Enhanced Chondrogenic Differentiation Activities in Human Bone Marrow Aspirates via sox9 Overexpression Mediated by pNaSS-Grafted PCL Film-Guided rAAV Gene Transfer
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Bone Marrow Aspirates
2.3. Preparation of the Poly(ε-caprolactone) Films
2.4. Preparation of the rAAV Vectors
2.5. rAAV Vector Immobilization on the PCL Films
2.6. rAAV-Mediated Gene Transfer
2.7. Transgene Expression
2.8. Biological Analyses
2.9. Histology and Immunohistochemistry
2.10. Histomorphometric Analyses
2.11. Real-Time RT-PCR Analysis
2.12. Statistical Analysis
3. Results
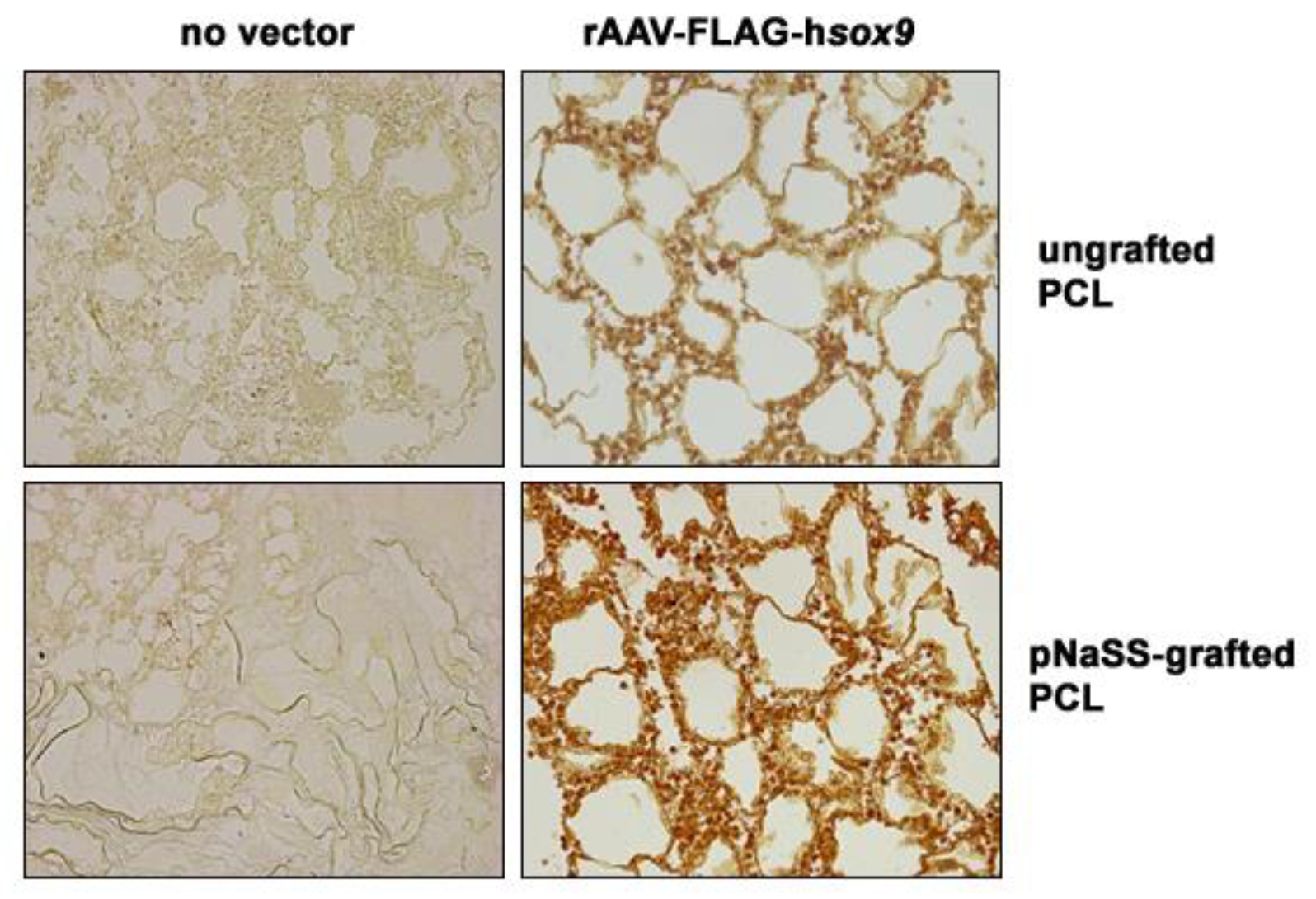
3.1. sox9/pNaSS-Grafted PCL Films Promote the Effective Overexpression of SOX9 in Human Bone Marrow Aspirates
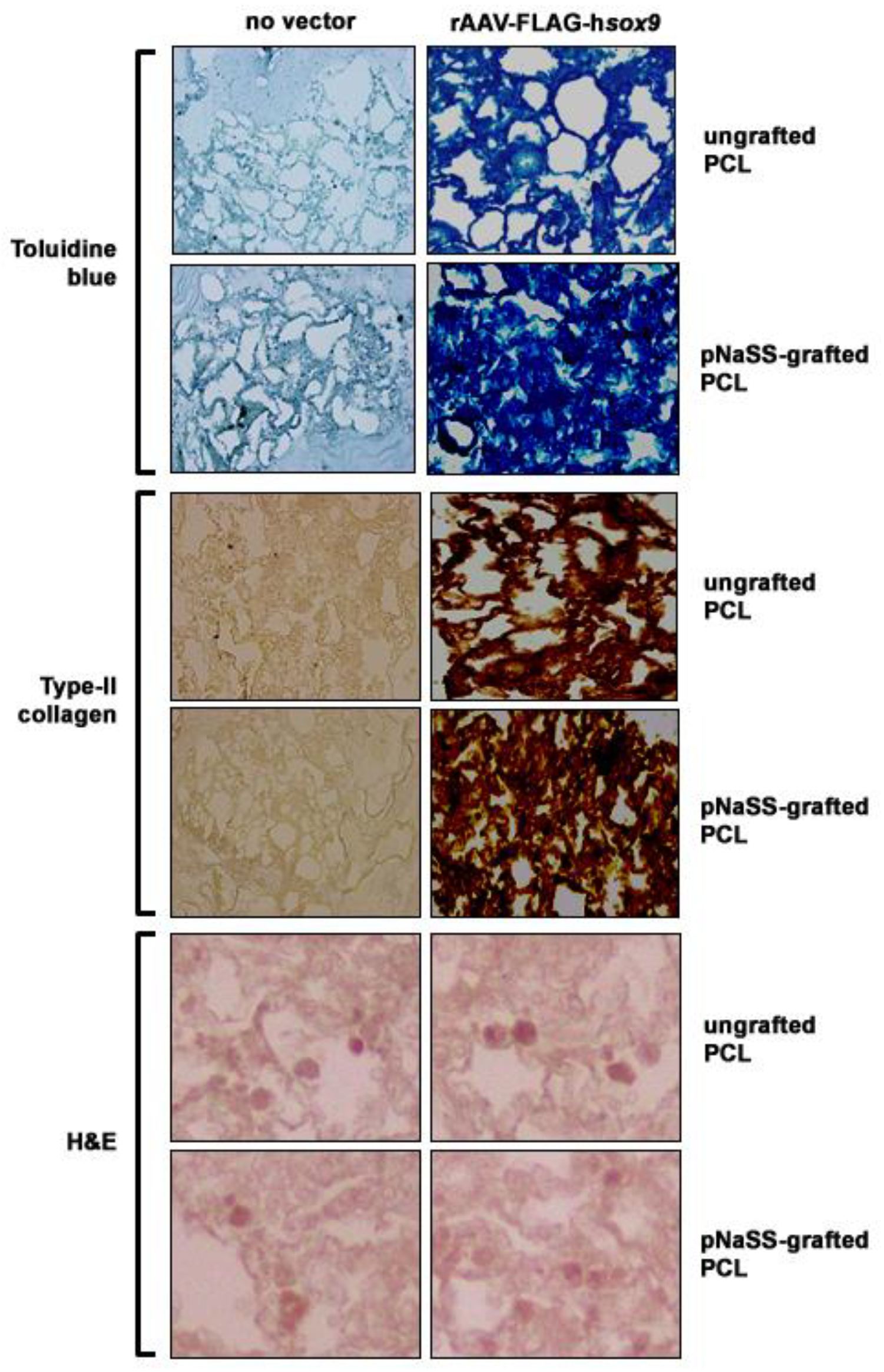
3.2. sox9/pNaSS-Grafted PCL Films Induce Proteoglycan and Type-II Collagen Deposition in Human Bone Marrow Aspirates
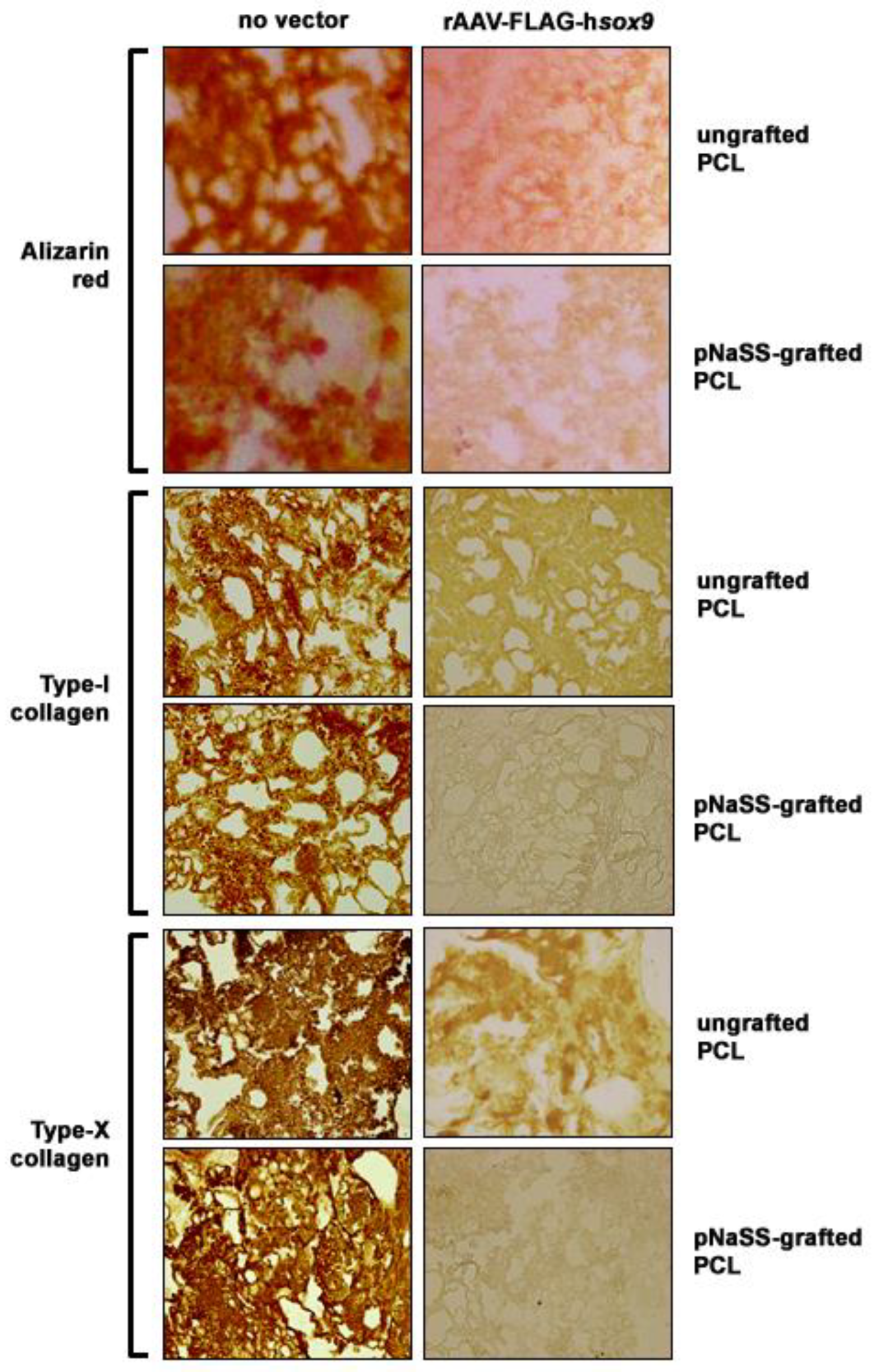
3.3. sox9/pNaSS-Grafted PCL Films Reduce Mineralization and Type-I and -X Collagen Deposition in Human Bone Marrow Aspirates
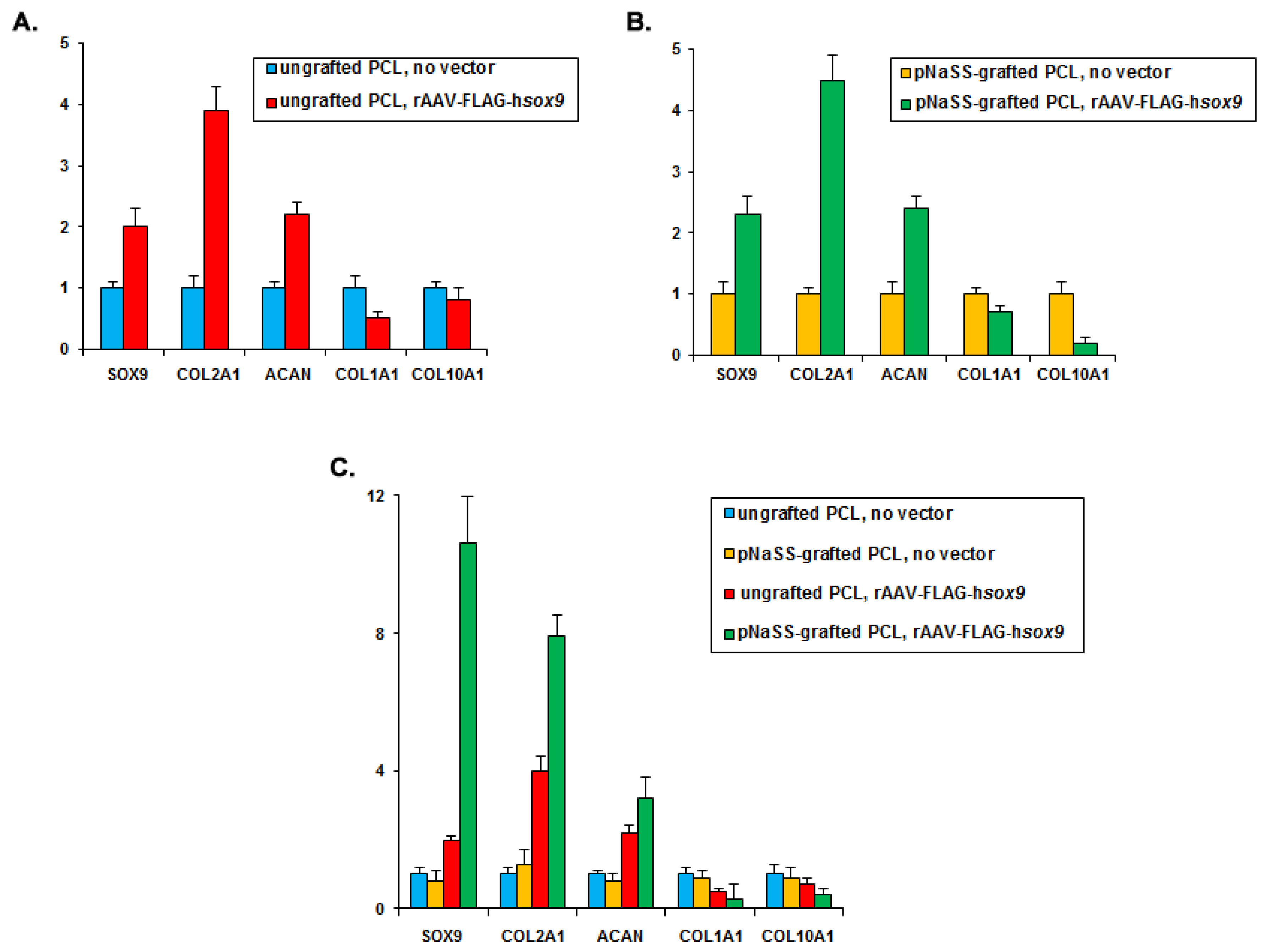
3.4. sox9/pNaSS-Grafted PCL Films Activate the Chondrogenic Expression Profiles and Reduce Osteogenic and Hypertrophic Expression in Human Bone Marrow Aspirates
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Buckwalter, J.A. Articular cartilage: Injuries and potential for healing. J. Orthop. Sports Phys. Ther. 1998, 28, 192–202. [Google Scholar] [CrossRef]
- O’Driscoll, S.W. The healing and regeneration of articular cartilage. J. Bone Joint Surg. Am. 1998, 80, 1795–1812. [Google Scholar] [CrossRef] [PubMed]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Madry, H.; Grün, U.W.; Knutsen, G. Cartilage repair and joint preservation: Medical and surgical treatment options. Dtsch. Arztebl. Int. 2011, 108, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, B.; Hering, T.M.; Caplan, A.I.; Goldberg, V.M.; You, J.U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp. Cell Res. 1998, 238, 265–272. [Google Scholar] [CrossRef]
