Blood Eosinophilia Is an on-Treatment Biomarker in Patients with Solid Tumors Undergoing Dendritic Cell Vaccination with Autologous Tumor-RNA
Abstract
1. Background
2. Methods
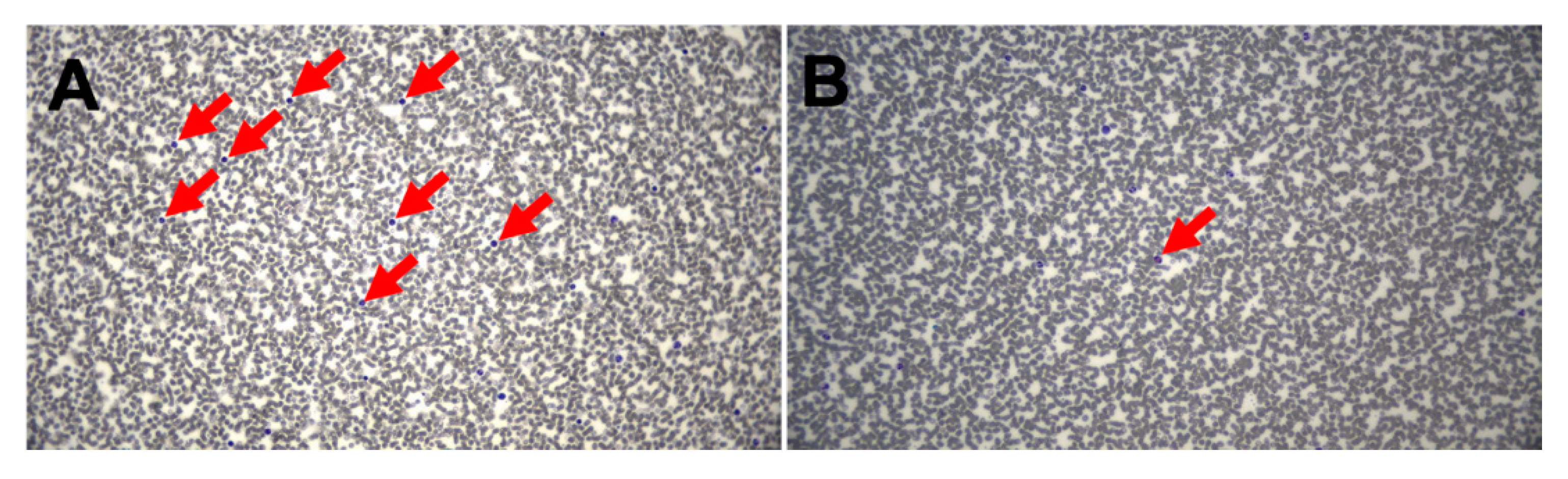
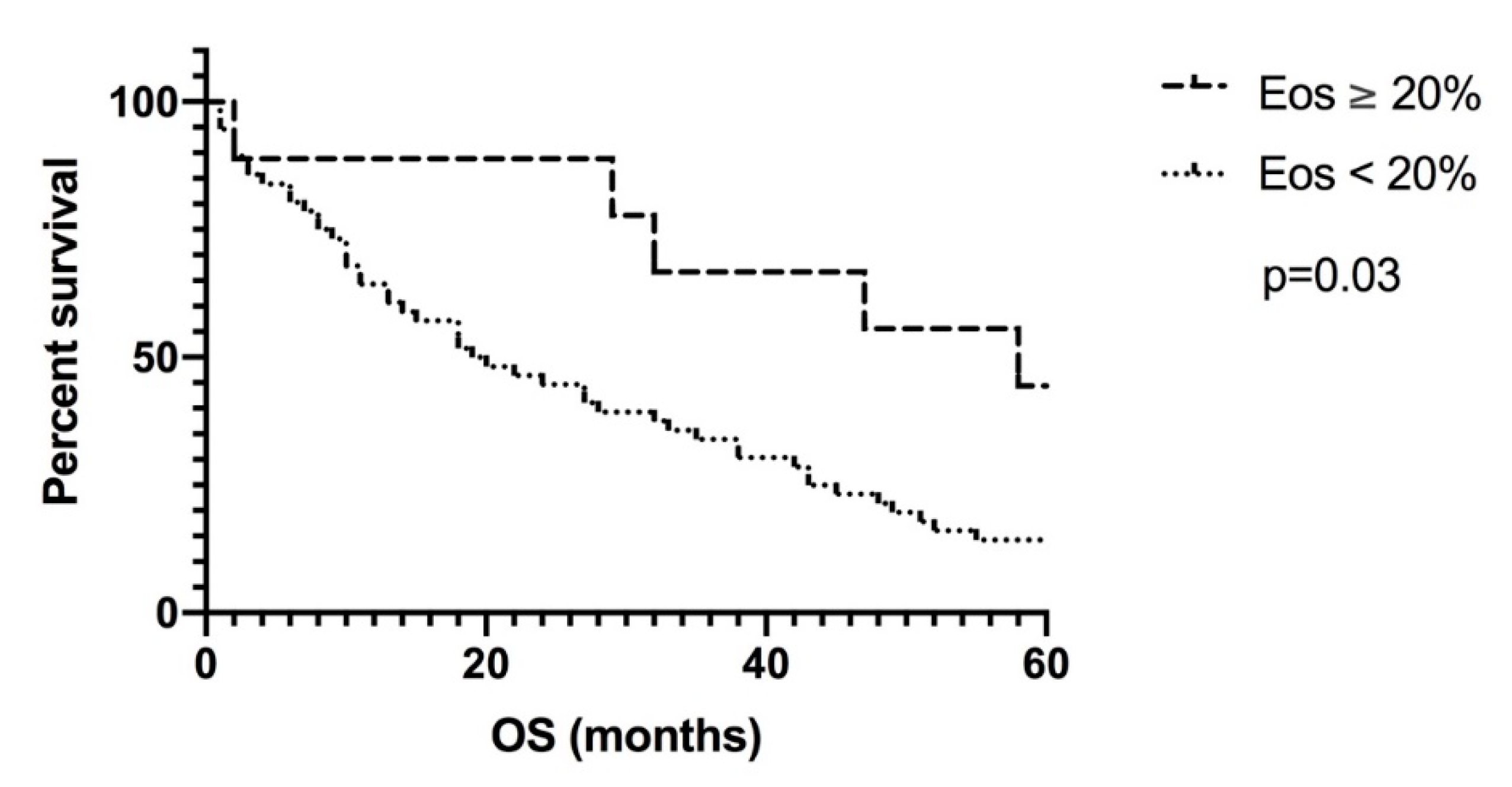
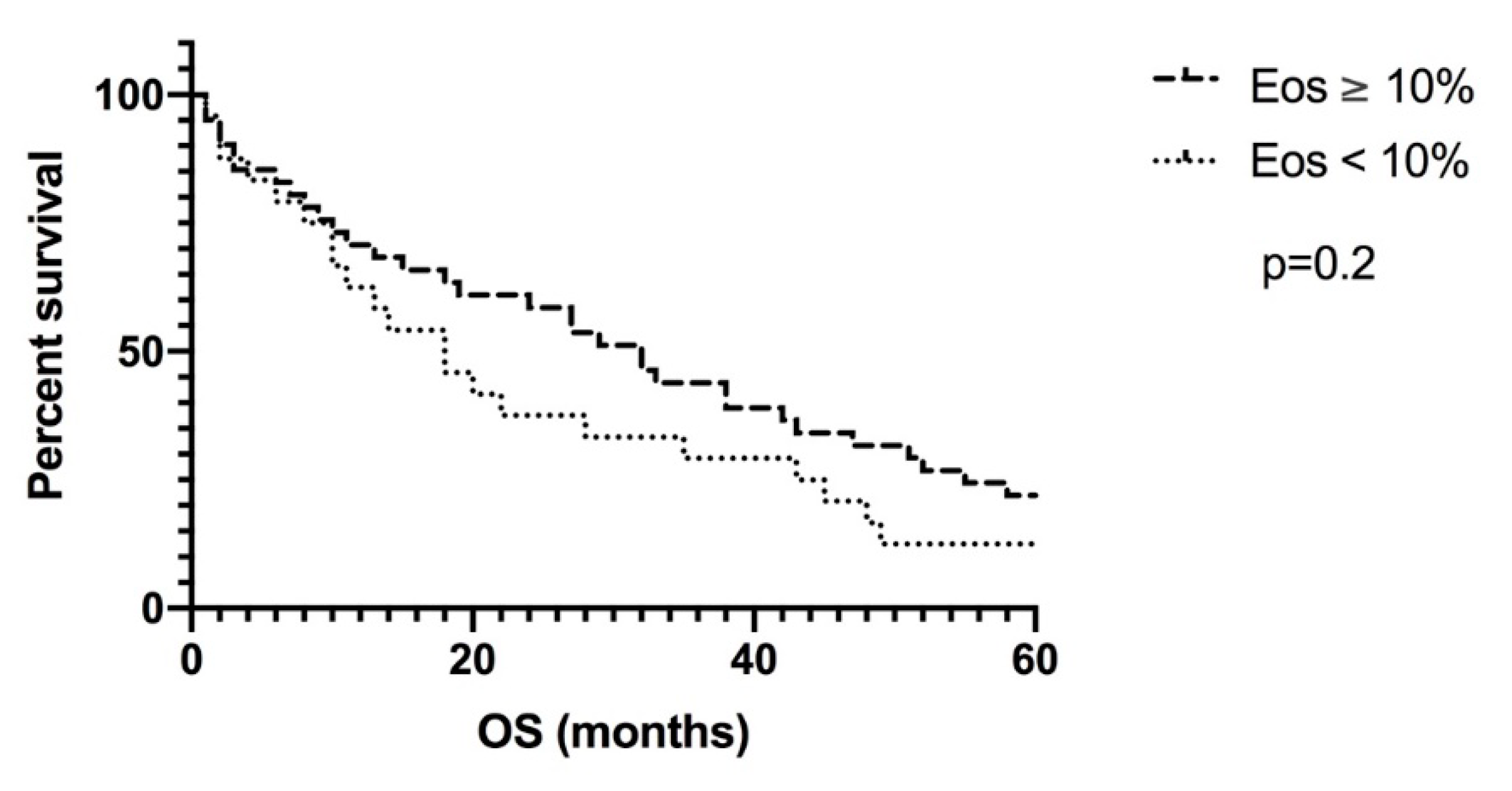
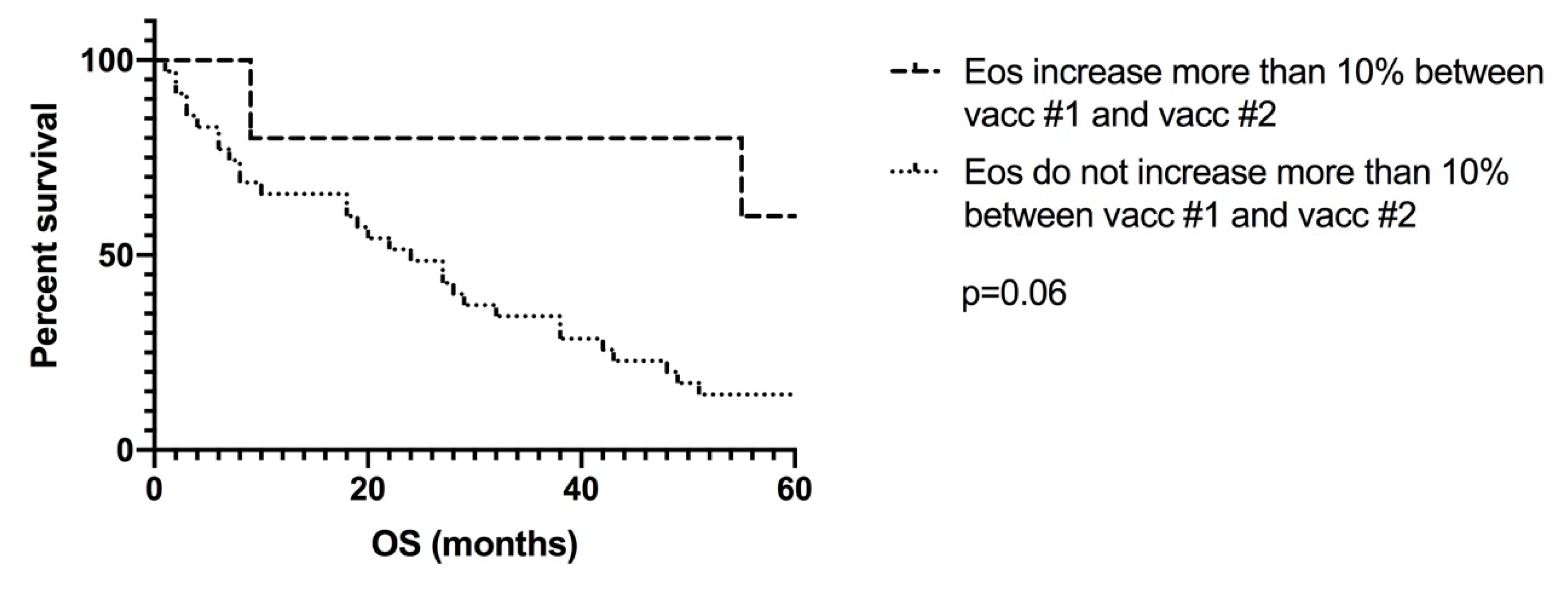
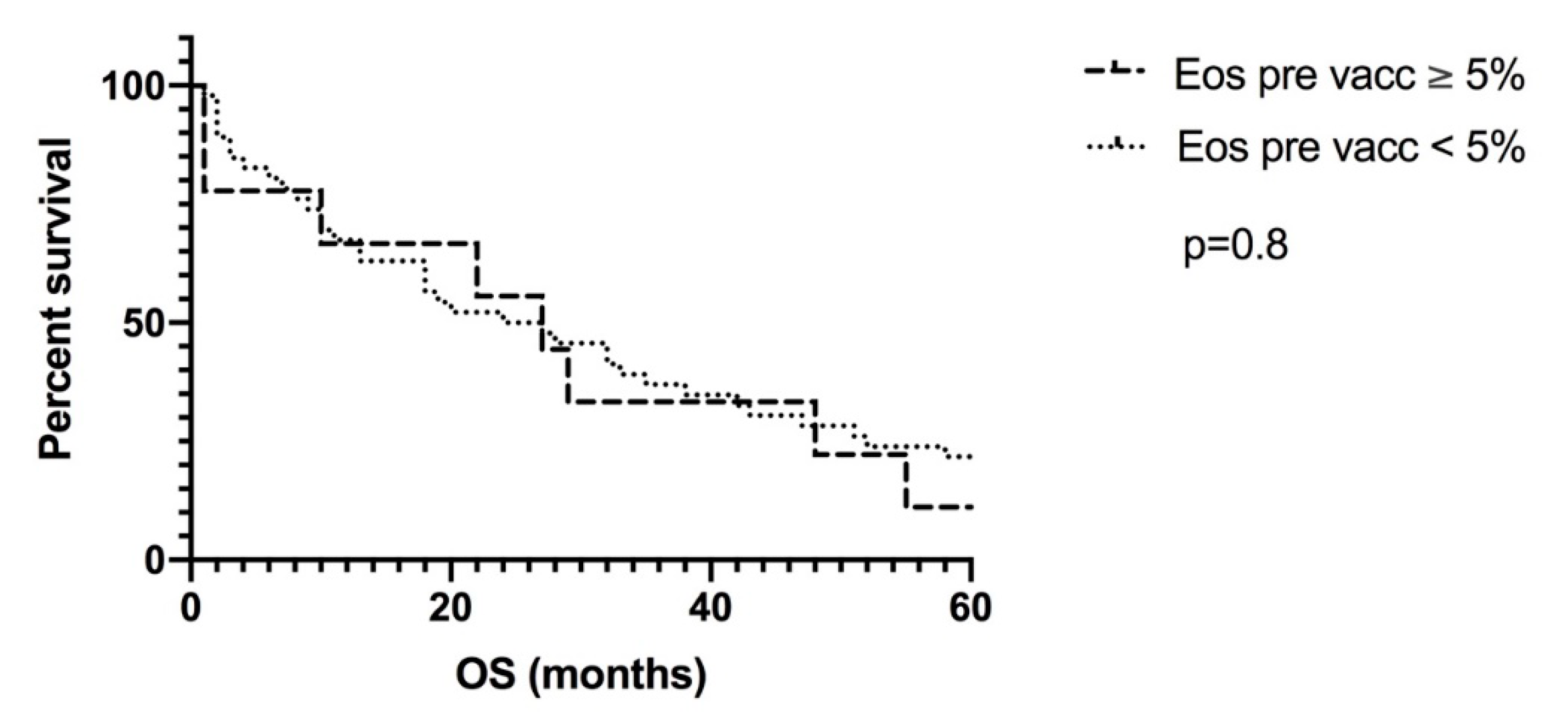
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saxena, M.; Bhardwaj, N. Re-Emergence of Dendritic Cell Vaccines for Cancer Treatment. Trends Cancer 2018, 4, 119–137. [Google Scholar] [CrossRef]
- Uslu, U.; Erdmann, M.; Wiesinger, M.; Schuler, G.; Schuler-Thurner, B. Automated Good Manufacturing Practice-compliant generation of human monocyte-derived dendritic cells from a complete apheresis product using a hollow-fiber bioreactor system overcomes a major hurdle in the manufacture of dendritic cells for cancer vaccines. Cytotherapy 2019, 21, 1166–1178. [Google Scholar] [CrossRef] [PubMed]
- Huber, A.; Dammeijer, F.; Aerts, J.; Vroman, H. Current State of Dendritic Cell-Based Immunotherapy: Opportunities for in vitro Antigen Loading of Different DC Subsets? Front. Immunol. 2018, 9, 2804. [Google Scholar] [CrossRef]
- Saxena, M.; Balan, S.; Roudko, V.; Bhardwaj, N. Towards superior dendritic-cell vaccines for cancer therapy. Nat. Biomed. Eng. 2018, 2, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Belderbos, R.A.; Aerts, J.; Vroman, H. Enhancing Dendritic Cell Therapy in Solid Tumors with Immunomodulating Conventional Treatment. Mol. Ther. Oncolytics 2019, 13, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Balan, S.; Finnigan, J.; Bhardwaj, N. Dendritic Cell Strategies for Eliciting Mutation-Derived Tumor Antigen Responses in Patients. Cancer J. 2017, 23, 131–137. [Google Scholar] [CrossRef]
- Galati, D.; Zanotta, S. Empowering dendritic cell cancer vaccination: The role of combinatorial strategies. Cytotherapy 2018, 20, 1309–1323. [Google Scholar] [CrossRef]
- Schuler, G.; Steinman, R.M. Dendritic cells as adjuvants for immune-mediated resistance to tumors. J. Exp. Med. 1997, 186, 1183–1187. [Google Scholar] [CrossRef]
- Hildner, K.; Edelson, B.T.; Purtha, W.E.; Diamond, M.; Matsushita, H.; Kohyama, M.; Calderon, B.; Schraml, B.U.; Unanue, E.R.; Diamond, M.S.; et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science 2008, 322, 1097–1100. [Google Scholar] [CrossRef]
