Abstract
The tendon is a highly aligned connective tissue that transmits force from muscle to bone. Each year, more than 32 million tendon injuries have been reported, in fact, tendinopathies represent at least 50% of all sports injuries, and their incidence rates have increased in recent decades due to the aging population. Current clinical grafts used in tendon treatment are subject to several restrictions and there is a significant demand for alternative engineered tissue. For this reason, innovative strategies need to be explored. Tendon replacement and regeneration are complex since scaffolds need to guarantee an adequate hierarchical structured morphology and mechanical properties to stand the load. Moreover, to guide cell proliferation and growth, scaffolds should provide a fibrous network that mimics the collagen arrangement of the extracellular matrix in the tendons. This review focuses on tendon repair and regeneration. Particular attention has been devoted to the innovative approaches in tissue engineering. Advanced manufacturing techniques, such as electrospinning, soft lithography, and three-dimensional (3D) printing, have been described. Furthermore, biological augmentation has been considered, as an emerging strategy with great therapeutic potential.
Keywords:
tendon; scaffolds; electrospinning; soft lithography; 3D printing; biological augmentation 1. Introduction
Tendon disorders are medical conditions, such as ruptures and overuse injuries, and inflammatory and degenerative disorders, such as tendinopathies. In the United States, 33 million musculoskeletal injuries have been reported per year, 50% involving tendon and ligament injuries. Tendon injuries, however, do not only occur in physically active adults and adolescents (especially males), but they also appear among the sedentary population (who conduct moderate physical activity). Next to sports, several intrinsic factors, including body weight, nutrition, and age, may be responsible for tendon injuries. Moreover, genetic diseases that affect connective tissue could have an impact on tendon/ligament. Tendinopathies could be accompanied by inflammation and pain (as tendonitis, peri-tendonitis, and retrocalcaneobursitis), whereas tendinosis and ruptures are caused by intertendinous degeneration without the evidence of inflammatory processes [1]. The incidence of Achilles tendon ruptures (one of the most frequent tendons injured), is up to 1%, typically in 30- to 50-year-old men [2].
Chronic, non-healing tendon injuries frequently require surgical treatment, and despite recent advancements in orthopedic surgery, many common tendon repair techniques yield less than optimal results [3,4,5]. Moreover, healed tendons tend to form scar tissue with different mechanical properties than healthy tendons and are prone to reinjury. Furthermore, at the tendon–bone interface, many common tools used in surgery, such as suture anchors, cannot regenerate the enthesitis, resulting in a high incidence of re-rupture [6,7]. This is due to the peculiarity of the tendon-to-bone interface (TBI), a hybrid complex tissue that serves as shock-absorber of transmission forces between the tendon and the bone. Therefore, alternative surgical procedures should enhance proper tendon healing. This review will focus on tendon structures and their junctions to bones or muscles, the injuries, common treatments to restore limb functions, and the recent strategies of tissue engineering based on three-dimensional (3D) scaffolds and biological augmentation.
2. Tendon Structure and Metabolism
Tendons are anatomic structures interposed between muscles and bones. Their function is to transmit the force created in the muscle to the bone, making joint movement possible and allowing the maintenance of body posture. Healthy tendons possess a fibro-elastic texture that shows a great resistance to mechanical loads with minimal deformations. They appear white because they are relatively avascular and they are mainly made of extracellular matrix (ECM), based on 30% collagen, 2% elastin, and 68% water. Elastin contributes to the flexibility of the tendon, while collagen is responsible for the resistance [8]. Tendon characteristics are related to their function in the muscle–tendon complex: short and broad tendons transmit powerful and resistive forces (such as in quadriceps), long and thin tendons transmit soft and delicate movements (such as in the finger flexors). Each muscle has two tendons, proximal and distal. The muscle to tendon joint is the myotendinous junction (MTJ), while the bone to tendon joint is called the osteotendinous junction (OTJ) [8,9].
Tendons are composed of fibrous structures, responsible for their biomechanical properties, and they possess non-linear viscoelastic properties that confer rigidity combined with flexibility, allowing the force transmission from muscle to bone [10].
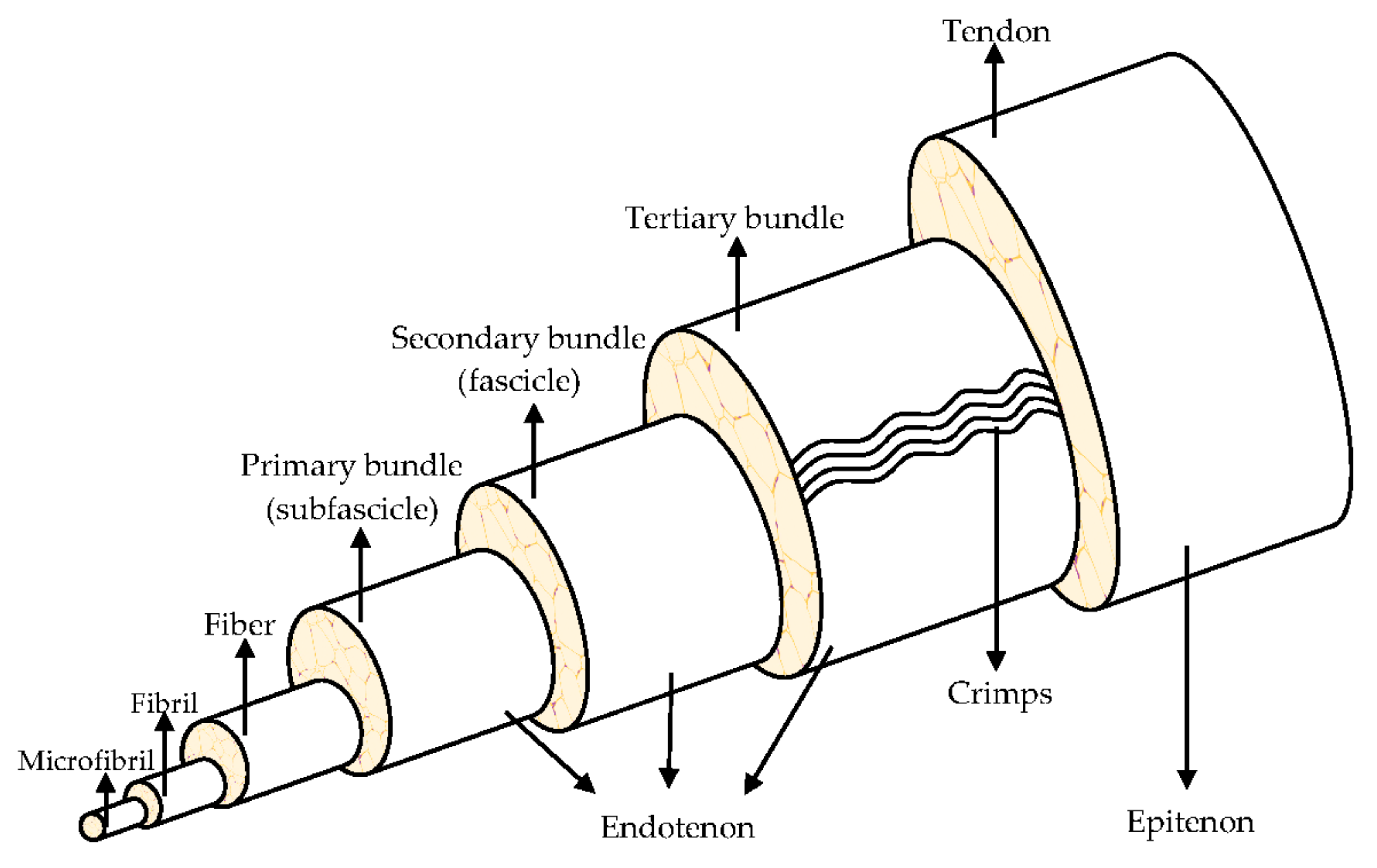
They are organized in a highly hierarchical structure (Figure 1) composed of collagen, which is bundled into larger progressive subunits [10,11]. The smallest tendon units are the collagen microfibril, arranged in larger fibrils from 10 nm to 500 nm in diameter. A bundle of fibrils forms a collagen fiber, which is the basic unit of a tendon, it is visible using light microscopy [8,12].
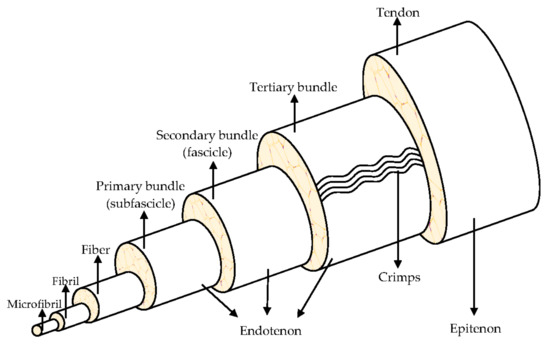
Figure 1.
Hierarchical structure of the tendon. Modified from [9].
Tendons consist of closely packed collagen fibers, parallel to one another, to ensure an optimal resistance to mechanical stresses that the tendon is subjected to, during the muscular tension. The high mechanical load the tendon supports, is due to the collagen fibers alignment in the direction of load application. Tropocollagen is a triple helix type I collagen (synthetized by tendon fibroblasts and tenocytes). Five tropocollagen molecules stacked in a quarter stage array form a myofibril and neighboring myofibrils interdigitate assemble a fibril, the smallest tendon structural unit with 10–500 nm diameter. Fibers are made of collagen fibrils, having a 3–7 μm diameter. The fibers are bound together to form fascicles, that are surrounded by the endotenon, a sheath of connective tissue with nerves and blood vessels. The fascicles are distinguished in primary bundles or subfascicles, with 15–400 mm diameter, secondary bundles or fascicles, with 150–1000 mm diameter, and tertiary bundles, with 1000–3000 mm diameter. Lastly, the tendon is enclosed by the epitenon, which encircles the periphery of the tendon, and it is surrounded by the paratenon, the outermost layer. A fluid is found between the paratenon and the epitenon to prevent friction and, therefore, to both facilitate and lubricate the tendon movement [8,10,13].
The collagen metabolism in tendons is relatively slow (compared to bone, connective tissue, and articular cartilage), and a balance between its synthesis and degradation is present. This is normally increased after an injury or an exercise [12].
3. Tendon Cellular Component and ECM
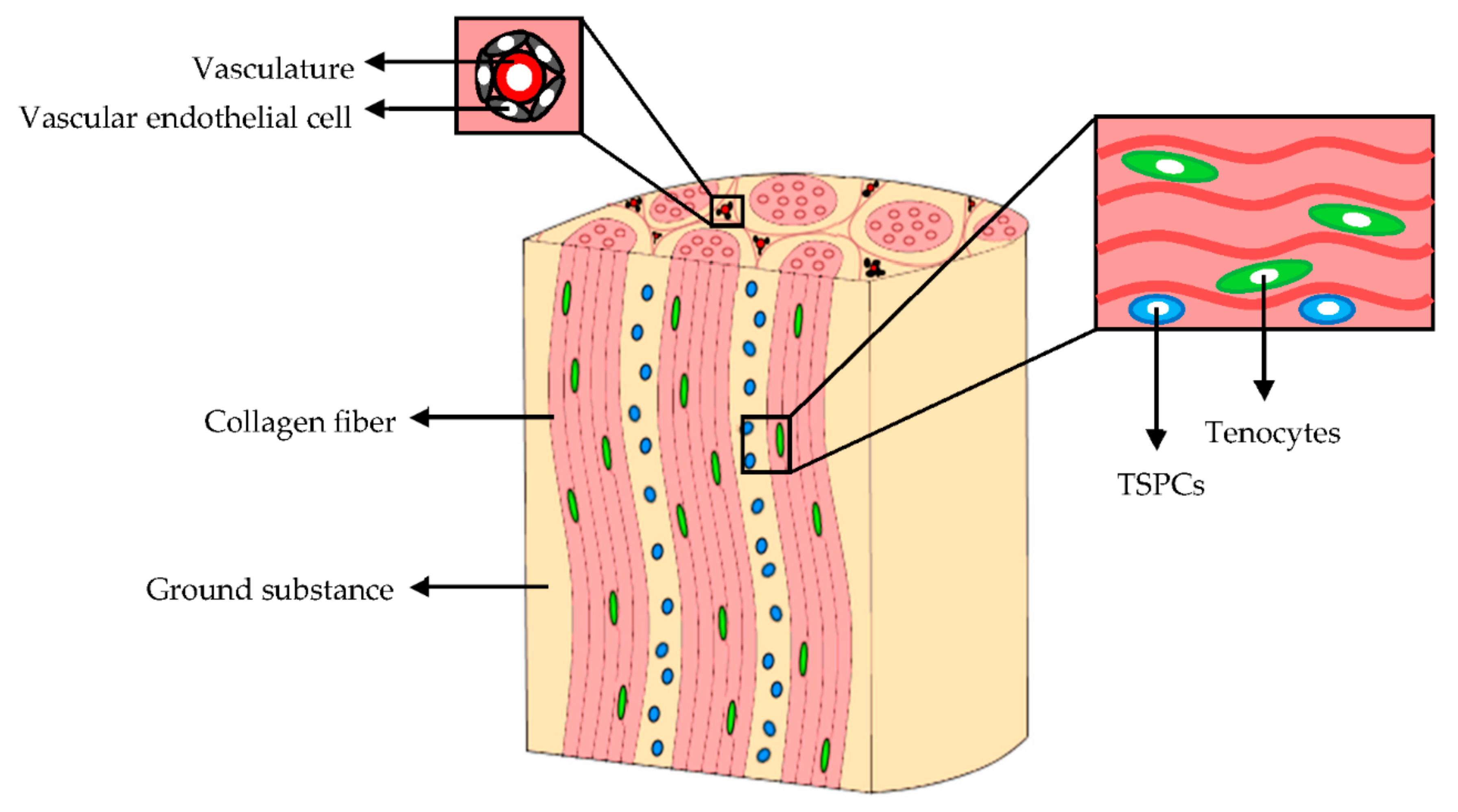
The cellular component of mature tendons (Figure 2) is composed by several cell types although the cellular content accounts for 20% of the overall tissue volume. Tenocytes, the primary cells, represent approximately 90% of the tendon cellular compartment. They are bipolar cells with elongated nuclei and spindle-shaped morphologies; they are arranged in columns along the collagen bundles. Tenocytes have a fundamental role in tendon homeostasis since they synthesize the collagen, and contribute to the maintenance of the macromolecular components of ECM [14,15].
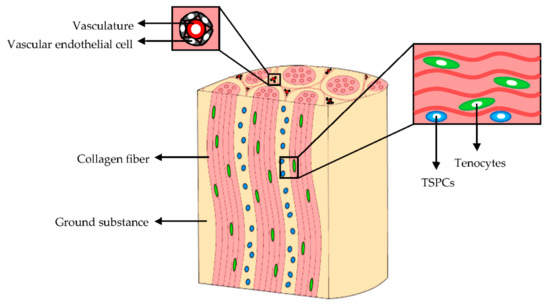
Figure 2.
Extracellular matrix (ECM) and cellular components of tendons. Modified from [15]. CC BY 4.0.
