Methiothepin Suppresses Human Ovarian Cancer Cell Growth by Repressing Mitochondrion-Mediated Metabolism and Inhibiting Angiogenesis In Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Cell Viability and Proliferation Test
2.4. Morphological Analysis
2.5. Spheroid Culture
2.6. Apoptosis Analysis
2.7. Western Blotting
2.8. Analysis of Mitochondrial Membrane Potential
2.9. Mitochondrial Calcium Analysis
2.10. Seahorse Analysis
2.11. Angiogenesis Validation In Vivo
2.12. Statistical Analysis
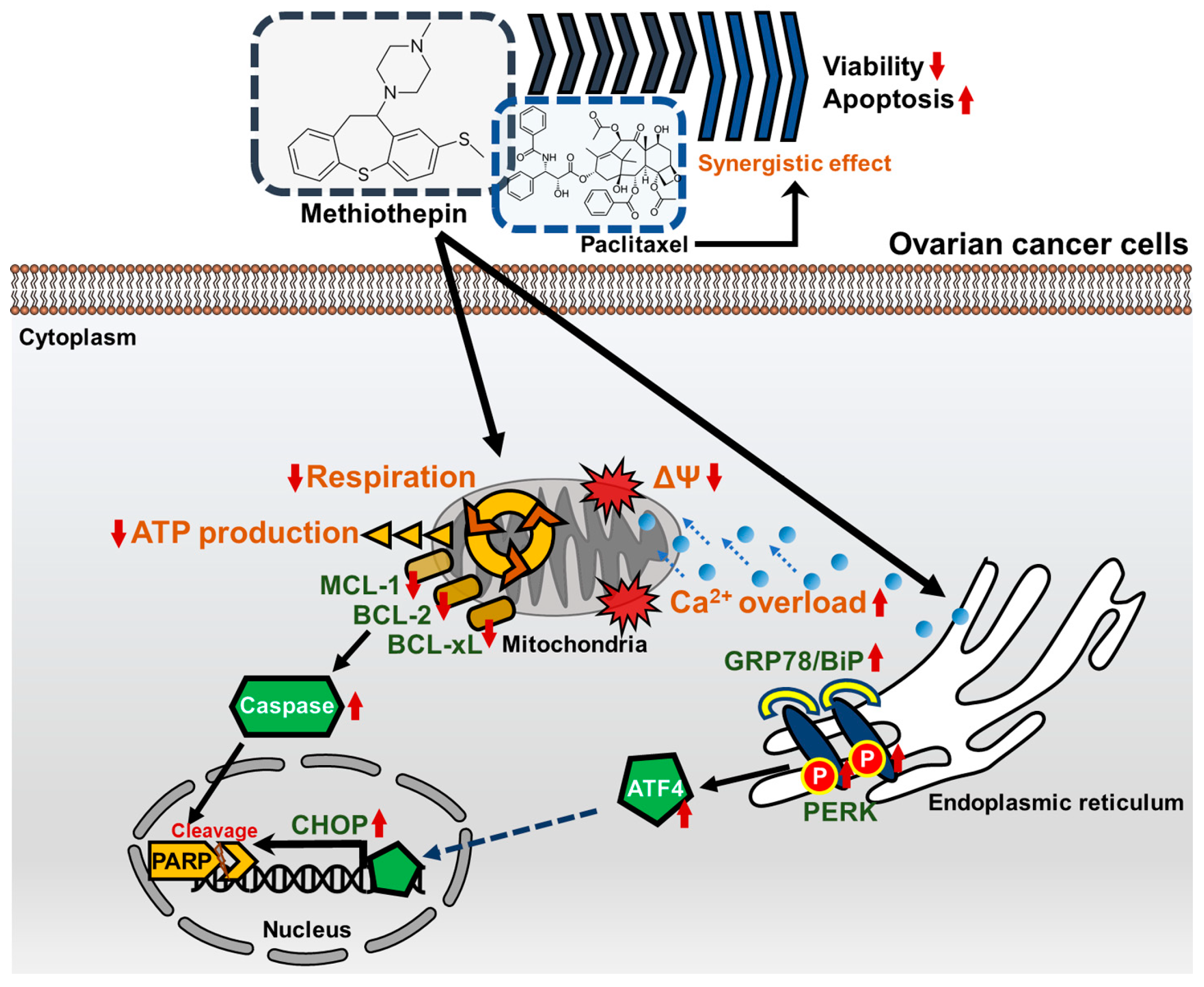
3. Results
3.1. Methiothepin Inhibits the Viability of Ovarian Cancer Cells
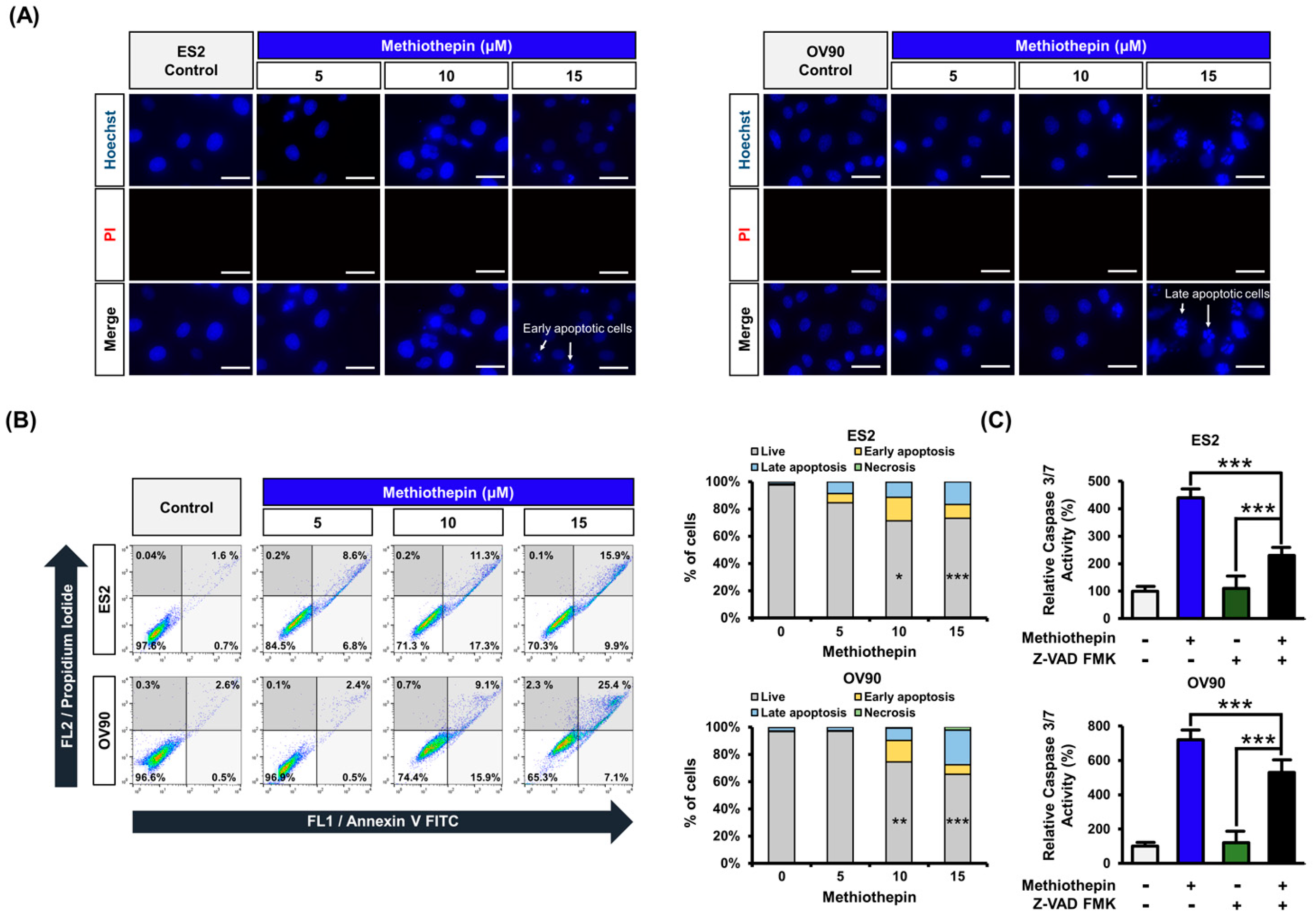
3.2. Methiothepin Induces Apoptotic Cell Death in Ovarian Cancer Cells
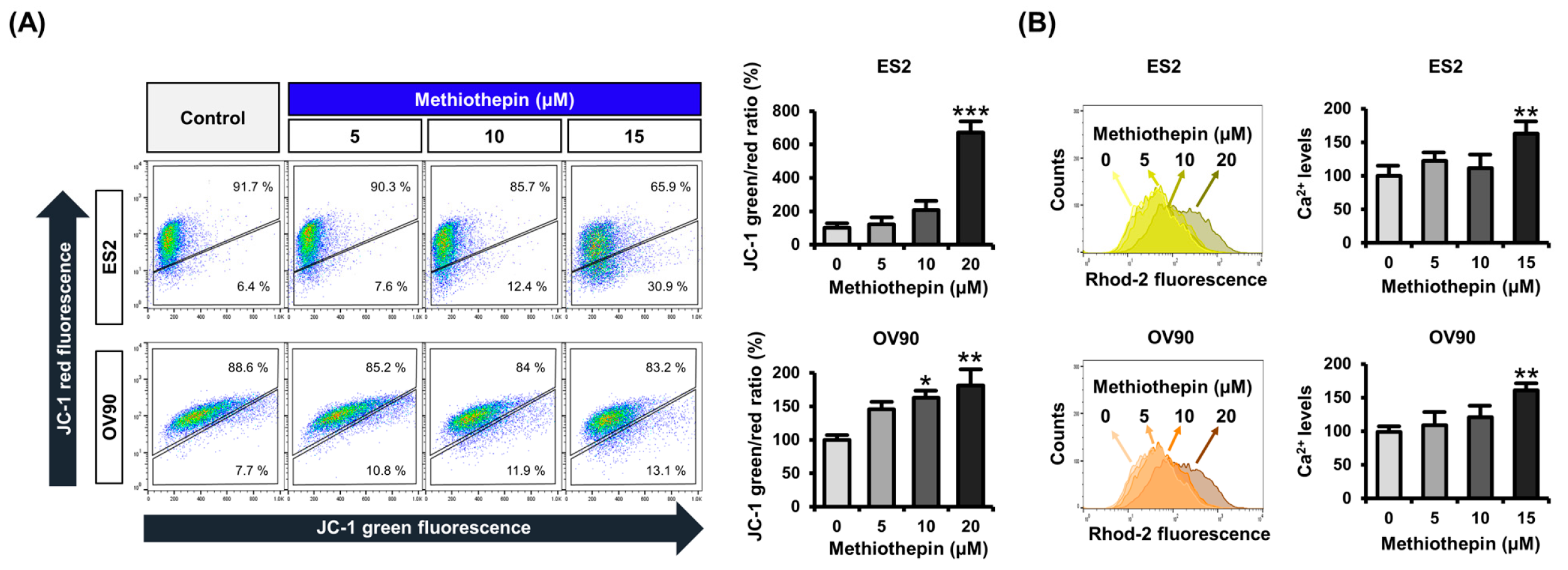
3.3. Methiothepin Induces Mitochondrial Disruption in Ovarian Cancer Cells
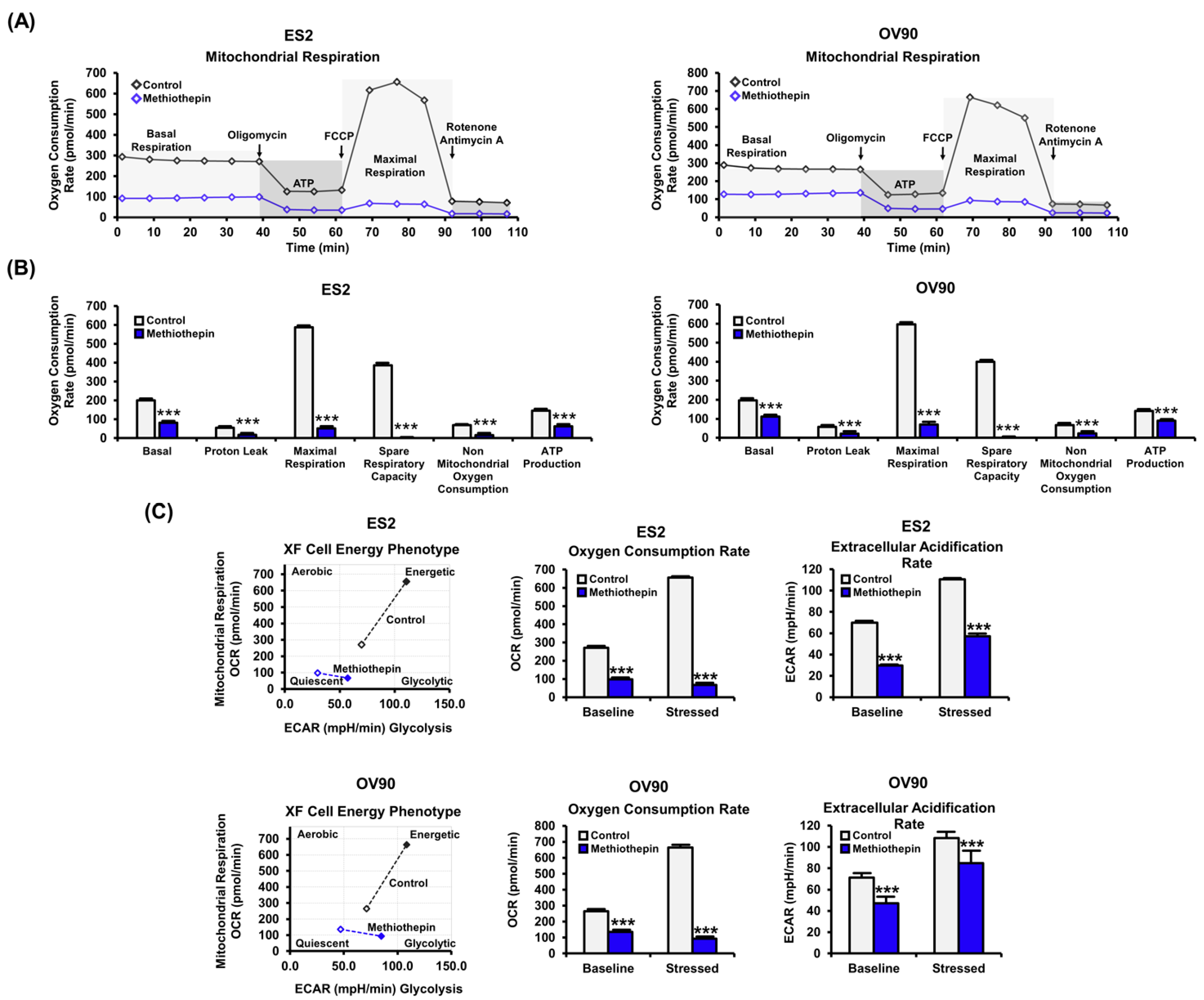
3.4. Methiothepin Interferes with Mitochondrion-Mediated Metabolic Status in Ovarian Cancer Cells
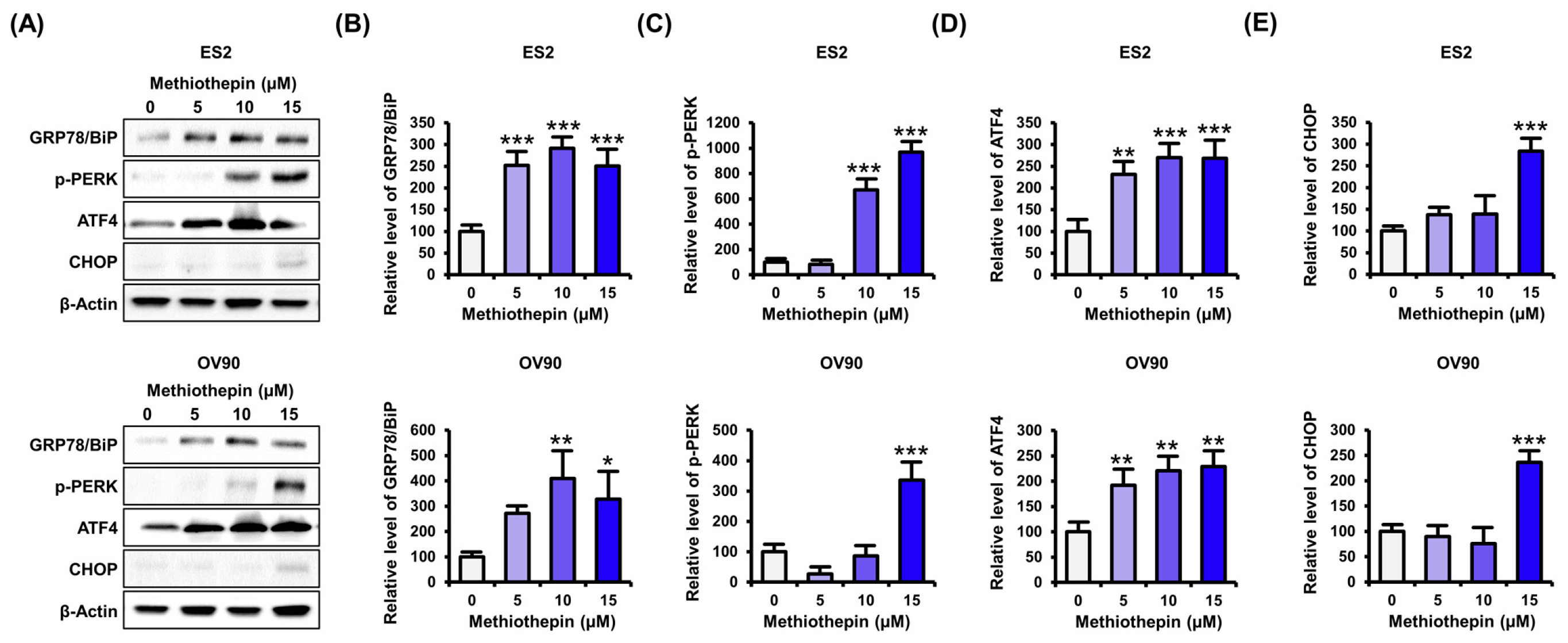
3.5. Methiothepin Regulates the Expression of Endoplasmic Reticulum Stress (ER)-Related Proteins in Ovarian Cancer Cells
3.6. Methiothepin Interferes with the Development of the Vascular System in Zebrafish Embryos
3.7. Methiothepin Enhances the Anti-Cancer Effects Induced by Paclitaxel
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Naishadham, D.; Jemal, A. Cancer statistics, 2013. CA Cancer J. Clin. 2013, 63, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, I.J.; Menon, U.; Ryan, A.; Gentry-Maharaj, A.; Burnell, M.; Kalsi, J.K.; Amso, N.N.; Apostolidou, S.; Benjamin, E.; Cruickshank, D.; et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): A randomised controlled trial. Lancet 2016, 387, 945–956. [Google Scholar] [CrossRef]