- Erdmann, M.; Uslu, U.; Wiesinger, M.; Bruning, M.; Altmann, T.; Strasser, E.; Schuler, G.; Schuler-Thurner, B. Automated closed-system manufacturing of human monocyte-derived dendritic cells for cancer immunotherapy. J. Immunol. Methods 2018, 463, 89–96. [Google Scholar] [CrossRef]
- Schuler-Thurner, B.; Bartz-Schmidt, K.U.; Bornfeld, N.; Cursiefen, C.; Fuisting, B.; Grisanti, S.; Heindl, L.M.; Holbach, L.; Keseru, M.; Knorr, H.; et al. Immunotherapy of uveal melanoma: Vaccination against cancer. Multicenter adjuvant phase 3 vaccination study using dendritic cells laden with tumor RNA for large newly diagnosed uveal melanoma. Der Ophthalmol. 2015, 112, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Kyi, C.; Roudko, V.; Sabado, R.; Saenger, Y.; Loging, W.; Mandeli, J.; Thin, T.H.; Lehrer, D.; Donovan, M.; Posner, M.; et al. Therapeutic Immune Modulation against Solid Cancers with Intratumoral Poly-ICLC: A Pilot Trial. Clin. Cancer Res. 2018, 24, 4937–4948. [Google Scholar] [CrossRef] [PubMed]
- Cancel, J.C.; Crozat, K.; Dalod, M.; Mattiuz, R. Are Conventional Type 1 Dendritic Cells Critical for Protective Antitumor Immunity and How? Front. Immunol. 2019, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Binnewies, M.; Mujal, A.M.; Pollack, J.L.; Combes, A.J.; Hardison, E.A.; Barry, K.C.; Tsui, J.; Ruhland, M.K.; Kersten, K.; Abushawish, M.A.; et al. Unleashing Type-2 Dendritic Cells to Drive Protective Antitumor CD4(+) T Cell Immunity. Cell 2019, 177, 556–571. [Google Scholar] [CrossRef] [PubMed]
- Sprooten, J.; Ceusters, J.; Coosemans, A.; Agostinis, P.; De Vleeschouwer, S.; Zitvogel, L.; Kroemer, G.; Galluzzi, L.; Garg, A.D. Trial watch: Dendritic cell vaccination for cancer immunotherapy. Oncoimmunology 2019, 8, e1638212. [Google Scholar] [CrossRef]
- Tanyi, J.L.; Bobisse, S.; Ophir, E.; Tuyaerts, S.; Roberti, A.; Genolet, R.; Baumgartner, P.; Stevenson, B.J.; Iseli, C.; Dangaj, D.; et al. Personalized cancer vaccine effectively mobilizes antitumor T cell immunity in ovarian cancer. Sci. Transl. Med. 2018, 10, eaao5931. [Google Scholar] [CrossRef]
- Wilgenhof, S.; Corthals, J.; Heirman, C.; van Baren, N.; Lucas, S.; Kvistborg, P.; Thielemans, K.; Neyns, B. Phase II Study of Autologous Monocyte-Derived mRNA Electroporated Dendritic Cells (TriMixDC-MEL) Plus Ipilimumab in Patients With Pretreated Advanced Melanoma. J. Clin. Oncol. 2016, 34, 1330–1338. [Google Scholar] [CrossRef]
