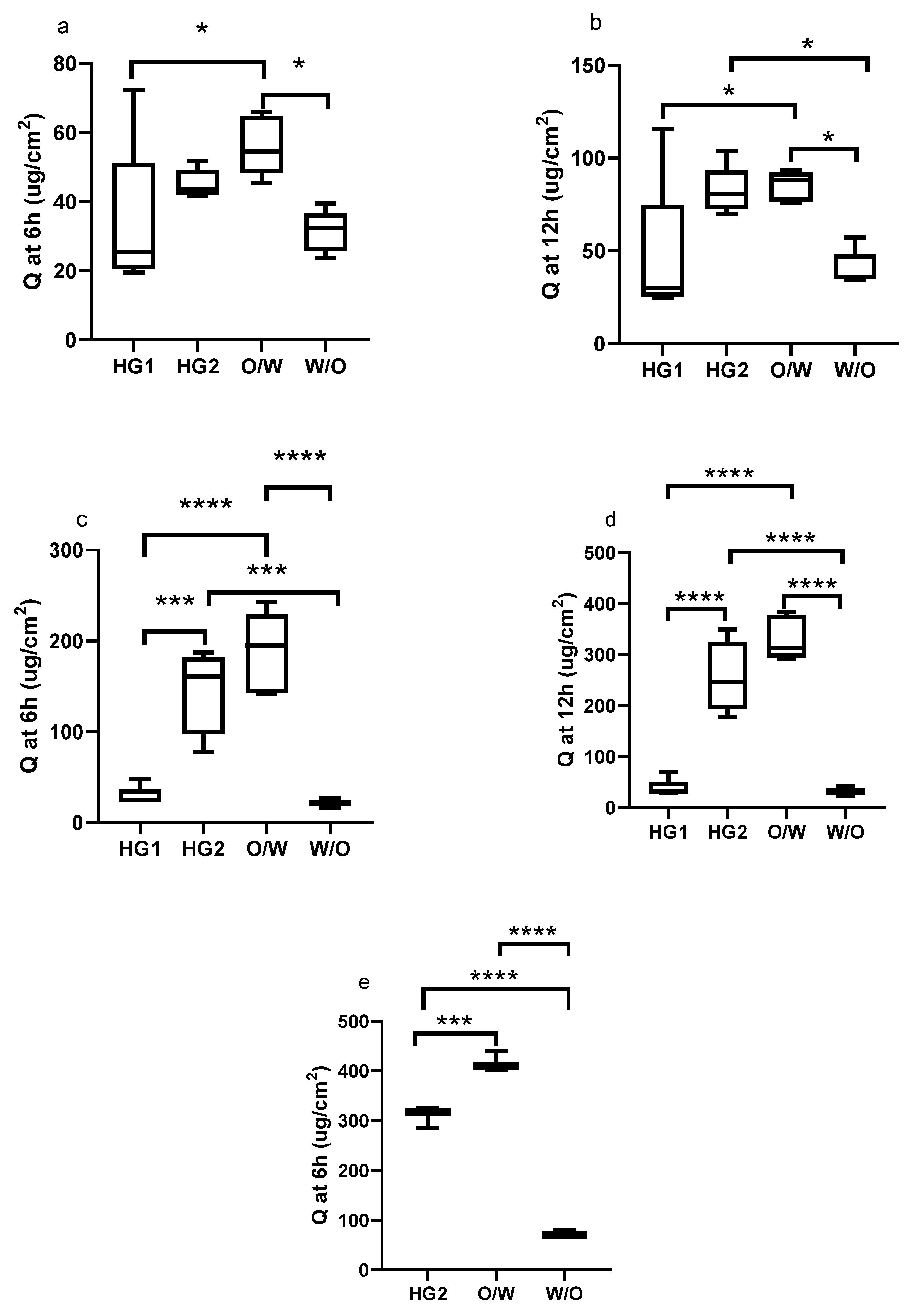
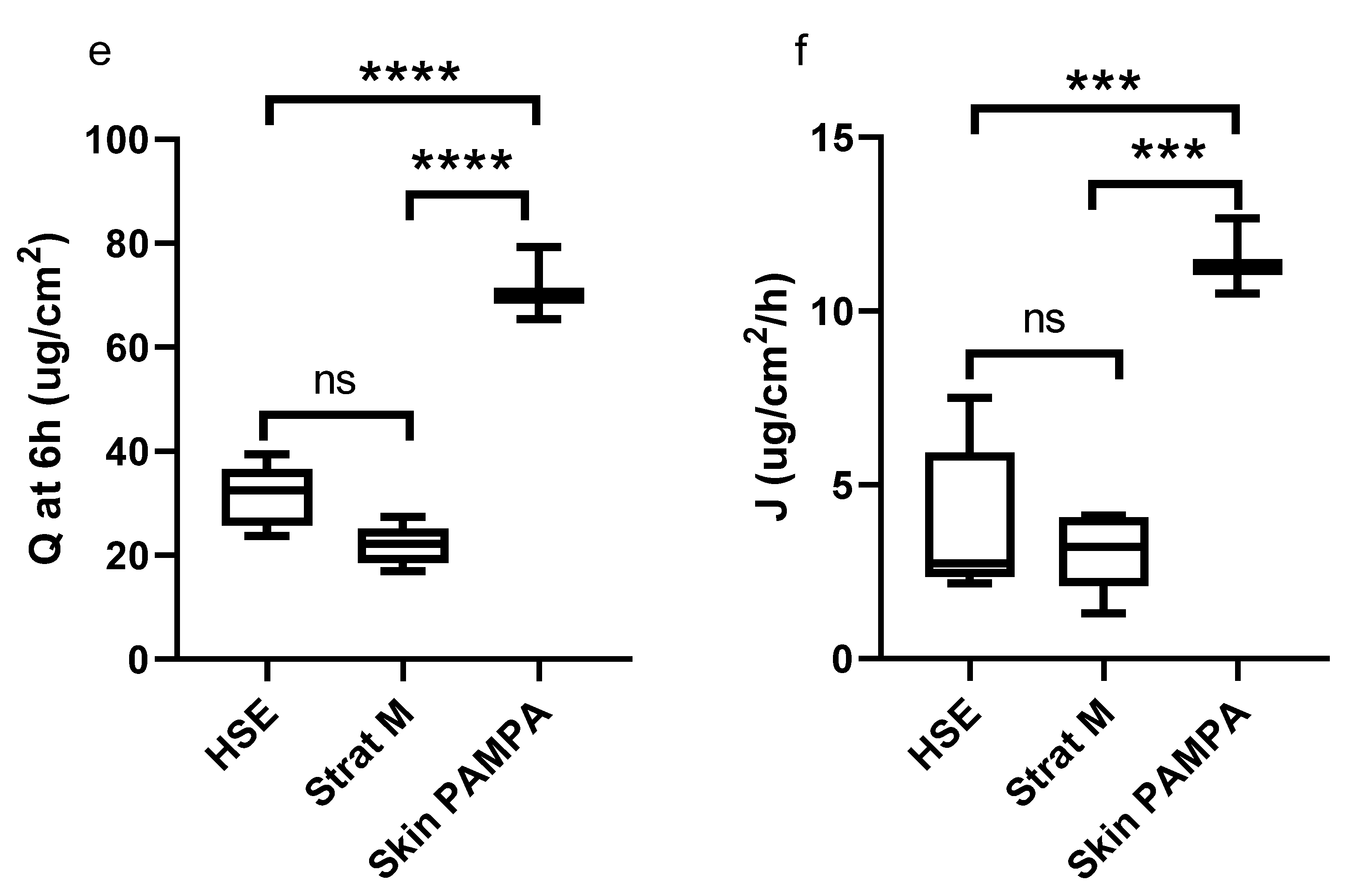
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dragicevic, N.; Maibach, H.I. (Eds.) Percutaneous Penetration Enhancers Drug Penetration into/through the Skin: Methodology and General Considerations; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 978-3-662-53268-3. [Google Scholar]
- Prausnitz, M.R.; Elias, P.M.; Franz, T.J.; Schmuth, M.; Tsai, J.-C.; Menon, G.K.; Holleran, W.M.; Feingold, K.R. 124. Skin Barrier and Transdermal Drug Delivery. Available online: https://drugdelivery.chbe.gatech.edu/Papers/2012/Prausnitz%20Derm%20Book%20Chapter%202012.pdf (accessed on 26 Novmber 2021).