The remaining 10% of the cellular component are synovial cells, chondrocytes, located in proximity of the tendon-to-bone insertions, and vascular endothelial cells [10]. Moreover, tendon stem and progenitor cells (TSPCs), which show self-renewal, clonogenicity, and trilineage differentiation capacity (adipogenic, osteogenic, and chondrogenic cells), have been identified in niches within the tendon fascicles, the epitenon, and among pericytes or perivascular cells derived from the surrounding vasculature [15,16,17,18]. TSPCs exposure to mechanical stimuli of the in vivo microenvironment causes their differentiation into tenocytes or non-tenocyte cells [19,20,21,22] and regulates their proliferation [22,23]. The TSPCs gene expression of matrix proteins, integrins, and metalloproteinases during the tendon development and repair, is strongly upregulated in vitro after 3 days of mechanical stimulation [22].
Next to cell component, ECM and its hierarchical structure is of fundamental importance and it is tightly linked to tendon function. Collagen is interposed between layers of a non-collagenous matrix rich in proteoglycans, and this contributes to the tendon non-linear and viscoelastic mechanical behavior and its normal function [24]. Other than collagen, ECM is made of elastin, ground substance, and inorganic components.
Collagen is the main ECM structural protein. Collagen type I constitutes 60% of the dry mass and 95% of the total collagen, but other collagen isotypes (III, V, VI, XI, XII, XIV) are present [10]. The abundance of these is also related to the pathophysiological state of the tendon, as in the injured tendon, collagen III increases [9,25].
Elastin represents 1–2% of the total dry mass of the tendon. It is organized in fibers forming a network and provides resilience and elasticity to ECM. In fact, it contributes to the recovery of the collagen fiber wavy configuration after tendon stretch and muscle tension, and it provides flexibility and extensibility to the tissue [9,10]. In some pathological conditions (such as Ehlers–Danlos syndrome) elastin fibers increase.
Ground substance surrounds collagen and elastin, and it is mainly made of proteoglycans (PGs) and glycosaminoglycans (GAGs). Interestingly, these possess a high water-binding capacity that improves the biomechanical properties of tendons, such as elasticity against compressive forces. PGs and GAGs also stabilize the collagen hierarchical structure and maintain the ion homeostasis. In particular, PGs are composed of a core where GAGs are covalently attached, and they are entrapped within collagen fibers [24]. They confer to the tendons high capacity to withstand the forces because of their rigidity, due to the charge-to-charge repulsion, and their high charge density. In fact, PG concentration, type, and quantity depends on the tendon tensile and the compressed regions [10,26]. Other glycoproteins, such as fibronectin, tenascin C (TNC), cartilage oligomeric matrix protein (COMP), tenomodulin (Tnmd), and thrombospondin-4 (TPS4) are also important components of the tendon. They possess a low molecular weight compared to PGs. Several of these proteins are able to regulate fibrillogenesis in terms of fibril diameter, alignment, and stability. Fibronectin and TNC enhance tendon mechanical stability and allow tendons to recover the pre-stretched length after physiological loading. TPS4 is abundant in mature tenocytes, it is associated to fibrillar structure, and regulates the collagen assembly, organization, and ECM remodeling. Moreover, small leucine rich proteoglycans (SLRPs) abundant in ECM act as regulators of collagen fibrils self-assembly. Furthermore, fibromodulin, one of the most expressed in tendons, is crucial for the organization of the collagen fibrils. Besides these, tenomodulin, highly expressed in developing and mature tendon, is a marker of tenocyte differentiation, and has been recently reported as a mechanosensitive one since its expression rapidly decreases in static culture, but it is restored upon axial stretching [27,28].
Lastly, inorganic components are less than the 0.2% of the tendon dry mass. Ca2+ is the most present (0.001–0.01% of tendon dry weight), and in pathological conditions, its concentration may increase. Ca2+ has a key role in the development of the osteotendinous junction. Other ions are Mg2+, Co3+, Zn2+, Cu2+, Mn2+, Ni2+, Cd3+, Li+, F−, Pb2+, Si2+, and PO43−, and they are involved in growth, development, and normal metabolism of the musculoskeletal structures [9,12,14].
4. Tendon-Bone Insertion (TBI) and Myotendinous Junction (MTJ)
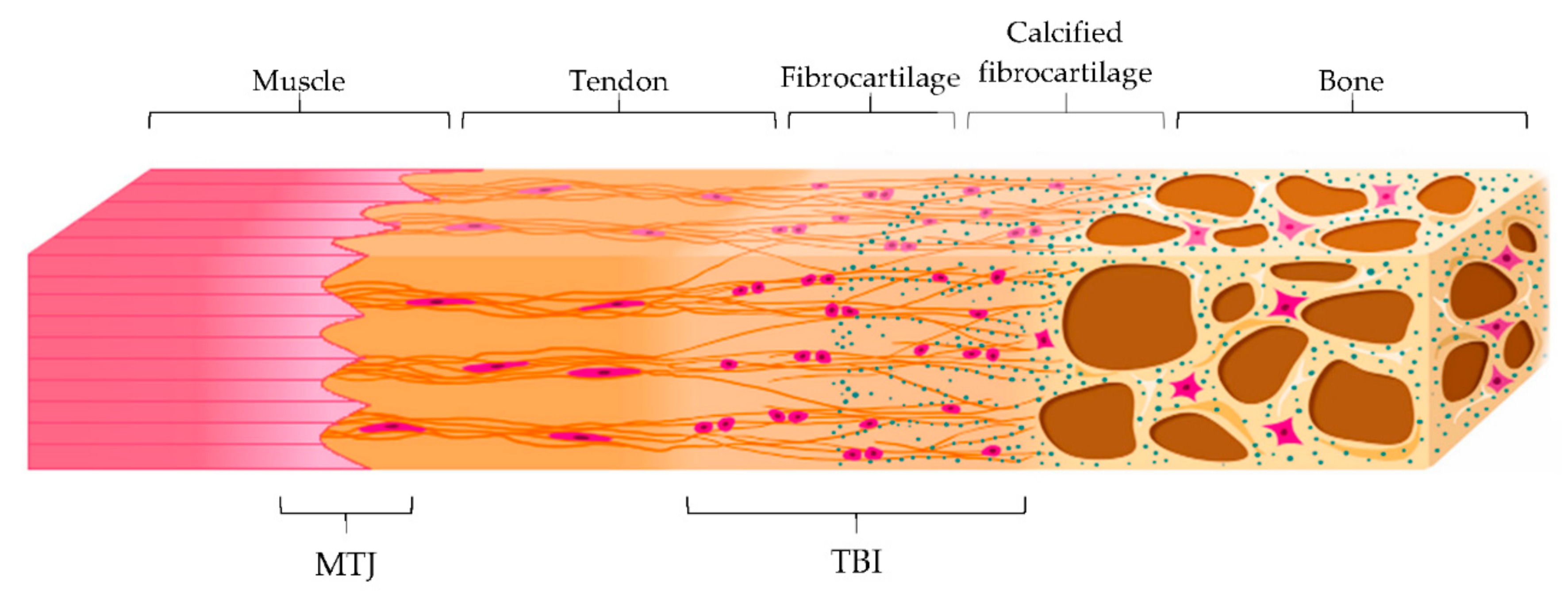
Tendon main function is the transmission of forces from muscle contraction to the bone to allow the movement, minimizing stress. Both tendon-bone insertion (TBI) and myotendinous junction (MTJ) are made of complex transitional tissue (Figure 3) that cannot be restored following injury, leading to high incidence of reoccurrence [29].
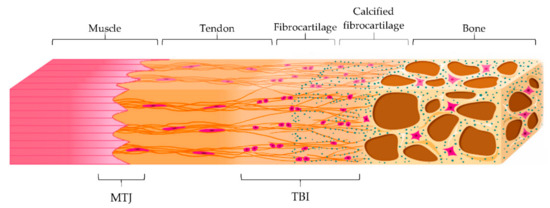
Figure 3.
Schematic representation of the myotendinous junction (MTJ) and the tendon-to-bone interface (TBI) with its different zones: tendon, fibrocartilage, calcified fibrocartilage and bone. Modified from [30]. CC BY 4.0.
MTJ is a specialized region located at the muscle-tendon interface, representing the primary site of force transmission. Structurally, it consists of subsarcolemmal, transmembrane, and extracellular protein complexes folded into invaginations and extensions in order to increase the interface area and, therefore, the resistance of the tissue to muscle contraction forces [29].
On the other hand, TBI is the region that connects the tendon to the bone. There are two different types of TBI, depending on the type of tissue present at the attachment site: dense fibrous connective tissue or fibrocartilage one. In the first type, the insertion is characterized by a dense fibrous connective tissue, similar to the tendon midsubstance, and it attacks directly to the bone. This insertion occurs over large surface areas perforating mineralized collagen fibers and is common in tendons attached to diaphyses of large bones, such as the deltoid, which inserts into the humerus.
In the second type of TBI, a layer of fibrocartilage, as a transition from the fibrous tendon tissue to bone, is found between the tendon and the bone. In this case, the tendon bundles are directly inserted into the bone and the tendon tissue close to the bone calcifies. This type of insertion is more common and prone to overuse injuries, such as in the case of the Achilles tendon, and it is characterized by four zones that creates a continuous gradient: tendon, fibrocartilage, calcified fibrocartilage, and bone. Particularly, the fibrocartilage zone, where chondrocytes and cartilage matrix are present between collagen fibers in the tendon, acts as a shock absorber to dissipate the stress generated by bending collagen fibers in the tendon. On the other hand, the calcified fibrocartilage is an avascular and irregular zone, populated by fibrochondrocytes and consisting of type I, II and X collagen, which represents the true junction to the bone. It provides the mechanical integrity of the insertion, allowing the mechanical transition of force across the insertion [30,31].
The healing process is based on a cascade of events starting from the recruitment of monocytes and macrophages and the cytokines and growth factors release (inflammatory phase) to the angiogenesis and tenocytes proliferation and ECM synthesis (proliferative phase), to ECM maturation (remodeling and modelling phases). Tendon injuries first repair by means of the deposition of an initial matrix to form a tissue template. The cells, taking part to this early event, seem to originate from extrinsic compartment (synovium like fascias—paratenon, epitenon, and endotenon) as the resident cells possess a limited reparative capacity, being in low number and with low metabolic rate [32].
Although numerous questions remain about the formation of transitional tissues and about the enhancement of the junction healing, tissue engineering, and biological augmentation, could have a significant impact on tendon injuries outcome and clinical practice.
5. Tendon Mechanical Properties
The tendon mechanical properties (directly related to ECM hierarchical structure) are fundamental for their function. In the force transmission from muscles to tendons, the ratio between the strength of muscular tension and the tendon resistance to tensile force should remain constant (this should be also constant throughout life) [9] although the aging and other degenerative processes could deteriorate tendon structure [30].
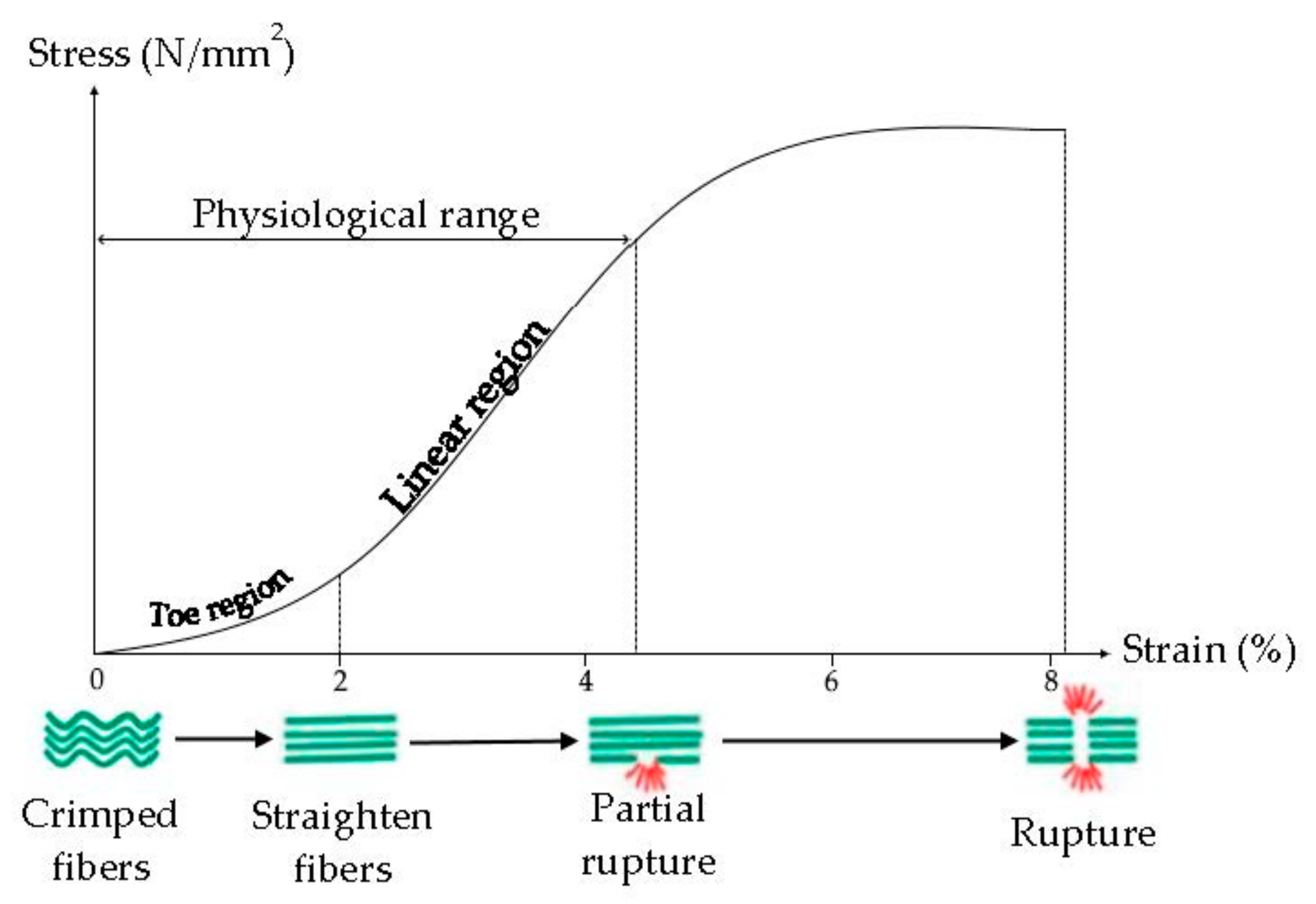
Tendon viscoelasticity, due to collagen fibers and elastin, possesses a time-dependent behavior, it is non-linear, and characterized by a Young modulus (the slope of the stress-strain curve) that increases from small values at small strains to high values at higher strains (Figure 4) [33]. Typically, the ultimate tensile strength of native tendon and ligament ranges from 5 to 100 MPa with a strain of failure 10–15% and Young’s Modulus from 20 to 1200 MPa [34].
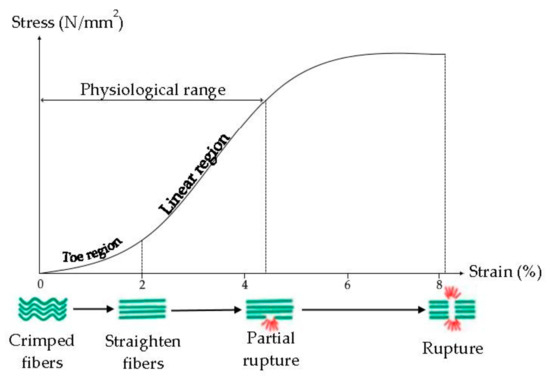
Figure 4.