- Carreno, B.M.; Magrini, V.; Becker-Hapak, M.; Kaabinejadian, S.; Hundal, J.; Petti, A.A.; Ly, A.; Lie, W.R.; Hildebrand, W.H.; Mardis, E.R.; et al. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science 2015, 348, 803–808. [Google Scholar] [CrossRef]
- McNeel, D.G.; Gardner, T.A.; Higano, C.S.; Kantoff, P.W.; Small, E.J.; Wener, M.H.; Sims, R.B.; DeVries, T.; Sheikh, N.A.; Dreicer, R. A transient increase in eosinophils is associated with prolonged survival in men with metastatic castration-resistant prostate cancer who receive sipuleucel-T. Cancer Immunol. Res. 2014, 2, 988–999. [Google Scholar] [CrossRef]
- Di Lorenzo, G.; Ferro, M.; Buonerba, C. Sipuleucel-T (Provenge(R)) for castration-resistant prostate cancer. BJU Int. 2012, 110, E99–E104. [Google Scholar] [CrossRef]
- Buschow, S.I.; Ramazzotti, M.; Reinieren-Beeren, I.M.J.; Heinzerling, L.M.; Westdorp, H.; Stefanini, I.; Beltrame, L.; Hato, S.V.; Ellebaek, E.; Gross, S.; et al. Survival of metastatic melanoma patients after dendritic cell vaccination correlates with expression of leukocyte phosphatidylethanolamine-binding protein 1/Raf kinase inhibitory protein. Oncotarget 2017, 8, 67439–67456. [Google Scholar] [CrossRef] [PubMed]
- Delyon, J.; Mateus, C.; Lefeuvre, D.; Lanoy, E.; Zitvogel, L.; Chaput, N.; Roy, S.; Eggermont, A.M.; Routier, E.; Robert, C. Experience in daily practice with ipilimumab for the treatment of patients with metastatic melanoma: An early increase in lymphocyte and eosinophil counts is associated with improved survival. Ann. Oncol. 2013, 24, 1697–1703. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, C.; Sevko, A.; Jiang, H.; Lichtenberger, R.; Reith, M.; Tarnanidis, K.; Holland-Letz, T.; Umansky, L.; Beckhove, P.; Sucker, A.; et al. Myeloid Cells and Related Chronic Inflammatory Factors as Novel Predictive Markers in Melanoma Treatment with Ipilimumab. Clin. Cancer Res. 2015, 21, 5453–5459. [Google Scholar] [CrossRef] [PubMed]