- Berns, E.M.J.J.; Bowtell, D.D. The Changing View of High-Grade Serous Ovarian Cancer. Cancer Res. 2012, 72, 2701–2704. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Kaye, S.B. Ovarian cancer: Strategies for overcoming resistance to chemotherapy. Nat. Rev. Cancer 2003, 3, 502–516. [Google Scholar] [CrossRef]
- Predescu, D.-V.; Pavelescu, L.A.; Suciu, N.; Radu, B.M.; Voinea, S.-C.; Crețoiu, S.M.; Crețoiu, D. G Protein-Coupled Receptors (GPCRs)-Mediated Calcium Signaling in Ovarian Cancer: Focus on GPCRs activated by Neurotransmitters and Inflammation-Associated Molecules. Int. J. Mol. Sci. 2019, 20, 5568. [Google Scholar] [CrossRef]
- Bar-Shavit, R.; Maoz, M.; Kancharla, A.; Nag, J.K.; Agranovich, D.; Grisaru-Granovsky, S.; Uziely, B. G Protein-Coupled Receptors in Cancer. Int. J. Mol. Sci. 2016, 17, 1320. [Google Scholar] [CrossRef]
- Fanburg, B.L.; Lee, S.L. A new role for an old molecule: Serotonin as a mitogen. Am. J. Physiol. Content 1997, 272, L795–L806. [Google Scholar] [CrossRef] [PubMed]
- Sonier, B.; Arseneault, M.; Lavigne, C.; Ouellette, R.J.; Vaillancourt, C. The 5-HT2A serotoninergic receptor is expressed in the MCF-7 human breast cancer cell line and reveals a mitogenic effect of serotonin. Biochem. Biophys. Res. Commun. 2006, 343, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Christensen, D.K.; Armaiz-Pena, G.N.; Ramirez, E.; Matsuo, K.; Zimmerman, B.; Zand, B.; Shinn, E.; Goodheart, M.J.; Bender, D.; Thaker, P.H.; et al. SSRI use and clinical outcomes in epithelial ovarian cancer. Oncotarget 2016, 7, 33179–33191. [Google Scholar] [CrossRef]