Mechanism of internal deformation of tendon. Modified from [37]. CC BY-NC-SA 3.0.
It was demonstrated that the tendon mechanical properties are related to the collagen fibril diameter: fibrils with a small diameter are elastic and they have more resistant to the plastic flow, due to their surface area and interconnections, while fibrils with a large diameter are stronger and more resistant to tensile force, due to greater density of intramolecular cross-links. The tendon is characterized by smaller and larger fibrils, to have resistance to tensile force and resistance to the plastic flow [33,35].
Crimps are also essential for the main tendon functions, because they act as a shock absorber avoiding the tissue damage and giving the tendons the ability to absorb and transmit the tension force. The greater the stress on the tendon, the greater the crimp angle, in fact, all ruptured tendons have low crimp angle values [36].
6. Tendon Injuries
Tendon injuries are generally acute or chronic. Acute injuries are mainly due to traumatic damage of previously healthy tissue, caused by an external and acute trauma (bruises, stab wounds, lacerations), and they are characterized by a disarrangement of the tendon bundles and a loss of the tensile strength, which render tendons prone to rupture. On the other hand, chronic injuries are degenerative and often the result of overuse or a repetitive mechanical load. Direct trauma includes contusions and lacerations, while indirect tendon ones are usually a consequence of tensile overload [38].
Acute injuries involve a sudden external disruption of originally healthy tendon. Although such injuries often heal with acceptable recovery of function, the preinjury state is rarely fully restored after healing [5,32]. Tendon ruptures may also occur spontaneously during daily living activities, but in these cases, those are attributable to underlying accumulated tissue damage associated with degenerative tissue remodeling processes. Tendon matrix damage can stem from many sources (including acute tearing or cutting, oxidative damage, accumulation of micro-tears, or de novo generation of aberrant matrix within the tendon). Tendon injuries may ultimately result in the mechanical and biological propagation of the lesion until structural disruption and little is known regarding the accumulation of tendon damages and the coordination of the tissue remodeling up to restore [32].
Injury may appear at any point of the muscle–tendon–bone system in the weakest point of the unit. The term tendinopathy refers to several diseases that affect the TBI, the MTJ, and the tendon. These are caused by tendon overload or overuse or by intratendinous degeneration, which is commonly due to aging [39]. The tendon structural damages take place from repetitive strain and loading, particularly in activities that require power or technique.
Tendons possess a basal reparative ability, but when they are overwhelmed by repetitive and traumatic processes, damage occurs. The earliest tendon injury is tendinitis, an inflammatory event. This could be greatly debilitating, with pain, loss of muscle function, joint instability, and abnormal movements, adversely affecting the patient’s life [12,39]. Although, for decades, tendinopathy was thought as non-inflammatory in nature, modern research confirmed the presence of inflammatory cells, including macrophages and lymphocytes in chronic tendinopathy [40]. In fact, it has been hypothesized that inflammation plays a role in the early initiation of tendon pathologies.
It is conceivable that inflammation begins earlier than fibrotic and other degenerative tendon changes. Tendon injuries are accompanied by the endogenous expression of various mediators of inflammation, including proinflammatory and anti-inflammatory cytokines, and some growth factors: tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, IL-10, vascular endothelial growth factor (VEGF), transforming growth factor beta (TGF-β), cyclooxygenase 2 (COX2) expression, and prostaglandin E2 (PGE2) [41].
Tendon inflammation is responsible of an inappropriate functionality that may increase the risk of tendon ruptures and re-ruptures [42].
Although degeneration and overuse seem to be the main causes for tendon injuries, it is unclear how different stresses induce different responses and, in many cases, the etiology remains unclear [43,44].
Tendon injuries are caused by intrinsic or extrinsic factors, also in combination, in fact, a multifactorial origin is generally found. In particular, intrinsic risk factors include demographic factors (sex, age, weight, and height), genetic polymorphisms, and local anatomical factors (leg length discrepancy, malalignment, and decreased flexibility), while extrinsic factors comprise therapeutic agents (corticosteroids, antibiotics), environmental conditions, and physical activity-related factors, including training patterns, techniques, and equipment. In acute traumas, the extrinsic factors are predominating; in chronic traumas, the combination between intrinsic and extrinsic factors is more common [12,45].
Anatomically, injured tendons show a degeneration that leads to an increase in vascularity, where blood vessels are randomly oriented and a disordered arrangement of fibers. Collagen matrix show unequal and irregular crimping, loosening, and increased waviness of collagen fibers with an increase of type III collagen (reparative collagen) [41]. Pain, edema, and inflammation are the first responses to injuries, and they are helpful in the early stages, leading to a restriction of activity and therefore of the damage.
The highest frequency of failure occurs in the over-30 age group, when tendons are more susceptible to injury due to a progressive deterioration of collagen. Moreover, elastin and proteoglycan matrix decrease, and the water content also decreases, leading to lower elasticity [45,46,47].
7. Traditional Approaches for the Treatment of Injuries
Tendons do not possess high regenerative potential, and they usually form scar tissue with scarce mechanical properties, increasing the risk of reoccurrence and leading to a long-term loss of fiber orientation [10]. Currently, two different approaches are used for the tendon injuries (acute or chronic): conservative, surgical, or a combination of the two. The healing time is primarily affected by the type of lesion, and the tendon involved rather than the approach considered. Whatever the method used, the treatment aims to eliminate pain, reduce inflammation, promote healing, and restore (as soon as possible) the joint function [48,49,50].
The conservative approach consists of rest, targeted exercises, cryotherapy with ice, anti-inflammatory drug therapy (nonsteroidal anti-inflammatory drugs (NSAIDs) and corticosteroids), orthopedic insoles, ultrasound, and laser therapy, to allow the tendon to heal naturally [48]. Due to the limited capacity of tendon self-healing, this approach requires long periods of treatment, a partial loss of function, and recurrent lesions. The source of the repair (intrinsic, involving matrix structure and composition, or extrinsic, involving tissue vascularity, state of inflammation, pain), is important, and a scar, mainly caused by extrinsic compartment, often results in an impaired range of joint motion. Moreover, a gap in the muscle–tendon complex could occur due to tendon retraction, and this further delays healing rate. Another key point is the extent of the wound: an extended injury (such as a total break) normally requires surgical treatment [49]. Although conservative treatment could avoid the complications related to surgery, such as infection, scar adhesion, tendon necrosis, and nerve injury, surgery has become the mainstay of therapy especially for acute injuries. The conventional surgical treatment aims to restore the tendon biomechanical properties [50,51,52,53,54,55] and involves the suturing of the wounds or the fixation of the tendon to the bone with multiple sutures, wire loops, and stainless steel anchors to the soft tissue. Both the “open air” technique and arthroscopic repair are equally considered [47]. These are often combined with the typical conservative treatments (rest, targeted exercises, cryotherapy with ice, anti-inflammatory drug therapy (NSAID and corticosteroids), ultrasound, and laser therapy) to accelerate the functional recovery (combined approach).
Tissue grafting is an alternative included in surgery approach. Autograft is the best choice to avoid tissue rejection; however, complication at the donor site could occur, and the reconstruction allows almost 50% of the ligament functions from the pre-injured state [34].
However, these approaches largely fail in presence of chronic injuries, since excessive tension could be present after the primary repair, and the failure reaches the 38%. This may occur because of tendon weakening, muscle atrophy, and contraction, decreased range of joint motion and postoperative alterations of the joint normal mechanics.
Since no consensus has yet been reached regarding the optimal treatment protocol [56], this highlights the need to find other innovative approaches to improve the repair strength [49].
8. New Strategies for the Treatment of Injuries: Tissue Engineering
Recently, many efforts have been attempted to find innovative approaches in tendon injuries. Tissue engineering is a multidisciplinary approach for creating a viable tissue by applying the principles and the methods of engineering and life sciences, with the ultimate aim to induce repair, and replacement or regeneration of injured tissue. Tissue engineering involves the use of cells combined to scaffolds and biologics (biological active molecules, mainly hemoderivatives and growth factors). Tissue engineered substitutes are the promising tissue replacement to overcome the limitations related to the traditional approaches although, to date, translation in clinic does not yet occur. However, in this perspective, cell-based therapy supported by scaffolds and cellular or acellular scaffolds have been proposed, both to enhance tendon regeneration and to control growth factor release to achieve tendon repair with minimal scar [48,57,58].
8.1. Tendon-Specific Stem and Progenitor Cells (TSPCs) Therapy
Cell-based therapy is based on direct injection of cells in the damage site: these are able to induce the in-situ production of ECM, effective in the healing process. In particular, TSPCs possess the ability to differentiate into tenocytes and into several non-tendon cell types, such as chondrocytes and osteocytes, demonstrating a tremendous potential to improve the healing of damaged tendons [57,59].
Specifically, it has been demonstrated that TSPCs are able to promote the tendon repair in a murine model of patellar tendon defect model, by increasing collagen production and by restoring collagen 3D structure, thus recovering tendon elasticity [60].
Similarly, it was stated that autologous tenocytes (a mixture of tenocytes and TSPCs) improve the histological outcomes and increase the tendon collagen content and its tensile strength, by healing chronic Achilles tendinopathy in a rabbit model [61].
Although many studies suggest that these cell therapy treatments increase the healing of the tendon injury, the translation in clinical practice needs to overcome some problems [57]. The major one is the identification of the suitable cell source and the appropriate cell number, both effective at recovering the tendon injuries. These are crucial, considering that, currently, no standard has been identified [57,62]. Along with this, the donor age seems to be another key issue [57,63].
The standardization of the therapeutic approach is a critical point, and this greatly influences the final outcome. Patient age, delivery techniques, and cell stemness, and their viability after injection or implant, are variables that should be considered. Furthermore, a carrier should be considered to prevent cell membrane breaking during the direct injection, and to facilitate cell survival and activities after implant [51]. Stem cell proliferation after implantation is an additional crucial aspect and the possible uncontrolled proliferation causes a certain degree of caution in the clinical translation. Although the use of TSPCs to improve tendon repair is an attractive option to treat diseases, such as tendinopathies, their potential clinical application is still an emerging field, and further research is required to establish a solid standard procedure to their administration.
8.2. 3D Scaffolds
The scaffolds, three-dimensional (3D) constructs considered the basis of tissue engineering [64,65], seem to be suitable to restore tendon injuries since they are able to recreate the spatial organization of the original tissue, to allow easier adhesion, survival, migration, proliferation, and differentiation of native cells, or seeded cells [65,66,67,68].
Resorbable scaffolds have been developed to overcome the problems arising from permanent implants and the rate of reabsorption should be planned considering the type of tissue involved. Scaffold degradation should be relatively slow to support the mechanical load until the regeneration of the new tissue. In the first phase of the repair process, the scaffold should protect the cells and the new tissue from high strains, and should allow gradual exposure to the loads in the later stages. Ideally, the system should degrade at the same speed of regeneration of the new tissue, and the degradation products should be biocompatible, without the onset of chronic inflammation or unwanted biological responses [69,70]. In particular, immune response should be negligible to prevent severe inflammatory reactions that delay the healing or cause a rejection [67,71].
Different (bio)materials have been proposed to achieve the tendon mechanical properties [48,67,71] and to resemble the tendon architecture. Porosity and pore size are key points, since cell penetration into the scaffolds and parallelly adequate nutrient diffusion should be assured. Moreover, scaffold architecture is crucial and influences the fate and the function of the implanted cells, for this reason, 3D constructs able to mimic the in vivo cell microenvironment seems critical [71].
Furthermore, during the design and the development of scaffolds, the manufacturing technology and the implant technique should be carefully evaluated to render them clinically and commercially available. Process scale-up, cost of production, and storage should be considered [67,68,69,71]. Among the various manufacturing technologies, electrospinning, soft lithography, and 3D printing have gained interest in the scientific community.
8.2.1. Emerging Manufacturing Methods
Electrospinning
Electrospinning is a method that allows the generation of nanofibrous porous scaffolds using a high voltage electric field. It is a simple, versatile, flexible, and cost-effective method to spin polymeric materials to generate ultra-thin fibers with diameters in the nanometric or micrometric range [72,73].
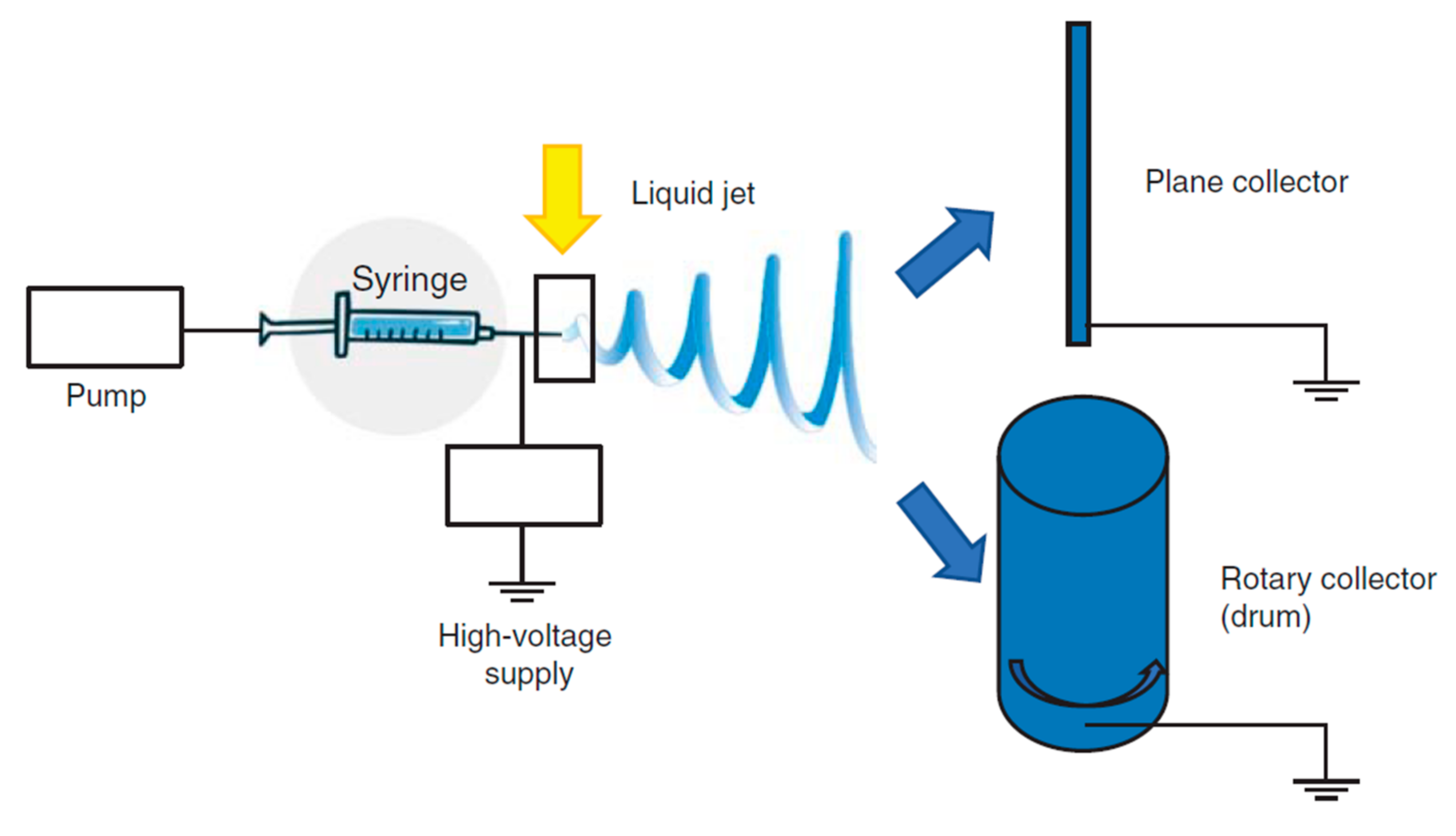
The basic experimental set up for electrospinning includes (Figure 5) a syringe, a spinneret (usually a needle or a capillary tube), a syringe pump, a high voltage power supply, a collector, generally planar or rotary.
