In Vitro Activity of Monofunctional Pt-II Complex Based on 8-Aminoquinoline against Human Glioblastoma
Abstract
:1. Introduction
2. Materials and Methods
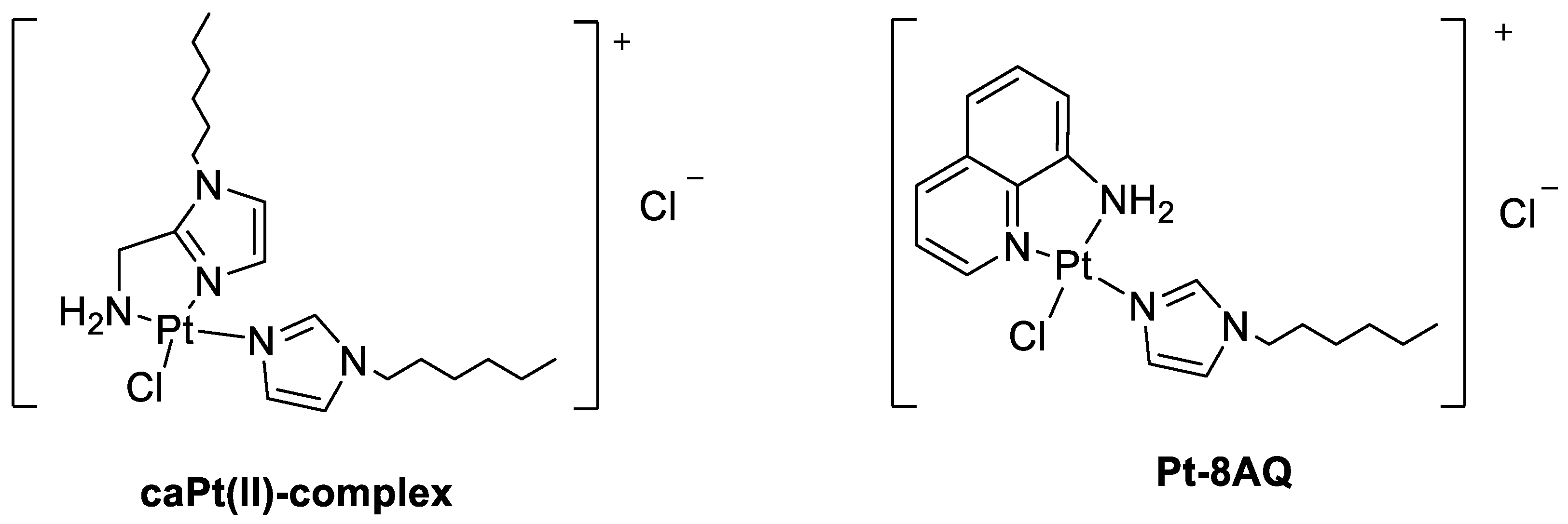
2.1. Drugs and Tumor Cells
2.2. Proliferation Assay, Cytotoxic Assay, and In Vitro Stability of Drugs
2.3. Interaction of Pt-8AQ with GSH, 9-EtG, and Mets7
2.4. RT-PCR Analysis and Primers
2.5. Cell Cycle Analysis
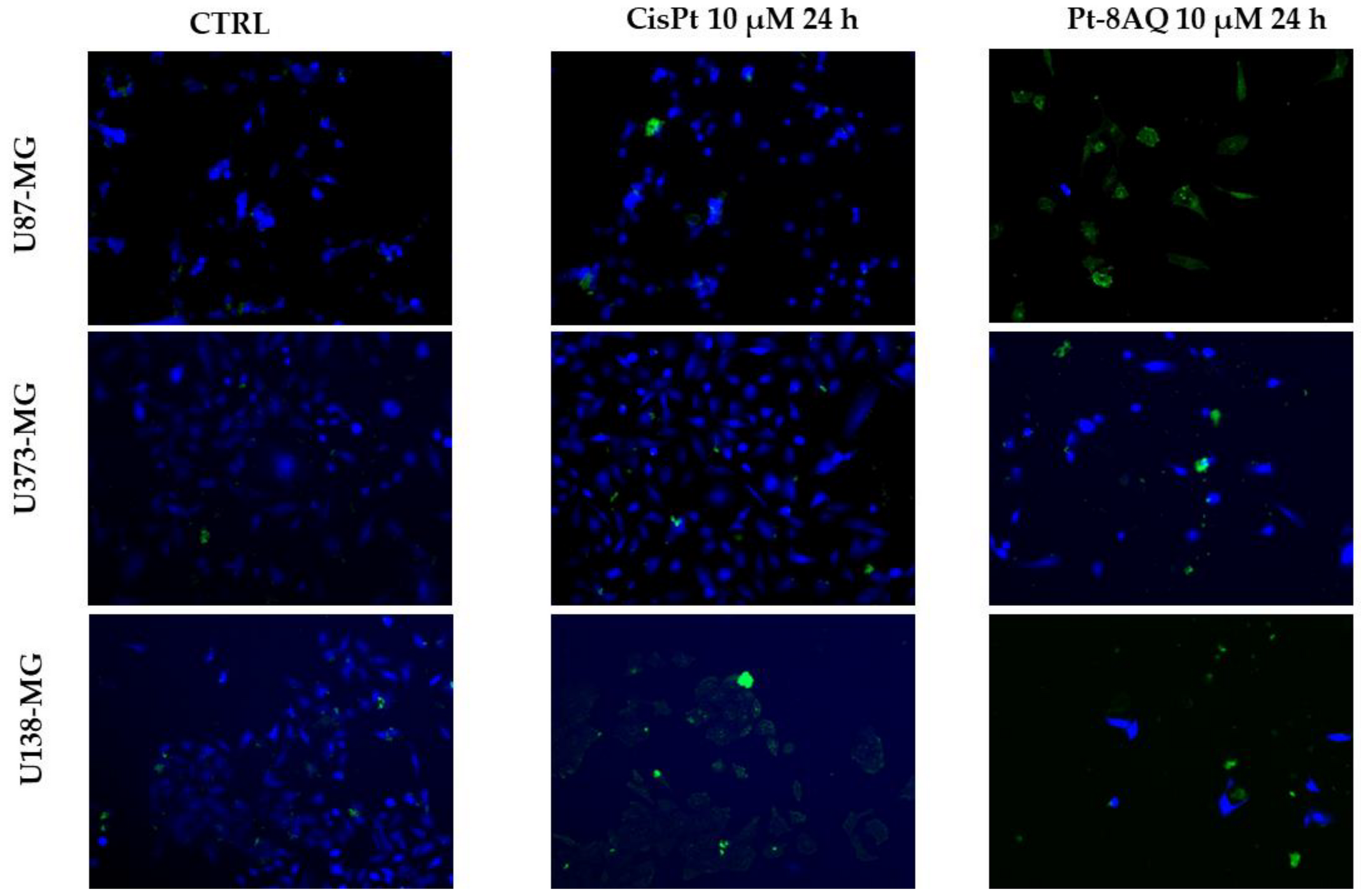
2.6. Apoptosis/Necrosis in Glioblastoma Cell Lines
2.7. Statistical Analysis
3. Results
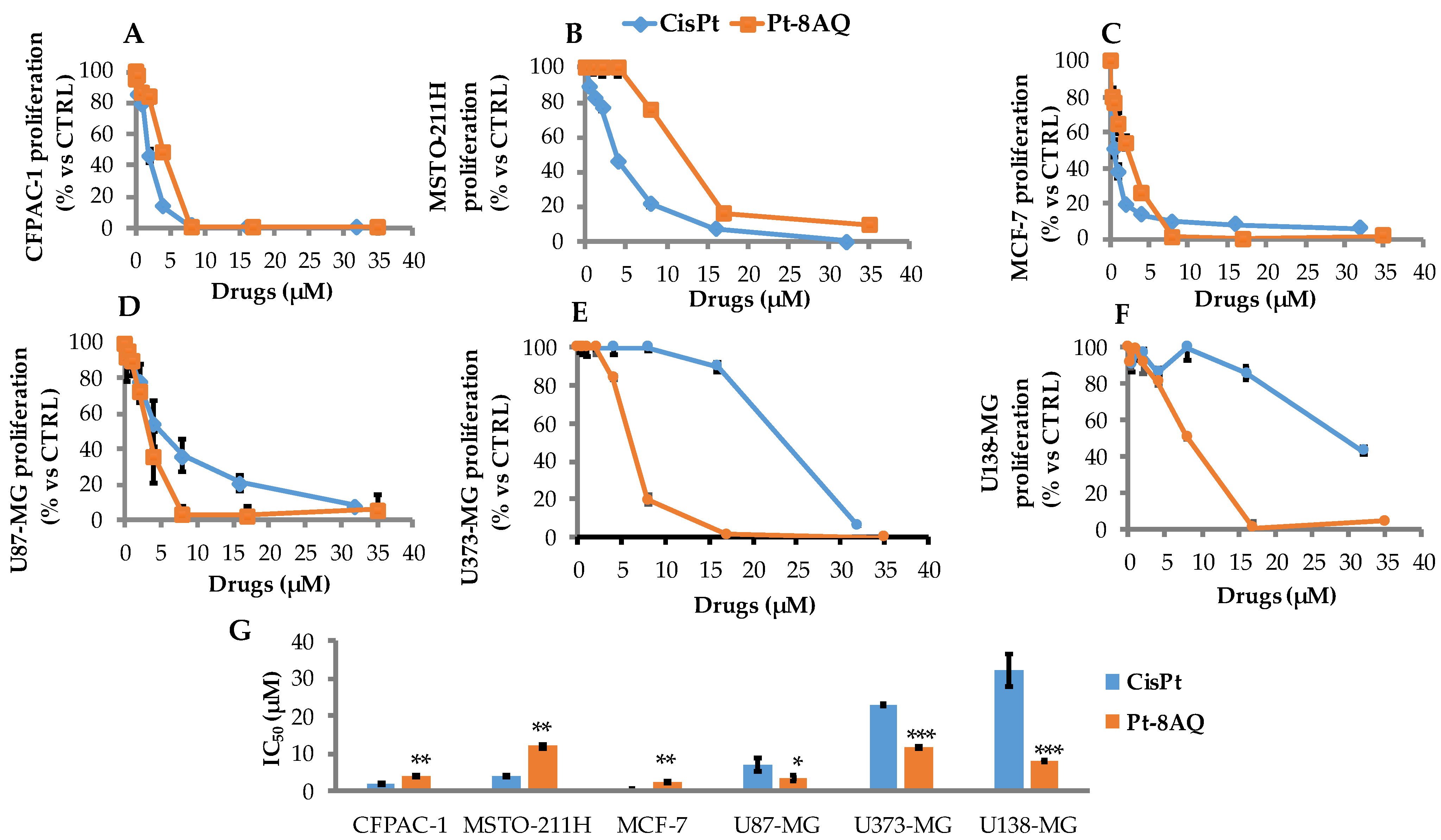
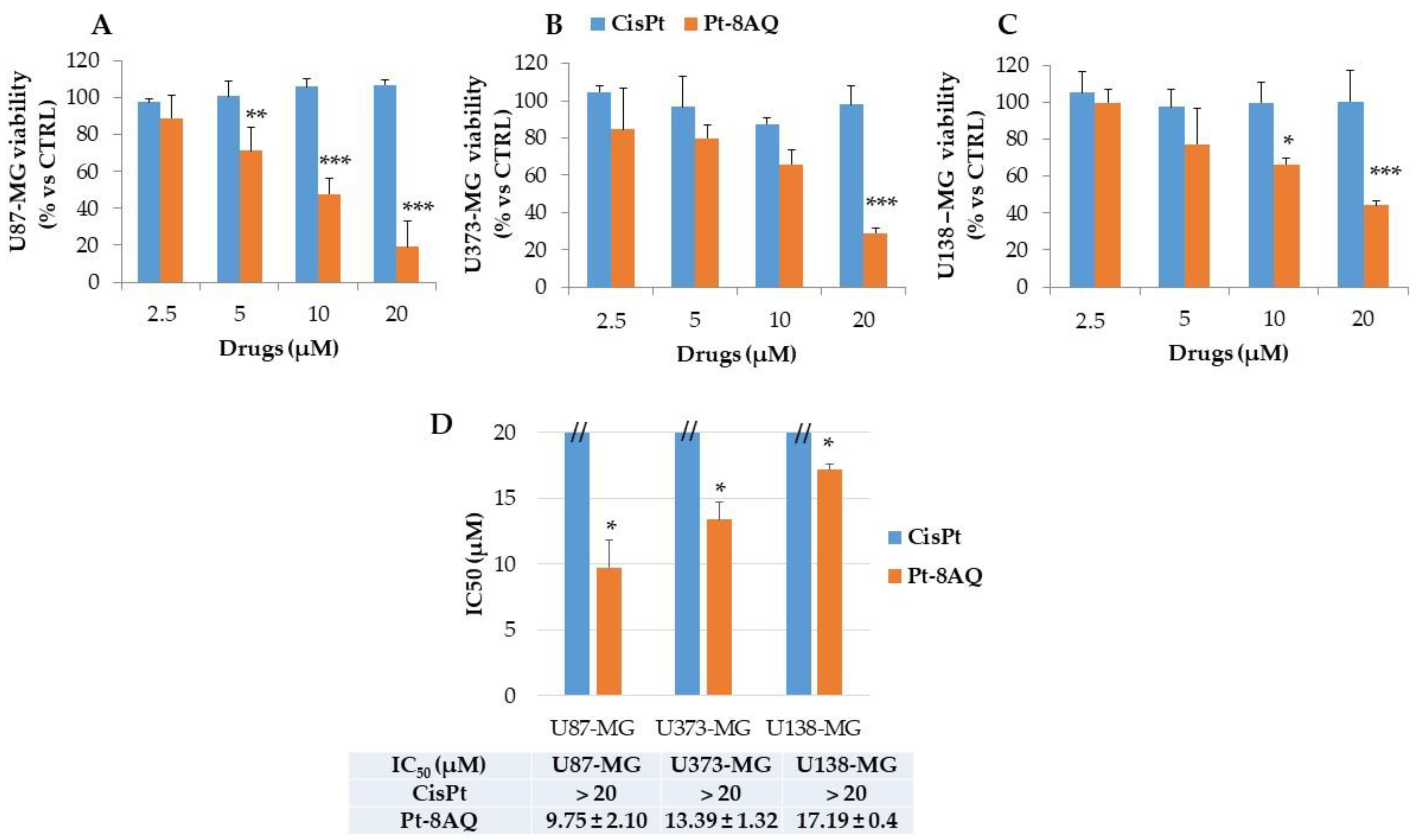
3.1. In Vitro Antitumor Activity of CisPt and Pt-8AQ on Different Cancer Cell Lines
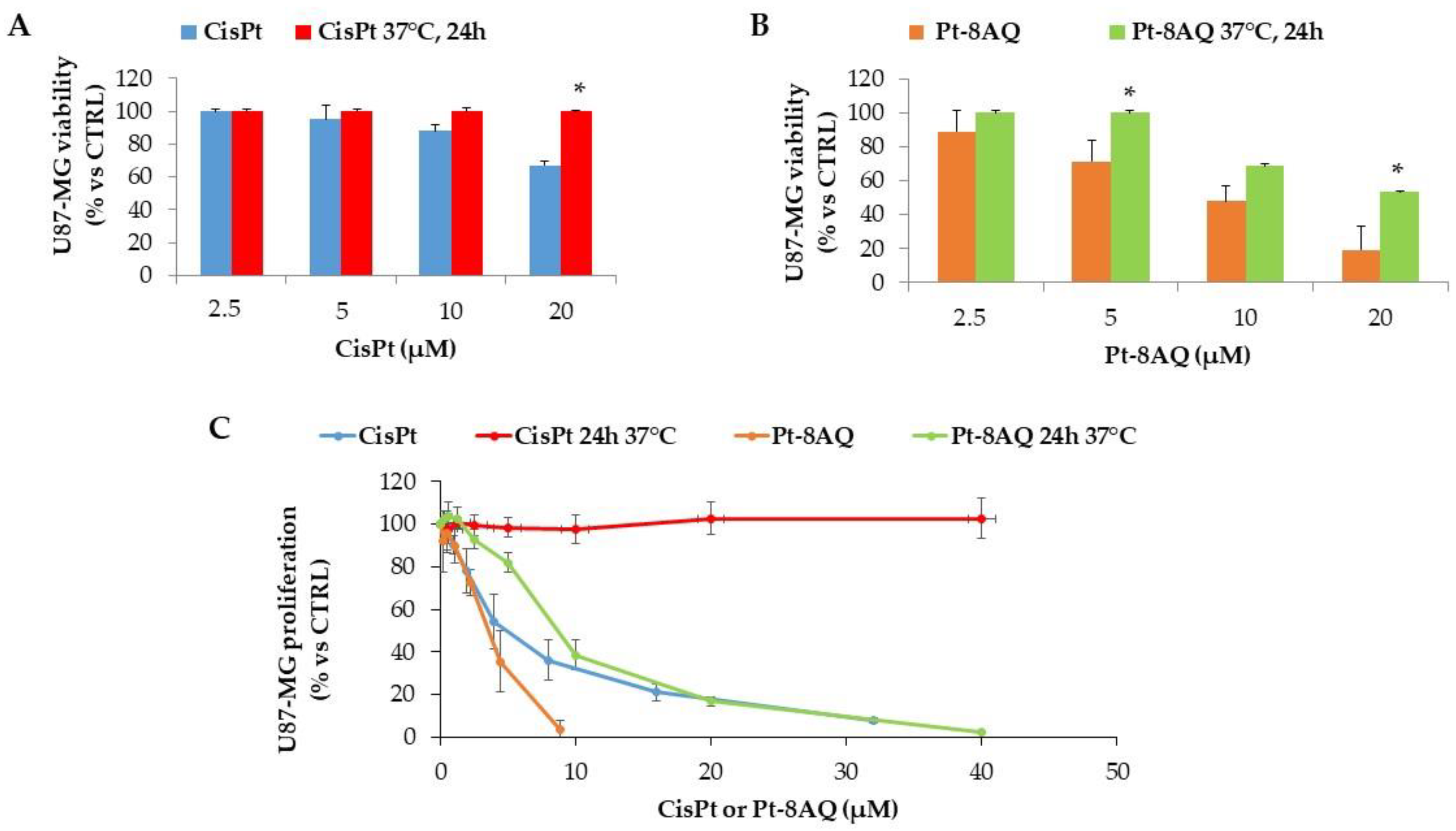
3.2. In Vitro Stability of CisPt and Pt-8AQ to Treatment at 37 °C
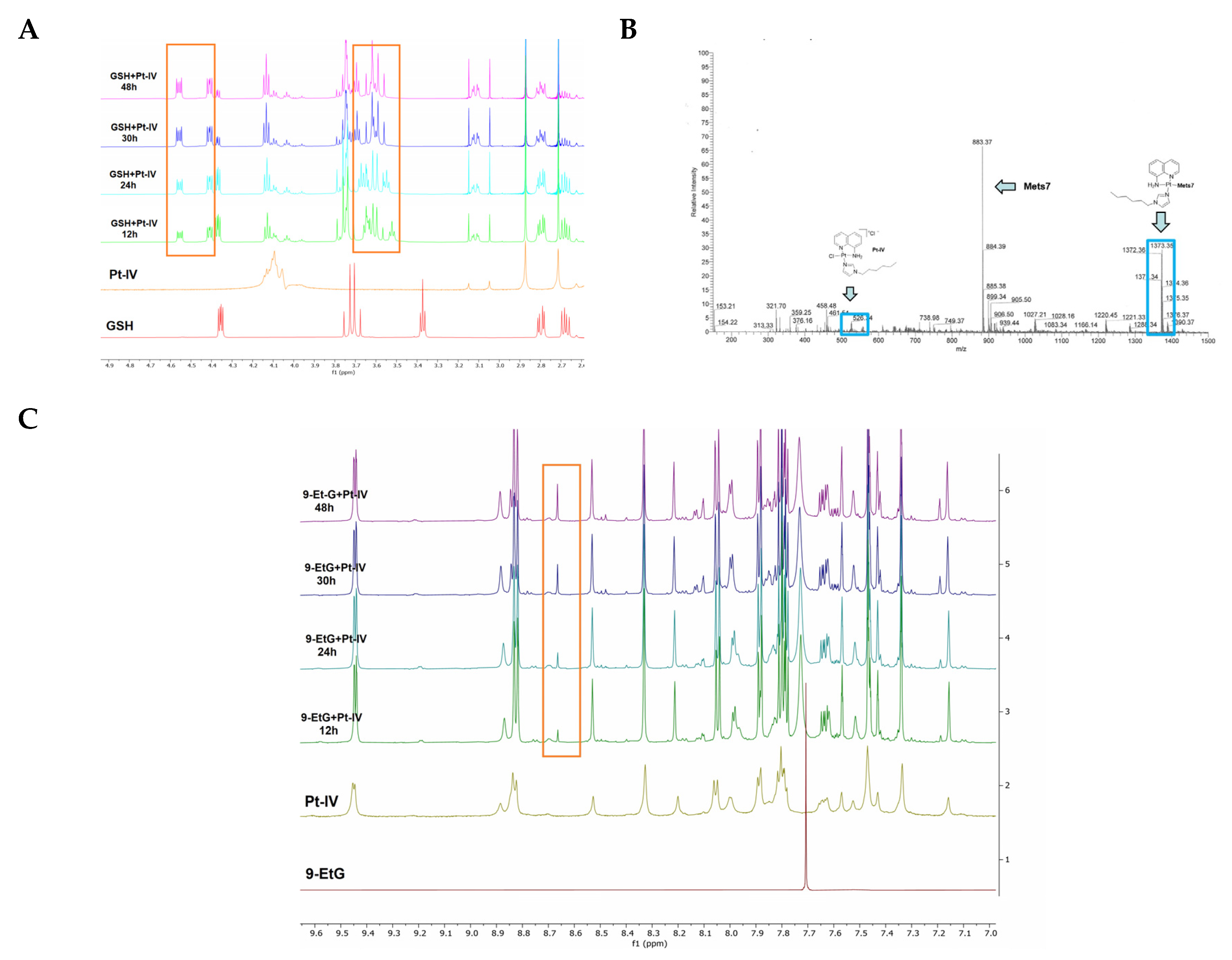
3.3. Study of Interaction between Pt-8AQ, GSH, 9-EtG, and Mets7
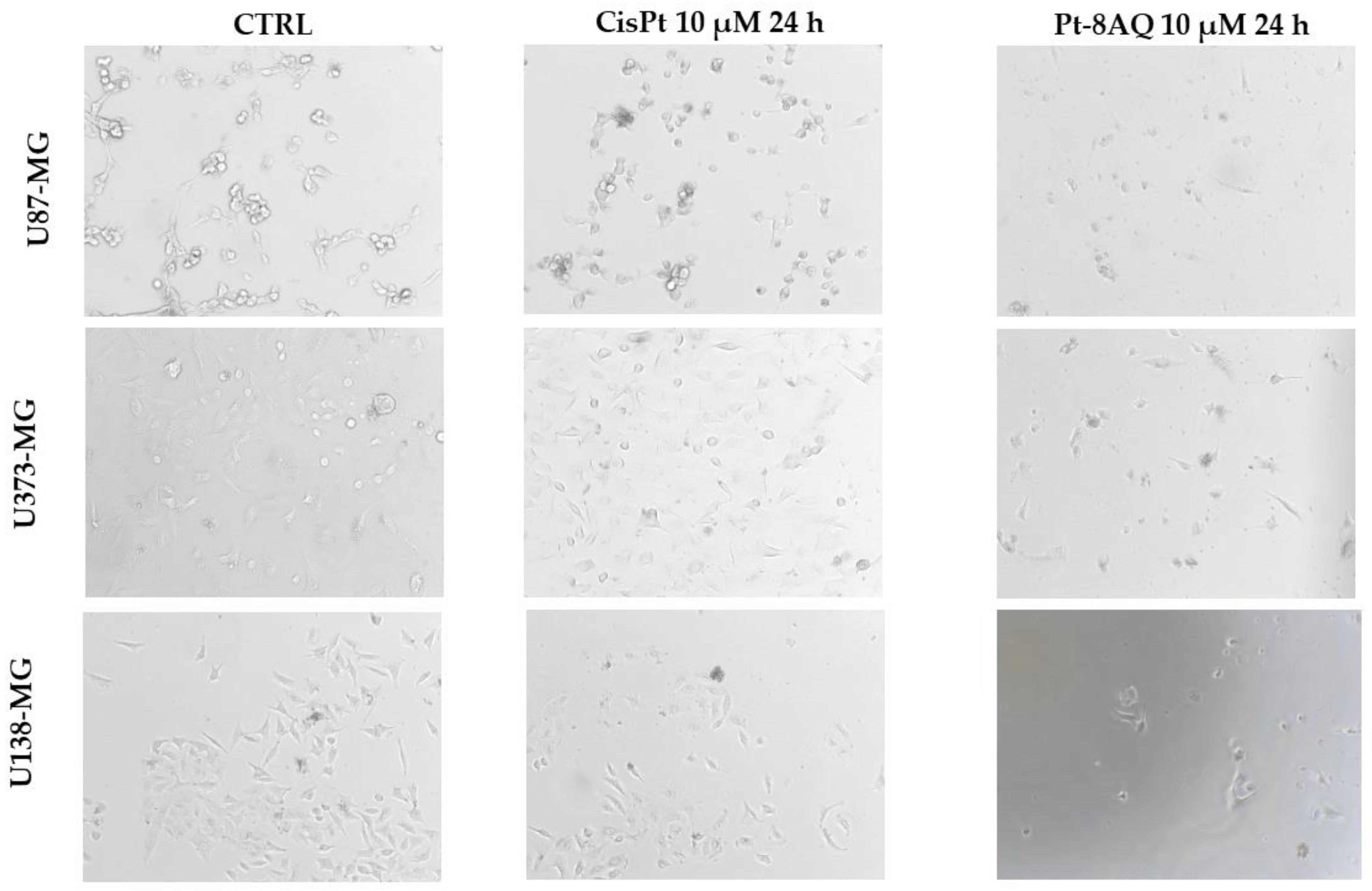
3.4. Cytotoxicity Activity of Pt-8AQ on Glioblastoma Cell Lines
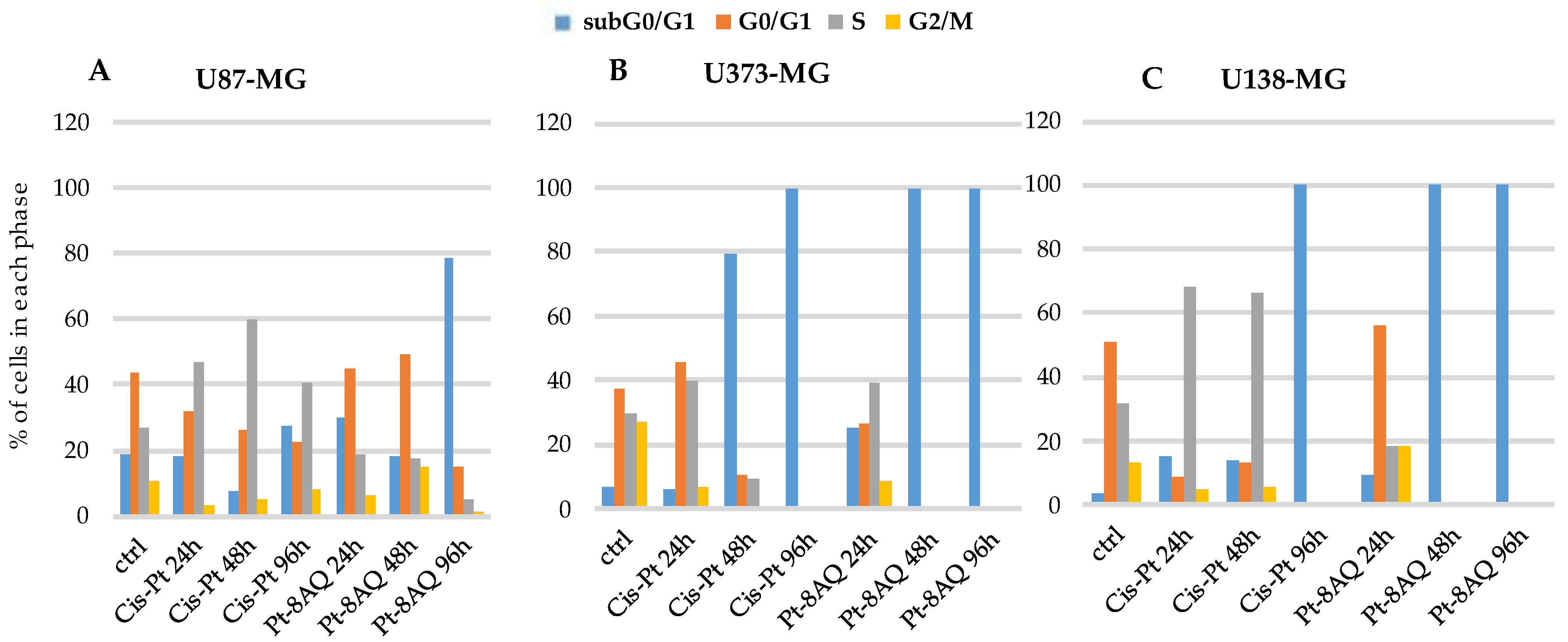
3.5. Effect on Cell Cycle of Glioblastoma Cell Lines
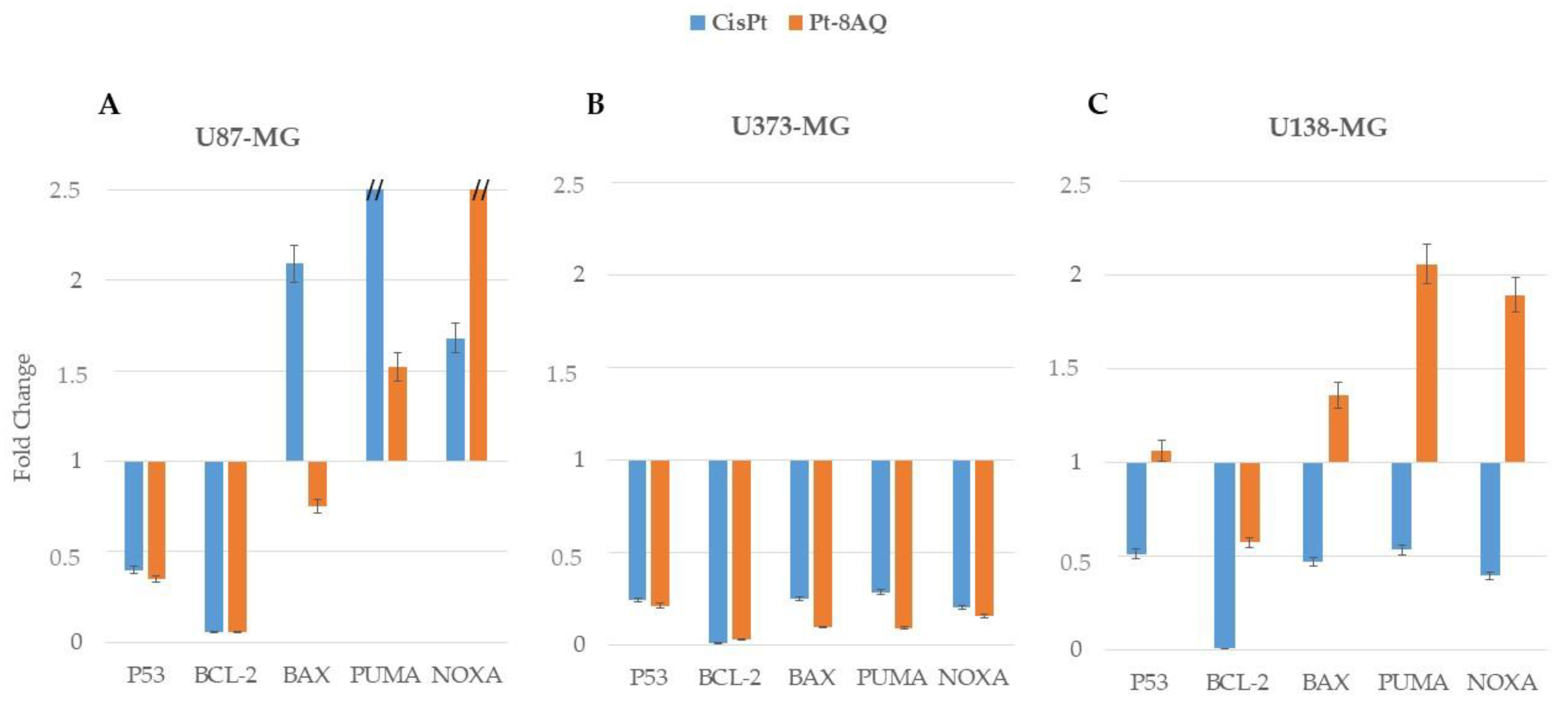
3.6. Pro-Apoptotic Gene Expression and Apoptosis in Glioblastoma Cell Lines
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ghosh, S. Cisplatin: The first metal based anticancer drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef]
- Rottenberg, S.; Disler, C.; Perego, P. The rediscovery of platinum-based cancer therapy. Nat. Rev. Cancer 2021, 21, 37–50. [Google Scholar] [CrossRef]