- Henriksen, R.; Dizeyi, N.; Abrahamsson, P. Expression of serotonin receptors 5-HT1A, 5-HT1B, 5-HT2B and 5-HT4 in ovary and in ovarian tumours. Anticancer Res. 2012, 32, 1361–1366. [Google Scholar] [PubMed]
- Mørch, L.S.; Dehlendorff, C.; Baandrup, L.; Friis, S.; Kjær, S.K.; Kjaer, S.K. Use of antidepressants and risk of epithelial ovarian cancer. Int. J. Cancer 2017, 141, 2197–2203. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Song, G.; Lim, W. Methiothepin mesylate causes apoptosis of human prostate cancer cells by mediating oxidative stress and mitochondrial dysfunction. Free Radic. Biol. Med. 2020, 150, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Lee, J.-Y.; Park, S.; Song, G.; Lim, W. Developmental toxicity and angiogenic defects of etoxazole exposed zebrafish (Danio rerio) larvae. Aquat. Toxicol. 2019, 217, 105324. [Google Scholar] [CrossRef]
- Choi, H.J.; Armaiz-Pena, G.N.; Pradeep, S.; Cho, M.S.; Coleman, R.L.; Sood, A.K. Anti-vascular therapies in ovarian cancer: Moving beyond anti-VEGF approaches. Cancer Metastasis Rev. 2015, 34, 19–40. [Google Scholar] [CrossRef]
- Delov, V.; Muth-Köhne, E.; Schäfers, C.; Fenske, M. Transgenic fluorescent zebrafish Tg(fli1:EGFP)y1 for the identification of vasotoxicity within the zFET. Aquat. Toxicol. 2014, 150, 189–200. [Google Scholar] [CrossRef]
