Phthalocyanine and Its Formulations: A Promising Photosensitizer for Cervical Cancer Phototherapy
Abstract
1. Introduction
2. Cervical Cancer
2.1. Basic Concepts
2.2. Human Cervical Cancer-Derived Cell Lines to In Vitro and In Vivo (Tumor-Bearing Mice) Studies
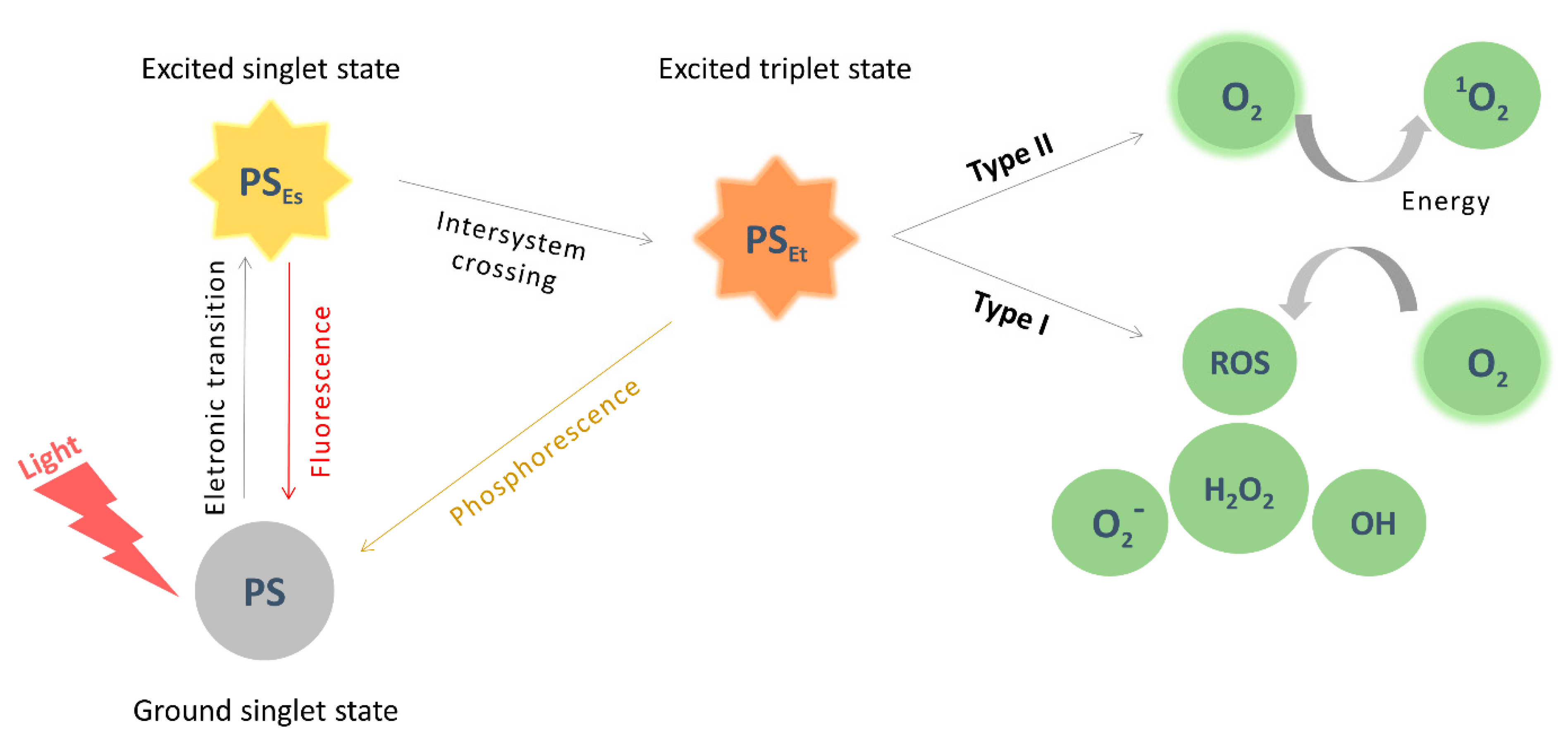
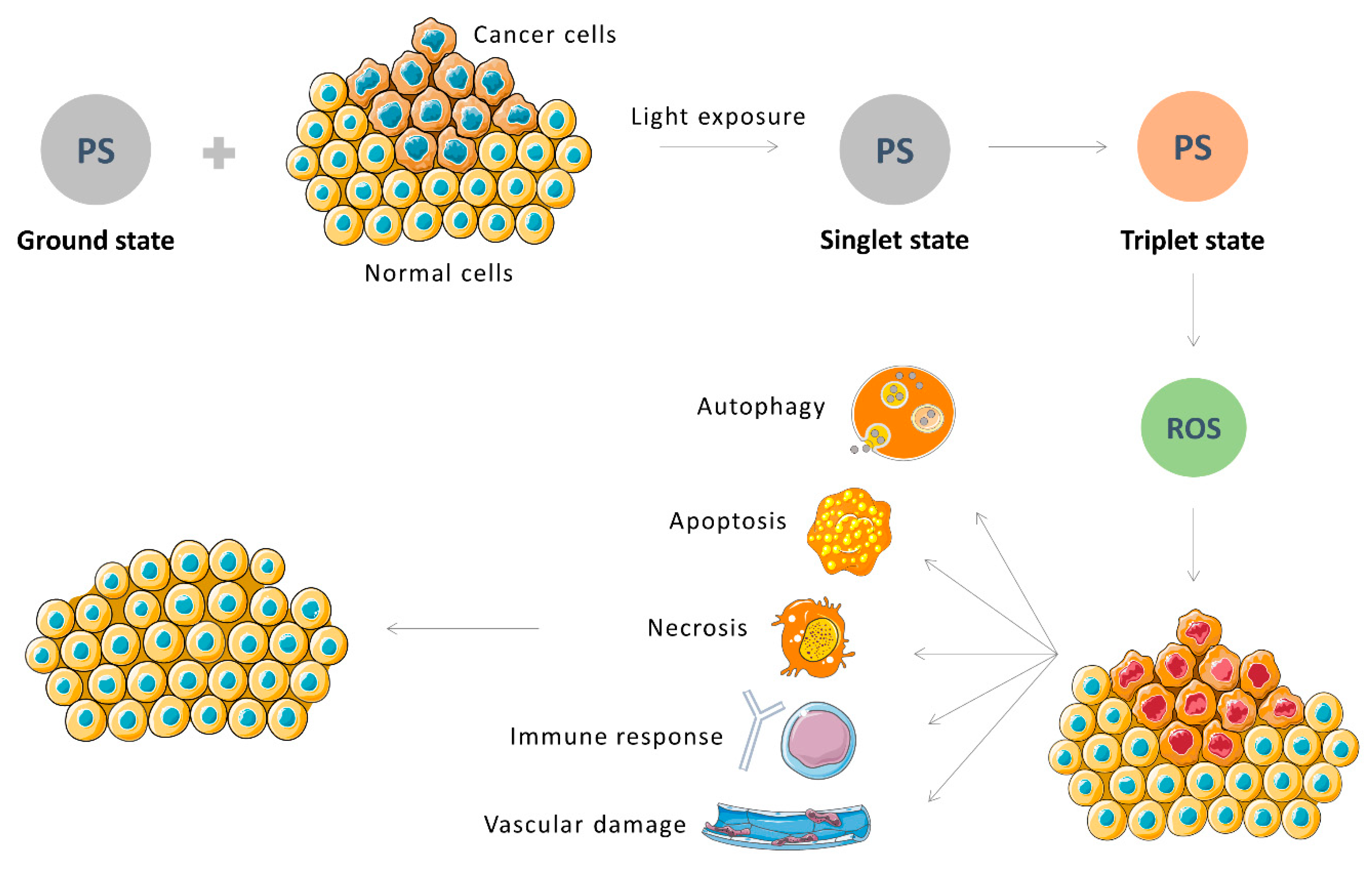
3. Photodynamic Therapy (PDT)
3.1. Principles and Applications
3.2. PDT on Cancer
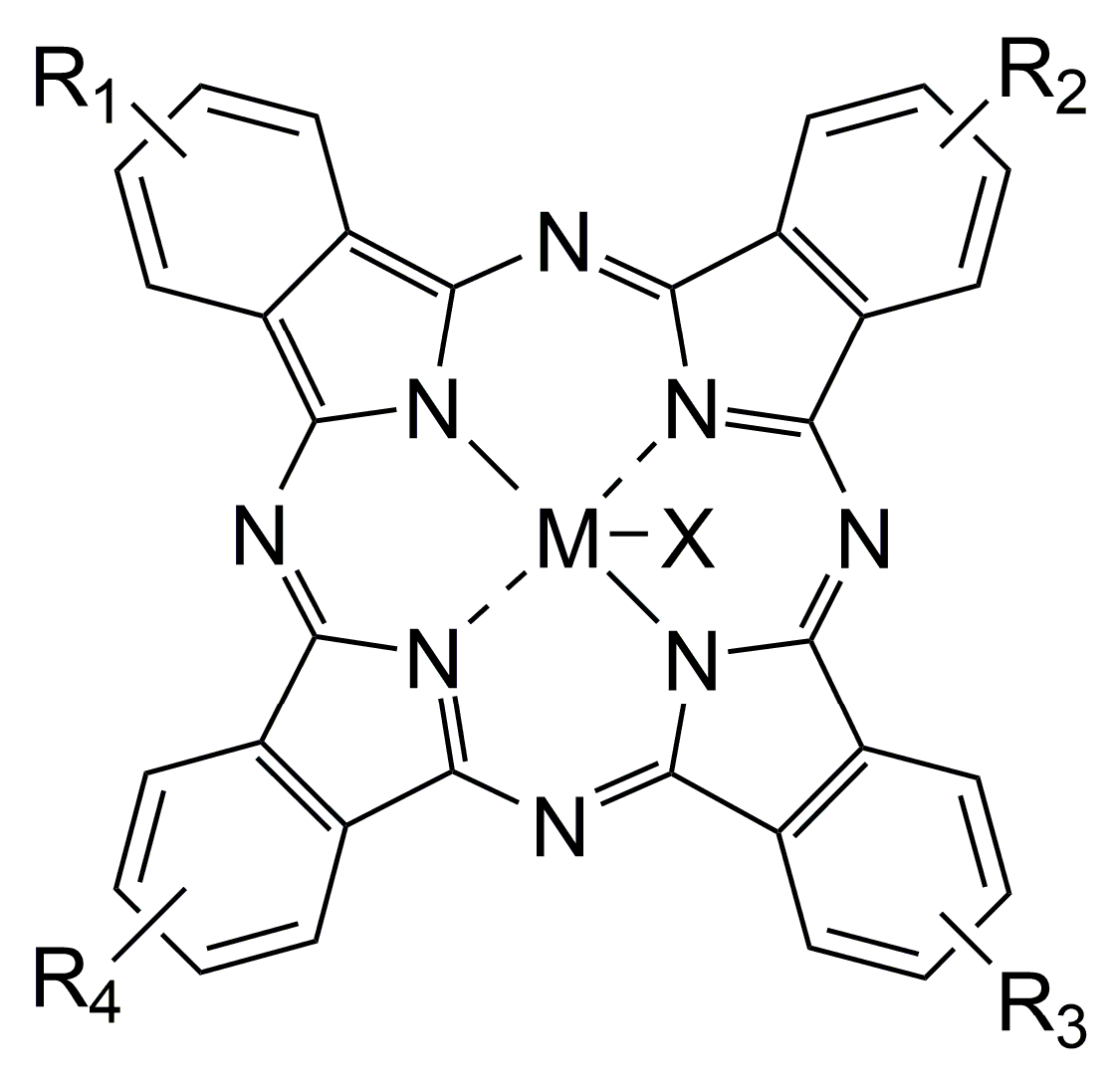
3.3. Pcs as Photosensitizers for Cancer PDT
3.4. Pcs Optimization of Photophysical Properties and Biocompatibility
4. Pcs as a PS for Cervical Cancer PDT
4.1. In Vitro Studies Evaluating Pcs on Hela Cells
4.1.1. MPcs
4.1.2. Silicon Pcs (SiPcs)
- Considering that the different studies included in Table 2 used different concentrations of MPCs, different light exposure times, and different light sources, it is very difficult to compare the results obtained between these studies.