- Zhou, J.; Kang, Y.; Chen, L.; Wang, H.; Liu, J.; Zeng, S.; Yu, L. The Drug-Resistance Mechanisms of Five Platinum-Based Antitumor Agents. Front. Pharmacol. 2020, 11, 343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Fang, L.; Zhao, J.; Gou, S. Insight into the antitumor actions of sterically hindered platinum(ii) complexes by a combination of STD NMR and LCMS techniques†. Metallomics 2020, 12, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, A.; Caligiuri, R.; Godbert, N.; Ricciardi, L.; La Deda, M.; Ghedini, M.; Ferri, N.; Lupo, M.G.; Facchetti, G.; Rimoldi, I.; et al. Cytotoxic performances of new anionic cyclometalated Pt(II) complexes bearing chelated O^O ligands. Appl. Organomet. Chem. 2020, 34, e5647. [Google Scholar] [CrossRef]
- Facchetti, G.; Rimoldi, I. Anticancer platinum(II) complexes bearing N-heterocycle rings. Bioorg. Med. Chem. Lett. 2019, 29, 1257–1263. [Google Scholar] [CrossRef]
- Rimoldi, I.; Facchetti, G.; Lucchini, G.; Castiglioni, E.; Marchianò, S.; Ferri, N. In vitro anticancer activity evaluation of new cationic platinum(II) complexes based on imidazole moiety. Bioorg. Med. Chem. 2017, 25, 1907–1913. [Google Scholar] [CrossRef] [PubMed]
- Facchetti, G.; Ferri, N.; Lupo, M.G.; Giorgio, L.; Rimoldi, I. Monofunctional PtII Complexes Based on 8-Aminoquinoline: Synthesis and Pharmacological Characterization. Eur. J. Inorg. Chem. 2019, 2019, 3389–3395. [Google Scholar] [CrossRef]