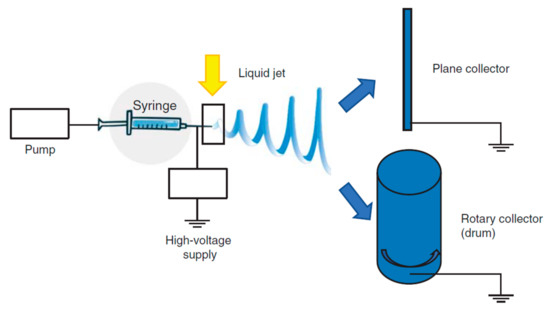
Figure 5.
Functioning scheme of electrospinning. Modified from [72].
The polymer solution is pumped through the syringe and it forms a droplet on the needle tip. The high voltage forces the droplet by adopting a conical shape, called “Taylor cone”, and a thin liquid jet forms as soon as the electrical field strength exceeds the solution surface tension. The liquid jet travels in spinning motions to the collector, simultaneously the solvent evaporates and the fiber is deposited onto the collector. Depending on the collector shape, such as the static or the rotating collectors, flat sheets or cylindrical structure are obtained with a great surface to volume ratio and a great porosity [72,74]. The mechanical properties are a key point and they are tuned by chemical composition and scaffold morphology (nanofiber dimension and alignment) [73,75,76,77,78] to form a 3D structure resembling tendon ECM. Nanofiber alignment obtained using a drum [71,79,80] better simulates the collagen network and this seems to favor an adequate environment for cell homing and proliferation and ECM synthesis. Morphology, combined with the high surface area to volume ratio, and mechanical properties, allow cells to maintain their phenotype and native orientation [72,81]. Moreover nanofibrous scaffolds could be easily loaded with drugs capable to enhance cell proliferation or to prevent/treat infections [82,83,84].
Aligned nanofibers based on Carbothane™ (a medical grade polyurethane) and reinforced with different percentages of multi-walled carbon nanotubes (0.06%, 0.33%, and 0.66%) demonstrated to possess better mechanical properties than random ones. Moreover, mouse embryonic fibroblasts (NIH 3T3) adhesion, and proliferation were assessed [85]. Analogously aligned fibers based on blends of poly (l-lactic acid), polyethylene oxide, and loaded with Trichostatin A, evidenced the capability to restore structural and mechanical properties of Achilles tendon in an in vivo preclinical model [86].
Soft Lithography
Soft lithography is an innovative strategy based on printing and molding and it represents a convenient, effective, and low-cost method for the manufacturing of micro- and nanostructures, such as scaffolds or microengineered hydrogels [87,88,89]. In soft lithography, an elastomeric stamp, with molded relief structures on its surface, ranging from 30 nm to 100 nm, is used to generate a 3D structure that reproduce the ECM [90,91,92,93].
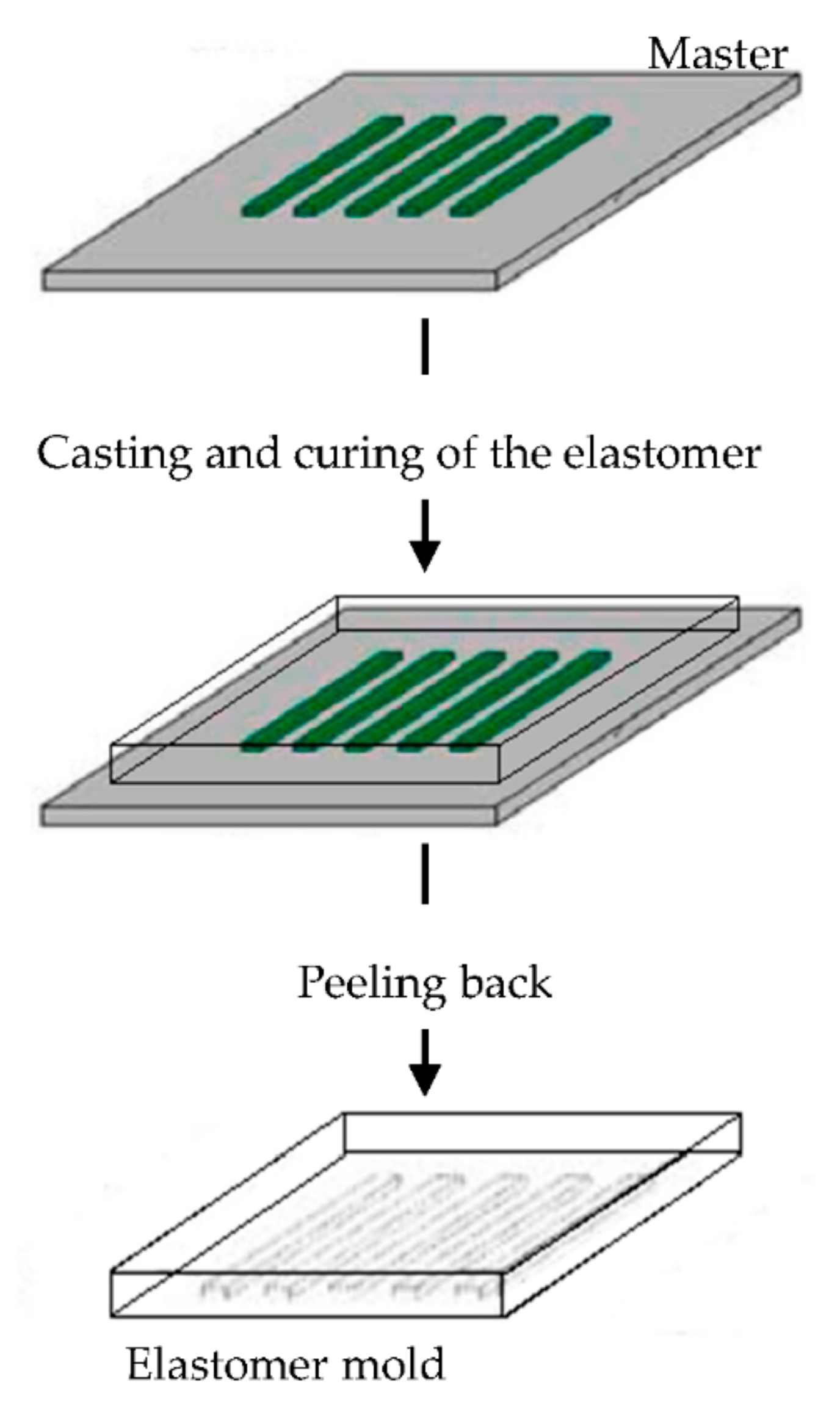
The elastomeric mold is fundamental in soft lithography and it is prepared by cast molding using elastomers, such as poly(dimethylsiloxane) (PDMS) or silicone rubbers, polyurethanes, and polymides. A prepolymer of the elastomer is poured over a master having relief structures on its surface, then cured and peeled off (Figure 6). The elastomers are preferentially used because they make a conformal contact with large surfaces and they are also released more easily from rigid masters or structures that are being molded [90,91,92,93,94,95,96].
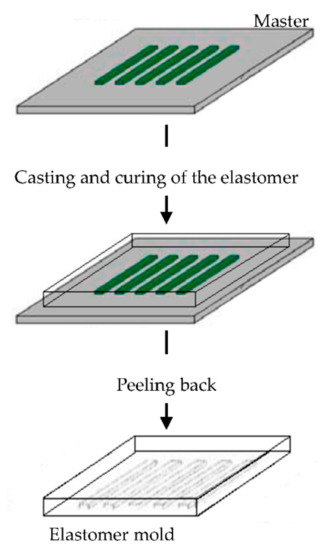
Figure 6.
Schematic illustration of the procedure for building poly(dimethylsiloxane) (PDMS) molds for soft lithography. Modified from [94]. CC BY-NC-ND 3.0.
Imprinted substrates with the same size range as those found in tendon has been used to investigate the relationship between the substrate topography, the tenocyte behavior, and their phenotype stability. Groove with a smaller wide than that of tenocytes promoted cell alignment, elongation, and support phenotype stability. Moreover, tenocytes with phenotype loss regained expression of tenogenic markers [97].
Different soft lithography techniques are available: microcontact printing (μCP), replica molding (REM), microtransfer molding (μTM), micromolding in capillaries (MIMIC), solvent-assisted micromolding (SAMIM) [90,91].
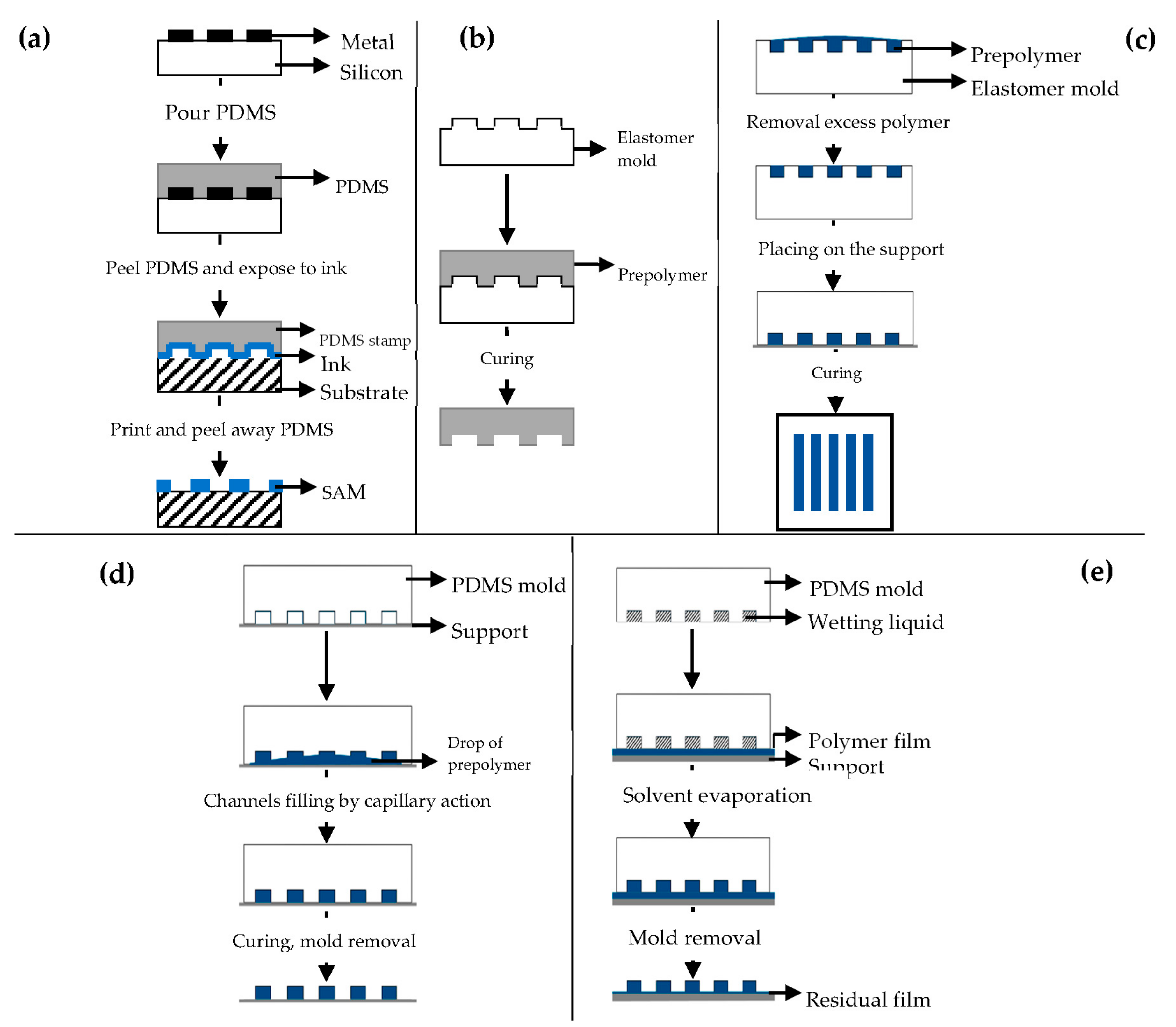
μCP (Figure 7a) offers the possibility to engineer the surface properties at a molecular level [98,99]. Self-assembled monolayers (SAMs) of alkanethiols on a substrate coated with a metal, such as gold (Au), copper (Cu), silver (Ag), platinum (Pt) or palladium (Pd), form microstructures of different materials [92], and they are easily prepared by physical vapor deposition, such as thermal or electron beam evaporation [91,92].
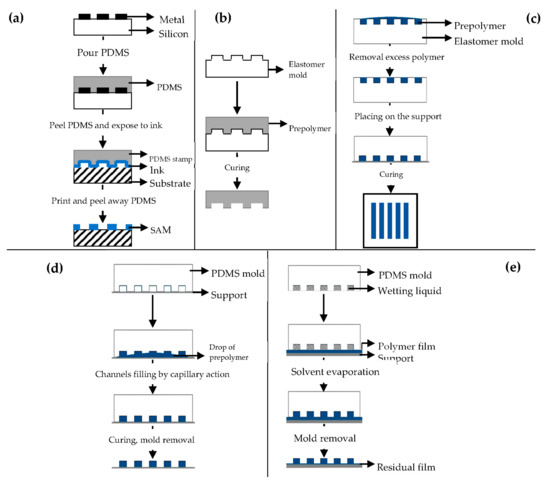
Figure 7.
Schematic illustration of: (a) microcontact printing (μCP); (b) replica molding (REM); (c) microtransfer molding (μTM); (d) micromolding in capillaries (MIMIC); (e) solvent-assisted micromolding (SAMIM). Modified from [99,101]. CC BY 3.0.
REM (Figure 7b) is an efficient method for the duplication, in a single step, of 3D structures with shape, and morphology of the mold surface [91,98] using PDMS based molds [91,92,93,94,100]. It is based on microfluidic technique.
μTM (Figure 7c) involves a mold filled with a prepolymer and a subsequent curing process to form a solid by means of UV light or heating [91,98].
MIMIC (Figure 7d) consists of placing an elastomer mold to form a network of empty capillaries. A drop of liquid prepolymer is then placed at the open end of the capillaries spontaneously filling them by capillary action. The prepolymer is then cured into a solid and the mold is removed to leave patterned microstructures of the polymer [91,98,101,102].
SAMIM (Figure 7e) is very similar to MIMIC.
The liquid prepolymer is dissolved in a suitable solvent and not melted without the need of high temperatures [91,93,98]. When the solvent evaporates, the liquid polymer solidifies and forms a molded structure complementary to the surface of the mold.
3D Printing
Three-dimensional (3D) printing is a versatile technique and scaffolds with defined shapes, controlled chemistry, and interconnected porous structures, able to mimic the ECM properties, can be manufactured [103,104,105,106,107,108].