- Martens, A.; Wistuba-Hamprecht, K.; Geukes Foppen, M.; Yuan, J.; Postow, M.A.; Wong, P.; Romano, E.; Khammari, A.; Dreno, B.; Capone, M.; et al. Baseline Peripheral Blood Biomarkers Associated with Clinical Outcome of Advanced Melanoma Patients Treated with Ipilimumab. Clin. Cancer Res. 2016, 22, 2908–2918. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.; Leisgang, W.; Schuler, G.; Heinzerling, L. Eosinophilic count as a biomarker for prognosis of melanoma patients and its importance in the response to immunotherapy. Immunotherapy 2017, 9, 115–121. [Google Scholar] [CrossRef]
- Blanchard, C.; Rothenberg, M.E. Biology of the eosinophil. Adv. Immunol. 2009, 101, 81–121. [Google Scholar]
- Cormier, S.A.; Taranova, A.G.; Bedient, C.; Nguyen, T.; Protheroe, C.; Pero, R.; Dimina, D.; Ochkur, S.I.; O’Neill, K.; Colbert, D.; et al. Pivotal Advance: Eosinophil infiltration of solid tumors is an early and persistent inflammatory host response. J. Leukoc. Biol. 2006, 79, 1131–1139. [Google Scholar] [CrossRef]
- Munitz, A.; Levi-Schaffer, F. Eosinophils: ‘new’ roles for ‘old’ cells. Allergy 2004, 59, 268–275. [Google Scholar] [CrossRef]
- Pesce, S.; Thoren, F.B.; Cantoni, C.; Prato, C.; Moretta, L.; Moretta, A.; Marcenaro, E. The Innate Immune Cross Talk between NK Cells and Eosinophils Is Regulated by the Interaction of Natural Cytotoxicity Receptors with Eosinophil Surface Ligands. Front. Immunol. 2017, 8, 510. [Google Scholar] [CrossRef]
- Hollande, C.; Boussier, J.; Ziai, J.; Nozawa, T.; Bondet, V.; Phung, W.; Lu, B.; Duffy, D.; Paradis, V.; Mallet, V.; et al. Inhibition of the dipeptidyl peptidase DPP4 (CD26) reveals IL-33-dependent eosinophil-mediated control of tumor growth. Nat. Immunol. 2019, 20, 257–264. [Google Scholar] [CrossRef]
- Andreone, S.; Spadaro, F.; Buccione, C.; Mancini, J.; Tinari, A.; Sestili, P.; Gambardella, A.R.; Lucarini, V.; Ziccheddu, G.; Parolini, I.; et al. IL-33 Promotes CD11b/CD18-Mediated Adhesion of Eosinophils to Cancer Cells and Synapse-Polarized Degranulation Leading to Tumor Cell Killing. Cancers 2019, 11, 1664. [Google Scholar] [CrossRef] [PubMed]
- Spencer, L.A.; Szela, C.T.; Perez, S.A.; Kirchhoffer, C.L.; Neves, J.S.; Radke, A.L.; Weller, P.F. Human eosinophils constitutively express multiple Th1, Th2, and immunoregulatory cytokines that are secreted rapidly and differentially. J. Leukoc. Biol. 2009, 85, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Legrand, F.; Landolina, N.; Zaffran, I.; Emeh, R.O.; Chen, E.; Klion, A.D.; Levi-Schaffer, F. Siglec-7 on peripheral blood eosinophils: Surface expression and function. Allergy 2019, 74, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.C.S.; Utikal, J.; Umansky, V. Opposing roles of eosinophils in cancer. Cancer Immunol. Immunother. 2019, 68, 823–833. [Google Scholar] [CrossRef]