- Still, for the same reasons, it is very difficult to assess which formulation presented the best therapeutic efficacy.
- The number of recent studies evaluating the combination of PDT and chemotherapy is very small and restricted to doxorubicin, limiting interpretations of its real benefit.
4.2. Both In Vitro and In Tumor-Bearing Mice Pcs Studies Based on HeLa Cells
4.3. Studies Evaluating Pcs in Other Cervical Cancer Cells than Hela
4.4. Clinical Studies
5. Conclusions
- Current evidence indicates that despite the increasing number of studies with a growing number of different Pcs formulations and their generally increased number of favorable aspects as mainly related to in vitro low effective concentration (mainly against the tumour cell line), low dark toxicity, increased photo cytotoxicity, and cellular uptake in a dose-dependent manner for cervical cancer, only a few Pcs were evaluated in cervical cancer cell lines other than HeLa. As a result, most studies assessed the activity of different Pcs formulations against cervical adenocarcinoma and not against squamous cervical cancer, which is the most common type of cervical cancer worldwide (approximately 70% of total) [40]. Additionally, few preclinical animal studies and no clinical studies with Pcs in invasive cervical cancer have been performed to date.
- Regarding pre-clinical animal models [203], there are only a few studies in tumor-bearing mice xenograft tumors based on HeLa cells. Therefore, there is an urgent need for more in vivo studies in tumor-bearing mice and primates based on HeLa cells and in other cervical cancer lineages to continue studies on the effectiveness of different Pc formulations against cervical cancer.
- Indeed, our review showed that in the field of Pcs in cervical cancer, the photophysical and photochemical properties, subcellular localization, phototoxic activity, mechanisms of cell death, and improved targeting to tumor tissues have been the main topics explored. However, up to this moment, there is an immediate need to increase the number of clinical trials evaluating Pcs in CIN and invasive cervical cancer. As a result, it would be possible to confirm preclinical studies and orient future research.
- Additionally, we showed that the combination of PDT with MPcs and chemotherapy using Dox can induce synergistic therapeutic effects, highlighting that MPcs could serve as a promising multifunctional platform in anticancer treatment by synergic chemo-PDT and superior tumor-targeting ability. However, the number of studies with this approach is still limited, so we strongly suggest implementing research in this area.
- Finally, the search for new possible mechanisms of action in different cervical cancer cells may consequently contribute to novel applications for single or combined Pcs treatments to CIN and invasive cervical cancer.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Tian, T.; Gong, X.; Gao, X.; Li, Y.; Ju, W.; Ai, Y. Comparison of survival outcomes of locally advanced cervical cancer by histopathological types in the surveillance, epidemiology, and end results (SEER) database: A propensity score matching study. Infect. Agent. Cancer 2020, 15, 33. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Schiffman, M.; Doorbar, J.; Wentzensen, N.; De Sanjosé, S.; Fakhry, C.; Monk, B.J.; Stanley, M.A.; Franceschi, S. Carcinogenic human papillomavirus infection. Nat. Rev. Dis. Prim. 2016, 2, 16086. [Google Scholar] [CrossRef]
- Zhao, F.; Qiao, Y. Cervical cancer prevention in China: A key to cancer control. Lancet 2019, 393, 969–970. [Google Scholar] [CrossRef]
- Ogilvie, G.S.; Krajden, M.; van Niekerk, D.; Smith, L.W.; Cook, D.; Ceballos, K.; Lee, M.; Gentile, L.; Gondara, L.; Elwood-Martin, R.; et al. HPV for cervical cancer screening (HPV FOCAL): Complete Round 1 results of a randomized trial comparing HPV-based primary screening to liquid-based cytology for cervical cancer. Int. J. Cancer 2017, 15, 440–448. [Google Scholar] [CrossRef]
- Cohen, P.A.; Jhingran, A.; Oaknin, A.; Denny, L. Cervical cancer. Lancet 2019, 393, 169–182. [Google Scholar] [CrossRef]
- American Cancer Society Cervical Cancer Early Detection, Diagnosis, and Staging. Available online: https://www.cancer.org/content/dam/CRC/PDF/Public/8601.00.pdf (accessed on 7 July 2021).
- Salvo, G.; Odetto, D.; Pareja, R.; Frumovitz, M.; Ramirez, P.T. Revised 2018 International Federation of Gynecology and Obstetrics (FIGO) cervical cancer staging: A review of gaps and questions that remain. Int. J. Gynecol. Cancer 2020, 30, 873–878. [Google Scholar] [CrossRef]
- Serrano-Olvera, A.; Cetina, L.; Coronel, J.; Dueñas-González, A. Emerging drugs for the treatment of cervical cancer. Expert Opin. Emerg. Drugs 2015, 20, 165–182. [Google Scholar] [CrossRef]
- American Cancer Society Cancer Facts & Figures. 2018. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2018/cancer-facts-and-figures-2018.pdf (accessed on 7 July 2021).
- American Cancer Society Treating Cervical Cancer. Available online: https://www.cancer.org/content/dam/CRC/PDF/Public/8602.00.pdf (accessed on 7 July 2021).
- Tao, X.H.; Guan, Y.; Shao, D.; Xue, W.; Ye, F.S.; Wang, M.; He, M.H. Efficacy and safety of photodynamic therapy for cervical intraepithelial neoplasia: A systemic review. Photodiagn. Photodyn. Ther. 2014, 11, 104–112. [Google Scholar] [CrossRef]
- Wolford, J.E.; Tewari, K.S. Rational design for cervical cancer therapeutics: Cellular and non-cellular based strategies on the horizon for recurrent, metastatic or refractory cervical cancer. Expert Opin. Drug Discov. 2018, 13, 445–457. [Google Scholar] [CrossRef]
- Gupta, S.; Maheshwari, A.; Parab, P.; Mahantshetty, U.; Hawaldar, R.; Sastri Chopra, S.; Kerkar, R.; Engineer, R.; Tongaonkar, H.; Ghosh, J.; et al. Neoadjuvant chemotherapy followed by radical surgery versus concomitant chemotherapy and radiotherapy in patients with stage IB2, IIA, or IIB squamous cervical cancer: A randomized controlled trial. J. Clin. Oncol. 2018, 36, 1548–1555. [Google Scholar] [CrossRef]
- Yang, H.; Wu, X.L.; Wu, K.H.; Zhang, R.; Ju, L.L.; Ji, Y.; Zhang, Y.W.; Xue, S.L.; Zhang, Y.X.; Yang, Y.F.; et al. MicroRNA-497 regulates cisplatin chemosensitivity of cervical cancer by targeting transketolase. Am. J. Cancer Res. 2016, 6, 2690–2699. [Google Scholar]
- Gadducci, A.; Sartori, E.; Maggino, T.; Landoni, F.; Zola, P.; Cosio, S.; Pasinetti, B.; Alessi, C.; Maneo, A.; Ferrero, A. The clinical outcome of patients with stage Ia1 and Ia2 squamous cell carcinoma of the uterine cervix: A Cooperation Task Force (CTF) study. Eur. J. Gynaecol. Oncol. 2003, 24, 513–516. [Google Scholar]
- Gadzinski, J.A.; Guo, J.; Philips, B.J.; Basse, P.; Craig, E.K.; Bailey, L.; Latoche, J.; Comerci, J.T.; Eiseman, J.L. Evaluation of silicon phthalocyanine 4 photodynamic therapy against human cervical cancer cells in vitro and in mice. Adv. Biol. Chem. 2016, 6, 193–215. [Google Scholar] [CrossRef]
- Dougherty, T.J.; Grindey, G.B.; Fiel, R.; Weishaupt, K.R.; Boyle, D.G. Photoradiation therapy. II. Cure of animal tumors with hematoporphyrin and light. J. Natl. Cancer Inst. 1975, 55, 115–121. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Photocure-The Bladder Cancer Company. Available online: https:// https://photocure.com/about/about-us/ (accessed on 10 October 2021).