The equipment is based on a regular inkjet print head that deposits a binder material onto a powder bed layer-by-layer [103,104]. Fused deposition modeling (FDM) coupled 3D printing has been recently developed, and in this, a fused filament is used as ink. Almost all types of materials, such as polymeric and composite materials (ceramic and metallic based), have been used [109,110].
The 3D printable models are generally created with a computer-aided design (CAD) package, via a 3D scanner, or by a plain digital camera and photogrammetry software, and these methods allow to realize complex 3D structure with highly organized hierarchical organization. The 3D scaffolds based on polycaprolactone (PCL) have been built to mimic tendon hierarchical structure in fiber to fascicle, organization, allowing the attachment and alignment of the human tenocytes in vitro [111]. Moreover reinforced geometry have been obtained, as in the case of acrylonitrile butadiene styrene matrices (ABS) in collagen scaffold, without changing scaffold bioactivity [112].
8.2.2. Materials
Numerous synthetic and natural materials have been considered for tendon tissue engineering.
Synthetic polymers are very attractive candidates as they are characterized by remarkable mechanical properties, and thanks to their chemico-physical properties, they are easily worked using emerging manufacturing technology as electrospinning and 3D printing. Moreover PCL (poly (-caprolactone), PLLA (poly-l-lactic acid), PLGA (poly (lactic-co-glycolic) acid), or poly urethanes (PUs) present tunable and reproducible mechanical and chemical properties (such as degradation) in spite of a marked hydrophobicity, and are biocompatible and non-toxic. Despite this, they possess limited capability to enhance cell adhesion and homing. On the contrary, natural polymers, such as collagen, gelatin, silk proteins (fibroin and sericin) alginate, chitosan, hyaluronan, are characterized by high hydrophilicity and superior bioactive properties, although it is difficult to obtain nanotopographic and reproducible structures and suitable mechanical properties. Considering this, natural polymers are often blended to synthetic polymers to obtain highly resistant systems able to interact with cells. This is the fundamental step toward intracellular signal stimulation to regulate cell migration, cell proliferation, and cell differentiation. Moreover, it has been proved that surface hydrophilicity or hydrophobicity could induce conformational changes of integrin-binding proteins, resulting in different adhesion strength [113].
9. Biological Augmentation for Tendon Healing
Besides the development of 3D hierarchical scaffolds, the use of biologicals is currently under investigation to enhance the scaffold performance without scar formation. Recently, various biologics combined with scaffolds have been considered and evaluated in preclinical animal studies to augment tendon repair [114,115,116]. The main goal is to achieve a complete functional recovery of the tendon injuries [10,117].
Recombinant growth factors (GFs) have been considered and nanoencapsulation was used as strategy to increase their stability and control their release. GFs are normally involved in the healing process and play an important role in the regulation of the tendon healing phases, since they tune cell proliferation, differentiation, chemotaxis, and matrix synthesis [109,118,119,120,121,122,123]. Nowadays, the use of hemoderivatives have been explored for tissue engineering applications as a simple and cost-effective source of GFs, cytokines, and structural proteins, which possess a key role in the regeneration of bone and soft tissues. In fact, the combination of different GFs is expected to have a synergistic effect on the regulation of tissue regeneration [124,125].
Despite the over 1600 clinical trials registered con ClinicalTrials.gov for the use of GFs, widespread regulatory approval and marketing are far from coming. Only a few, single GF-based formulations have been approved, by US-FDA (Food and Drug Administration) bone morphogenic protein (BMP)-2 and BMP-7 for lumbar spine fusion and bone fracture, platelet derived growth factor in BB isoform (PDGF)-BB for enhancement of granulation tissue formation and keratinocyte growth factor (KGF) for the prevention and treatment of mucositis in cancer patients; and by Japan and China, fibroblast growth factor (FGF)-2; and by Latin America and part of Asia, epidermal growth factor (EGF), all for wound healing [126].
In fact, hemoderivatives are rich in both GFs and cytokines: they provide multiple signals required to complete the regeneration process, all fundamental to modulate and eventually accelerate the healing process. Moreover, hemoderivatives may contain β-lysin with antimicrobial properties [127,128,129] and structural proteins, such as fibrinogen and fibronectin which act as matrix for cell adhesion and migration [130].
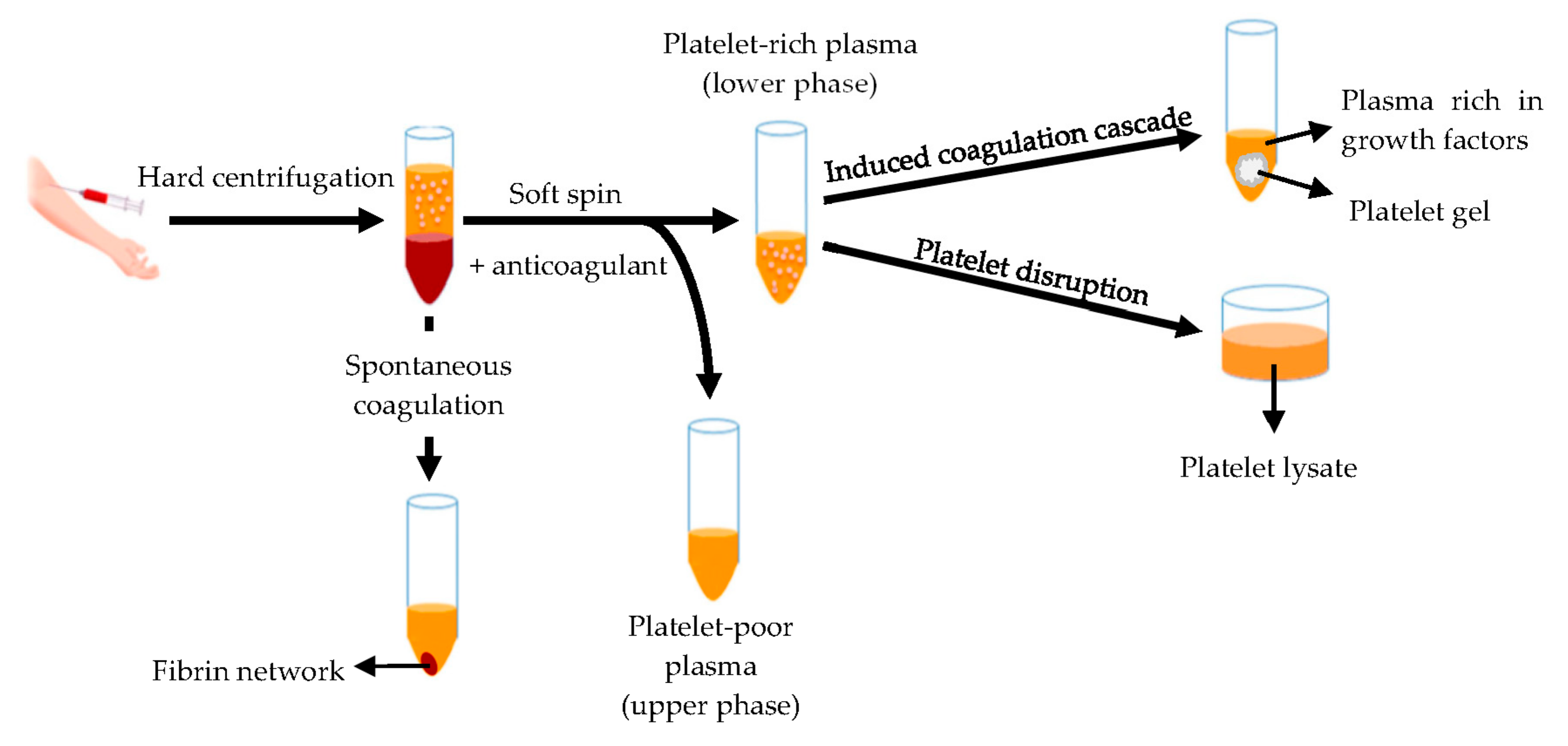
Hemoderivatives are prepared starting from whole blood or apheresis. The fastest production of blood derivatives involves the separation of blood components by means of the double centrifugation technique (Figure 8) to produce two different fractions: the platelet-poor plasma and the platelet-rich plasma (PRP) [131,132].
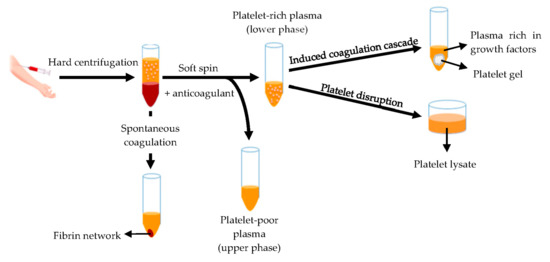
Figure 8.
Schematic representation of the blood derivatives production by means of double centrifugation technique. Modified from [141]. CC BY 4.0.
The platelet-rich plasma is defined as a volume of plasma that contains a platelet concentration above blood normal baseline (15–35 × 104/μL), with an average of 20 × 104/µL [132,133]. Autologous origin renders it inherently safe and free from transmissible diseases, such as HIV and hepatitis. Moreover, it can be used as liquid formulation or as platelet gel [132,134,135,136,137,138,139,140]. Otherwise platelets are disrupted originating the platelet lysate [141,142,143] rich of bioactive components involved in tissue regeneration, such as platelet derived growth factor (PDGF), epidermal growth factor (EGF), platelet derived epidermal growth factor (PDEGF), vascular endothelial growth factor (VEGF), transforming growth factor beta (TGF-β), fibroblast growth factor (FGF), insulin-like growth factor (IGF), interleukin 8 (IL-8) and tumor necrosis factor (TNF)-α [58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146]. Platelet lysate provides various advantages for therapeutic applications: it is liquid since the clot and platelet are removed, its production is easy to standardize, and it can be frozen and stored to be quickly available for use [142].
In recent years, hemoderivatives for tendon healing have been administered via direct injection: in situ gelling is led to tissue collagen and thromboplastin, leading to gel formation, or coagulation cascade induction, thus forming in vivo a fibrin matrix-based scaffold that mimics the natural healing process [147]. However, despite the numerous positive reports on the use of blood derivatives in the tendon regeneration field, the literature frequently shows contradictory results, and it is complicated to establish a clear cause-effect relationship due to the complexity of the formulations, showing that the development and the standardization of new strategies is of paramount importance to overcome these limitations.
Despite this, clinical evidence suggests the effectiveness of hemoderivatives in improving tendon activity, reducing pain and, lastly, tissue reparation [131].
Recent clinical trials have shown midterm positive outcomes (improved activity level and reduced pain) on multiple platelet rich plasma injections for the treatment of various tendinopathies as Achilles and rotator cuff. In the first one [148], eight studies were considered and the clinical outcomes of rotator cuff repair with and without platelet rich plasma were analyzed, while in the second one (eight studies) [149], PRP was compared to steroid treatment on lateral epicondylitis. Both the meta-analyses suggest that platelet rich plasma was effective in reducing pain and improving tendon function in the intermediate term (12 weeks), and long-term (1 year), although there were no particular differences in tissue integrity [150].
However, newer meta-analyses aimed to evidence the effectiveness of platelet-rich plasma treatments in tissue healing. In the first one [151], 11 studies (randomized controlled trials) compared 355 patients treated with PRP at the tendon-bone interface to 351 patients treated with a control in rotator cuff repair, as they reported the tendon healing rate. A statistical difference in favor of PRP was reported, in fact, with PRP, 17.2% of patients showed incomplete tendon healing, while in the control, 30.5% of patients showed incomplete tendon healing. The difference was statistically in favor of PRP, both in small-medium and medium-large tears, demonstrating that PRP possesses clinical benefits in improving the tendon healing rates of all tears, independently of their dimensions. It also significantly reduces pain levels in the immediate postoperative period and increases the functional outcomes when compared with a control. In the second meta-analysis of randomized controlled trials [152], the efficacy of PRP applied to the tendon-bone interface in the arthroscopic rotator cuff repair was also demonstrated. It considered seven studies, which included 233 patients treated with PRP and 231 patients treated with a control, which reported the retear rate, which was significantly lower in the PRP group compared to the control. Moreover, these studies reported the efficacy of PRP in improving the functional outcomes, especially in the short-term.
However, the absence of standardization of the preparation methods, such as the donor number and the anticoagulant agents involved, generally leads to considerable differences in the composition and concentration of the blood derivatives. For this reason, the preparation is a key point to control and accomplish the desired therapeutic effect [135]. Another key point is the GF concentration (effective and safe concentration and ratio between different factors) and delivery rate at the target, since in vivo ECM regulates GF availability and signaling [153,154]. This highlights the need of a standard method for hemoderivative administration through the conjugation of scaffolds able to mimic the ECM matrix.
Furthermore, scaffolds should stabilize GFs from external stress, light, temperature, pH and ionic strength that may induce protein conformational change and, consequently, reduce their biological activity [155,156].
Table 1 reports examples of the in vivo studies carried out so far on scaffolds combined with biologics for tendon repair, underlying the possible advantages in the treatment of tendon pathologies.

Table 1.
In vivo studies using growth factors (GFs) and blood derivatives for tendon repair.
10. Conclusions and Future Perspectives
Chronic, non-healing tendon injuries frequently require surgical treatment, and despite recent advancements in orthopedic surgery, they present several limitations with a high (38%) percentage of failure, thus leading to the loss of the tendon and joint correct function.
Nowadays, in a biomimetic regenerative medicine approaches, an important challenge is the development of 3D scaffolds with hierarchical structure for the control of the spatiotemporal and selective delivery of blood derivatives, and many attempts have been made to identify suitable carriers to deliver GFs in the injury site. This strategy should increase their therapeutic effects, enhancing healing and leading to the formation of a functional tissue without scar formation [109]. Biomaterials have a crucial role: their intrinsic properties may increase the controlled delivery of hemoderivatives and may synergistically potentiate the healing process [166,167,168]. The control of the spatial-temporal release profiles should potentiate the effectiveness of future treatments [117], avoiding GF degradation and uncontrolled cell proliferation growth, and prolonging GFs short half-life in vivo [156,169,170,171,172].
Innovative strategies focus on the development of scaffolds to enhance tendon restore. Electrospinning, soft lithography, and 3D printing are emerging techniques used to manufacture 3D scaffolds with suitable geometry and structure to replace damaged tissue and support tendon reparation. Although great advancements have been achieved in manufacture techniques, biomaterials or biomimetic structures are not enough performing to efficiently restore tendon and joint functions. Many attempts have been made to enhance scaffold effectiveness and the biological augmentation seems the more convenient option to obtain both tissue healing and fast translation towards clinic.
Healing of the tendon-to-bone interface remains a challenge due to the peculiar anatomy and structure, and the association of inorganic materials with biologics in 3D scaffolds should be a strategy to both enhance hard/soft tissue interface.
Current research activities point toward finding an optimal 3D structure with suitable mechanical properties, capable of delivering biologics, having a synergic response on tendon recovery.
Author Contributions
Conceptualization, G.S.; visualization, D.M., B.V.; writing—original draft preparation, E.B., M.R.; writing—review and editing, G.S.; supervision, G.S.; project administration, G.S.; funding acquisition, G.S., F.F., M.C.B., S.R. All authors have read and agreed to the published version of the manuscript.