- Mackay, A.M.; Beck, S.C.; Murphy, J.M.; Barry, F.P.; Chichester, C.O.; Pittenger, M.F. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998, 4, 415–428. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Slynarski, K.; Deszczynski, J.; Karpinski, J. Fresh bone marrow and periosteum transplantation for cartilage defects of the knee. Transplant. Proc. 2006, 38, 318–319. [Google Scholar] [CrossRef]
- Gigante, A.; Cecconi, S.; Calcagno, S.; Busilacchi, A.; Enea, A. Arthroscopic knee cartilage repair with covered microfracture and bone marrow concentrate. Arthrosc. Tech. 2012, 1, e175–e180. [Google Scholar] [CrossRef]
- Cucchiarini, M. Human gene therapy: Novel approaches to improve the current gene delivery systems. Discov. Med. 2016, 21, 495–506. [Google Scholar]
- Cucchiarini, M.; Madry, H. Biomaterial-guided delivery of gene vectors for targeted articular cartilage repair. Nat. Rev. Rheumatol. 2019, 15, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.C.; Raftery, R.M.; Curtin, C.M.; O’Driscoll, C.M.; O’Brien, F.J. Scaffold-based delivery of nucleic acid therapeutics for enhanced bone and cartilage repair. J. Orthop. Res. 2019, 37, 1671–1680. [Google Scholar] [CrossRef] [PubMed]
- Diao, H.; Wang, J.; Shen, C.; Xia, S.; Guo, T.; Dong, L.; Zhang, C.; Chen, J.; Zhao, J.; Zhang, J. Improved cartilage regeneration utilizing mesenchymal stem cells in TGF-beta1 gene-activated scaffolds. Tissue Eng. Part A 2009, 15, 2687–2698. [Google Scholar] [CrossRef] [PubMed]
- Im, G.I.; Kim, H.J.; Lee, J.H. Chondrogenesis of adipose stem cells in a porous PLGA scaffold impregnated with plasmid DNA containing SOX trio (SOX-5,-6 and -9) genes. Biomaterials 2011, 32, 4385–4392. [Google Scholar] [CrossRef]
- Li, B.; Yang, J.; Ma, L.; Li, F.; Tu, Z.; Gao, C. Fabrication of poly(lactide-co-glycolide) scaffold filled with fibrin gel, mesenchymal stem cells, and poly(ethylene oxide)-b-poly(L-lysine)/TGF-β1 plasmid DNA complexes for cartilage restoration in vivo. J. Biomed. Mater. Res. A 2013, 101, 3097–31008. [Google Scholar] [CrossRef]