- Shishkova, N.; Kuznetsova, O.; Berezov, T. Photodynamic therapy for gynecological diseases and breast cancer. Cancer Biol. Med. 2012, 9, 9–17. [Google Scholar] [CrossRef]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef]
- Ochsner, M. Photophysical and photobiological processes in the photodynamic therapy of tumours. J. Photochem. Photobiol. B 1997, 39, 1–18. [Google Scholar] [CrossRef]
- Vendette, A.C.F.; Piva, H.L.; Muehlmann, L.A.; de Souza, D.A.; Tedesco, A.C.; Azevedo, R.B. Clinical treatment of intra-epithelia cervical neoplasia with photodynamic therapy. Int. J. Hyperth. 2020, 37, 50–58. [Google Scholar] [CrossRef]
- Mroz, P.; Yaroslavsky, A.; Kharkwal, G.B.; Hamblin, M.R. Cell death pathways in photodynamic therapy of cancer. Cancers 2011, 3, 2516–2539. [Google Scholar] [CrossRef]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in photodynamic therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef]
- Fan, W.; Huang, P.; Chen, X. Overcoming the Achilles’ heel of photodynamic therapy. Chem. Soc. Rev. 2016, 45, 6488–6519. [Google Scholar] [CrossRef]
- Hu, F.; Xu, S.; Liu, B. Photosensitizers with aggregation-induced emission: Materials and biomedical applications. Adv. Mater. 2018, 30, 1801350. [Google Scholar] [CrossRef]
- Li, X.; Fan, H.; Guo, T.; Bai, H.; Kwon, N.; Kim, K.H.; Yu, S.; Cho, Y.; Kim, H.; Nam, K.T.; et al. Sequential protein-responsive nanophotosensitizer complex for enhancing tumor-specific therapy. ACS Nano 2019, 13, 6702–6710. [Google Scholar] [CrossRef]
- Kou, J.; Dou, D.; Yang, L. Porphyrin photosensitizers in photodynamic therapy and its applications. Oncotarget 2017, 8, 81591–81603. [Google Scholar] [CrossRef]
- Reddi, E.; Lo Castro, G.; Biolo, R.; Jori, G. Pharmacokinetic studies with zinc(II)-phthalocyanine in tumour-bearing mice. Br. J. Cancer 1987, 56, 597–600. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Fang, Y.; Jiang, Z.; Wang, A.; Xue, J. Improved photodynamic anticancer activity and mechanisms of a promising zinc(II) phthalocyanine-quinoline conjugate photosensitizer in vitro and in vivo. Biomed. Opt. Express. 2020, 11, 3900–3912. [Google Scholar] [CrossRef]
- Wasielewski, M.R. Self-assembly strategies for integrating light harvesting and charge separation in artificial photosynthetic systems. Acc. Chem. Res. 2009, 42, 1910–1921. [Google Scholar] [CrossRef]
- Revuelta-Maza, M.A.; Hally, C.; Nonell, S.; de la Torre, G.; Torres, T. Crosswise phthalocyanines with collinear functionalization: New paradigmatic derivatives for efficient singlet oxygen photosensitization. ChemPlusChem 2019, 84, 673–679. [Google Scholar] [CrossRef]
- Dumoulin, F.; Durmuş, M.; Ahsen, V.; Nyokong, T. Synthetic pathways to water-soluble phthalocyanines and close analogs. Coord. Chem. Rev. 2010, 254, 2792–2847. [Google Scholar] [CrossRef]
- Rak, J.; Pouckova, P.; Benes, J.; Vetvicka, D. Drug delivery systems for phthalocyanines for photodynamic therapy. Anticancer Res. 2019, 39, 3323–3339. [Google Scholar] [CrossRef]
- Castle, P.; Murokora, D.; Perez, C.; Alvarez, M.; Quek, S.; Campbell, C. Treatment of cervical intraepithelial lesions. Int. J. Gynaecol. Obstet. 2017, 138, 20–25. [Google Scholar] [CrossRef]
- Nene, B.M.; Hiremath, P.S.; Kane, S.; Fayette, J.M.; Shastri, S.S.; Sankaranarayanan, R. Effectiveness, safety, and acceptability of cryotherapy by midwives for cervical intraepithelial neoplasia in Maharashtra, India. Int. J. Gynaecol. Obstet. 2008, 103, 232–236. [Google Scholar] [CrossRef]
- Mizuno, M.; Mitsui, H.; Kajiyama, H.; Teshigawara, T.; Inoue, K.; Takahashi, K.; Ishii, T.; Ishizuka, M.; Nakajima, M.; Kikkawa, F. Efficacy of 5-aminolevulinic acid and LED photodynamic therapy in cervical intraepithelial neoplasia: A clinical trial. Photodiagn. Photodyn. Ther. 2020, 32, 102004. [Google Scholar] [CrossRef]
- Hu, K.; Wang, W.; Liu, X.; Meng, Q.; Zhang, F. Comparison of treatment outcomes between squamous cell carcinoma and adenocarcinoma of cervix after definitive radiotherapy or concurrent chemoradiotherapy. Radiat. Oncol. 2018, 13, 1–7. [Google Scholar] [CrossRef]
- Walboomers, J.M.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.F.; Peto, J.; Meijer, C.J.L.M.; Muñoz, N.J.M.M. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999, 189, 12–19. [Google Scholar] [CrossRef]
- Castellsagué, X.; Diaz, M.; de Sanjosé, S.; Muñoz, N.; Herrero, R.; Franceschi, S.; Peeling, R.W.; Ashley, R.; Smith, J.S.; Snijders, P.J.F.; et al. Worldwide human papillomavirus etiology of cervical adenocarcinoma and its cofactors: Implications for screening and prevention. J. Natl. Cancer Inst. 2006, 98, 303–315. [Google Scholar] [CrossRef]
- De Sanjose, S.; Quint, W.G.V.; Alemany, L.; Geraets, D.T.; Klaustermeier, J.E.; Lloveras, B.; Tous, S.; Felix, A.; Bravo, L.E.; Shin, H.R.; et al. Human papillomavirus genotype attribution in invasive cervical cancer: A retrospective cross-sectional worldwide study. Lancet Oncol. 2010, 11, 1048–1056. [Google Scholar] [CrossRef]
- Ho, G.Y.F.; Bierman, R.; Beardsley, L.; Chang, C.J.; Burk, R.D. Natural History of cervicovaginal papillomavirus infection in young women. N. Engl. J. Med. 1998, 338, 423–428. [Google Scholar] [CrossRef]
- Koh, W.J.; Greer, B.E.; Abu-Rustum, N.R.; Apte, S.M.; Campos, S.M.; Cho, K.R.; Chu, C.; Cohn, D.; Crispens, M.A.; Dorigo, O.; et al. Cervical cancer, version 2.2015: Featured updates to the NCCN guidelines featured updates to the NCCN guidelines. J. Natl. Compr. Cancer Netw. 2015, 13, 395–404. [Google Scholar] [CrossRef]
- Tota, J.E.; Chevarie-Davis, M.; Richardson, L.A.; DeVries, M.; Franco, E.L. Epidemiology and burden of HPV infection and related diseases: Implications for prevention strategies. Prev. Med. 2011, 53, S12–S21. [Google Scholar] [CrossRef]
- Monsonego, J.; Cox, J.T.; Behrens, C.; Sandri, M.; Franco, E.L.; Yap, P.S.; Huh, W. Prevalence of high-risk human papilloma virus genotypes and associated risk of cervical precancerous lesions in a large U.S. screening population: Data from the ATHENA trial. Gynecol. Oncol. 2015, 137, 47–54. [Google Scholar] [CrossRef]
- De Abreu, A.L.P.; Malaguti, N.; Souza, R.P.; Uchimura, N.S.; Ferreira, É.C.; Pereira, M.W.; Carvalho, M.D.B.; Pelloso, S.M.; Bonini, M.G.; Gimenes, F.; et al. Association of human papillomavirus, Neisseria gonorrhoeae and Chlamydia trachomatis co-infections on the risk of high-grade squamous intraepithelial cervical lesion. Am. J. Cancer Res. 2016, 6, 1371–1383. [Google Scholar]
- Bodily, J.; Laimins, L.A. Persistence of human papillomavirus infection: Keys to malignant progression. Trends Microbiol. 2011, 19, 33–39. [Google Scholar] [CrossRef]
- Herrero, R.; Murillo, R. Cervical Cancer. In Cancer Epidemiology and Prevention; Thun, M., Linet, M.S., Cerhan, J.R., Haiman, C.A., Schottenfeld, D., Eds.; Oxford University Press: New York, NY, USA, 2018; pp. 925–946. ISBN 13:9780190238667. [Google Scholar]
- Egawa, N.; Egawa, K.; Griffin, H.; Doorbar, J. Human papillomaviruses; Epithelial tropisms, and the development of neoplasia. Viruses 2015, 7, 3863–3890. [Google Scholar] [CrossRef]
- Mac, M.; Moody, C.A. Epigenetic regulation of the human papillomavirus life cycle. Pathogens 2020, 9, 483. [Google Scholar] [CrossRef]
- PaVE: Papilloma Virus Genome Database. Available online: https://pave.niaid.nih.gov/ (accessed on 6 January 2020).
- Huh, W.K.; Joura, E.A.; Giuliano, A.R.; Iversen, O.E.; de Andrade, R.P.; Ault, K.A.; Bartholomew, D.; Cestero, R.M.; Fedrizzi, E.N.; Hirschberg, A.L.; et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16–26 years: A randomised, double-blind trial. Lancet 2017, 390, 2143–2159. [Google Scholar] [CrossRef]
- WHO. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Human Papillomaviruses; WHO: Lyon, France, 2007; Volume 90, ISBN 9789283212904. [Google Scholar]
- Pyeon, D.; Pearce, S.M.; Lank, S.M.; Ahlquist, P.; Lambert, P.F. Establishment of human papillomavirus infection requires cell cycle progression. PLoS Pathog. 2009, 5, e1000318. [Google Scholar] [CrossRef]
- Ozbun, M.A.; Meyers, C. Temporal usage of multiple promoters during the life cycle of human papillomavirus type 31b. J. Virol. 1998, 72, 2715–2722. [Google Scholar] [CrossRef]
- Moody, C.A. Mechanisms by which HPV induces a replication competent environment in differentiating keratinocytes. Viruses 2017, 9, 261. [Google Scholar] [CrossRef]
- Hummel, M.; Hudson, J.B.; Laimins, L.A. Differentiation-induced and constitutive transcription of human papillomavirus type 31b in cell lines containing viral episomes. J. Virol. 1992, 66, 6070–6080. [Google Scholar] [CrossRef]
- Klumpp, D.; Laimins, L. Differentiation-induced changes in promoter usage for transcripts encoding the human papillomavirus type 31 replication protein E1. Virology 1999, 25, 239–246. [Google Scholar] [CrossRef]
- Bedell, M.A.; Hudson, J.B.; Golub, T.R.; Turyk, M.E.; Hosken, M.; Wilbanks, G.D.; Laimins, L.A. Amplification of human papillomavirus genomes in vitro is dependent on epithelial differentiation. J. Virol. 1991, 65, 2254–2260. [Google Scholar] [CrossRef]
- Hummel, M.; Lim, H.B.; Laimins, L.A. Human papillomavirus type 31b late gene expression is regulated through protein kinase C-mediated changes in RNA processing. J. Virol. 1995, 69, 3381–3388. [Google Scholar] [CrossRef]
- Ozbun, M.A.; Meyers, C. Characterization of late gene transcripts expressed during vegetative replication of human papillomavirus type 31b. J. Virol. 1997, 71, 5161–5172. [Google Scholar] [CrossRef]
- Verteramo, R.; Pierangeli, A.; Calzolari, E.; Patella, A.; Recine, N.; Mancini, E.; Marcone, V.; Masciangelo, R.; Bucci, M.; Antonelli, G.; et al. Direct sequencing of HPV DNA detected in gynaecologic outpatients in Rome, Italy. Microbes Infect. 2006, 8, 2517–2521. [Google Scholar] [CrossRef]
- Lehtinen, M.; Ault, K.; Lyytikainen, E.; Dillner, J.; Garland, S.; Ferris, D.; Koutsky, L.; Sings, H.; Lu, S.; Haupt, R.; et al. Chlamydia trachomatis infection and risk of cervical intraepithelial neoplasia. Sex. Transm. Infect. 2011, 87, 372–376. [Google Scholar] [CrossRef][Green Version]