Funding
Authors thank Horizon 2020 Research and Innovation Program under Grant Agreement No 814607 for partial funding the research project.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sigal, I.R.; Grande, D.A.; Dines, D.M.; Dines, J.; Drakos, M. Biologic and Tissue Engineering Strategies for Tendon Repair. Regen. Eng. Transl. Med. 2016, 2, 107–125. [Google Scholar] [CrossRef]
- Wu, F.; Nerilich, M.; Docheva, D. Tendon injuries: Basic science and new repair proposals. Effort. Open Rev. 2017, 2, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Lian, Ø.B.; Engebretsen, L.; Bahr, R. Prevalence of jumper’s knee among elite athletes from different sports: A cross-sectional study. Am. J. Sports Med. 2005, 33. [Google Scholar] [CrossRef] [PubMed]
- Peers, K.H.E.; Lysens, R.J.J. Patellar tendinopathy in athletes. Sports Med. 2005, 35, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Tonoli, C.; Cumps, E.; Aerts, I.; Meeusen, R. Incidence, risk factors and prevention of running related injuries in long distance running: A systematic review. Sport Geneeskd. 2010, 43, 12–18. [Google Scholar] [CrossRef]
- Min, H.K.; Oh, S.H.; Lee, J.M.; Im, G.I.; Lee, J.H. Porous membrane with reverse gradients of PDGF-BB and BMP-2 for tendon-to-bone repair: In vitro evaluation on adipose-derived stem cell differentiation. Acta Biomater. 2014, 10, 1272–1279. [Google Scholar] [CrossRef]
- Zumstein, M.A.; Jost, B.; Hempel, J.; Hodler, J.; Gerber, C. The clinical and structural long-term results of open repair of massive tears of the rotator cuff. J. Bone Jt. Surg. 2008, 90, 2423–2431. [Google Scholar] [CrossRef]
- Benjamin, M.; Ralphs, J.R. The cell and developmental biology of tendons and ligaments. Int. Rev. Cytol. 2000, 196, 85–130. [Google Scholar] [CrossRef]
- Kannus, P. Structure of the tendon connective tissue. Scand J. Med. Sci. Sports 2000, 10, 312–320. [Google Scholar] [CrossRef]
- Tempfer, H.; Lehner, C.; Grütz, M.; Gehwolf, R.; Traweger, A. Biological Augmentation for Tendon Repair: Lessons to be Learned from Development, Disease, and Tendon Stem Cell Research. Cell Eng. Regen. 2017, 1–31. [Google Scholar] [CrossRef]
- Canty, E.G.; Kadler, K. Collagen fibril biosynthesis in tendon: A review and recent insights. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002, 133, 979–985. [Google Scholar] [CrossRef]
- Sharma, P.; Maffulli, N. Basic biology of tendon injury and healing. Surg. J. R Coll. Surg. E 2005, 3, 309–316. [Google Scholar] [CrossRef]
- Franchi, M.; Trirè, A.; Quaranta, M.; Orsini, E.; Ottani, V. Collagen Structure of Tendon Relates to Function. Sci. World J. 2007, 7, 404–420. [Google Scholar] [CrossRef] [PubMed]
- Roshan, J.; Kesturu, S.G.; Balian, G.; Chhabra, B. Tendon: Biology, Biomechanics, Repair, Growth Factors, and Evolving Treatment Options. J. Hand Surg. 2008, 33, 102–112. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, J.; Zhou, Y.; Thampatty, B.P.; Wang, J.H.-C. Tendon Stem/Progenitor Cells and Their Interactions with Extracellular Matrix and Mechanical Loading. Stem Cells Int. 2019, 2019. [Google Scholar] [CrossRef]
- Bi, Y.; Ehirchiou, D.; Kilts, T.M.; Inkson, C.A.; Embree, M.C.; Sonoyama, W.; Li, L.; Leet, A.I.; Seo, B.M.; Zhang, L.; et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat. Med. 2007, 13, 1219–1227. [Google Scholar] [CrossRef]
- Salingcarnboriboon, R.; Yoshitake, H.; Tsuji, K.; Obinata, M.; Amagasa, T.; Nifuji, A.; Noda, M. Establishment of tendon-derived cell lines exhibiting pluripotent mesenchymal stem cell-like property. Exp. Cell Res. 2003, 287, 289–300. [Google Scholar] [CrossRef]
- Tempfer, H.; Wagner, A.; Gehwolf, R.; Lehner, C.; Tauber, M.; Resch, H.; Bauer, H.C. Perivascular cells of the supraspinatus tendon express both tendon- and stem cell-related markers. Histochem. Cell Biol. 2009, 131, 733–741. [Google Scholar] [CrossRef]
- Stanley, R.L.; Fleck, R.A.; Becker, D.L.; Goodship, A.E.; Ralphs, J.R.; Patterson-Kane, J.C. Gap junction protein expression and cellularity: Comparison of immature and adult equine digital tendons. J. Anat. 2007, 211, 325–334. [Google Scholar] [CrossRef]
- Schiele, N.R.; Marturano, J.E.; Kuo, C.K. Mechanical factors in embryonic tendon development: Potential cues for stem cell tenogenesis. Curr. Opin. Biotechnol. 2013, 24, 834–840. [Google Scholar] [CrossRef]
- Schwartz, M.A. Integrins and Extracellular Matrix in Mechanotransduction. Cold Spring Harb. Perspect Biol. 2010, 2, a005066. [Google Scholar] [CrossRef] [PubMed]
- Popov, C.; Burggraf, M.; Kreja, L.; Ignatius, A.; Schieker, M.; Docheva, D. Mechanical stimulation of human tendon stem/progenitor cells results in upregulation of matrix proteins, integrins and MMPs, and activation of p38 and ERK1/2 kinases. BMC Mol. Biol. 2015, 16. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.H. Mechanobiological response of tendon stem cells: Implications of tendon homeostasis and pathogenesis of tendinopathy. J. Orthop. Res. 2010, 28, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, C.T.; Birch, H.L.; Clegg, P.D.; Screen, H.R. The role of the non-collagenous matrix in tendon function. Int. J. Exp. Pathol. 2013, 94, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Pajala, A.; Melkko, J.; Leppilahti, J.; Ohtonen, P.; Soini, Y.; Risteli, J. Tenascin-C and type I and III collagen expression in total Achilles tendon rupture. An immunohistochemical study. Histol. Histopathol. 2009, 24, 1207–1211. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, H.F.; Felisbino, S.L.; Covizi, D.Z.; Della Colleta, H.H.; Gomes, L. Structure and proteoglycan composition of specialized regions of the elastic tendon of the chicken wing. Cell Tissue Res. 2000, 300, 435–446. [Google Scholar] [CrossRef]
- Smith, R.K.; Zunino, L.; Webbon, P.M.; Heinegard, D. The distribution of cartilage oligomeric matrix protein (COMP) in tendon and its variation with tendon site, age and load. Matrix Biol. 1997, 16, 255–271. [Google Scholar] [CrossRef]
- Matos, A.M.; Gonçalves, A.I.; El Haj, A.J.; Gomes, M.E. Magnetic biomaterials and nano-instructive tools as mediators of tendon mechanotransduction. Nanoscale Adv. 2020, 2, 140. [Google Scholar] [CrossRef]
- Felsenthal, N.; Zelzer, E. Mechanical regulation of musculoskeletal system development. Development 2017, 144, 4271–4283. [Google Scholar] [CrossRef]
- Friese, N.; Gierschner, M.B.; Schadzek, P.; Roger, Y.; Hoffmann, A. Regeneration of damaged tendon-bone junctions (entheses)—TAK1 as a potential node factor. Int. J. Mol. Sci. 2020, 21, 5177. [Google Scholar] [CrossRef]
- Apostolakos, J.; Durant, T.J.S.; Dwyer, C.R.; Russell, R.P.; Weinreb, J.H.; Alaee, F.; Beitzel, K.; McCarthy, M.B.; Cote, M.P.; Mazzocca, A.D. The enthesis: A review of the tendon-to-bone insertion. Muscle Ligaments Tendons J. 2014, 4, 333–342. [Google Scholar] [CrossRef]
- Snedeker, J.G.; Foolen, J. Tendon injury and repair—A perspective on the basic mechanisms of tendon disease and future clinical therapy. Acta Biomater. 2017, 63, 18–36. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, P.; Hansen, P.; Kjær, M. Tendon properties in relation to muscular activity and physical training. Scand J. Med. Sci. Sports 2002, 13, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.L.; Liau, L.L.; Ng, M.H.; Chowdhury, S.R.; Law, J.X. Current progress in tendon and ligament tissue engineering. Tissue Eng. Regen. Med. 2019, 16, 549–571. [Google Scholar] [CrossRef]
- Screen, H.R.C.; Bader, D.L.; Lee, D.A.; Shelton, J.C. Local Strain Measurement within Tendon. Strain 2004, 40, 157–163. [Google Scholar] [CrossRef]
- Tero, A.H.J.; Teppo, L.N.J.; Pekka, K.; Làszò, J.; Markku, J. Collagen fibres of the spontaneously ruptured human tendons display decreased thickness and crimp angle. J. Orthop. 2006, 22, 1303–1309. [Google Scholar] [CrossRef]
- Andrades, J.A.; Claros, S.; Jiménez-Palomo, P.; López-Puertas, J.M.; Zamora-Navas, P.; Guerado, E.; Monleón, M.; Araque, M.C.; Becerra, J. Skeletal Regeneration by Mesenchymal Stem Cells: What Else? Regen. Med. Tissue Eng. Cells Biomater. 2011. Available online: https://www.intechopen.com/books/regenerative-medicine-and-tissue-engineering-cells-and-biomaterials/skeletal-regeneration-by-mesenchymal-stem-cells-what-else-?cid=social_20150505_45207456&adbid=595591418732683264&adbpl=tw&adbpr=38225654 (accessed on 24 September 2020). [CrossRef]
- Lin, T.W.; Cardenas, L.; Soslowsky, L.J. Biomechanics of tendon injury and repair. J. Biomech. 2004, 37, 865–877. [Google Scholar] [CrossRef]
- Voleti, P.B.; Buckley, M.R.; Soslowsky, L.J. Tendon Healing: Repair and Regeneration. Annu. Rev. Biomed. Eng. 2012, 14, 47–71. [Google Scholar] [CrossRef]
- Rees, J.D.; Stride, M.; Scott, A. Tendons—Time to revisit inflammation. Br. J. Sports Med. 2014, 48, 1553–1557. [Google Scholar] [CrossRef]
- D’Addona, A.; Maffulli, N.; Formisano, S.; Rosa, D. Inflammation in tendinopathy. Surgeon 2017, 15, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Morita, W.; Snelling, S.J.; Dakin, S.G.; Carr, A.J. Profibrotic mediators in tendon disease: A systematic review. Arthritis Res. 2016, 18, 269. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.K.H.; Ng, G.Y.F.; Mak, A.F.T. Effects of knee bracing on the sensorimotor function of subjects with Anterior Cruciate Ligament reconstruction. Am. J. Sports Med. 2001, 29, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Abate, M.; Silbernagel, K.G.; Siljeholm, C.; Di Iorio, A.; De Amicis, D.; Salini, V.; Werner, S.; Paganelli, R. Pathogenesis of tendinopathies: Inflammation or degeneration? Arthritis Res. 2009, 11, 235. [Google Scholar] [CrossRef]
- Weiler, A.; Scheffler, S.; Apreleva, M. Healing of Ligament and Tendon to Bone. In Repair and Regeneration of Ligaments, Tendons, and Joint Capsule; Humana Press: Totowa, NJ, USA, 2006; pp. 201–231. [Google Scholar]
- Szczeny, S.E.; Peloquin, J.M.; Cortes, D.H.; Kadlowec, J.A.; Soslowky, L.J.; Elliott, D.M. Biaxial Tensile Testing and Constitutive Modeling of Human Supraspinatus Tendon. J. Biomech. Eng. 2012, 134, 21004. [Google Scholar] [CrossRef] [PubMed]
- Weiler, A.; Hoffmann, R.F.G.; Bail, H.J.; Rehm, O.; Südkamp, N.P. Tendon healing in a bone tunnel. Part II: Histologic analysis after biodegradable interference fit fixation in a model of anterior cruciate ligament reconstruction in sheep. Arthroscopy 2002, 18, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Walden, G.; Liao, X.; Donell, S.; Raxworthy, M.J.; Riley, G.P.; Saeed, A.A. Clinical, Biological, and Biomaterials Perspective into Tendon Injuries and Regeneration. Tissue Eng. Part B Rev. 2017, 23, 44–58. [Google Scholar] [CrossRef]
- Butler, D.L.; Juncosa, N.; Dressler, M.R. Functional Efficacy of Tendon Repair Processes. Annu. Rev. Biomed. Eng. 2004, 6, 303–329. [Google Scholar] [CrossRef]
- Reed, S.A.; Leahy, E.R. Growth and development symposium: Stem cell therapy in equine tendon injury. J. Anim. Sci. 2013, 91, 59–65. [Google Scholar] [CrossRef]
- Xie, R.G.; Zhang, S.; Tang, J.B.; Chen, F. Biomechanical studies of 3 different 6-strand flexor tendon repair techniques. J. Hand Surg. 2002, 27, 621–627. [Google Scholar] [CrossRef]
- Tang, J.B.; Wang, B.; Chen, F.; Pan, C.Z.; Xie, R.G. Biomechanical evaluation of flexor tendon repair techniques. Clin. Orthop. Relat. Res. 2001, 386, 252–259. [Google Scholar] [CrossRef]
- Veitch, A.; Firoozbakhsh, K.; Pribyl, C.R.; McNally, T. In vitro biomechanical evaluation of the double loop suture for flexor tendon repair. Clin. Orthop. 2000, 377, 228–234. [Google Scholar] [CrossRef]
- Silva, M.J.; Boyer, M.I.; Ditsios, K.; Burns, M.E.; Harwood, F.L.; Amiel, D.; Gelberman, R.H. The insertion site of the canine flexor digitorum profundus tendon heals slowly following injury and suture repair. J. Orthop. Res. 2002, 20, 447–453. [Google Scholar] [CrossRef]
- Zhao, C.; Amadio, P.C.; Momose, T.; Couvreur, P.; Zobitz, M.E.; An, K.N. The effect of suture technique on adhesion formation after flexor tendon repair for partial lacerations in a canine model. J. Trauma 2001, 51, 917–921. [Google Scholar] [CrossRef]
- Deng, S.; Sun, Z.; Zhang, C.; Chen, G.; Li, J. Surgical Treatment Versus Conservative Management for Acute Achilles Tendon Rupture: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Foot Ankle Surg. 2017, 56, 1236–1243. [Google Scholar] [CrossRef]
- Wang, J.H.-C.; Nirmala, X. Application of Tendon Stem/Progenitor Cells and Platelet-Rich Plasma to Treat Tendon Injuries. Oper. Tech. Orthop. 2016, 26, 68–72. [Google Scholar] [CrossRef][Green Version]
- Malgarim Cordenonsi, L.; Faccendini, A.; Rossi, S.; Bonferoni, M.C.; Malavasi, L.; Raffin, R.; Scherman Schapoval, E.E.; del Fante, C.; Vigani, B.; Miele, D.; et al. Platelet lysate loaded electrospun scaffolds: Effect of nanofiber types on wound healing. Eur. J. Pharm. Biopharm. 2019, 142, 247–257. [Google Scholar] [CrossRef]
- Rui, Y.F.; Lui, P.P.; Li, G.; Fu, S.C.; Lee, Y.W.; Chan, K.M. Isolation and characterization of multipotent rat tendon-derived stem cells. Tissue Eng. Part A 2010, 16, 1549–1558. [Google Scholar] [CrossRef]