- Ueda, C.T.; Shah, V.P.; Derdzinski, K.; Ewing, G.; Flynn, G.; Maibach, H.; Marques, M.; Rytting, H.; Shaw, S.; Thakker, K.; et al. Topical and Transdermal Drug Products. Dissolution Technol. 2010, 17, 12–25. [Google Scholar] [CrossRef]
- Draft Guideline on Quality and Equivalence of Topical Products EMA/CHMP/QWP/708282/2018; Committee for Medicinal Products for Human Use: Amsterdam, The Netherlands, 2018.
- OECD. Guidance Notes on Dermal Absorption, Series on Testing and Assessment No. 156, 2011th ed.; OECD: Paris, France, 2011. [Google Scholar]
- OECD. Test Guideline 427: Skin Absorption: In Vivo Method, 2004th ed.; OECD: Paris, France, 2004. [Google Scholar]
- OECD. Test Guideline 428: Skin Absorption: In Vitro Method, 2004th ed.; OECD: Paris, France, 2004. [Google Scholar]
- OECD. Guidance Document for the Conduct of Skin Absorption Studies No. 28, 2004th ed.; OECD: Paris, France, 2004. [Google Scholar]
- Franz, T.J. Percutaneous Absorption on the Relevance of in Vitro Data. J. Investig. Dermatol. 1975, 64, 190–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godin, B.; Touitou, E. Transdermal Skin Delivery: Predictions for Humans from In Vivo, Ex Vivo and Animal Models. Adv. Drug Deliv. Rev. 2007, 59, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Kamel, R. Transdermal Drug Delivery: Benefits and Challenges. J. App. Pharm. 2016, 8, e103. [Google Scholar] [CrossRef]
- Abd, E.; Yousuf, S.A.; Pastore, M.N.; Telaprolu, K.; Mohammed, Y.H.; Namjoshi, S.; Grice, J.E.; Roberts, M.S. Skin Models for the Testing of Transdermal Drugs. CPAA Clin. Pharmacol. Adv. Appl. 2016, 8, 163–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flaten, G.E.; Palac, Z.; Engesland, A.; Filipović-Grčić, J.; Vanić, Ž.; Škalko-Basnet, N. In Vitro Skin Models as a Tool in Optimization of Drug Formulation. Eur. J. Pharm. Sci. 2015, 75, 10–24. [Google Scholar] [CrossRef] [Green Version]
- Haq, A.; Dorrani, M.; Goodyear, B.; Joshi, V.; Michniak-Kohn, B. Membrane Properties for Permeability Testing: Skin versus Synthetic Membranes. Int. J. Pharm. 2018, 539, 58–64. [Google Scholar] [CrossRef]
- Neupane, R.; Boddu, S.H.S.; Renukuntla, J.; Babu, R.J.; Tiwari, A.K. Alternatives to Biological Skin in Permeation Studies: Current Trends and Possibilities. Pharmaceutics 2020, 12, 152. [Google Scholar] [CrossRef] [Green Version]
- Wiedersberg, S.; Guy, R.H. Transdermal Drug Delivery: 30+ Years of War and Still Fighting! J. Control. Release 2014, 190, 150–156. [Google Scholar] [CrossRef] [Green Version]
- Zsikó, S.; Csányi, E.; Kovács, A.; Budai-Szűcs, M.; Gácsi, A.; Berkó, S. Methods to Evaluate Skin Penetration In Vitro. Sci. Pharm. 2019, 87, 19. [Google Scholar] [CrossRef] [Green Version]
- Arce, F.J.; Asano, N.; See, G.L.; Itakura, S.; Todo, H.; Sugibayashi, K. Usefulness of Artificial Membrane, Strat-M®, in the Assessment of Drug Permeation from Complex Vehicles in Finite Dose Conditions. Pharmaceutics 2020, 12, 173. [Google Scholar] [CrossRef] [Green Version]
- Bolla, P.K.; Clark, B.A.; Juluri, A.; Cheruvu, H.S.; Renukuntla, J. Evaluation of Formulation Parameters on Permeation of Ibuprofen from Topical Formulations Using Strat-M® Membrane. Pharmaceutics 2020, 12, 151. [Google Scholar] [CrossRef] [Green Version]