- Longworth, M.S.; Wilson, R.; Laimins, L.A. HPV31 E7 facilitates replication by activating E2F2 transcription through its interaction with HDACs. EMBO J. 2005, 24, 1821–1830. [Google Scholar] [CrossRef]
- Howie, H.L.; Katzenellenbogen, R.A.; Galloway, D.A. Papillomavirus E6 proteins. Virology 2009, 384, 324–334. [Google Scholar] [CrossRef]
- Munger, K.; Werness, B.A.; Dyson, N.; Phelps, W.C.; Harlow, E.; Howley, P.M. Complex formation of c-myc papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J. 1989, 8, 4099–4105. [Google Scholar] [CrossRef] [PubMed]
- Dyson, N.; Howley, P.M.; Münger, K.; Harlow, E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science 1989, 243, 934–937. [Google Scholar] [CrossRef] [PubMed]
- Scheffner, M.; Werness, B.A.; Huibregtse, J.M.; Levine, A.J.; Howley, P.M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 1990, 63, 1129–1136. [Google Scholar] [CrossRef]
- Huibregtse, J.M.; Scheffner, M.; Howley, P.M. A cellular protein mediates association of p53 with the E6 oncoprotein of human papillomavirus types 16 or 18. EMBO J. 1991, 10, 4129–4135. [Google Scholar] [CrossRef] [PubMed]
- Scheffner, M.; Huibregtse, J.M.; Vierstra, R.D.; Howley, P.M. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 1993, 75, 495–505. [Google Scholar] [CrossRef]
- Sharma, S.V.; Haber, D.A.; Settleman, J. Cell line-based platforms to evaluate the therapeutic efficacy of candidate anticancer agents. Nat. Rev. Cancer 2010, 10, 241–253. [Google Scholar] [CrossRef]
- Campo, M.S. Animal models of papillomavirus pathogenesis. Virus Res. 2002, 89, 249–261. [Google Scholar] [CrossRef]
- Boshart, M.; Gissmann, L.; Ikenberg, H.; Kleinheinz, A.; Scheurlen, W.; zur Hausen, H. A new type of papillomavirus DNA, its presence in genital cancer biopsies and in cell lines derived from cervical cancer. EMBO J. 1984, 3, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, E.; Freese, U.K.; Gissmann, L.; Mayer, W.; Roggenbuck, B.; Stremlau, A.; Zur Hausen, H. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature 1985, 314, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Pater, M.M.; Pater, A. Human papillomavirus types 16 and 18 sequences in carcinoma cell lines of the cervix. Virology 1985, 145, 313–318. [Google Scholar] [CrossRef]
- Rosa, M.N.; Evangelista, A.F.; Leal, L.F.; De Oliveira, C.M.; Silva, V.A.O.; Munari, C.C.; Munari, F.F.; Matsushita, G.D.M.; Dos Reis, R.; Andrade, C.E.; et al. Establishment, molecular and biological characterization of HCB-514: A novel human cervical cancer cell line. Sci. Rep. 2019, 9, 1913. [Google Scholar] [CrossRef] [PubMed]
- Van Straten, D.; Mashayekhi, V.; de Bruijn, H.S.; Oliveira, S.; Robinson, D.J. Oncologic photodynamic therapy: Basic principles, current clinical status and future directions. Cancers 2017, 9, 19. [Google Scholar] [CrossRef]
- Levy, J.G. Photodynamic therapy. Trends Biotechnol. 1995, 13, 14–18. [Google Scholar] [CrossRef]
- Wolf, P. Photodynamic therapy in dermatology: State of the art. J. Eur. Acad. Dermatol. Venereol. 2001, 15, 508–509. [Google Scholar] [CrossRef] [PubMed]
- Ghodasra, D.H.; Demirci, H. Photodynamic therapy for choroidal metastasis. Am. J. Ophthalmol. 2016, 161, 104–109. [Google Scholar] [CrossRef]
- Kharkwal, G.B.; Sharma, S.K.; Huang, Y.Y.; Dai, T.; Hamblin, M.R. Photodynamic therapy for infections: Clinical applications. Lasers Surg. Med. 2011, 43, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Gannon, M.J.; Brown, S.B. Photodynamic therapy and its applications in gynaecology. Br. J. Obstet. Gynaecol. 1999, 106, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Konan, Y.N.; Gurny, R.; Allémann, E. State of the art in the delivery of photosensitizers for photodynamic therapy. J. Photochem. Photobiol. B Biol. 2002, 66, 89–106. [Google Scholar] [CrossRef]
- Kalka, K.; Merk, H.; Mukhtar, H. Photodynamic therapy in dermatology. J. Am. Acad. Dermatol. 2000, 42, 389–413. [Google Scholar] [CrossRef]
- Calixto, G.M.F.; Bernegossi, J.; De Freitas, L.M.; Fontana, C.R.; Chorilli, M.; Grumezescu, A.M. Nanotechnology-based drug delivery systems for photodynamic therapy of cancer: A review. Molecules 2016, 21, 342. [Google Scholar] [CrossRef]
- Moan, J.; Streckyte, G.; Bagdonas, S.; Bech, O.; Berg, K. The pH dependency of protoporphyrin IX formation in cells incubated with 5-aminolevulinic acid. Cancer Lett. 1997, 113, 25–29. [Google Scholar] [CrossRef]
- Tedesco, A.; Rotta, J.; Lunardi, C. Synthesis, photophysical and photochemical aspects of phthalocyanines for photodynamic therapy. Curr. Org. Chem. 2003, 7, 187–196. [Google Scholar] [CrossRef]
- Tokumaru, K. Photochemical and photophysical behaviour of porphyrins and phthalocyanines irradiated with violet or ultraviolet light. J. Porphyr. Phthalocyanines 2001, 5, 77–86. [Google Scholar] [CrossRef]
- Ahmad, N.; Mukhtar, H. Mechanism of photodynamic therapy-lnduced cell death. Methods Enzym. 2000, 319, 342–358. [Google Scholar] [CrossRef]
- Allison, R.R.; Downie, G.H.; Cuenca, R.; Hu, X.H.; Childs, C.J.H.; Sibata, C.H. Photosensitizers in clinical PDT. Photodiagn. Photodyn. Ther. 2004, 1, 27–42. [Google Scholar] [CrossRef]
- Nagata, J.Y.; Hioka, N.; Kimura, E.; Batistela, V.R.; Terada, R.S.S.; Graciano, A.X.; Baesso, M.L.; Hayacibara, M.F. Antibacterial photodynamic therapy for dental caries: Evaluation of the photosensitizers used and light source properties. Photodiagn. Photodyn. Ther. 2012, 9, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Andrzejak, M.; Price, M.; Kessel, D.H. Apoptotic and autophagic responses to photodynamic therapy in 1c1c7 murine hepatoma cells. Autophagy 2011, 7, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Chiaviello, A.; Postiglione, I.; Palumbo, G. Targets and mechanisms of photodynamic therapy in lung cancer cells: A brief overview. Cancers 2011, 3, 1014–1041. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lim, W.; Kim, S.; Jeon, S.; Hui, Z.; ni, K.; Kim, C.; Im, Y.; Choi, H.; Kim, O. Photodynamic therapy (PDT) resistance by PARP1 regulation on PDT-induced apoptosis with autophagy in head and neck cancer cells. J. Oral Pathol. Med. 2014, 43, 675–684. [Google Scholar] [CrossRef]
- Buytaert, E.; Dewaele, M.; Agostinis, P. Molecular effectors of multiple cell death pathways initiated by photodynamic therapy. Biochim. Biophys. Acta-Rev. Cancer 2007, 1776, 86–107. [Google Scholar] [CrossRef]
- Taylor, E.L.; Brown, S.B. The advantages of aminolevulinic acid photodynamic therapy in dermatology. J. Dermatolog. Treat. 2002, 13, s3–s11. [Google Scholar] [CrossRef]
- Calzavara-Pinton, P.G.; Venturini, M.; Sala, R. Photodynamic therapy: Update 2006 Part 1: Photochemistry and photobiology. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef]
- Issa, M.C.A.; Manela-Azulay, M. Photodynamic therapy: A review of the literature and image documentation. An. Bras. Dermatol. 2010, 85, 501–511. [Google Scholar] [CrossRef]
- Tedesco, A.C.; Primo, F.L.; Beltrame, M. Phtalocyanines: Synthesis, characterization and biological applications of photodynamic therapy (PDT), nanobiotechnology, magnetohypertermia and photodiagnosis (theranostics). In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Dos Santos, A.F.; De Almeida, D.R.Q.; Terra, L.F.; Baptista, M.S.; Labriola, L. Photodynamic therapy in cancer treatment—An update review. J. Cancer Metastasis Treat. 2019, 5, 25. [Google Scholar] [CrossRef]
- Tsubone, T.M.; Martins, W.K.; Pavani, C.; Junqueira, H.C.; Itri, R.; Baptista, M.S. Enhanced efficiency of cell death by lysosome-specific photodamage. Sci. Rep. 2017, 7, 6734. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.S.; Turchiello, R.; Kowaltowski, A.J.; Indig, G.L.; Baptista, M.S. Major determinants of photoinduced cell death: Subcellular localization versus photosensitization efficiency. Free Radic. Biol. Med. 2011, 51, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Kessel, D.; Reiners, J.J. Promotion of proapoptotic signals by lysosomal photodamage. Photochem. Photobiol. 2015, 91, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Shi, Y.; Xie, L.; Zhang, K.; Wang, X.; Liu, Q.; Wang, P. Synthesis, Characterization, and Biological Evaluation of a Porphyrin-Based Photosensitizer and Its Isomer for Effective Photodynamic Therapy against Breast Cancer. J. Med. Chem. 2018, 61, 7189–7201. [Google Scholar] [CrossRef]
- Agarwal, M.L.; Clay, M.E.; Harvey, E.J.; Evans, H.H.; Antunez, A.R.; Oleinick, L.N. Photodynamic therapy induces rapid cell death by apoptosis in LSI78Y mouse lymphoma cells. Cancer Res. 1991, 51, 5993–5996. [Google Scholar] [PubMed]
- Kessel, D. Death pathways associated with photodynamic therapy. Med. Laser Appl. 2006, 15, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Kessel, D.; Oleinick, N.L. Cell death pathways associated with photodynamic therapy: An update. Photochem. Photobiol. 2018, 94, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Kushibiki, T.; Hirasawa, T.; Okawa, S.; Ishihara, M. Responses of cancer cells induced by photodynamic therapy. J. Healthc. Eng. 2013, 4, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Nowis, D.; Golab, J.; Vandenabeele, P.; Krysko, D.; Agostinis, P. Immunogenic cell death, DAMPs and anticancer therapeutics: An emerging amalgamation. Biochim. Biophys. Acta 2010, 1805, 53–71. [Google Scholar] [CrossRef]
- Reiners, J.J.; Agostinis, P.; Berg, K.; Oleinick, N.L.; Kessel, D. Assessing autophagy in the context of photodynamic therapy. Autophagy 2010, 6, 7–18. [Google Scholar] [CrossRef]
- Galluzzi, L.; Bravo-San Pedro, J.M.; Kroemer, G. Organelle-specific initiation of cell death. Nat. Cell Biol. 2014, 16, 728–736. [Google Scholar] [CrossRef]
- Babilas, P.; Schreml, S.; Landthaler, M.; Szeimies, R.M. Photodynamic therapy in dermatology: State-of-the-art. Photodermatol. Photoimmunol. Photomed. 2010, 26, 118–132. [Google Scholar] [CrossRef]
- Baldeia, I.; Filip, A. Photodynamic therapy in melanoma––an update. J. Physiol. Pharmacol. 2012, 63, 109–118. [Google Scholar]