- Simpkins, F.; Hevia-Paez, P.; Sun, J.; Ullmer, W.; Gilbert, C.A.; Da Silva, T.; Pedram, A.; Levin, E.R.; Reis, I.M.; Rabinovich, B.; et al. Src Inhibition with Saracatinib Reverses Fulvestrant Resistance in ER-Positive Ovarian Cancer Models In Vitro and In Vivo. Clin. Cancer Res. 2012, 18, 5911–5923. [Google Scholar] [CrossRef]
- Zagon, I.S.; Donahue, R.; McLaughlin, P.J. Targeting the opioid growth factor: Opioid growth factor receptor axis for treatment of human ovarian cancer. Exp. Biol. Med. 2013, 238, 579–587. [Google Scholar] [CrossRef]
- Liu, J.; Agopiantz, M.; Poupon, J.; Wu, Z.; Just, P.-A.; Borghese, B.; Ségal-Bendirdjian, E.; Gauchotte, G.; Gompel, A.; Forgez, P. Neurotensin Receptor 1 Antagonist SR48692 Improves Response to Carboplatin by Enhancing Apoptosis and Inhibiting Drug Efflux in Ovarian Cancer. Clin. Cancer Res. 2017, 23, 6516–6528. [Google Scholar] [CrossRef]
- Li, L.; Li, X.; Han, X.; Yang, T.; Fu, J.; Zhang, Y.; Gou, W. An ovarian cancer model with positive ER: Reversion of ER antagonist resistance by Src blockade. Oncol. Rep. 2014, 32, 943–950. [Google Scholar] [CrossRef]
- Gilchrist, A. Modulating G-protein-coupled receptors: From traditional pharmacology to allosterics. Trends Pharmacol. Sci. 2007, 28, 431–437. [Google Scholar] [CrossRef]
- Nichols, D.E.; Nichols, C.D. Serotonin Receptors. Chem. Rev. 2008, 108, 1614–1641. [Google Scholar] [CrossRef] [PubMed]
- Brandes, L.J.; Arron, R.J.; Bogdanovic, R.P.; Tong, J.; Zaborniak, C.L.; Hogg, G.R.; Warrington, R.C.; Fang, W.; Labella, F.S. Stimulation of malignant growth in rodents by antidepressant drugs at clinically relevant doses. Cancer Res. 1992, 52, 3796–3800. [Google Scholar] [PubMed]