- Sacco, F.; Tarchi, M.; Ferraro, G.; Merlino, A.; Facchetti, G.; Rimoldi, I.; Messori, L.; Massai, L. Reactions with Proteins of Three Novel Anticancer Platinum(II) Complexes Bearing N-Heterocyclic Ligands. Int. J. Mol. Sci. 2021, 22, 10551. [Google Scholar] [CrossRef]
- Ratchanok, P.; Apilak, W.; Veda, P.; Supaluk, P.; Somsak, R.; Virapong, P. Transition Metal Complexes of 8-Aminoquinoline-5-Substituted Uracils with Antioxidative and Cytotoxic Activities. Lett. Drug Des. Discov. 2013, 10, 859–864. [Google Scholar] [CrossRef]
- Casado-Sánchez, A.; Martín-Santos, C.; Padrón, J.M.; Mas-Ballesté, R.; Navarro-Ranninger, C.; Alemán, J.; Cabrera, S. Effect of electronic and steric properties of 8-substituted quinolines in gold(III) complexes: Synthesis, electrochemistry, stability, interactions and antiproliferative studies. J. Inorg. Biochem. 2017, 174, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Fogh, J.; Fogh, J.M.; Orfeo, T. One Hundred and Twenty-Seven Cultured Human Tumor Cell Lines Producing Tumors in Nude Mice23. JNCI: J. Natl. Cancer Inst. 1977, 59, 221–226. [Google Scholar] [CrossRef]
- Gomez-Manzano, C.; Fueyo, J.; Kyritsis, A.P.; Steck, P.A.; Roth, J.A.; McDonnell, T.J.; Steck, K.D.; Levin, V.A.; Alfred Yung, W.K. Adenovirus-mediated Transfer of the, p53, Gene Produces Rapid and Generalized Death of Human Glioma Cells via Apoptosis. Cancer Res. 1996, 56, 694–699. [Google Scholar] [PubMed]