- Marmo Moreira, L.; Fábio Vieira dos Santos, B.; Juliana Pereira Lyon, A.; Maira Maftoum-Costa, A.; Cristina Pacheco-Soares, A.; Soares da Silva, N.A. Photodynamic therapy: Porphyrins and phthalocyanines as photosensitizers. Aust. J. Chem. 2008, 61, 741–754. [Google Scholar] [CrossRef]
- Crescenzi, E.; Chiaviello, A.; Canti, G.; Reddi, E.; Veneziani, B.M.; Palumbo, G. Low doses of cisplatin or gemcitabine plus Photofrin/photodynamic therapy: Disjointed cell cycle phase-related activity accounts for synergistic outcome in metastatic non-small cell lung cancer cells (H1299). Mol. Cancer Ther. 2006, 5, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Aniogo, E.C.; George, B.P.A.; Abrahamse, H. Phthalocyanine induced phototherapy coupled with Doxorubicin; a promising novel treatment for breast cancer. Expert Rev. Anticancer Ther. 2017, 17, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Henderson, V.W.; Busch, T.M.; Snyder, J.W. Fluence rate as a modulator of PDT mechanisms. Lasers Surg. Med. 2006, 38, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Spring, B.Q.; Rizvi, I.; Xu, N.; Hasan, T. The role of photodynamic therapy in overcoming cancer drug resistance. Photochem. Photobiol. Sci. 2015, 14, 1476–1491. [Google Scholar] [CrossRef]
- Cid, J.J.; Yum, J.H.; Jang, S.R.; Nazeeruddin, M.K.; Martínez-Ferrero, E.; Palomares, E.; Ko, J.; Grätzel, M.; Torres, T. Molecular cosensitization for efficient panchromatic dye-sensitized solar cells. Angew. Chem.-Int. Ed. 2007, 46, 8358–8362. [Google Scholar] [CrossRef] [PubMed]
- Brasseur, N. Sensitizers for PDT: Phthalocyanines. In Photodynamic Therapy; Royal Society of Chemistry: London, UK, 2007; pp. 105–118. ISBN 978-1-84755-165-8. [Google Scholar]
- Ogura, S.I.; Tabata, K.; Fukushima, K.; Kamachi, T.; Okura, I. Development of phthalocyanines for photodynamic therapy. J. Porphyr. Phthalocyanines 2006, 10, 1116–1124. [Google Scholar] [CrossRef]
- Mantareva, V.; Kussovski, V.; Angelov, I.; Borisova, E.; Avramov, L.; Schnurpfeil, G.; Wöhrle, D. Photodynamic activity of water-soluble phthalocyanine zinc(II) complexes against pathogenic microorganisms. Bioorg. Med. Chem. 2007, 15, 4829–4835. [Google Scholar] [CrossRef]
- Ali, H.; Van Lier, J.E. Metal complexes as photo- and radiosensitizers. Chem. Rev. 1999, 99, 2379–2450. [Google Scholar] [CrossRef]
- Nyman, E.S.; Hynninen, P.H. Research advances in the use of tetrapyrrolic photosensitizers for photodynamic therapy. J. Photochem. Photobiol. B Biol. 2004, 73, 1–28. [Google Scholar] [CrossRef]
- Ball, D.J.; Wood, S.R.; Vernon, D.I.; Griffiths, J.; Dubbelman, T.M.A.R.; Brown, S.B. The characterisation of three substituted zinc phthalocyanines of differing charge for use in photodynamic therapy. A comparative study of their aggregation and photosensitising ability in relation to mTHPC and polyhaematoporphyrin. J. Photochem. Photobiol. B Biol. 1998, 45, 28–35. [Google Scholar] [CrossRef]
- Kadish, K.M.; Smith, K.M.; Guilard, R. The Porphyrin Handbook; Academic Press: San Diego, CA, USA, 2003. [Google Scholar]
- Pinzón, J.R.; Plonska-Brzezinska, M.E.; Cardona, C.M.; Athans, A.J.; Gayathri, S.S.; Guldi, D.M.; Herranz, M.Á.; Martín, N.; Torres, T.; Echegoyen, L. Sc3N@C80-ferrocene electron-donor/acceptor conjugates as promising materials for photovoltaic applications. Angew. Chemie-Int. Ed. 2008, 47, 4173–4176. [Google Scholar] [CrossRef] [PubMed]
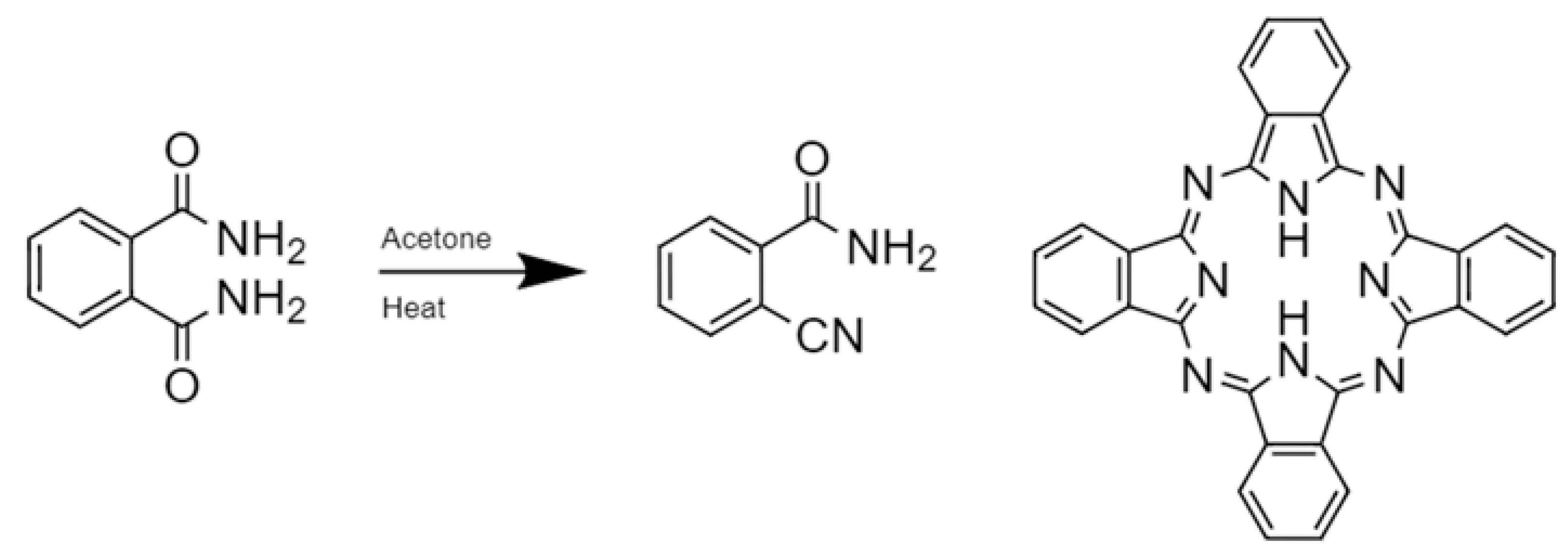
- Braun, A.; Tcherniac, J. Über die produkte der einwirkung von acetanhydrid auf phthalamid. Ber. Dtsch. Chem. Ges. 1907, 40, 2709–2714. [Google Scholar] [CrossRef]
- Kharisov, B.I.; Mendez, U.O.; Garza, J.L.A.; Rodrı´guez, J.R.A. Synthesis of non-substituted phthalocyanines by standard and non-standard techniques. Influence of solvent nature in phthalocyanine preparation at low temperature by UV-treatment of the reaction system. New J. Chem. 2005, 29, 686–692. [Google Scholar] [CrossRef]
- De Diesbach, H.; von der Weid, E. Quelques sels complexes des o-dinitriles avec le cuivre et la pyridine. Helv. Chim. Acta 1927, 10, 886–888. [Google Scholar] [CrossRef]
- Linstead, R.P. Phthalocyanines. Part I. A new type of synthetic colouring matters. J. Chem. Soc. 1934, 95, 1016–1017. [Google Scholar] [CrossRef]
- Linstead, R.P.; Lowe, A.R. Phthalocyanines. Part III. Preliminary experiments on the preparation of phthalocyanines from phthalonitrile. J. Chem. Soc. 1934, 1022–1027. [Google Scholar] [CrossRef]
- Dent, C.E.; Linstead, R.P.; Lowe, A.R. Phthalocyanines. Part VI. The structure of the phthalocyanines. J. Chem. Soc. 1934, 1033, 184. [Google Scholar] [CrossRef]
- Sharman, W.M.; Allen, C.M.; Van Lier, J.E. Photodynamic therapeutics: Basic principles and clinical applications. Drug Discov. Today 1999, 4, 507–517. [Google Scholar] [CrossRef]
- Medina, W.S.G.; dos Santos, N.A.G.; Curti, C.; Tedesco, A.C.; dos Santos, A.C. Effects of zinc phthalocyanine tetrasulfonate-based photodynamic therapy on rat brain isolated mitochondria. Chem. Biol. Interact. 2009, 179, 402–406. [Google Scholar] [CrossRef]
- Staicu, A.; Pascu, A.; Nuta, A.; Sorescu, A.; Raditoiu, V.; Pascu, M.L. Studies about phthalocyanine photosensitizers to be used in photodynamic therapy. Rom. Rep. Phys. 2013, 65, 1032–1051. [Google Scholar]
- Spikes, J.D.; Jori, G. Photodynamic therapy of tumours and other diseases using porphyrins. Laser Med. Sci. 1987, 2, 3–15. [Google Scholar] [CrossRef]
- Whitacre, C.M.; Feyes, D.K.; Satoh, T.; Grossmann, J.; Mulvihill, J.W.; Mukhtar, H.; Oleinick, N.L. Photodynamic therapy with the phthalocyanine photosensitizer Pc 4 of SW480 human colon cancer xenografts in athymic mice. Clin. Cancer Res. 2000, 6, 2021–2027. [Google Scholar] [PubMed]
- Vieira Velloso, N.; Muehlmann, L.A.; Longo, P.F.; Rodrigues Da Silva, J.; Zancanela, D.C.; Tedesco, A.C.; Bentes De Azevedo, R. Aluminum-phthalocyanine chloride-based photodynamic therapy inhibits pi3k/akt/mtor pathway in oral squamous cell carcinoma cells in vitro. Chemotherapy 2012, 2012, 5. [Google Scholar] [CrossRef]
- Abrahamse, H.; Kresfelder, T.; Horne, T.; Cronje, M.; Nyokong, T. Apoptotic inducing ability of a novel photosensitizing agent, Ge sulfophthalocyanine, on oesophageal and breast cancer cell lines. Opt. Methods Tumor Treat. Detect. Mech. Tech. Photodyn. Ther. XV 2006, 6139, 613904. [Google Scholar] [CrossRef]
- Seotsanyana-Mokhosi, I.; Kresfelder, T.; Abrahamse, H.; Nyokong, T. The effect of Ge, Si and Sn phthalocyanine photosensitizers on cell proliferation and viability of human oesophageal carcinoma cells. J. Photochem. Photobiol. B Biol. 2006, 83, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Manoto, S.L.; Sekhejane, P.R.; Houreld, N.N.; Abrahamse, H. Localization and phototoxic effect of zinc sulfophthalocyanine photosensitizer in human colon (DLD-1) and lung (A549) carcinoma cells (in vitro). Photodiagn. Photodyn. Ther. 2012, 9, 52–59. [Google Scholar] [CrossRef]
- Manoto, S.L.; Houreld, N.N.; Abrahamse, H. Phototoxic effect of photodynamic therapy on lung cancer cells grown as a monolayer and three dimensional multicellular spheroids. Lasers Surg. Med. 2013, 45, 186–194. [Google Scholar] [CrossRef]
- Love, W.G.; Duk, S.; Biolo, R.; Jori, G.; Taylor, P.W. Liposome-mediated delivery of photosensitizers: Localization of zinc (II)-phthalocyanine within implanted tumors after intravenous administration. Photochem. Photobiol. 1996, 63, 656–661. [Google Scholar] [CrossRef]
- Shao, J.; Xue, J.; Dai, Y.; Liu, H.; Chen, N.; Jia, L.; Huang, J. Inhibition of human hepatocellular carcinoma HepG2 by phthalocyanine photosensitiser photocyanine: ROS production, apoptosis, cell cycle arrest. Eur. J. Cancer 2012, 48, 2086–2096. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.D.; Baron, E.D.; Scull, H.; Hsia, A.; Berlin, J.C.; McCormick, T.; Colussi, V.; Kenney, M.E.; Cooper, K.D.; Oleinick, N.L. Photodynamic therapy with the phthalocyanine photosensitizer Pc 4: The case experience with preclinical mechanistic and early clinical-translational studies. Toxicol. Appl. Pharmacol. 2007, 224, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Baran, T.M.; Giesselman, B.R.; Hu, R.; Biel, M.A.; Foster, T.H. Factors influencing tumor response to photodynamic therapy sensitized by intratumor administration of methylene blue. Lasers Surg. Med. 2010, 42, 728–735. [Google Scholar] [CrossRef]
- Spikes, J.D. Phthalocyanines as photosensitizers in biological systems and for the photodynamic therapy of tumors. Photochem. Photobiol. 1986, 43, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.I.A.; Agarwal, R.; Eichler, G.; Rihter, B.D.; Kenney, M.E.; Mukhtar, H. Photodynamic effects of new silicon phthalocyanines: In vitro studies utilizing rat hepatic microsomes and human erythrocyte ghosts as model membrane sources. Photochem. Photobiol. 1993, 58, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Athar, M.; Elmets, C.A.; Bickers, D.R.; Mukhtar, H. Photodynamic therapy of chemically- and ultraviolet B radiation-induced murine skin papillomas by chloroaluminum phthalocyanine tetrasulfonate. Photochem. Photobiol. 1992, 56, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Brasseur, N.; Ali, H.; Autenrieth, D.; Langlois, R.; van Liert, J.E. Biological activities of Phthalocyanines—iii. Photoinactivation of v-79 chinese hamster cells by tetrasulfophthalocyanines. Photochem. Photobiol. 1985, 42, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.J.; Chambrier, I.; Cracknell, S.J.; Mayes, D.A.; Russell, D.A. Octa-alkyl zinc phthalocyanines: Potential photosensitizers for use in the photodynamic therapy of cancer. Photochem. Photobiol. 1995, 62, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Stilts, C.E.; Nelen, M.I.; Hilmey, D.G.; Davies, S.R.; Gollnick, S.O.; Oseroff, A.R.; Gibson, S.L.; Hilf, R.; Detty, M.R. Water-soluble, core-modified porphyrins as novel, longer-wavelength- absorbing sensitizers for photodynamic therapy. J. Med. Chem. 2000, 43, 2403–2410. [Google Scholar] [CrossRef] [PubMed]