- Needham, C.J.; Shah, S.R.; Dahlin, R.L.; Kinard, L.A.; Lam, J.; Watson, B.M.; Lu, S.; Kasper, F.K.; Mikos, A.G. Osteochondral tissue regeneration through polymeric delivery of DNA encoding for the SOX trio and RUNX2. Acta Biomater. 2014, 10, 4103–4112. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, T.; Tierney, E.G.; Cunniffe, G.M.; O’Brien, F.J.; Kelly, D.J. Gene delivery of TGF-β3 and BMP2 in an MSC-laden alginate hydrogel for articular cartilage and endochondral bone tissue engineering. Tissue Eng. Part A 2016, 22, 776–787. [Google Scholar] [CrossRef]
- Lee, Y.H.; Wu, H.C.; Yeh, C.W.; Kuan, C.H.; Liao, H.T.; Hsu, H.C.; Tsai, J.C.; Sun, J.S.; Wang, T.W. Enzyme-crosslinked gene-activated matrix for the induction of mesenchymal stem cells in osteochondral tissue regeneration. Acta Biomater. 2017, 63, 210–226. [Google Scholar] [CrossRef]
- Park, J.S.; Yi, S.W.; Kim, H.J.; Kim, S.M.; Kim, J.H.; Park, K.H. Construction of PLGA nanoparticles coated with polycistronic SOX5, SOX6, and SOX9 genes for chondrogenesis of human mesenchymal stem cells. ACS Appl. Mater. Interfaces 2017, 9, 1361–1372. [Google Scholar] [CrossRef]
- Brunger, J.M.; Huynh, N.P.; Guenther, C.M.; Parez-Pinera, P.; Moutos, F.T.; Sanchez-Adams, J.; Gersbach, C.A.; Guilak, F. Scaffold-mediated lentiviral transduction for functional tissue engineering of cartilage. Proc. Natl. Acad. Sci. USA 2014, 111, E798–E806. [Google Scholar] [CrossRef]
- Glass, K.A.; Link, J.M.; Brunger, J.M.; Moutos, F.T.; Gersbach, C.A.; Guilak, F. Tissue-engineered cartilage with inducible and tunable immunomodulatory properties. Biomaterials 2014, 35, 5921–5931. [Google Scholar] [CrossRef] [PubMed]
- Moutos, F.T.; Glass, K.A.; Compton, S.A.; Ross, A.K.; Gersbach, C.A.; Guilak, F.; Estes, B.T. Anatomically shaped tissue-engineered cartilage with tunable and inducible anticytokine delivery for biological joint resurfacing. Proc. Natl. Acad. Sci. USA 2016, 113, E4513–E4522. [Google Scholar] [CrossRef] [PubMed]