- Balzarotti, M.; Ciusani, E.; Calatozzolo, C.; Croci, D.; Boiardi, A.; Salmaggi, A. Effect of Association of Temozolomide With Other Chemotherapic Agents on Cell Growth Inhibition in Glioma Cell Lines. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2004, 14, 325–330. [Google Scholar] [CrossRef]
- Schoumacher, R.A.; Ram, J.; Iannuzzi, M.C.; Bradbury, N.A.; Wallace, R.W.; Hon, C.T.; Kelly, D.R.; Schmid, S.M.; Gelder, F.B.; Rado, T.A. A cystic fibrosis pancreatic adenocarcinoma cell line. Proc. Natl. Acad. Sci. USA 1990, 87, 4012–4016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugarman, B.; Aggarwal, B.; Hass, P.; Figari, I.; Palladino, M.; Shepard, H. Recombinant human tumor necrosis factor-alpha: Effects on proliferation of normal and transformed cells in vitro. Science 1985, 230, 943–945. [Google Scholar] [CrossRef] [PubMed]
- Bepler, G.; Koehler, A. Multiple chromosomal aberrations and 11p allelotyping in lung cancer cell lines. Cancer Genet. Cytogenet. 1995, 84, 39–45. [Google Scholar] [CrossRef]
- Pessina, A.; Bonomi, A.; Coccè, V.; Invernici, G.; Navone, S.; Cavicchini, L.; Sisto, F.; Ferrari, M.; Viganò, L.; Locatelli, A.; et al. Mesenchymal stromal cells primed with paclitaxel provide a new approach for cancer therapy. PLoS ONE 2011, 6, e28321. [Google Scholar] [CrossRef] [Green Version]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A Simple Method of Estimating Fifty per Cent Endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Porta, F.; Facchetti, G.; Ferri, N.; Gelain, A.; Meneghetti, F.; Villa, S.; Barlocco, D.; Masciocchi, D.; Asai, A.; Miyoshi, N.; et