- Hilakivi-Clarke, L.; Wright, A.; Lippman, M.E. DMBA-induced mammary tumor growth in rats exhibiting increased or decreased ability to cope with stress due to early postnatal handling or antidepressant treatment. Physiol. Behav. 1993, 54, 229–236. [Google Scholar] [CrossRef]
- Cosgrove, L.; Shi, L.; Creasey, D.E.; Anaya-McKivergan, M.; Myers, J.A.; Huybrechts, K.F. Antidepressants and Breast and Ovarian Cancer Risk: A Review of the Literature and Researchers’ Financial Associations with Industry. PLoS ONE 2011, 6, e18210. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, E.M.; Franci, C.R. Involvement of serotonin 5HT1 and 5HT2 receptors and nitric oxide synthase in the medial preoptic area on gonadotropin secretion. Brain Res. Bull. 2004, 63, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Kannisto, P.; Owman, C.; Schmidt, G.; Sjöberg, N.-O. Characterization of presynaptic 5-HT receptors on adrenergic nerves supplying the bovine ovarian follicle. Br. J. Pharmacol. 1987, 92, 487–497. [Google Scholar] [CrossRef]
- Steingart, A.B.; Cotterchio, M. Do antidepressants cause, promote, or inhibit cancers? J. Clin. Epidemiol. 1995, 48, 1407–1412. [Google Scholar] [CrossRef]
- Tutton, P.J.; Barkla, D.H. Influence of inhibitors of serotonin uptake on intestinal epithelium and colorectal carcinomas. Br. J. Cancer 1982, 46, 260–265. [Google Scholar] [CrossRef]
- Grygier, B.; Arteta, B.; Kubera, M.; Basta-Kaim, A.; Budziszewska, B.; Leśkiewicz, M.; Curzytek, K.; Duda, W.; Lasoń, W.; Maes, M. Inhibitory effect of antidepressants on B16F10 melanoma tumor growth. Pharmacol. Rep. 2013, 65, 672–681. [Google Scholar] [CrossRef]