- Rigoni, A.; Colombo, M.P.; Pucillo, C. Mast cells, basophils and eosinophils: From allergy to cancer. Semin. Immunol. 2018, 35, 29–34. [Google Scholar] [CrossRef]
- McDuffie, H.H.; Cockcroft, D.W.; Talebi, Z.; Klaassen, D.J.; Dosman, J.A. Lower prevalence of positive atopic skin tests in lung cancer patients. Chest 1988, 93, 241–246. [Google Scholar] [CrossRef]
- McDuffie, H.H. Atopy and primary lung cancer. Histol. Sex Distrib. Chest 1991, 99, 404–407. [Google Scholar] [CrossRef]
- Turner, M.C.; Chen, Y.; Krewski, D.; Ghadirian, P.; Thun, M.J.; Calle, E.E. Cancer mortality among US men and women with asthma and hay fever. Am. J. Epidemiol. 2005, 162, 212–221. [Google Scholar] [CrossRef]
- Ghadirian, P.; Lacroix, A.; Perret, C.; Maisonneuve, P.; Boyle, P. Sociodemographic characteristics, smoking, medical and family history, and breast cancer. Cancer Detect. Prev. 1998, 22, 485–494. [Google Scholar] [CrossRef][Green Version]
- Vesterinen, E.; Pukkala, E.; Timonen, T.; Aromaa, A. Cancer incidence among 78,000 asthmatic patients. Int. J. Epidemiol. 1993, 22, 976–982. [Google Scholar] [CrossRef]
- Ohrui, T.; Yamaya, M.; Sato, T.; Matsui, T.; Sasaki, H.; Namima, T. Risk of prostate cancer in older Japanese asthmatics. J. Am. Geriatr. Soc. 2002, 50, 202. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, A.L.; Carneiro, F. New Insights into the Role of Tissue Eosinophils in the Progression of Colorectal Cancer: A Literature Review. Acta Med. Port. 2018, 31, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Reichman, H.; Itan, M.; Rozenberg, P.; Yarmolovski, T.; Brazowski, E.; Varol, C.; Gluck, N.; Shapira, S.; Arber, N.; Qimron, U.; et al. Activated Eosinophils Exert Antitumorigenic Activities in Colorectal Cancer. Cancer Immunol. Res. 2019, 7, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.L.; Siersma, V.D.; Hasselbalch, H.C.; Lindegaard, H.; Vestergaard, H.; Felding, P.; Olivarius Nde, F.; Bjerrum, O.W. Eosinophilia in routine blood samples as a biomarker for solid tumor development—A study based on The Copenhagen Primary Care Differential Count (CopDiff) Database. Acta Oncol. 2014, 53, 1245–1250. [Google Scholar] [CrossRef]