- Yates, N.C.; Moan, J.; Western, A. Water-soluble metal naphthalocyanines-near-IR photosensitizers: Cellular uptake, toxicity and photosensitizing properties in nhik 3025 human cancer cells. J. Photochem. Photobiol. B Biol. 1990, 4, 379–390. [Google Scholar] [CrossRef]
- Cruse-Sawyer, J.E.; Griffiths, J.; Dixon, B.; Brown, S.B. The photodynamic response of two rodent tumour models to four zinc (II)-substituted phthalocyanines. Br. J. Cancer 1998, 77, 965–972. [Google Scholar] [CrossRef]
- Sharman, W.M.; Kudrevich, S.V.; Van Lier, J.E. Novel water-soluble phthalocyanines substituted with phosphonate moieties on the benzo rings. Tetrahedron Lett. 1996, 37, 5831–5834. [Google Scholar] [CrossRef]
- Wöhrle, D. Phthalocyanines: Properties and applications, volume 3. Edited by C. C. Leznoff and A. B. P. Lever, VCH, Weinheim 1993, 303 pp., hardcover, DM 198, ISBN3-527-89638-4. Adv. Mater. 1993, 5, 943–944. [Google Scholar] [CrossRef]
- Lo, P.C.; Rodríguez-Morgade, M.S.; Pandey, R.K.; Ng, D.K.P.; Torres, T.; Dumoulin, F. The unique features and promises of phthalocyanines as advanced photosensitisers for photodynamic therapy of cancer. Chem. Soc. Rev. 2020, 49, 1041–1056. [Google Scholar] [CrossRef]
- Brasseur, N.; Ouellet, R.; La Madeleine, C.; Van Lier, J. Water soluble aluminium phthalocyanine-polymer conjugates for PDT: Photodynamic activities and pharmacokinetics in tumour bearing mice. Br. J. Cancer 1999, 80, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, T.; Tabata, Y.; Ikada, Y. Distribution and tissue uptake of poly(ethylene glycol) with different molecular weights after intravenous administration to mice. J. Pharm. Sci. 1994, 83, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.; Larocca, M.; Wu, W.; Babu, V.; Padrutt, R.; Slyshkina, E.; König, C.; Ferrari, S.; Spingler, B. Exocyclically metallated tetrapyridinoporphyrazine as a potential photosensitizer for photodynamic therapy. Photochem. Photobiol. Sci. 2019, 18, 2792–2803. [Google Scholar] [CrossRef] [PubMed]
- Md, S.; Haque, S.; Madheswaran, T.; Zeeshan, F.; Meka, V.S.; Radhakrishnan, A.K.; Kesharwani, P. Lipid based nanocarriers system for topical delivery of photosensitizers. Drug Discov. Today 2017, 22, 1274–1283. [Google Scholar] [CrossRef]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy—Current Limitations and Novel Approaches. Front. Chem. 2021, 9, 691697. [Google Scholar] [CrossRef] [PubMed]
- Rahbari, R.; Sheahan, T.; Modes, V.; Collier, P.; Macfarlane, C.; Badge, R.M. A novel L1 retrotransposon marker for HeLa cell line identification. Biotechniques 2009, 46, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Scherer, W.F.; Syverton, J.T.; Gey, G.O. Studies on the propagation in vitro of poliomyelitis viruses: IV. Viral multiplication in a stable strain of human malignant epithelial cells (strain HeLa) derived from an epidermoid carcinoma of the cervix. J. Exp. Med. 1953, 97, 695–710. [Google Scholar] [CrossRef] [PubMed]
- Capes-Davis, A.; Theodosopoulos, G.; Atkin, I.; Drexler, H.G.; Kohara, A.; MacLeod, R.A.F.; Masters, J.R.; Nakamura, Y.; Reid, Y.A.; Reddel, R.R.; et al. Check your cultures! A list of cross-contaminated or misidentified cell lines. Int. J. Cancer 2010, 127, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Halaskova, M.; Rahali, A.; Almeida-Marrero, V.; Machacek, M.; Kucera, R.; Jamoussi, B.; Torres, T.; Novakova, V.; de la Escosura, A.; Zimcik, P. Peripherally crowded cationic phthalocyanines as efficient photosensitizers for photodynamic therapy. ACS Med. Chem. Lett. 2021, 12, 502–507. [Google Scholar] [CrossRef]
- Chu, J.C.H.; Fong, W.-P.; Wong, C.T.T.; Ng, D.K.P. Facile synthesis of cyclic peptide–phthalocyanine conjugates for epidermal growth factor receptor-targeted photodynamic therapy. J. Med. Chem. 2021, 64, 2064–2076. [Google Scholar] [CrossRef] [PubMed]
- Göksel, M.; Durmuş, M.; Biyiklioglu, Z. Synthesis and photodynamic activities of novel silicon(IV) phthalocyanines axially substituted with water soluble groups against HeLa cancer cell line. Dalt. Trans. 2021, 50, 2570–2584. [Google Scholar] [CrossRef] [PubMed]
- Pola, M.; Kolarova, H.; Ruzicka, J.; Zholobenko, A.; Modriansky, M.; Mosinger, J.; Bajgar, R. Effects of zinc porphyrin and zinc phthalocyanine derivatives in photodynamic anticancer therapy under different partial pressures of oxygen in vitro. Investig. New Drugs 2020, 39, 89–97. [Google Scholar] [CrossRef]
- Revuelta-Maza, M.; Mascaraque, M.; González-Jiménez, P.; González-Camuñas, A.; Nonell, S.; Juarranz, Á.; de la Torre, G.; Torres, T. Assessing amphiphilic ABAB Zn(II) phthalocyanines with enhanced photosensitization abilities in in vitro photodynamic therapy studies against cancer. Molecules 2020, 25, 213. [Google Scholar] [CrossRef]
- Ermakov, A.; Verkhovskii, R.; Babushkina, I.; Trushina, D.; Inozemtseva, O.; Lukyanets, E.; Ulyanov, V.; Gorin, D.; Belyakov, S.; Antipina, M. In vitro bioeffects of polyelectrolyte multilayer microcapsules post-loaded with water-soluble cationic photosensitizer. Pharmaceutics 2020, 12, 610. [Google Scholar] [CrossRef] [PubMed]
- Balçik-Erçin, P.; Çetin, M.; Göksel, M.; Durmuş, M. Improved targeting for photodynamic therapy: Via a biotin-phthalocyanine conjugate: Synthesis, photophysical and photochemical measurements, and in vitro cytotoxicity assay. New J. Chem. 2020, 44, 3392–3401. [Google Scholar] [CrossRef]
- Kollar, J.; Machacek, M.; Halaskova, M.; Lenco, J.; Kucera, R.; Demuth, J.; Rohlickova, M.; Hasonova, K.; Miletin, M.; Novakova, V.; et al. Cationic versus anionic phthalocyanines for photodynamic therapy: What a difference the charge makes. J. Med. Chem. 2020, 63, 7616–7632. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhan, Q.; Li, Y.; Zhou, L.; Wei, S. Multiple functions integrated inside a single molecule for amplification of photodynamic therapy activity. Mol. Pharm. 2020, 17, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, M.; Pesce, L.; Pezzuoli, D.; Montali, C.; Brancaleon, L.; Cavanna, L.; Abbruzzetti, S.; Diaspro, A.; Bianchini, P.; Viappiani, C. Apomyoglobin is an efficient carrier for zinc phthalocyanine in photodynamic therapy of tumors. Biophys. Chem. 2019, 253, 106228. [Google Scholar] [CrossRef]
- Ma, J.; Li, Y.; Liu, G.; Li, A.; Chen, Y.; Zhou, X.; Chen, D.; Hou, Z.; Zhu, X. Novel theranostic zinc phthalocyanine–phospholipid complex self-assembled nanoparticles for imaging-guided targeted photodynamic treatment with controllable ROS production and shape-assisted enhanced cellular uptake. Colloids Surf. B Biointerfaces 2018, 162, 76–89. [Google Scholar] [CrossRef]
- Ma, J.; Chen, D.; Li, Y.; Chen, Y.; Liu, Q.; Zhou, X.; Qian, K.; Li, Z.; Ruan, H.; Hou, Z.; et al. Zinc phthalocyanine-soybean phospholipid complex based drug carrier for switchable photoacoustic/fluorescence image, multiphase photothermal/photodynamic treatment and synergetic therapy. J. Control. Release 2018, 284, 1–14. [Google Scholar] [CrossRef]
- Ma, J.; Wu, H.; Li, Y.; Liu, Z.; Liu, G.; Guo, Y.; Hou, Z.; Zhao, Q.; Chen, D.; Zhu, X. Novel core-interlayer-shell DOX/ZnPc co-loaded MSNs@ pH-sensitive CaP@PEGylated liposome for enhanced synergetic chemo-photodynamic therapy. Pharm. Res. 2018, 35, 57. [Google Scholar] [CrossRef] [PubMed]
- Gülmez, A.D.; Göksel, M.; Durmuş, M. Silicon(IV) phthalocyanine-biotin conjugates: Synthesis, photophysicochemical properties and in vitro biological activity for photodynamic therapy. J. Porphyr. Phthalocyanines 2017, 21, 547–554. [Google Scholar] [CrossRef]
- Wang, A.; Zhou, R.; Zhou, L.; Sun, K.; Jiang, J.; Wei, S. Positively charged phthalocyanine-arginine conjugates as efficient photosensitizer for photodynamic therapy. Bioorg. Med. Chem. 2017, 25, 1643–1651. [Google Scholar] [CrossRef]
- Avşar, G.; Sari, F.A.; Yuzer, A.C.; Soylu, H.M.; Er, O.; Ince, M.; Lambrecht, F.Y. Intracellular uptake and fluorescence imaging potential in tumor cell of zinc phthalocyanine. Int. J. Pharm. 2016, 505, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Franchi, L.P.; Amantino, C.F.; Melo, M.T.; de Lima Montaldi, A.P.; Primo, F.L.; Tedesco, A.C. In vitro effects of photodynamic therapy induced by chloroaluminum phthalocyanine nanoemulsion. Photodiagn. Photodyn. Ther. 2016, 16, 100–105. [Google Scholar] [CrossRef]
- Göksel, M. Synthesis of asymmetric zinc(II) phthalocyanines with two different functional groups & spectroscopic properties and photodynamic activity for photodynamic therapy. Bioorg. Med. Chem. 2016, 24, 4152–4164. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yu, H.; Lv, H.; Zhang, H.; Ma, D.; Yang, H.; Xie, S.; Peng, Y. Triblock copolymers encapsulated poly (aryl benzyl ether) dendrimer zinc(II) phthalocyanine nanoparticles for enhancement in vitro photodynamic efficacy. Photodiagn. Photodyn. Ther. 2016, 16, 124–131. [Google Scholar] [CrossRef]
- Lu, S.; Wang, A.; Ma, Y.J.; Xuan, H.Y.; Zhao, B.; Li, X.D.; Zhou, J.H.; Zhou, L.; Wei, S.H. Cyclodextrin type dependent host-guest interaction mode with phthalocyanine and their influence on photodynamic activity to cancer. Carbohydr. Polym. 2016, 148, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Jin, W.; Chen, E.; Zhou, J.; Zhou, L.; Wei, S. Drug delivery function of carboxymethyl-β-cyclodextrin modified upconversion nanoparticles for adamantine phthalocyanine and their NIR-triggered cancer treatment. Dalt. Trans. 2016, 45, 3853–3862. [Google Scholar] [CrossRef]
- Young, J.; Yee, M.; Kim, H.; Cheung, J.; Chino, T.; Düzgüneş, N.; Konopka, K. Phototoxicity of liposomal Zn- and Al-phthalocyanine against cervical and oral squamous cell carcinoma cells in vitro. Med. Sci. Monit. Basic Res. 2016, 22, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Yurt, F.; Ocakoglu, K.; Ince, M.; Colak, S.G.; Er, O.; Soylu, H.M.; Gunduz, C.; Biray Avci, C.; Caliskan Kurt, C. Photodynamic therapy and nuclear imaging activities of zinc phthalocyanine-integrated TiO2 nanoparticles in breast and cervical tumors. Chem. Biol. Drug Des. 2018, 91, 789–796. [Google Scholar] [CrossRef]