- Rowland, C.R.; Glass, K.A.; Ettyreddy, A.R.; Gloss, C.C.; Matthews, J.R.L.; Huynh, N.P.T.; Guilak, F. Regulation of decellularized tissue remodeling via scaffold-mediated lentiviral delivery in anatomically-shaped osteochondral constructs. Biomaterials 2018, 177, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Cottard, V.; Valvason, C.; Falgarone, G.; Lutomski, D.; Boissier, M.C.; Bessis, N. Immune response against gene therapy vectors: Influence of synovial fluid on adeno-associated virus mediated gene transfer to chondrocytes. J. Clin. Immunol. 2004, 24, 162–169. [Google Scholar] [CrossRef]
- Lee, H.H.; Haleem, A.M.; Yao, V.; Li, J.; Xiao, X.; Chu, C.R. Release of bioactive adeno-associated virus from fibrin scaffolds: Effects of fibrin glue concentrations. Tissue Eng. Part A 2011, 17, 1969–1978. [Google Scholar] [CrossRef]
- Díaz-Rodríguez, P.; Rey-Rico, A.; Madry, H.; Landin, M.; Cucchiarini, M. Effective genetic modification and differentiation of hMSCs upon controlled release of rAAV vectors using alginate/poloxamer composite systems. Int. J. Pharm. 2015, 496, 614–626. [Google Scholar] [CrossRef]
- Rey-Rico, A.; Venkatesan, J.K.; Frisch, J.; Rial-Hermida, I.; Schmitt, G.; Concheiro, A.; Madry, H.; Alvarez-Lorenzo, C.; Cucchiarini, M. PEO-PPO-PEO micelles as effective rAAV-mediated gene delivery systems to target human mesenchymal stem cells without altering their differentiation potency. Acta Biomater. 2015, 27, 42–50. [Google Scholar] [CrossRef]
- Rey-Rico, A.; Venkatesan, J.K.; Frisch, J.; Schmitt, G.; Monge-Marcet, A.; Lopez-Chicon, P.; Mata, A.; Semino, C.; Madry, H.; Cucchiarini, M. Effective and durable genetic modification of human mesenchymal stem cells via controlled release of rAAV vectors from self-assembling peptide hydrogels with a maintained differentiation potency. Acta Biomater. 2015, 18, 118–127. [Google Scholar] [CrossRef]
- Rey-Rico, A.; Frisch, J.; Venkatesan, J.K.; Schmitt, G.; Rial-Hermida, I.; Taboada, P.; Concheiro, A.; Madry, H.; Alvarez-Lorenzo, C.; Cucchiarini, M. PEO-PPO-PEO carriers for rAAV-mediated transduction of human articular chondrocytes in vitro and in a human osteochondral defect model. ACS Appl. Mater. Interfaces 2016, 8, 20600–20613. [Google Scholar] [CrossRef]