- Cao, C.; Gu, Y.; Zhu, C.; Palmai-Pallag, T.; Lan, F.; Chen, Z.; Li, W.; Shen, H.; Ying, S. Potential roles of eosinophils in cancer therapy: Epidemiological studies, experimental models, and clinical pathology. Recent Pat. Anticancer Drug Discov. 2014, 9, 241–248. [Google Scholar] [CrossRef]
- Mackensen, A.; Meidenbauer, N.; Vogl, S.; Laumer, M.; Berger, J.; Andreesen, R. Phase I study of adoptive T-cell therapy using antigen-specific CD8+ T cells for the treatment of patients with metastatic melanoma. J. Clin. Oncol. 2006, 24, 5060–5069. [Google Scholar] [CrossRef]
- Rivoltini, L.; Viggiano, V.; Spinazze, S.; Santoro, A.; Colombo, M.P.; Takatsu, K.; Parmiani, G. In vitro anti-tumor activity of eosinophils from cancer patients treated with subcutaneous administration of interleukin 2. Role of interleukin 5. Int. J. Cancer 1993, 54, 8–15. [Google Scholar] [CrossRef]
- Lotze, M.T.; Matory, Y.L.; Rayner, A.A.; Ettinghausen, S.E.; Vetto, J.T.; Seipp, C.A.; Rosenberg, S.A. Clinical effects and toxicity of interleukin-2 in patients with cancer. Cancer 1986, 58, 2764–2772. [Google Scholar] [CrossRef]
- Ramirez, G.A.; Yacoub, M.R.; Ripa, M.; Mannina, D.; Cariddi, A.; Saporiti, N.; Ciceri, F.; Castagna, A.; Colombo, G.; Dagna, L. Eosinophils from Physiology to Disease: A Comprehensive Review. BioMed Res. Int. 2018, 2018. [Google Scholar] [CrossRef]
- Gross, S.; Erdmann, M.; Haendle, I.; Voland, S.; Berger, T.; Schultz, E.; Strasser, E.; Dankerl, P.; Janka, R.; Schliep, S.; et al. Twelve-year survival and immune correlates in dendritic cell-vaccinated melanoma patients. JCI Insight 2017, 2, e91438. [Google Scholar] [CrossRef]
- Schaft, N.; Dorrie, J.; Thumann, P.; Beck, V.E.; Muller, I.; Schultz, E.S.; Kampgen, E.; Dieckmann, D.; Schuler, G. Generation of an optimized polyvalent monocyte-derived dendritic cell vaccine by transfecting defined RNAs after rather than before maturation. J. Immunol. 2005, 174, 3087–3097. [Google Scholar] [CrossRef] [PubMed]
- Boczkowski, D.; Nair, S.K.; Nam, J.H.; Lyerly, H.K.; Gilboa, E. Induction of tumor immunity and cytotoxic T lymphocyte responses using dendritic cells transfected with messenger RNA amplified from tumor cells. Cancer Res. 2000, 60, 1028–1034. [Google Scholar] [PubMed]