- Hodgkinson, N.; Kruger, C.A.; Mokwena, M.; Abrahamse, H. Cervical cancer cells (HeLa) response to photodynamic therapy using a zinc phthalocyanine photosensitizer. J. Photochem. Photobiol. B Biol. 2017, 177, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Pazos, M.D.C.; Nader, H.B. Effect of photodynamic therapy on the extracellular matrix and associated components. Braz. J. Med. Biol. Res. 2007, 40, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Bacellar, I.; Tsubone, T.; Pavani, C.; Baptista, M. Photodynamic efficiency: From molecular photochemistry to cell death. Int. J. Mol. Sci. 2015, 16, 20523–20559. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Wang, Z.; Shen, W.; Liang, R.; Yan, D.; Wei, M. Recent advances in innovative strategies for enhanced cancer photodynamic therapy. Theranostics 2021, 11, 3278–3300. [Google Scholar] [CrossRef]
- Mitra, K.; Hartman, M.C.T. Silicon phthalocyanines: Synthesis and resurgent applications. Org. Biomol. Chem. 2021, 19, 1168–1190. [Google Scholar] [CrossRef]
- Li, K.; Dong, W.; Liu, Q.; Lv, G.; Xie, M.; Sun, X.; Qiu, L.; Lin, J. A biotin receptor-targeted silicon(IV) phthalocyanine for in vivo tumor imaging and photodynamic therapy. J. Photochem. Photobiol. B Biol. 2019, 190, 1–7. [Google Scholar] [CrossRef]
- Liang, X.; Xie, Y.; Wu, J.; Wang, J.; Petković, M.; Stepić, M.; Zhao, J.; Ma, J.; Mi, L. Functional titanium dioxide nanoparticle conjugated with phthalocyanine and folic acid as a promising photosensitizer for targeted photodynamic therapy in vitro and in vivo. J. Photochem. Photobiol. B Biol. 2021, 215, 112122. [Google Scholar] [CrossRef]
- Wang, J.; Zhuo, X.; Xiao, X.; Mao, R.; Wang, Y.; Wang, J.; Liu, J. AlPcS-loaded gold nanobipyramids with high two-photon efficiency for photodynamic therapy: In vivo. Nanoscale 2019, 11, 3397. [Google Scholar] [CrossRef]
- Hillemanns, P.; Garcia, F.; Petry, K.U.; Dvorak, V.; Sadovsky, O.; Iversen, O.; Einstein, M.H. A randomized study of hexaminolevulinate photodynamic therapy in patients with cervical intraepithelial neoplasia 1/2. Am. J. Obstet. Gynecol. 2015, 212, 465.e1-7. [Google Scholar] [CrossRef]
- Larmour, L.I.; Jobling, T.W.; Gargett, C.E. A review of current animal models for the study of cervical dysplasia and cervical carcinoma. Int. J. Gynecol. Cancer 2015, 25, 1345–1352. [Google Scholar] [CrossRef]
| Cell Line | Histologic Type | Donor Age | HPV | p53 | pRb |
|---|---|---|---|---|---|
| HeLa (ATCC® CCL-2™) | Adenocarcinoma | 31 | HPV-18 | Positive (low) | Positive (normal) |
| SiHa (ATCC® HTB-35™) | Squamous cell carcinoma | 55 | HPV-16, 1 to 2 copies per cell | Positive | Positive |
| CasKi (ATCC® CRM-CRL-1550™) | Squamous cell carcinoma | 40 | HPV-16, about 600 copies per cell, and HPV-18 | NA | NA |
| C-33A (ATCC® HTB-31™) | Squamous cell carcinoma | 66 | Negative | Positive | Positive |
| MS751 (ATCC® HTB-34™) | Squamous cell carcinoma | 47 | HPV-18 HPV-45 | NA | NA |
| ME-180 (ATCC® HTB-33™) | Squamous cell carcinoma | 66 | HPV DNA with higher homology to HPV-68 than HPV-18 | Positive | Positive |
| C-4 I (ATCC® CRL-1594™) | Squamous cell carcinoma | 41 | HPV-18 | NA | NA |
| Title | Reference | PS/Concentration/ Time of Exposure to PS | Light Source/ Wavelength/Fluence/ Power-Density/ Time of Exposure | Main Outcomes |
|---|---|---|---|---|
| Exocyclically metallated tetrapyridinoporphyrazine as a potential photosensitizer for photodynamic therapy | [165] | Exocyclically metallated tetrapyridinoporphyrazine [tetrakis-(trans-Pt(NH3)2Cl)-tetra(3,4-pyrido)porphyrazine-zinc(II)](NO3)4, 1.6 μM, and 4 h | 600 nm, 5.8 mW/cm−2, 6.96 J/cm−2, and 20 min | Single digit micromolar concentrations are able to induce photocytotoxicity while maintaining low toxicity in the dark. The compound mainly accumulates in the nucleus, suggesting that interacts with DNA, leading to subsequent DNA damage and resulting in photocytotoxicity. |
| Peripherally crowded cationic phthalocyanines as efficient photosensitizers for photodynamic therapy | [171] | Zinc phthalocyanine bearing four or eight bulky 2,6-di(pyridin-3-yl)phenoxy substituents, 0–10 μM, and 12 h | 570 nm, 12.4 mW/cm2, 11.2 J/cm2, and 15 min | High photodynamic activity against cancer cells while maintaining low toxicity in the dark. Localization in the lysosomes, inducing an apoptotic cell death pathway with secondary necrosis. |
| Facile synthesis of cyclic peptide−phthalocyanine conjugates for epidermal growth factor receptor-targeted photodynamic therapy | [172] | Cyclic peptide-conjugated zinc(II) phthalocyanine, 0–60 nM, and 2 h | 610 nm, 23 mW/cm2, 28 J/cm2, and 20 min | The intensity of cell uptake in EGFR-positive HT29 and HCT116 cells is up to 25 times higher than against EGFR-negative HeLa and HEK293 cells. This conjugate also shows high photo cytotoxicity for HT29 and HCT116 cells. |
| Synthesis and photodynamic activities of novel silicon(IV) phthalocyanines axially substituted | [173] | Quaternized cationic silicon(IV) phthalocyanine (SiPc) derivatives, 0–10 μM, and 24 h | 680 ± 10 nm, 2/J cm2, and NR | High photodynamic activity against cancer cells while maintaining low toxicity in the dark. |
| Effects of zinc porphyrin and zinc phthalocyanine derivatives in photodynamic anticancer therapy under different partial pressures of oxygen in vitro | [174] | Disulphonated zinc phthalocyanine (ZnPcS2) and tetrasulphonated zinc tetraphenylporphyrin (ZnTPPS4), 0–10 μM, and 24 h | ZnTPPS4: 415 ± 10 nm ZnPcS2: 660 ± 15 nm; 7 mW/cm2, 4.2 J/cm2, and 10 min | ZnTPPS4 was internalized in the cytosol and lysosomes, whereas ZnPcS2 was attached to membrane structures and was photodynamically effective at a minimal level of oxygen, with a higher effect on mitochondrial respiration. |
| Assessing amphiphilic ABAB Zn(II) phthalocyanines with enhanced photosensitization abilities in in vitro photodynamic therapy studies against cancer | [175] | Triethylene glycol (TEG)-containing Zn(II)Pcs, namely, ABAB-1, A3B-1, and A4-1 | ABAB-1, A3B-1, and A4-1; 637 nm ± 17 nm; different red light doses (3, 6 and 9 J/cm2), NR | ABAB-1 and A3B-1 presented high photodynamic activity on cancer cells while maintaining low toxicity in the dark. |
| In vitro bioeffects of polyelectrolyte multilayer microcapsules post-loaded with water-soluble cationic photosensitizer | [176] | Dextran sulfate (DS) and poly-l-arginine (PArg) PMC ([DS/PAgr]4) capsules loaded with zinc phthalocyanine choline derivative (cholosens), NR | - | High drug release rate, internalization, light toxicities, and low dark effects. |
| Improved targeting for photodynamic therapy via a biotin–phthalocyanine conjugate: synthesis, photophysical and photochemical measurements, and in vitro cytotoxicity assay | [177] | Peripherally biotin-substituted zinc(II) phthalocyanine (Pc2), 0.25–5 μM, and 24 h | 690 ± 10 nm, 1/J cm2 and 2/J cm2, and NR | The biotin-conjugated zinc(II) phthalocyanine derivative presented a higher cytotoxic effect than the amino functionalized zinc(II) phthalocyanine derivative. Pcs were located in the cytoplasm, leading to cell death by apoptosis and reduction of colony capacity after PDT. |
| Cationic versus anionic phthalocyanines for photodynamic therapy: what a difference the charge makes | [178] | Anionic and cationic zinc(II) phthalocyanines, 1–10 mM, and 12 h | 570 nm, 12.4 mW/cm2, 11.2 J/cm2, and 15 min | Hydrophilic compounds were localized into lysosomes and amphiphilic compounds were also detected in the cellular membrane. Hydrophilic cationic Pcs were relocalized into the cytoplasm upon irradiation and damaged the nuclear membrane. A high dose of Pcs induced morphological changes and phototoxicity. |
| Multiple functions integrated inside a single molecule for amplification of photodynamic therapy activity | [179] | Arg and Lys zinc phthalocyanines, 0–10 μM, and 48 h | 665 nm, 5 W, 0.4 W/cm2, and 4 min | The phototoxic effects were more accentuated and the percentage of apoptotic cells was higher in the cells treated with Arg-ZnPc. |
| Apomyoglobin is an efficient carrier for zinc phthalocyanine in photodynamic therapy of tumors | [180] | Zinc phthalocyanine carried by apomyoglobin, 500 nM, and NR | 647 nm, 2.5 mW, 130 mW/cm2, 40 J/cm2, and 5 min | The uptake of ZnPc by cells was efficient, with no dark toxicity. When illuminated, a moderate fluence and low concentrations were sufficient to induce extensive cell death. |
| Novel theranostic zinc phthalocyanine–phospholipid complex self-assembled nanoparticles for imaging-guided targeted photodynamic treatment with controllable ROS production and shape-assisted enhanced cellular uptake | [181] | Zinc phthalocyanine-soybean phosphatidylcholine (ZnPc-SPC) complex, 0.3–10 µg/mL, and 12 or 24 h | 630 nm, NR, and 5 min | Pcs could target folate receptors-overexpressed cancer cells and internalized in the cytoplasm. Apoptosis rate increased after PDT. |
| Zinc phthalocyanine-soybean phospholipid complex based drug carrier for switchable photoacoustic/fluorescence image, multiphase photothermal/ photodynamic treatment and synergetic therapy | [182] | Zinc phthalocyanine-soybean phospholipid complex with doxorubicin (DZSM), 0.3–10 µg/mL, and 12 h | 638 nm, 1 W/cm2, NR, and 5 min | ZnPc was distributed in the cytoplasm. Dox was almost located in the nucleus. DZSM presented high selectivity for FRα over-expressed tumor cells as HeLa, excellent switchable image, significant multiphase photothermal therapy (PTT)/PDT effect, and great synergetic therapy potential, leading to notable inhibition of tumor growth. |
| Novel core-interlayer-shell DOX/ZnPc Co-loaded MSNs@ pH-sensitive CaP@PEGylated liposome for enhanced synergetic chemo-photodynamic therapy | [183] | DOX/ZnPc co-loaded MSNs@CaP@PEGylated liposome, NR, and 24 h | 630 nm, 0.05 W/cm2, NR, and 5 min | ZnPc in the nanoparticles successfully produced the intracellular singlet oxygen under the light that could eventually induce the cytotoxicity of PDT in the cells and could be a promising candidate for PDT besides serving as a chemotherapeutic agent. |