- Rey-Rico, A.; Babicz, H.; Madry, H.; Concheiro, A.; Alvarez-Lorenzo, C.; Cucchiarini, M. Supramolecular polypseudorotaxane gels for controlled delivery of rAAV vectors in human mesenchymal stem cells for regenerative medicine. Int. J. Pharm. 2017, 531, 492–503. [Google Scholar] [CrossRef]
- Rey-Rico, A.; Venkatesan, J.K.; Schmitt, G.; Concheiro, A.; Madry, H.; Alvarez-Lorenzo, C.; Cucchairini, M. rAAV-mediated overexpression of TGF-β via vector delivery in polymeric micelles stimulates the biological and reparative activities of human articular chondrocytes in vitro and in a human osteochondral defect model. Int. J. Nanomed. 2017, 12, 6985–6996. [Google Scholar] [CrossRef] [PubMed]
- Rey-Rico, A.; Venkatesan, J.K.; Schmitt, G.; Speicher-Mentges, S.; Madry, H.; Cucchiarini, M. Effective remodelling of human osteoarthritic cartilage by sox9 gene transfer and overexpression upon delivery of rAAV vectors in polymeric micelles. Mol. Pharm. 2018, 15, 2816–2826. [Google Scholar] [CrossRef] [PubMed]
- Madry, H.; Gao, L.; Rey-Rico, A.; Venkatesan, J.K.; Müller-Brandt, K.; Cai, X.; Goebel, L.; Schmitt, G.; Speicher-Mentges, S.; Zurakowski, D.; et al. Thermosensitive hydrogel based on PEO-PPO-PEO poloxamers for a controlled in situ release of recombinant adeno-associated viral vectors for effective gene therapy of cartilage defects. Adv. Mater. 2020, 32, 1906508. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.K.; Falentin-Daudré, C.; Leroux, A.; Migonney, V.; Cucchiarini, M. Controlled release of gene therapy constructs from solid scaffolds for therapeutic applications in orthopedics. Discov. Med. 2018, 25, 195–203. [Google Scholar]
- Moutos, F.T.; Guilak, F. Functional properties of cell-seeded three-dimensionally woven poly(epsilon-caprolactone) scaffolds for cartilage tissue engineering. Tissue Eng. Part A 2010, 16, 1291–1301. [Google Scholar] [CrossRef]
- Dash, T.K.; Konkimalla, V.B. Poly-ε-caprolactone based formulations for drug delivery and tissue engineering: A review. J. Control. Release 2012, 158, 15–33. [Google Scholar] [CrossRef]