- Kaur, L.; Singh, K.; Paul, S.; Singh, S.; Singh, S.; Jain, S.K. A Mechanistic Study to Determine the Structural Similarities between Artificial Membrane Strat-MTM and Biological Membranes and Its Application to Carry out Skin Permeation Study of Amphotericin B Nanoformulations. AAPS PharmSciTech 2018, 19, 1606–1624. [Google Scholar] [CrossRef]
- Uchida, T.; Kadhum, W.R.; Kanai, S.; Todo, H.; Oshizaka, T.; Sugibayashi, K. Prediction of Skin Permeation by Chemical Compounds Using the Artificial Membrane, Strat-MTM. Eur. J. Pharm. Sci. 2015, 67, 113–118. [Google Scholar] [CrossRef] [Green Version]
- Sinkó, B.; Garrigues, T.M.; Balogh, G.T.; Nagy, Z.K.; Tsinman, O.; Avdeef, A.; Takács-Novák, K. Skin-PAMPA: A New Method for Fast Prediction of Skin Penetration. Eur. J. Pharm. Sci. 2012, 45, 698–707. [Google Scholar] [CrossRef]
- Lee, W.-R.; Hsiao, C.-Y.; Huang, T.-H.; Wang, C.-L.; Alalaiwe, A.; Chen, E.-L.; Fang, J.-Y. Post-Irradiation Recovery Time Strongly Influences Fractional Laser-Facilitated Skin Absorption. Int. J. Pharm. 2019, 564, 48–58. [Google Scholar] [CrossRef]
- Zsikó, S.; Csányi, E.; Kovács, A.; Budai-Szűcs, M.; Gácsi, A.; Berkó, S. Novel In Vitro Investigational Methods for Modeling Skin Permeation: Skin PAMPA, Raman Mapping. Pharmaceutics 2020, 12, 803. [Google Scholar] [CrossRef]
- Kligman, A.M.; Christophers, E. Preparation of Isolated Sheets of Human Stratum Corneum. Arch. Dermatol. 1963, 88, 702–705. [Google Scholar] [CrossRef] [PubMed]
- Zsikó, S.; Cutcher, K.; Kovács, A.; Budai-Szűcs, M.; Gácsi, A.; Baki, G.; Csányi, E.; Berkó, S. Nanostructured Lipid Carrier Gel for the Dermal Application of Lidocaine: Comparison of Skin Penetration Testing Methods. Pharmaceutics 2019, 11, 310. [Google Scholar] [CrossRef] [Green Version]
- Simon, A.; Amaro, M.I.; Healy, A.M.; Cabral, L.M.; de Sousa, V.P. Comparative Evaluation of Rivastigmine Permeation from a Transdermal System in the Franz Cell Using Synthetic Membranes and Pig Ear Skin with In Vivo-In Vitro Correlation. Int. J. Pharm. 2016, 512, 234–241. [Google Scholar] [CrossRef]
- Milanowski, B.; Wosicka-Frąckowiak, H.; Główka, E.; Sosnowska, M.; Woźny, S.; Stachowiak, F.; Suchenek, A.; Wilkowski, D. Optimization and Evaluation of the In Vitro Permeation Parameters of Topical Products with Non-Steroidal Anti-Inflammatory Drugs through Strat-M® Membrane. Pharmaceutics 2021, 13, 1305. [Google Scholar] [CrossRef]
- Nair, R.S.; Billa, N.; Leong, C.-O.; Morris, A.P. An Evaluation of Tocotrienol Ethosomes for Transdermal Delivery Using Strat-M® Membrane and Excised Human Skin. Pharm. Dev. Technol. 2021, 26, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Vizserálek, G.; Berkó, S.; Tóth, G.; Balogh, R.; Budai-Szűcs, M.; Csányi, E.; Sinkó, B.; Takács-Novák, K. Permeability Test for Transdermal and Local Therapeutic Patches Using Skin PAMPA Method. Eur. J. Pharm. Sci. 2015, 76, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Patel, A.; Sinko, B.; Bell, M.; Wibawa, J.; Hadgraft, J.; Lane, M.E. A Comparative Study of the in Vitro Permeation of Ibuprofen in Mammalian Skin, the PAMPA Model and Silicone Membrane. Int. J. Pharm. 2016, 505, 14–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Smeden, J.; Hoppel, L.; van der Heijden, R.; Hankemeier, T.; Vreeken, R.J.; Bouwstra, J.A. LC/MS Analysis of Stratum Corneum Lipids: Ceramide Profiling and Discovery. J. Lipid Res. 2011, 52, 1211–1221. [Google Scholar] [CrossRef] [Green Version]