| Silicon(IV) phthalocyanine-biotin conjugates: synthesis, photo physicochemical properties and in vitro biological activity for photodynamic therapy | [184] | Axially biotin substituted silicon(IV) phthalocyanine, 0–10 μM, and 24 h | NR, 1 J/cm2 or 2 J/cm2, and NR | Both axially mono- and bis-biotin substituted silicon(IV) phthalocyanines presented high photo cytotoxicity against HeLa cancer cells with the cell survival degree ranging from 13% to 50%. The photosensitivity and the intensity of damage were found to be directly related to the concentration of the used photosensitizers. |
| Positively charged phthalocyanine-arginine conjugates as efficient photosensitizer for photodynamic therapy | [185] | Arginine substituted zinc phthalocyanines (ArgEZnPc and ArgZnPc), 0–4 µM, and 4 h | 665 nm, 96 mW/cm2, 28.8 J/cm2, 5 min, and NR | ArgEZnPc presented higher cellular uptake, high water solubility and ROSs generation ability. HeLa cells showed shrinkage and cell scatter, membrane deformation, and chromatin damage. ArgEZnPc can target the lysosomes and exhibited high cytotoxicity. |
| Intracellular uptake and fluorescence imaging potential in tumor cell of zinc phthalocyanine | [186] | Zinc phthalocyanine (ZnPc), 10–90 mM, and 24 h | NR | The IC50 values were observed to be 35 mM in HeLa cells, maximum uptake was determined at 6 h, and the uptake was decreased at 24 h. |
| In vitro effects of photodynamic therapy induced by chloro aluminum phthalocyanine nanoemulsion | [187] | Chloroaluminum phthalocyanine nanoemulsion (ClAlPc/NE) or MX+ ClAlPc/NE (methoxyamine), NR, and 3 h | 670 nm, 0.1, 0.5 and 1.0 J/cm2, and NR | A dose-dependent cell death reduced clonogenic survival rates, and sub-G1 accumulation and apoptosis induction were observed in HeLa cells. MX increased PDT effects. |
| Synthesis of asymmetric zinc(II) phthalocyanines with two different functional groups and spectroscopic properties and photodynamic activity for photodynamic therapy | [188] | Zinc(II) phthalocyanine functionalized, 0–10 µM, and 24 h | 690 ± 10 nm, 2 J/cm2, and NR | The photodynamic efficiency is micromolar. Biotin conjugated zinc(II) phthalocyanine displayed a higher photo cytotoxicity relative to amino phthalocyanine, probably attributed to its high triplet quantum yield of 1O2. |
| Triblock copolymers encapsulated poly (aryl benzyl ether) dendrimer zinc(II) phthalocyanine nanoparticles for enhancement in vitro photodynamic efficacy | [189] | Zinc (II) phthalocyanines nanoparticles with triblock copolymer (G2-DPcZn), 0.02–10 µM, and 24 h | 670 nm, 25, 50, and 100 mW/cm2, 0–6 J/cm2, and 2 min | The nanocarriers enhanced intracellular uptake, phototoxicity, and ROS production. The nanoparticle surface with positive charge seems to localize G2-DPcZn in mitochondria. |
| Cyclodextrin type dependent host-guest interaction mode with phthalocyanine and their influence on photodynamic activity against cancer | [190] | Phthalocyanines (Pc) with cyclodextrins (CDs), 5 µM, and 4 h | 665 nm, NR, and 5 min. | The aggregation degree of Pcs was decreased, the water solubility and photodynamic activity were increased. The cellular uptake and ROS generation efficiency of (-CD)4-ZnPc was higher. PDT induced morphology changes, such as chromatin condensation, shrinkage, and fragmentation. |
| Drug delivery function of carboxymethyl-β-cyclodextrin modified upconversion nanoparticles for adamantine phthalocyanine and their NIR-triggered cancer treatment | [191] | UCNP/COOH-β-CD/Ad-ZnPc, 2–8 μM, and 4 h | 980 nm, NR, and 1–4 sessions of 2 min | The complex was mainly located in the cytoplasm and after irradiation induced morphology changes, such as chromatin condensation, shrinkage, and fragmentation. The UCNPs and Ad-ZnPc and UCNP/COOH-β-CD/Ad-ZnPc treated cell survival percent sharply decreased with the increasing of drug concentration and light dose. |
| Phototoxicity of liposomal Zn- and Al-phthalocyanine against cervical and oral squamous cell carcinoma cells in vitro | [192] | Liposomal Zn- and Al-phthalocyanine, 0.1–1 μM, and 24 h | ZnPc: 350–800 nm, 43.2 J/cm2; AlPc: 690 nm, 3.6 J/cm2; 20 min | Liposome-embedded ZnPc and AlPc were more effective than free ZnPc and AlPc in reducing cell viability. HeLa cervical adenocarcinoma cells were more sensitive to AlPc. |
| Photodynamic therapy and nuclear imaging activities of zinc phthalocyanine-integrated TiO2 nanoparticles in breast and cervical tumors | [193] | Zinc phthalocyanine integrated to the TiO2 nanoparticle (ZnPc- TiO2), 1–6.25 μM, and 3h | LED light source, 10 mW/cm2, 30, 60, and 90 J/cm2, and NR | TiO2 nanoparticles increased the cytotoxicity of ZnPc in the HeLa cell line. Phototoxic effects increased depending on the dose of light. Cellular localization of the Pcs was found especially in cytoplasm but not nuclei. |
| Cervical cancer cells (HeLa) response to photodynamic therapy using a zinc phthalocyanine photosensitizer | [194] | Sulphonated zinc phthalocyanine PS (ZnPcSmix), 0.25–1 μM, and 24 h | 673 nm diode laser, 96 mW, 2, 4 and 8 J/cm2, and NR | The PS was located in the cytoplasm and perinuclear region of HeLa cells. PDT induced dose-dependent structural changes, with decreased cell viability and proliferation, as well as membrane damage. |
| Title | Reference | Animal Model | PS/Concentration/ Time of Exposure to PS | Light Source/Wavelength/Fluence/Power/Density/Time of Exposure | Main In Vitro Outcomes | Main In Vivo Outcomes |
|---|---|---|---|---|---|---|
| Functional titanium dioxide nanoparticle conjugated with phthalocyanine and folic acid as a promising photosensitizer for targeted photodynamic therapy in vitro and in vivo | [200] | Female BALB/c nude mice inoculated subcutaneously on the right armpit with 100 μL HeLa cells (1 × 107 cells) in PBS. | TiO2 nanoparticle conjugated with folic acid (FA), and Al (III) phthalocyanine chloride tetrasulfonic acid (FA-TiO2-Pc), 0.52 μmol/kg, 6 h | 420–800 nm, 0.75 W/cm2, 10 min. | FA-TiO2-Pc presented high therapeutic drug efficiency at a low concentration dose and short incubation time under one-photon excitation. | Tumor growth of the FA-TiO2-Pc treated mice was significantly inhibited. The survival rates were 100%, and no significant physiological morphology changes were observed in heart, liver, spleen, lungs, and kidney, indicating no toxic effects after treatment. |
| A biotin receptor-targeted silicon(IV) phthalocyanine for in vivo tumor imaging and photodynamic therapy | [199] | Female BALB/c nude mice inoculated subcutaneously on the right foreleg armpit with 100 μL of PBS containing Hela cells (1 × 107) | Biotin receptor-targeted silicon(IV) phthalocyanine, 2 μmol/kg, 2 h | 670 nm, 10 mW/cm2, 30 min | High photodynamic activity on cancer cells while maintaining low toxicity in the dark. | The compound specifically accumulated in tumor tissue through the biotin receptor-mediated process, allowing for the targeted imaging of the tumor tissue in vivo. Moreover, under-irradiation induced clear necrosis of the tumor tissues and the tumor’s growth was inhibited. |
| Sequential protein-responsive nanophotosensitizer complex for enhancing tumor-specific therapy | [29] | Male NOD-SCID mice injected subcutaneously with HeLa cells (2 × 107 cells) | zinc(II) phthalocyanine derivative entrapped mesoporous silica nanoparticles (MSNs) and a wrapping DNA (O1) (PcC4-MSN-O1), 200 μM, 24 h | 670 nm, 0.5 W/cm2, 20 min | PcC4-MSN-O1 displayed selective phototoxicity against HeLa over normal cells (HEK-293). | There was an accumulation in HeLa tumors of xenograft-bearing mice, and irradiation induced the inhibition of tumor growth and apoptosis. The time-modulated activation process in tumors and the relatively fast excretion of PcC4-MSN-O1 indicated its advantages in reducing potential side effects. |
| AlPcS-loaded gold nanobipyramids with high two-photon efficiency for photodynamic therapy in vivo | [201] | Nude mice inoculated subcutaneously with HeLa cells | Sulfonated Al-phthalocyanine (AlPcS)-loaded by gold nanobipyramids, 5 nM, 2 h | 800 nm 2.8 W/cm2., 30 min | High photodynamic activity against cancer cells. | An evident inhibition in tumor growth and extensive necrosis was observed in mice after PDT treatment with GBP-AlPcS. Moreover, no side effect or toxicity to normal tissues was observed. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carobeli, L.R.; Meirelles, L.E.d.F.; Damke, G.M.Z.F.; Damke, E.; Souza, M.V.F.d.; Mari, N.L.; Mashiba, K.H.; Shinobu-Mesquita, C.S.; Souza, R.P.; Silva, V.R.S.d.; et al. Phthalocyanine and Its Formulations: A Promising Photosensitizer for Cervical Cancer Phototherapy. Pharmaceutics 2021, 13, 2057. https://doi.org/10.3390/pharmaceutics13122057
Carobeli LR, Meirelles LEdF, Damke GMZF, Damke E, Souza MVFd, Mari NL, Mashiba KH, Shinobu-Mesquita CS, Souza RP, Silva VRSd, et al. Phthalocyanine and Its Formulations: A Promising Photosensitizer for Cervical Cancer Phototherapy. Pharmaceutics. 2021; 13(12):2057. https://doi.org/10.3390/pharmaceutics13122057
Chicago/Turabian StyleCarobeli, Lucimara R., Lyvia E. de F. Meirelles, Gabrielle M. Z. F. Damke, Edilson Damke, Maria V. F. de Souza, Natália L. Mari, Kayane H. Mashiba, Cristiane S. Shinobu-Mesquita, Raquel P. Souza, Vânia R. S. da Silva, and et al. 2021. "Phthalocyanine and Its Formulations: A Promising Photosensitizer for Cervical Cancer Phototherapy" Pharmaceutics 13, no. 12: 2057. https://doi.org/10.3390/pharmaceutics13122057
APA StyleCarobeli, L. R., Meirelles, L. E. d. F., Damke, G. M. Z. F., Damke, E., Souza, M. V. F. d., Mari, N. L., Mashiba, K. H., Shinobu-Mesquita, C. S., Souza, R. P., Silva, V. R. S. d., Gonçalves, R. S., Caetano, W., & Consolaro, M. E. L. (2021). Phthalocyanine and Its Formulations: A Promising Photosensitizer for Cervical Cancer Phototherapy. Pharmaceutics, 13(12), 2057. https://doi.org/10.3390/pharmaceutics13122057