- Li, Z.; Tan, B.H. Towards the development of polycaprolactone based amphiphilic block copolymers: Molecular design, self-assembly and biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 45, 620–634. [Google Scholar] [CrossRef]
- Rohman, G.; Huot, S.; Vilas-Boas, M.; Radu-Bostan, G.; Castner, D.G.; Migonney, V. The grafting of a thin layer of poly(sodium styrene sulfonate) onto poly(ε-caprolactone) surface can enhance fibroblast behavior. J. Mater. Sci. Mater. Med. 2015, 26, 206–215. [Google Scholar] [CrossRef][Green Version]
- Venkatesan, J.K.; Falentin-Daudré, C.; Leroux, A.; Migonney, V.; Cucchiarini, M. Biomaterial-guided recombinant adeno-associated virus delivery from poly(sodium styrene sulfonate)-grafted poly(ε-caprolactone) films to target human bone marrow aspirates. Tissue Eng. Part A 2019. [Google Scholar] [CrossRef]
- Bi, W.; Deng, J.M.; Zhang, Z.; Behringer, R.R.; de Crombrugghe, B. Sox9 is required for cartilage formation. Nat. Genet. 1999, 22, 85–89. [Google Scholar] [CrossRef]
- Cucchiarini, M.; Thurn, T.; Weimer, A.; Kohn, D.; Terwilliger, E.F.; Madry, H. Restoration of the extracellular matrix in human osteoarthritic articular cartilage by overexpression of the transcription factor SOX9. Arthritis Rheum. 2007, 56, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Rey-Rico, A.; Frisch, J.; Venkatesan, J.K.; Schmitt, G.; Madry, H.; Cucchiarini, M. Determination of effective rAAV-mediated gene transfer conditions to support chondrogenic differentiation processes in human primary bone marrow aspirates. Gene Ther. 2015, 22, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.K.; Moutos, F.T.; Rey-Rico, A.; Estes, B.T.; Frisch, J.; Schmitt, G.; Madry, H.; Guilak, F.; Cucchiarini, M. Chondrogenic differentiation processes in human bone-marrow aspirates seeded in three-dimensional-woven poly(ε-caprolactone) scaffolds enhanced by recombinant adeno-associated virus-mediated SOX9 gene transfer. Hum. Gene Ther. 2018, 29, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, V. Roles and regulation of SOX transcription factors in skeletogenesis. Curr. Top. Dev. Biol. 2019, 133, 171–193. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Chaboissier, M.C.; Martin, J.F.; Schedl, A.; de Crombrugghe, B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002, 16, 2813–2828. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.M.; Leung, K.K.; Wheatly, S.C.; Ng, L.J.; Zhou, S.; Ling, K.W.; Sham, M.H.; Koopman, P.; Tam, P.P.; Cheah, K.S. SOX9 directly regulates the type-II collagen gene. Nat. Genet. 1997, 16, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, I.; Tsuji, K.; Koopman, P.; Watanabe, H.; Yamada, Y.; Shinomiya, K.; Nifuji, A.; Noda, M. SOX9 enhances aggrecan gene promoter/enhancer activity and is up-regulated by retinoic acid in a cartilage-derived cell line, TC6. J. Biol. Chem. 2000, 275, 10738–10744. [Google Scholar] [CrossRef]
- Samulski, R.J.; Chang, L.S.; Shenk, T. A recombinant plasmid from which an infectious adeno-associated virus genome can be excised in vitro and its use to study viral replication. J. Virol. 1987, 61, 3096–3101. [Google Scholar] [CrossRef]
- Samulski, R.J.; Chang, L.S.; Shenk, T. Helper-free stocks of recombinant adeno-associated viruses: Normal integration does not require viral gene expression. J. Virol. 1989, 63, 3822–3828. [Google Scholar] [CrossRef]
- Akiyama, H.; Lyons, J.P.; Mori-Akiyama, Y.; Yang, X.; Zhang, R.; Zhang, Z.; Deng, J.M.; Taketo, M.M.; Nakamura, T.; Behringer, R.R.; et al. Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev. 2004, 18, 1072–1087. [Google Scholar] [CrossRef]
- Cucchiarini, M.; Orth, P.; Madry, H. Direct rAAV SOX9 administration for durable articular cartilage repair with delayed terminal differentiation and hypertrophy in vivo. J. Mol. Med. 2013, 91, 625–636. [Google Scholar] [CrossRef] [PubMed]
| Parameters | No Vector/Ungrafted PCL | No Vector/pNaSS-grafted PCL | sox9/Ungrafted PCL | sox9/pNaSS-Grafted PCL |
|---|---|---|---|---|
| SOX9 | 5.6 ± 2.7 | 6.1 ± 2.9 | 78.6 ± 2.4 a,b | 94.4 ± 2.2 a,b,c |
| Toluidine blue | 1.4 ± 0.5 | 1.6 ± 0.5 | 3.3 ± 0.5 a,b | 3.9 ± 0.4 a,b,c |
| Proteoglycans | 0.040 ± 0.002 | 0.039 ± 0.002 | 0.070 ± 0.003 a,b | 0.083 ± 0.001 a,b,c |