- Genaro, A.M.; Genaro, A.M.; Sterin-Borda, L. Differential effects of fluoxetine on murine B-cell proliferation depending on the biochemical pathways triggered by distinct mitogens. Biochem. Pharmacol. 2000, 60, 1279–1283. [Google Scholar] [CrossRef]
- Slamon, N.D.; Mead, C.; Morgan, C.; Mitchell, A.; Pentreath, V.W. The Involvement of Calcium in the Protective and Toxic (Nonlinear) Responses of Rodent and Human Astroglial Cells. Nonlinearity Biol. Toxicol. Med. 2005, 3, 79–95. [Google Scholar] [CrossRef] [PubMed]
- Orrenius, S.; Zhivotovsky, B.; Nicotera, P. Regulation of cell death: The calcium–apoptosis link. Nat. Rev. Mol. Cell Biol. 2003, 4, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, J.; Das, M.K.; Yang, Z.-Y.; Lew, R. Evaluation of the binding of the radiolabeled antidepressant drug, 18F-fluoxetine in the rodent brain: An in vitro and in vivo study. Nucl. Med. Biol. 1998, 25, 605–610. [Google Scholar] [CrossRef]
- Charles, E.; Hammadi, M.; Kischel, P.; Delcroix, V.; Demaurex, N.; Castelbou, C.; Vacher, A.-M.; Devin, A.; Ducret, T.; Nunes-Hasler, P.; et al. The antidepressant fluoxetine induces necrosis by energy depletion and mitochondrial calcium overload. Oncotarget 2016, 8, 3181–3196. [Google Scholar] [CrossRef]
- Madhok, B.M.; Yeluri, S.; Perry, S.L.; Hughes, T.A.; Jayne, D.G. Targeting Glucose Metabolism. Am. J. Clin. Oncol. 2011, 34, 628–635. [Google Scholar] [CrossRef]
- Mondal, S.; Roy, D.; Bhattacharya, S.S.; Jin, L.; Jung, D.; Zhang, S.; Kalogera, E.; Staub, J.; Wang, Y.; XuYang, W.; et al. Therapeutic targeting of PFKFB3 with a novel glycolytic inhibitor PFK158 promotes lipophagy and chemosensitivity in gynecologic cancers. Int. J. Cancer 2018, 144, 178–189. [Google Scholar] [CrossRef]