- Ni, M.; Lui, P.P.Y.; Rui, Y.F.; Lee, Y.W.; Lee, Y.W.; Tan, Q.; Wong, Y.M.; Kong, S.K.; Lau, P.M.; Li, G.; et al. Tendon-derived stem cells (TDSCs) promote tendon repair in a rat patellar tendon window defect model. J. Orthop. Res. 2011, 30, 613–619. [Google Scholar] [CrossRef]
- Chen, J.; Yu, Q.; Wu, B.; Lin, Z.; Pavlos, N.J.; Xu, J.; Ouyang, H.; Wang, A.; Zheng, M.H. Autologous Tenocyte Therapy for Experimental Achilles Tendinopathy in a Rabbit Model. Tissue Eng. Part A 2011, 17, 2037–2048. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.H. Prostaglandin E2 (PGE2) exerts biphasic effects on human tendon stem cells. PLoS ONE 2014, 9, e87706. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Akinbiyi, T.; Xu, L.; Ramcharan, M.; Leong, D.J.; Ros, S.J.; Colvin, A.C.; Schaffler, M.B.; Majeska, R.J.; Flatow, E.L.; et al. Tendon-derived stem/progenitor cell aging: Defective self-renewal and altered fate. Aging Cell 2010, 9, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Butler, D.; Goldstein, S.; Guilak, F. Functional tissue engineering: The role of biomechanics. J. Biomech. Eng. 2000, 122, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Guilak, F.; Butler, D.L.; Goldstein, S.A.; Mooney, D. (Eds.) Functional Tissue Engineering; Springer: New York, NY, USA, 2003. [Google Scholar]
- Lanza, R.P.; Langer, R.S.; Vacanti, J. (Eds.) Principles of Tissue Engineering; Academic Press: San Diego, CA, USA, 2000. [Google Scholar]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Caramella, C.; Conti, B.; Modena, T.; Ferrari, F.; Bonferoni, M.C.; Genta, I.; Rossi, S.; Torre, M.L.; Sandri, G.; Sorrenti, M.; et al. Controlled delivery systems for tissue repair and regeneration. J. Drug. Deliv. Sci. Technol. 2016, 32, 206–228. [Google Scholar] [CrossRef]
- Verdiyeva, G.; Koshy, K.; Glibbery, N.; Mann, H.; Seifalian, A.M. Tendon Reconstruction with Tissue Engineering Approach: A Review. J. Biomed. Nanotechnol. 2015, 11, 1495–1523. [Google Scholar] [CrossRef]
- Sandri, G.; Bonferoni, M.C.; Rossi, S.; Ferrari, F.; Aguzzi, C.; Viseras, C.; Caramella, C. Clay minerals for tissue regeneration, repair, and engineering. Wound Health Biomater. 2016, 2, 385–402. [Google Scholar]
- Sensini, A.; Cristofolini, L. Biofabrication of Electrospun Scaffolds for the Regeneration of Tendons and Ligaments. Materials 2018, 11, 1963. [Google Scholar] [CrossRef]
- Sandri, G.; Rossi, S.; Bonferoni, M.C.; Caramella, C.; Ferrari, F. Electrospinning Technologies in Wound Dressing Applications. In Therapeutic Dressings and Wound Healing Applications, 1st ed.; Boateng, J., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; Volume 14, pp. 315–336. [Google Scholar] [CrossRef]
- Ruggeri, M.; Bianchi, E.; Rossi, S.; Vigani, B.; Bonferoni, M.C.; Caramella, C.; Sandri, G.; Ferrari, F. Nanotechnology-Based Medical Devices for the Treatment of Chronic Skin Lesions: From Research to the Clinic. Pharmaceutics 2020, 12, 815. [Google Scholar] [CrossRef]
- Baji, A.; Mai, Y.-W.; Wang, S.-C.; Abtahi, M.; Pei, C. Electrospinning of Polymer Nanofibers: Effects on Oriented Morphology, Structures and Tensile Properties. Compos. Sci. Technol. 2010, 70, 703–718. [Google Scholar] [CrossRef]
- Ding, B.; Kim, H.-Y.; Lee, S.-C.; Shao, C.-L.; Lee, D.-R.; Park, S.-J.; Kwag, G.-B.; Choi, K.-J. Preparation and characterization of a nanoscale poly(vinyl alcohol) fiber aggregate produced by an electrospinning method. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 1261–1268. [Google Scholar] [CrossRef]
- Li, D.; Wang, Y.; Xia, Y. Electrospinning Nanofibers as Uniaxially Aligned Arrays and Layer-by-Layer Stacked Films. Adv. Mater. 2004, 16, 361–366. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Electrospinning of nanofibers: Reinventing the wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Dan, L.; McCann, J.T.; Younan, X.; Marquez, M. Electrospinning: A Simple and Versatile Technique for Producing Ceramic Nanofibers and Nanotubes. J. Am. Ceram. Soc. 2006, 89, 1861–1869. [Google Scholar] [CrossRef]
- Mit-uppatham, C.; Nithitanakul, M.; Supaphol, P. Ultrafine electrospun polyamide-6 fibers: Effect of solution conditions on morphology and Mean fiber diameter. Macromol. Chem. Phys. 2004, 205, 2327–2338. [Google Scholar] [CrossRef]
- Kumbar, S.G.; James, R.; Nukavarapu, S.P.; Laurencin, C.T. Electrospun nanofiber scaffolds: Engineering soft tissues. Biomed. Mater. 2008, 3. [Google Scholar] [CrossRef]
- Li, W.-J.; Laurencin, C.T.; Caterson, E.J.; Tuan, R.S.; Ko, F.K. Electrospun nanofibrous structure: A novel scaffold for tissue engineering. J. Biomed. Mater. Res. 2002, 60, 613–621. [Google Scholar] [CrossRef]
- Beachley, V.; Wen, X. Effect of electrospinning parameters on the nanofiber diameter and length. Mater. Sci. Eng. C 2008, 29, 663–668. [Google Scholar] [CrossRef]
- Sandri, G.; Miele, D.; Faccendini, A.; Bonferoni, M.C.; Rossi, S.; Grisoli, P.; Taglietti, A.; Ruggeri, M.; Bruni, G.; Vigani, B.; et al. Chitosan/Glycosaminoglycan Scaffolds: The Role of Silver Nanoparticles to Control Microbial Infections in Wound Healing. Polymers 2019, 11, 1207. [Google Scholar] [CrossRef]
- Smith, L.A.; Ma, P.X. Nano-fibrous scaffolds for tissue engineering. Colloids Surf. B Biointerfaces 2004, 39, 125–131. [Google Scholar] [CrossRef]
- Sheikh, F.A.; Macossay, J.; Cantu, T.; Zhang, X.; Shamshi Hassan, M.; Esther Salinas, M.; Farhangi, C.S.; Ahmad, H.; Kim, H.; Bowlin, G.L. Imaging, spectroscopy, mechanical, alignment and biocompatibility studies of electrospun medical grade polyurethane (Carbothane™ 3575A) nanofibers and composite nanofibers containing multiwalled carbon nanotubes. J. Mech. Behav. Biomed. Mater. 2014, 41, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, X.; Zhang, E.; Wang, L.; Yuan, H.; Tu, W.; Zhang, H.; Yin, Z.; Shen, W.; Chen, X.; et al. An epigenetic bioactive composite scaffold with well-aligned nanofibers for functional tendon tissueengineering. Acta Biomater. 2017, 66, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Domingues, R.M.A.; Goncalves, A.I.; Costa-Almeida, R.; Rodrigues, M.T.; Reis, R.L.; Gomes, M.E. Fabrication of Hierarchical and Biomimetic Fibrous Structures to Support the Regeneration of Tendon Tissues. Tendon Regen. Underst. Tissue Physiol. Dev. Eng. Funct. Substit. 2015, 259–277. [Google Scholar]
- Khademhosseini, A.; Langer, R. Microengineered hydrogels for tissue engineering. Biomaterials 2007, 28, 5087–5092. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.G.; Lee, K.-H.; Khademhosseini, A.; Lee, S.-H. Microfluidic fabrication of microengineered hydrogels and their application in tissue engineering. Lab. Chip 2010, 12, 45–59. [Google Scholar] [CrossRef]
- Vozzi, G.; Flaim, C.; Ahluwalia, A.; Bhatia, S. Fabrication of PLGA scaffolds using soft lithography and microsyringe deposition. Biomaterials 2003, 24, 2533–2540. [Google Scholar] [CrossRef]
- Xia, Y.; Whitesides, G.M. Soft Lithography. Annu. Rev. Mater. Sci. 1998, 28, 153–184. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Ostuni, E.; Takayama, S.; Jiang, X.; Ingber, D.E. Soft Lithography in biology and biochemistry. Annu. Rev. Biomed. Eng. 2001, 3, 335–373. [Google Scholar] [CrossRef]
- Qin, D.; Xin, Y.; Whitesides, G.M. Soft Lithography for micro-and nanoscale patterning. Nat. Protoc. 2010, 5, 491–502. [Google Scholar] [CrossRef]
- Rogers, J.A.; Nuzzo, R.G. Recent progress in Soft Lithography. Mater. Today 2005, 8, 50–56. [Google Scholar] [CrossRef]
- Odom, T.W.; Love, J.C.; Wolfe, D.B.; Paul, K.E.; Whitesides, G.M. Improved pattern transfer in Soft Lithography using composite stamps. Langmuir 2002, 18, 5314–5320. [Google Scholar] [CrossRef]
- Lomas, A.; English, A.; Biggs, M.; Pandit, A.; Zeugolis, D.I. Engineering Anisotropic 2D and 3D Structures for Tendon Repair and Regeneration. Tendon Regen. 2015, 225–242. [Google Scholar] [CrossRef]
- Zhu, J.; Li, J.; Wang, B.; Zhang, W.J.; Zhou, G.; Cao, Y.; Liu, W. The regulation of phenotype of cultured tenocytes by microgrooved surface structure. Biomaterials 2010, 31, 6952–6958. [Google Scholar] [CrossRef]
- Lima, M.J.; Correlo, V.M.; Reis, R.L. Micro/nano replication and 3D assembling techniques for scaffold fabrication. Mater. Sci Eng. C Mater. Biol. Appl. 2014, 42, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, K.; Masaru, T.; Sheikh, R.A. Fabrication of polymeric biomaterials: A strategy for tissue engineering and medical devices. J. Mater. Chem. 2015, 3, 8224–8249. [Google Scholar] [CrossRef]
- Borenstein, J.T.; Terai, H.; King, K.R.; Weinberg, E.J.; Kaazempur-Mofrad, M.R.; Vacanti, J.P. Microfabrication Technology for Vascularized Tissue Engineering. Biomed. Microdevices 2002, 4, 167–175. [Google Scholar] [CrossRef]
- Zhao, X.-M.; Xia, Y.; Whitesides, G.M. Soft lithographic methods for nano-fabrication. J. Mater. Chem. 1997, 7, 1069–1074. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Z.; Xing, R.; Luan, S.; Han, Y. Fabrication of structures with tunable morphologies and sizes by soft molding. Appl. Surf. Sci. 2005, 252, 1947–1953. [Google Scholar] [CrossRef]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Leukers, B.; Gülkan, H.; Irsen, S.H.; Milz, S.; Tille, C.; Schieker, M.; Seitz, H. Hydroxyapatite scaffolds for bone tissue engineering made by 3D printing. J. Mater. Sci. Mater. Med. 2005, 16, 1121–1124. [Google Scholar] [CrossRef]
- Ahmed, T.A.E.; Dare, E.V.; Hincke, M. Fibrin: A Versatile Scaffold for Tissue Engineering Applications. Tissue Eng. Part B Rev. 2008, 14, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.J.; Pirraco, R.P.; Sousa, R.A.; Neves, N.M.; Marques, A.P.; Bhattacharya, M.; Correlo, V.M.; Reis, R.L. Bottom-up approach to construct microfabricated multi-layer scaffolds for bone tissue engineering. Biomed. Microdevices 2014, 16, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Inzana, J.A.; Olvera, D.; Fuller, S.M.; Kelly, J.P.; Graeve, O.A.; Schwarz, E.M.; Kates, S.L.; Awada, H.A. 3D Printing of Composite Calcium Phosphate and Collagen Scaffolds for Bone Regeneration. Biomaterials 2014, 35, 4026–4034. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.J.; West, J.L. Vascularization of Engineered Tissues: Approaches to Promote Angio-genesis in Biomaterials. Curr. Top. Med. Chem. 2008, 8, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wen, F.; Lim, P.N.; Zhang, Q.; Konishi, T.; Wang, D.; Teoh, S.H.; Thian, E.S. Nanomaterial scaffolds to regenerate musculoskeletal tissue: Signals from within for neovessel formation. Drug Discov. Today 2017, 22, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
- Tarafder, S.; Balla, V.K.; Davies, N.M.; Bandyopadhyay, A.; Bose, S. Microwave-sintered 3D printed tricalcium phosphate scaffolds for bone tissue engineering. J. Tissue Eng. Regen. Med. 2012, 7, 631–641. [Google Scholar] [CrossRef]
- Wu, Y.; Fuh, J.Y.H.; Wong, Y.S.; Sun, J. Fabrication of 3D scaffolds via e-jet printing for tendon tissue repair. ASME Int. Manuf. Sci. Eng. Conf. 2015, 56833, V002T03A005. [Google Scholar] [CrossRef]
- Mozdzen, L.C.; Rodgers, R.; Banks, J.M.; Bailey, R.C.; Harley, B.A.C. Increasing the strength and bioactivity of collagen scaffolds using customizable arrays of 3D-printed polymer fibers. Acta Biomater. 2016, 33, 25–33. [Google Scholar] [CrossRef]
- Lin, J.; Zhou, W.; Han, S.; Bunpetch, V.; Zhao, K.; Liu, C.; Yin, Z.; Ouyang, H. Cell-material interactions in tendon tissue engineering. Acta Biomater. 2018, 70, 11. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, J.; Weir, M.D.; Zhang, N.; Zhang, L.; Xie, X.; Zhang, C.; Zhang, K.; Bai, Y.; Xu, H.H.K. Human periodontal ligament stem cells on calcium phosphate scaffold delivering platelet lysate to enhance bone regeneration. RSC Adv. 2019, 9, 41161–41172. [Google Scholar] [CrossRef]
- Santo, V.E.; Gomes, M.E.; Mano, J.F.; Reis, R.L. Chitosan-chondroitin sulphate nanoparticles for controlled delivery of platelet lysates in bone regenerative medicine. J. Tissue Eng. Regen. Med. 2012, 6, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Babo, P.S.; Cai, X.; Plachokova, A.S.; Reis, R.L.; Jansen, J.A.; Gomes, M.E.; Walboomers, X.F. The role of a platelet lysate-based compartmentalized system as a carrier of cells and platelet-origin cytokines for tissue regeneration. Tissue Eng. Part A 2016, 22, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Calejo, I.; Costa-Almeida, R.; Reis, R.L.; Gomes, M.E. Enthesis tissue engineering: Biological requirements meet at the interface. Tissue Eng. Part B Rev. 2019, 25, 330–356. [Google Scholar] [CrossRef] [PubMed]
- Longo, U.G.; Lamberti, A.; Maffulli, N.; Denaro, V. Tissue engineered biological augmentation for tendon healing: A systematic review. Br. Med. Bull. 2010, 98, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Gulotta, L.V.; Kovacevic, D.; Ehteshami, J.R.; Dagher, E.; Packer, J.D.; Rodeo, S.A. Application of bone marrow-derived mesenchymal stem cells in a rotator cuff repair model. Am. J. Sports Med. 2009, 37, 2126–2133. [Google Scholar] [CrossRef] [PubMed]