- Shi, H.Z. Eosinophils function as antigen-presenting cells. J. Leukoc. Biol. 2004, 76, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Kruckel, A.; Moreira, A.; Frohlich, W.; Schuler, G.; Heinzerling, L. Eosinophil-cationic protein—A novel liquid prognostic biomarker in melanoma. BMC Cancer 2019, 19, 207. [Google Scholar] [CrossRef] [PubMed]
- Sakkal, S.; Miller, S.; Apostolopoulos, V.; Nurgali, K. Eosinophils in Cancer: Favourable or Unfavourable? Curr. Med. Chem. 2016, 23, 650–666. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Galdiero, M.R.; Loffredo, S.; Lucarini, V.; Marone, G.; Mattei, F.; Schiavoni, G. Eosinophils: The unsung heroes in cancer? Oncoimmunology 2018, 7, e1393134. [Google Scholar] [CrossRef] [PubMed]
- Munitz, A.; Hogan, S.P. Alarming eosinophils to combat tumors. Nat. Immunol. 2019, 20, 250–252. [Google Scholar] [CrossRef]
- Amini-Vaughan, Z.J.; Martinez-Moczygemba, M.; Huston, D.P. Therapeutic strategies for harnessing human eosinophils in allergic inflammation, hypereosinophilic disorders, and cancer. Curr. Allergy Asthma Rep. 2012, 12, 402–412. [Google Scholar] [CrossRef]
- Carretero, R.; Sektioglu, I.M.; Garbi, N.; Salgado, O.C.; Beckhove, P.; Hammerling, G.J. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat. Immunol. 2015, 16, 609–617. [Google Scholar] [CrossRef]
- Mattes, J.; Hulett, M.; Xie, W.; Hogan, S.; Rothenberg, M.E.; Foster, P.; Parish, C. Immunotherapy of cytotoxic T cell-resistant tumors by T helper 2 cells: An eotaxin and STAT6-dependent process. J. Exp. Med. 2003, 197, 387–393. [Google Scholar] [CrossRef]
- Lucarini, V.; Ziccheddu, G.; Macchia, I.; La Sorsa, V.; Peschiaroli, F.; Buccione, C.; Sistigu, A.; Sanchez, M.; Andreone, S.; D’Urso, M.T.; et al. IL-33 restricts tumor growth and inhibits pulmonary metastasis in melanoma-bearing mice through eosinophils. Oncoimmunology 2017, 6, e1317420. [Google Scholar] [CrossRef] [PubMed]
- Legrand, F.; Driss, V.; Delbeke, M.; Loiseau, S.; Hermann, E.; Dombrowicz, D.; Capron, M. Human eosinophils exert TNF-alpha and granzyme A-mediated tumoricidal activity toward colon carcinoma cells. J. Immunol. 2010, 185, 7443–7451. [Google Scholar] [CrossRef] [PubMed]