- Talekar, M.; Boreddy, S.R.; Singh, A.; Amiji, M. Tumor aerobic glycolysis: New insights into therapeutic strategies with targeted delivery. Expert Opin. Biol. Ther. 2014, 14, 1145–1159. [Google Scholar] [CrossRef]
- Taylor, C.; Mannion, D.; MirandA, F.M.; KaramiNejadRanjbar, M.; Herrero-Gonzalez, S.; Hellner, K.; Zheng, Y.; Bartholomeusz, G.; Bast, R.C., Jr.; Ahmed, A.A. Loss of PFKFB4 induces cell death in mitotically arrested ovarian cancer cells. Oncotarget 2017, 8, 17960–17980. [Google Scholar] [CrossRef]
- Hsu, P.P.; Sabatini, D.M. Cancer Cell Metabolism: Warburg and Beyond. Cell 2008, 134, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Kanakkanthara, A.; Kurmi, K.; Ekstrom, T.L.; Hou, X.; Purfeerst, E.R.; Heinzen, E.P.; Correia, C.; Huntoon, C.J.; O’Brien, D.; Hendrickson, A.E.W.; et al. BRCA1 Deficiency Upregulates NNMT, Which Reprograms Metabolism and Sensitizes Ovarian Cancer Cells to Mitochondrial Metabolic Targeting Agents. Cancer Res. 2019, 79, 5920–5929. [Google Scholar] [CrossRef]
- Opferman, J.T.; Letai, A.; Beard, C.; Sorcinelli, M.D.; Ong, C.C.; Korsmeyer, S.J. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature 2003, 426, 671–676. [Google Scholar] [CrossRef]
- Arbour, N.; Vanderluit, J.L.; Le Grand, J.N.; Jahani-Asl, A.; Ruzhynsky, V.A.; Cheung, E.C.C.; Kelly, M.A.; MacKenzie, A.E.; Park, D.S.; Opferman, J.T.; et al. Mcl-1 is a key regulator of apoptosis during CNS development and after DNA damage. J. Neurosci. 2008, 28, 6068–6078. [Google Scholar] [CrossRef]