al. An in vivo active 1,2,5-oxadiazole Pt(II) complex: A promising anticancer agent endowed with STAT3 inhibitory properties. Eur. J. Med. Chem. 2017, 131, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Ferri, N.; Facchetti, G.; Pellegrino, S.; Pini, E.; Ricci, C.; Curigliano, G.; Rimoldi, I. Promising antiproliferative platinum(II) complexes based on imidazole moiety: Synthesis, evaluation in HCT-116 cancer cell line and interaction with Ctr-1 Met-rich domain. Bioorg. Med. Chem. 2015, 23, 2538–2547. [Google Scholar] [CrossRef] [PubMed]
- Schuldes, H.; Bade, S.; Knobloch, J.; Jonas, D. Loss of in vitro cytotoxicity of cisplatin after storage as stock solution in cell culture medium at various temperatures. Cancer 1997, 79, 1723–1728. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, B.; Hu, X.; Kim, H.; Squatrito, M.; Scarpace, L.; de Carvalho, A.C.; Lyu, S.; Li, P.; Li, Y.; et al. Tumor Evolution of Glioma-Intrinsic Gene Expression Subtypes Associates with Immunological Changes in the Microenvironment. Cancer Cell 2017, 32, 42–56.e46. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Nishioka, W.; Th’ng, J.; Bradbury, E.; Litchfield, D.; Greenberg, A. Premature p34cdc2 activation required for apoptosis. Science 1994, 263, 1143–1145. [Google Scholar] [CrossRef]