| Type-II collagen | 0.3 ± 0.5 | 0.4 ± 0.5 | 3.4 ± 0.5 a,b | 3.9 ± 0.4 a,b,c |
| Cell densities | 2481 ± 57 | 2523 ± 72 | 2520 ± 53 | 2515 ± 58 |
| WST-1 | 0.51 ± 0.07 | 0.63 ± 0.12 | 0.61 ± 0.10 | 0.70 ± 0.07 |
| Alizarin red | 3.7 ± 0.5 | 3.9 ± 0.4 | 1.9 ± 0.4 a,b | 0.9 ± 0.4 a,b,c |
| Type-I collagen | 3.7 ± 0.5 | 3.7 ± 0.5 | 1.3 ± 0.5 a,b | 0.7 ± 0.5 a,b,c |
| Type-X collagen | 3.9 ± 0.4 | 3.9 ± 0.4 | 1.3 ± 0.8 a,b | 0.6 ± 0.5 a,b,c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkatesan, J.K.; Meng, W.; Rey-Rico, A.; Schmitt, G.; Speicher-Mentges, S.; Falentin-Daudré, C.; Leroux, A.; Madry, H.; Migonney, V.; Cucchiarini, M. Enhanced Chondrogenic Differentiation Activities in Human Bone Marrow Aspirates via sox9 Overexpression Mediated by pNaSS-Grafted PCL Film-Guided rAAV Gene Transfer. Pharmaceutics 2020, 12, 280. https://doi.org/10.3390/pharmaceutics12030280
Venkatesan JK, Meng W, Rey-Rico A, Schmitt G, Speicher-Mentges S, Falentin-Daudré C, Leroux A, Madry H, Migonney V, Cucchiarini M. Enhanced Chondrogenic Differentiation Activities in Human Bone Marrow Aspirates via sox9 Overexpression Mediated by pNaSS-Grafted PCL Film-Guided rAAV Gene Transfer. Pharmaceutics. 2020; 12(3):280. https://doi.org/10.3390/pharmaceutics12030280
Chicago/Turabian StyleVenkatesan, Jagadeesh K., Weikun Meng, Ana Rey-Rico, Gertrud Schmitt, Susanne Speicher-Mentges, Céline Falentin-Daudré, Amélie Leroux, Henning Madry, Véronique Migonney, and Magali Cucchiarini. 2020. "Enhanced Chondrogenic Differentiation Activities in Human Bone Marrow Aspirates via sox9 Overexpression Mediated by pNaSS-Grafted PCL Film-Guided rAAV Gene Transfer" Pharmaceutics 12, no. 3: 280. https://doi.org/10.3390/pharmaceutics12030280
APA StyleVenkatesan, J. K., Meng, W., Rey-Rico, A., Schmitt, G., Speicher-Mentges, S., Falentin-Daudré, C., Leroux, A., Madry, H., Migonney, V., & Cucchiarini, M. (2020). Enhanced Chondrogenic Differentiation Activities in Human Bone Marrow Aspirates via sox9 Overexpression Mediated by pNaSS-Grafted PCL Film-Guided rAAV Gene Transfer. Pharmaceutics, 12(3), 280. https://doi.org/10.3390/pharmaceutics12030280