- Armstrong, J.L.; Flockhart, R.; Veal, G.J.; Lovat, P.E.; Redfern, C.P.F. Regulation of Endoplasmic Reticulum Stress-induced Cell Death by ATF4 in Neuroectodermal Tumor Cells. J. Biol. Chem. 2009, 285, 6091–6100. [Google Scholar] [CrossRef] [PubMed]
- Oyadomari, S.; Mori, M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2003, 11, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Dhani, N.; Oza, A.M. Targeting Angiogenesis. Hematol. Clin. N. Am. 2018, 32, 1041–1055. [Google Scholar] [CrossRef] [PubMed]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-Y.; Yang, C.; Lim, W.; Song, G. Methiothepin Suppresses Human Ovarian Cancer Cell Growth by Repressing Mitochondrion-Mediated Metabolism and Inhibiting Angiogenesis In Vivo. Pharmaceutics 2020, 12, 686. https://doi.org/10.3390/pharmaceutics12070686
Lee J-Y, Yang C, Lim W, Song G. Methiothepin Suppresses Human Ovarian Cancer Cell Growth by Repressing Mitochondrion-Mediated Metabolism and Inhibiting Angiogenesis In Vivo. Pharmaceutics. 2020; 12(7):686. https://doi.org/10.3390/pharmaceutics12070686
Chicago/Turabian StyleLee, Jin-Young, Changwon Yang, Whasun Lim, and Gwonhwa Song. 2020. "Methiothepin Suppresses Human Ovarian Cancer Cell Growth by Repressing Mitochondrion-Mediated Metabolism and Inhibiting Angiogenesis In Vivo" Pharmaceutics 12, no. 7: 686. https://doi.org/10.3390/pharmaceutics12070686
APA StyleLee, J.-Y., Yang, C., Lim, W., & Song, G. (2020). Methiothepin Suppresses Human Ovarian Cancer Cell Growth by Repressing Mitochondrion-Mediated Metabolism and Inhibiting Angiogenesis In Vivo. Pharmaceutics, 12(7), 686. https://doi.org/10.3390/pharmaceutics12070686