- Costain, D.J.; Guha, A.K.; Liwski, R.S.; Lee, T.D. Murine hypodense eosinophils induce tumour cell apoptosis by a granzyme B-dependent mechanism. Cancer Immunol. Immunother. 2001, 50, 293–299. [Google Scholar] [CrossRef]
- Gatault, S.; Delbeke, M.; Driss, V.; Sarazin, A.; Dendooven, A.; Kahn, J.E.; Lefevre, G.; Capron, M. IL-18 Is Involved in Eosinophil-Mediated Tumoricidal Activity against a Colon Carcinoma Cell Line by Upregulating LFA-1 and ICAM-1. J. Immunol. 2015, 195, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Simson, L.; Ellyard, J.I.; Dent, L.A.; Matthaei, K.I.; Rothenberg, M.E.; Foster, P.S.; Smyth, M.J.; Parish, C.R. Regulation of carcinogenesis by IL-5 and CCL11: A potential role for eosinophils in tumor immune surveillance. J. Immunol. 2007, 178, 4222–4229. [Google Scholar] [CrossRef] [PubMed]
- Untersmayr, E.; Bax, H.J.; Bergmann, C.; Bianchini, R.; Cozen, W.; Gould, H.J.; Hartmann, K.; Josephs, D.H.; Levi-Schaffer, F.; Penichet, M.L.; et al. Allergo Oncology: Microbiota in allergy and cancer-A European Academy for Allergy and Clinical Immunology position paper. Allergy 2019, 74, 1037–1051. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira, A.; Erdmann, M.; Uslu, U.; Vass, V.; Schuler, G.; Schuler-Thurner, B. Blood Eosinophilia Is an on-Treatment Biomarker in Patients with Solid Tumors Undergoing Dendritic Cell Vaccination with Autologous Tumor-RNA. Pharmaceutics 2020, 12, 210. https://doi.org/10.3390/pharmaceutics12030210
Moreira A, Erdmann M, Uslu U, Vass V, Schuler G, Schuler-Thurner B. Blood Eosinophilia Is an on-Treatment Biomarker in Patients with Solid Tumors Undergoing Dendritic Cell Vaccination with Autologous Tumor-RNA. Pharmaceutics. 2020; 12(3):210. https://doi.org/10.3390/pharmaceutics12030210
Chicago/Turabian StyleMoreira, Alvaro, Michael Erdmann, Ugur Uslu, Verona Vass, Gerold Schuler, and Beatrice Schuler-Thurner. 2020. "Blood Eosinophilia Is an on-Treatment Biomarker in Patients with Solid Tumors Undergoing Dendritic Cell Vaccination with Autologous Tumor-RNA" Pharmaceutics 12, no. 3: 210. https://doi.org/10.3390/pharmaceutics12030210
APA StyleMoreira, A., Erdmann, M., Uslu, U., Vass, V., Schuler, G., & Schuler-Thurner, B. (2020). Blood Eosinophilia Is an on-Treatment Biomarker in Patients with Solid Tumors Undergoing Dendritic Cell Vaccination with Autologous Tumor-RNA. Pharmaceutics, 12(3), 210. https://doi.org/10.3390/pharmaceutics12030210