- Köllmer, M.; Mossahebi, P.; Sacharow, E.; Gorissen, S.; Gräfe, N.; Evers, D.-H.; Herbig, M.E. Investigation of the Compatibility of the Skin PAMPA Model with Topical Formulation and Acceptor Media Additives Using Different Assay Setups. AAPS PharmSciTech 2019, 20, 89. [Google Scholar] [CrossRef]


| Hydrogel No1 HG1 | Hydrogel No2 HG2 | o/w Cream O/W | w/o Cream W/O | ||||
|---|---|---|---|---|---|---|---|
| Component | % | Component | % | Component | % | Component | % |
| Diclofenac sodium | 1 | Diclofenac sodium | 1 | Diclofenac sodium | 1 | Diclofenac sodium | 1 |
| Methocel E4M | 3 | Methocel E4M | 3 | Cetostearyl alcohol | 4 | White beeswax | 10 |
| Propylene glycol | 50 | Ethanol 96 w/w% | 30 | Liquid paraffin | 12 | Wool fat | 10 |
| Purified water | 46 | Purified water | 66 | Polysorbate 60 | 4 | Oleyl oleate | 5 |
| White Petrolatum | 20 | Castor oil | 40 | ||||
| Purified water | 59 | Purified water | 29 | ||||
| Pearson r | |||
| HG1 | |||
| HSE | Strat-M | Skin PAMPA | |
| HSE | - | 0.9890 | - |
| Strat-M | 0.9890 | - | - |
| Skin PAMPA | - | - | - |
| HG2 | |||
| HSE | Strat-M | Skin PAMPA | |
| HSE | - | 0.9970 | 0.9964 |
| Strat-M | 0.9970 | - | 0.9981 |
| Skin PAMPA | 0.9964 | 0.9981 | - |
| O/W | |||
| HSE | Strat-M | Skin PAMPA | |
| HSE | - | 0.9670 | 0.9956 |
| Strat-M | 0.9670 | - | 0.9521 |
| Skin PAMPA | 0.9956 | 0.9521 | - |
| W/O | |||
| HSE | Strat-M | Skin PAMPA | |
| HSE | - | 0.9993 | 0.99583 |
| Strat-M | 0.9993 | - | 0.9967 |
| Skin PAMPA | 0.9958 | 0.9967 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovács, A.; Zsikó, S.; Falusi, F.; Csányi, E.; Budai-Szűcs, M.; Csóka, I.; Berkó, S. Comparison of Synthetic Membranes to Heat-Separated Human Epidermis in Skin Permeation Studies In Vitro. Pharmaceutics 2021, 13, 2106. https://doi.org/10.3390/pharmaceutics13122106
Kovács A, Zsikó S, Falusi F, Csányi E, Budai-Szűcs M, Csóka I, Berkó S. Comparison of Synthetic Membranes to Heat-Separated Human Epidermis in Skin Permeation Studies In Vitro. Pharmaceutics. 2021; 13(12):2106. https://doi.org/10.3390/pharmaceutics13122106
Chicago/Turabian StyleKovács, Anita, Stella Zsikó, Fanni Falusi, Erzsébet Csányi, Mária Budai-Szűcs, Ildikó Csóka, and Szilvia Berkó. 2021. "Comparison of Synthetic Membranes to Heat-Separated Human Epidermis in Skin Permeation Studies In Vitro" Pharmaceutics 13, no. 12: 2106. https://doi.org/10.3390/pharmaceutics13122106
APA StyleKovács, A., Zsikó, S., Falusi, F., Csányi, E., Budai-Szűcs, M., Csóka, I., & Berkó, S. (2021). Comparison of Synthetic Membranes to Heat-Separated Human Epidermis in Skin Permeation Studies In Vitro. Pharmaceutics, 13(12), 2106. https://doi.org/10.3390/pharmaceutics13122106