- Vitale, I.; Galluzzi, L.; Castedo, M.; Kroemer, G. Mitotic catastrophe: A mechanism for avoiding genomic instability. Nat. Rev. Mol. Cell Biol. 2011, 12, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Aubrey, B.J.; Kelly, G.L.; Janic, A.; Herold, M.J.; Strasser, A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 2018, 25, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Yakovlev, A.G.; Di Giovanni, S.; Wang, G.; Liu, W.; Stoica, B.; Faden, A.I. BOK and NOXA Are Essential Mediators of p53-dependent Apoptosis. J. Biol. Chem. 2004, 279, 28367–28374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibue, T.; Suzuki, S.; Okamoto, H.; Yoshida, H.; Ohba, Y.; Takaoka, A.; Taniguchi, T. Differential contribution of Puma and Noxa in dual regulation of p53-mediated apoptotic pathways. EMBO J. 2006, 25, 4952–4962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, T.; Sadler, P.J. Speciation of precious metal anti-cancer complexes by NMR spectroscopy. Drug Discov. Today: Technol. 2015, 16, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Harvey, C.J.; Thimmulappa, R.K.; Singh, A.; Blake, D.J.; Ling, G.; Wakabayashi, N.; Fujii, J.; Myers, A.; Biswal, S. Nrf2-regulated glutathione recycling independent of biosynthesis is critical for cell survival during oxidative stress. Free Radic. Biol. Med. 2009, 46, 443–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.H.; Kuo, M.T. Role of glutathione in the regulation of Cisplatin resistance in cancer chemotherapy. Met. Based Drugs 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- Kasherman, Y.; Sturup, S.; Gibson, D. Is glutathione the major cellular target of cisplatin? A study of the interactions of cisplatin with cancer cell extracts. J. Med. Chem. 2009, 52, 4319–4328. [Google Scholar] [CrossRef] [PubMed]