- Gulotta, L.V.; Rodeo, S.A. Growth factors for rotator cuff repair. Clin. Sports Med. 2009, 28, 13–23. [Google Scholar] [CrossRef]
- Ali, I.H.; Brazil, D.P. Bone morphogenetic proteins and their antagonists: Current and emerging clinical uses. Br. J. Pharm. 2014, 171, 3620. [Google Scholar] [CrossRef]
- Sharma, P.; Maffulli, N. Tendinopathy and tendon injury: The future. Disabil. Rehabil. 2008, 30, 1733–1745. [Google Scholar] [CrossRef]
- Sharma, P.; Maffulli, N. Biology of tendon injury: Healing, modeling and remodelling. J. Musculoskelet. Neuronal Interact. 2006, 6, 181–190. [Google Scholar]
- Babo, P.S.; Reis, R.L.; Gomes, M.E. Periodontal tissue engineering: Current strategies and the role of platelet rich hemoderivatives. J. Mater. Chem. B 2017, 5, 3617–3628. [Google Scholar] [CrossRef]
- Mori, M.; Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Sandri, G.; Riva, F.; Tenci, M.; Del Fante, C.; Nicoletti, G.; Caramella, C. Sponge-Like Dressings Based on the Association of Chitosan and Sericin for the Treatment of Chronic Skin Ulcers. II. Loading of the Hemoderivative Platelet Lysate. J. Pharm. Sci. 2016, 105, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, S.P.B.; Domingues, R.M.A.; Shevchuk, M.; Gomes, M.E.; Peppas, N.A.; Reis, R.L. Biomaterials for Sequestration of Growth Factors and Modulation of Cell Behavior. Adv. Funct. Mater. 2020, 30. [Google Scholar] [CrossRef]
- Andia, I.; Maffulli, N. Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nat. Rev. Rheumatol. 2013, 9, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Burnouf, T.; Chou, M.-L.; Wu, Y.-W.; Su, C.-Y.; Lee, L.-W. Antimicrobial activity of platelet (PLT)-poor plasma, PLT-rich plasma, PLT gel, and solvent/detergent-treated PLT lysate biomaterials against wound bacteria. Transfusion 2013, 53, 138–146. [Google Scholar] [CrossRef]
- Yeaman, M.R.; Bayer, A.S. Antimicrobial peptides from platelets. Drug Resist. Updat. 1999, 2, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Copland, I.B.; Garcia, M.A.; Waller, E.K.; Roback, J.D.; Galipeau, J. The effect of platelet lysate fibrinogen on the functionality of MSCs in immunotherapy. Biomaterials 2013, 34, 7840–7850. [Google Scholar] [CrossRef]
- Mishra, A.; Woodall, J.; Vieira, A. Treatment of tendon and muscle using platelet-rich plasma. Clin. Sports Med. 2009, 28, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E. Platelet-rich plasma (PRP): What is PRP and what is not PRP? Implant. Dent. 2001, 10, 225–228. [Google Scholar] [CrossRef]
- Amable, P.R.; Carias, R.B.; Teixeira, M.V.; da Cruz Pacheco, I.; Correa do Amaral, R.J.; Granjeiro, J.M.; Borojevic, R. Platelet-rich plasma preparation for regenerative medicine: Optimization and quantification of cytokines and growth factors. Stem Cell Res. Ther. 2013, 4, 67. [Google Scholar] [CrossRef]
- Seidel, S.R.T.; Vendruscolo, C.P.; Moreira, J.J.; Fülber, J.; Ottaiano, T.F.; Oliva, M.L.V.; Michelacci, Y.M.; Baccarin, R.Y.A. Does Double Centrifugation Lead to Premature Platelet Aggregation and Decreased TGF-β1 Concentrations in Equine Platelet-Rich Plasma? Vet. Sci. 2019, 6, 68. [Google Scholar] [CrossRef]
- Mendes, B.B.; Gómez-Florit, M.; Babo, P.S.; Domingues, R.M.; Reis, R.L.; Gomes, M.E. Blood derivatives awaken in regenerative medicine strategies to modulate wound healing. Avd. Drug Deliv. Rev. 2018, 129, 376–393. [Google Scholar] [CrossRef] [PubMed]
- Perazzi, A.; Busetto, R.; Martinello, T.; Drigo, M.; Pasotto, D.; Cian, F.; Patruno, M.; Iacopetti, I. Description of a double centrifugation tube method for concentrating canine platelets. BMC Vet. Res. 2013, 9, 146. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M.; Rasmusson, L.; Albrektsson, T. Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009, 27, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M.; Andia, I.; Zumstein, M.A.; Zhang, C.Q.; Pinto, N.R.; Bielecki, T. Classification of platelet concentrates (Platelet-Rich Plasma-PRP, Platelet-Rich Fibrin- PRF) for topical and infiltrative use in orthopedic and sports medicine: Current consensus, clinical implications and perspectives. Muscles Ligaments Tendons J. 2014, 4, 3–9. [Google Scholar] [CrossRef]
- Martineau, I.; Lacoste, E.; Gagnon, G. Effects of calcium and thrombin on growth factor release from platelet concentrates: Kinetics and regulation of endothelial cell proliferation. Biomaterials 2004, 25, 4489–4502. [Google Scholar] [CrossRef]
- Wang, H.-L.; Avila, G. Platelet rich plasma: Myth or reality? Eur. J. Dent. 2007, 1, 192–194. [Google Scholar] [CrossRef]
- Fortunato, T.M.; Beltrami, C.; Emanueli, C.; De Bank, P.A.; Pula, G. Platelet lysate gel and endothelial progenitors stimulate microvascular network formation in vitro: Tissue engineering implications. Sci. Rep. 2016, 6, 25326. [Google Scholar] [CrossRef]
- Crespo-Diaz, R.; Behfar, A.; Butler, G.W.; Padley, D.J.; Sarr, M.G.; Bartunek, J.; Dietz, A.B.; Terzic, A. Platelet lysate consisting of a natural repair proteome supports human mesenchymal stem cell proliferation and chromosomal stability. Cell Transplant. 2011, 20, 797–811. [Google Scholar] [CrossRef]
- Fekete, N.; Gadelorge, M.; Furst, D.; Maurer, C.; Dausend, J.; Fleury-Cappellesso, S.; Mailander, V.; Lotfi, R.; Ignatius, A.; Sensebe, L.; et al. Platelet lysate from whole blood-derived pooled platelet concentrates and apheresis-derived platelet concentrates for the isolation and expansion of human bone marrow mesenchymal stromal cells: Production process, content and identification of active components. Cytotherapy 2010, 14, 540–554. [Google Scholar] [CrossRef]
- Brown, A.C.; Barker, T.H. Fibrin-based biomaterials: Modulation of macroscopic properties through rational design at the molecular level. Acta Biomater. 2014, 10, 1502–1514. [Google Scholar] [CrossRef]
- Capila, I.; Linhardt, R.J. Heparin–protein interactions. Angew. Chem. Int. Ed. Eng. 2002, 41, 390–412. [Google Scholar] [CrossRef]
- Macri, L.; Silverstein, D.; Clark, R.A.F. Growth factor binding to the pericellular matrix and its importance in tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 1366–1381. [Google Scholar] [CrossRef] [PubMed]
- Caliari, S.R.; Weisgerber, D.W.; Grier, W.K.; Mahmassani, Z.; Boppart, M.D.; Harley, B.A.C. Collagen scaffolds incorporating coincident gradations of instructive structural and biochemical cues for osteotendinous junction engineering. Adv. Healthc. Mater. 2015, 4, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Sun, Y.; Xu, P.; Cheng, B. Can patients get better clinical outcomes by using PRP in rotator cuff repair: A metaanalysis of randomized controlled trials. J. Sports Med. Phys. Fit. 2016, 56, 1359–1367. [Google Scholar]
- Mi, B.; Liu, G.; Zhou, W.; Lv, H.; Liu, Y.; Wu, Q.; Liu, J. Platelet rich plasma versus steroid on lateral epicondylitis: Meta-analysis of randomized clinical trials. Phys. Sports Med. 2017, 45, 97–104. [Google Scholar] [CrossRef]
- Costa-Almeida, R.; Babo, P.S.; Reis, R.L.; Gomes, M.E. Platelet-rich Blood Derivatives for Tendon Regeneration. J. Am. Acad Orthop. Surg. 2020, 28, e202–e205. [Google Scholar] [CrossRef]
- Hurley, E.T.; Fat, D.L.; Moran, C.J.; Mullett, H. The Efficacy of Platelet-Rich Plasma and Platelet-Rich Fibrin in Arthroscopic Rotator Cuff Repair: A Meta-analysis of Randomized Controlled Trials. Am. J. Sports Med. 2019, 47, 753–761. [Google Scholar] [CrossRef]
- Yang, F.-A.; Liao, C.-D.; Wu, C.-W.; Shih, Y.-C.; Wu, L.-C.; Chen, H.-C. Effects of applying platelet-rich plasma during arthroscopic rotator cuff repair: A systematic review and meta-analysis of randomised controlled trials. Sci. Rep. 2020, 10, 17171. [Google Scholar] [CrossRef]
- Lee, K.; Silva, E.A.; Mooney, D.J. Growth factor delivery-based tissue engineering: General approaches and a review of recent developments. J. R Soc. Interface 2010, 8, 153–170. [Google Scholar] [CrossRef]
- Mitchell, A.C.; Briquez, P.S.; Hubbell, J.A.; Cochran, J.R. Engineering growth factors for regenerative medicine applications. Acta Biomater. 2016, 30, 1–12. [Google Scholar] [CrossRef]
- Bu, M.; Tang, J.; Wei, Y.; Sun, Y.; Wang, X.; Wu, L.; Liu, H. Enhanced bioavailability of nerve growth factor with phytantriol lipid-based crystalline nanoparticles in cochlea. Int. J. Nanomed. 2015, 10, 6879–6889. [Google Scholar] [CrossRef]
- Liu, S.; Qin, M.; Hu, C.; Wu, F.; Cui, W.; Jin, T.; Fan, C. Tendon healing and anti-adhesion properties of electrospun fibrous membranes containing bFGF loaded nanoparticles. Biomaterials 2013, 34, 4690–4701. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.M.; Willers, C.; Xu, J.; Wang, A.; Zheng, M.H. Autologous tenocyte therapy using porcine derived bioscaffolds for massive rotator cuff defect in rabbits. Tissue Eng. 2007, 13, 1479–1491. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.P.; Lee, C.-H.; Jung, J.W.; Lee, H.-J.; Lee, Y.-S.; Kim, J.-Y.; Park, G.Y.; Choi, J.H.; Chung, S.W. Sustained delivery of transforming growth factor b1 by use of absorbable alginate scaffold enhances rotator cuff healing in a rabbit model. Am. J. Sports Med. 2018, 46, 1441–1450. [Google Scholar] [CrossRef]
- Manning, C.N.; Kim, H.M.; Sakiyama-Elbert, S.; Galatz, L.M.; Havlioglu, N.; Thomopoulos, S. Sustained delivery of transforming growth factor beta three enhances tendon-to-bone healing in a rat model. J. Orthop. Res. 2011, 29, 1099–1105. [Google Scholar] [CrossRef]
- Zhao, S.; Zhao, J.; Dong, S.; Huangfu, X.; Li, B.; Yang, H.; Zhao, J.; Cui, W. Biological augmentation of rotator cuff repair using bFGF-loaded electrospun poly(lactide-co-glycolide) fibrous membranes. Int. J. Nanomed. 2014, 9, 2373–2385. [Google Scholar] [CrossRef]
- Ide, J.; Kikukawa, K.; Hirose, J.; Iyama, K.; Sakamoto, H.; Fujimoto, T.; Mizuta, H. The effect of a local application of fibroblast growth factor-2 on tendon-to-bone remodeling in rats with acute injury and repair of the supraspinatus tendon. J. Shoulder Elb. Surg. 2009, 18, 391–398. [Google Scholar] [CrossRef]
- Rickert, M.; Jung, M.; Adiyaman, M.; Richter, W.; Simank, H.G. A Growth and Differentiation Factor-5 (GDF-5)-coated Suture Stimulates Tendon Healing in an Achilles Tendon Model in Rats. Growth Factors 2001, 19, 115–126. [Google Scholar] [CrossRef]
- Kabuto, Y.; Morihara, T.; Sukenari, T.; Kida, Y.; Oda, R.; Arai, Y.; Sawada, K.; Matsuda, K.-I.; Kawata, M.; Tabata, Y.; et al. Stimulation of Rotator Cuff Repair by Sustained Release of Bone Morphogenetic Protein-7 Using a Gelatin Hydrogel Sheet. Tissue Eng. Part A 2015, 21, 2025–2033. [Google Scholar] [CrossRef]
- Lee, K.-W.; Lee, J.-S.; Kim, Y.-S.; Shim, Y.-B.; Jang, J.-W.; Lee, K.-I. Effective healing of chronic rotator cuff injury using recombinant bone morphogenetic protein-2 coated dermal patch in vivo. J. Biomed. Mater. Res. B 2016, 105, 1840–1846. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, T.; Zheng, N.; Zhou, Y.; Hogan, M.V.; Wang, J.H.-C. The combined use of kartogenin and platelet-rich plasma promotes fibrocartilage formation in the wounded rat Achilles tendon entheses. Bone Jt. Res. 2017, 6, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.; Zhou, C.; Xu, D.; Bi, F. Combination of platelet-rich plasma and bone marrow mesenchymal stem cells enhances tendon–bone healing in a rabbit model of anterior cruciate ligament reconstruction. J. Orthop. Surg. Res. 2016, 11, 96. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.; Kawase, T.; Momose, M.; Murata, M.; Saito, Y.; Suzuki, H.; Wolff, L.F.; Yoshie, H. Platelet-rich plasma contains high levels of platelet-derived growth factor and transforming growth factor-beta and modulates the proliferation of periodontally related cells in vitro. J. Periodontol. 2003, 74, 849–857. [Google Scholar] [CrossRef]
- Ranzato, E.; Patrone, M.; Mazzucco, L.; Burlando, B. Platelet lysate stimulates wound repair of HaCaT keratinocytes. Br. J. Derm. 2008, 159, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Backly, R.E.; Ulivi, V.; Tonachini, L.; Cancedda, R.; Descalzi, F.; Mastrogiacomo, M. Platelet lysate induces in vitro wound healing of human keratinocytes associated with a strong proinflammatory response. Tissue Eng. Part A 2011, 17, 1787–1800. [Google Scholar] [CrossRef]
- Witting, M.; Obst, K.; Friess, W.; Hedtrich, S. Recent advances in topical delivery of proteins and peptides mediated by soft matter nanocarriers. Biotechnol. Adv. 2015, 33, 1355–1369. [Google Scholar] [CrossRef]
- Mottaghitalab, F.; Farokhi, M.; Shokrgozar, M.A.; Atyabi, F.; Hosseinkhani, H. Silk fibroin nanoparticle as a novel drug delivery system. J. Control. Release 2015, 206, 161–176. [Google Scholar] [CrossRef]
- Smith, B.D.; Grande, D.A. The current state of scaffolds for musculoskeletal regenerative applications. Nat. Rev. Rheumatol. 2015, 11, 213–222. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).