- Rimoldi, I.; Coccè, V.; Facchetti, G.; Alessandri, G.; Brini, A.T.; Sisto, F.; Parati, E.; Cavicchini, L.; Lucchini, G.; Petrella, F.; et al. Uptake-release by MSCs of a cationic platinum(II) complex active in vitro on human malignant cancer cell lines. Biomed. Pharmacother. 2018, 108, 111–118. [Google Scholar] [CrossRef]
- Enríquez Pérez, J.; Fritzell, S.; Kopecky, J.; Visse, E.; Darabi, A.; Siesjö, P. The effect of locally delivered cisplatin is dependent on an intact immune function in an experimental glioma model. Sci. Rep. 2019, 9, 5632. [Google Scholar] [CrossRef] [Green Version]
- Pessina, A.; Leonetti, C.; Artuso, S.; Benetti, A.; Dessy, E.; Pascucci, L.; Passeri, D.; Orlandi, A.; Berenzi, A.; Bonomi, A.; et al. Drug-releasing mesenchymal cells strongly suppress B16 lung metastasis in a syngeneic murine model. J Exp Clin Cancer Res 2015, 34, 82. [Google Scholar] [CrossRef] [Green Version]
| GENE | Sequence (5′-3′) | Ta (°C) |
|---|---|---|
| GAPDH | FW: GGAGTCAACGGATTTGGTCG REV: CTTCCCGTTCTCAGCCTTGA | 60 |
| NOXA | FW: GCTGGAAGTCGAGTGTGCTA REV: GGAGTCCCCTCATGCAAGTT | 60 |
| BCL2 | FW: ATCGCCCTGTGGATGACTGAGT REV GCCAGGAGAAATCAAACAGAGGC | 60 |
| PUMA | FW: ATGCCTGCCTCACCTTCATC REV: TCAGCCAAAATCTCCCACCC | 60 |
| BAX | FW: TGGCAGCTGACATGTTTTCTGAC REV: TCACCCAACCACCCTGGTCTT | 60 |
| P53 | FW: GAGCTGAATGAGGCCTTGGA REV: CTGAGTCAGGCCCTTCTGTCTT | 60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coccè, V.; Rimoldi, I.; Facchetti, G.; Ciusani, E.; Alessandri, G.; Signorini, L.; Sisto, F.; Giannì, A.; Paino, F.; Pessina, A. In Vitro Activity of Monofunctional Pt-II Complex Based on 8-Aminoquinoline against Human Glioblastoma. Pharmaceutics 2021, 13, 2101. https://doi.org/10.3390/pharmaceutics13122101
Coccè V, Rimoldi I, Facchetti G, Ciusani E, Alessandri G, Signorini L, Sisto F, Giannì A, Paino F, Pessina A. In Vitro Activity of Monofunctional Pt-II Complex Based on 8-Aminoquinoline against Human Glioblastoma. Pharmaceutics. 2021; 13(12):2101. https://doi.org/10.3390/pharmaceutics13122101
Chicago/Turabian StyleCoccè, Valentina, Isabella Rimoldi, Giorgio Facchetti, Emilio Ciusani, Giulio Alessandri, Lucia Signorini, Francesca Sisto, Aldo Giannì, Francesca Paino, and Augusto Pessina. 2021. "In Vitro Activity of Monofunctional Pt-II Complex Based on 8-Aminoquinoline against Human Glioblastoma" Pharmaceutics 13, no. 12: 2101. https://doi.org/10.3390/pharmaceutics13122101
APA StyleCoccè, V., Rimoldi, I., Facchetti, G., Ciusani, E., Alessandri, G., Signorini, L., Sisto, F., Giannì, A., Paino, F., & Pessina, A. (2021). In Vitro Activity of Monofunctional Pt-II Complex Based on 8-Aminoquinoline against Human Glioblastoma. Pharmaceutics, 13(12), 2101. https://doi.org/10.3390/pharmaceutics13122101










