Immunoregulatory and Antimicrobial Activity of Bovine Neutrophil β-Defensin-5-Loaded PLGA Nanoparticles against Mycobacterium bovis
Abstract
:1. Introduction
2. Methods
2.1. Mice
2.2. Bacterial Culture
2.3. Preparation of B5-NPs
2.4. Determination of B5-Encapsulation and -Loading Efficiency
2.5. In Vitro Release Kinetics of B5-NPs
2.6. Cell Culture and Stimulation
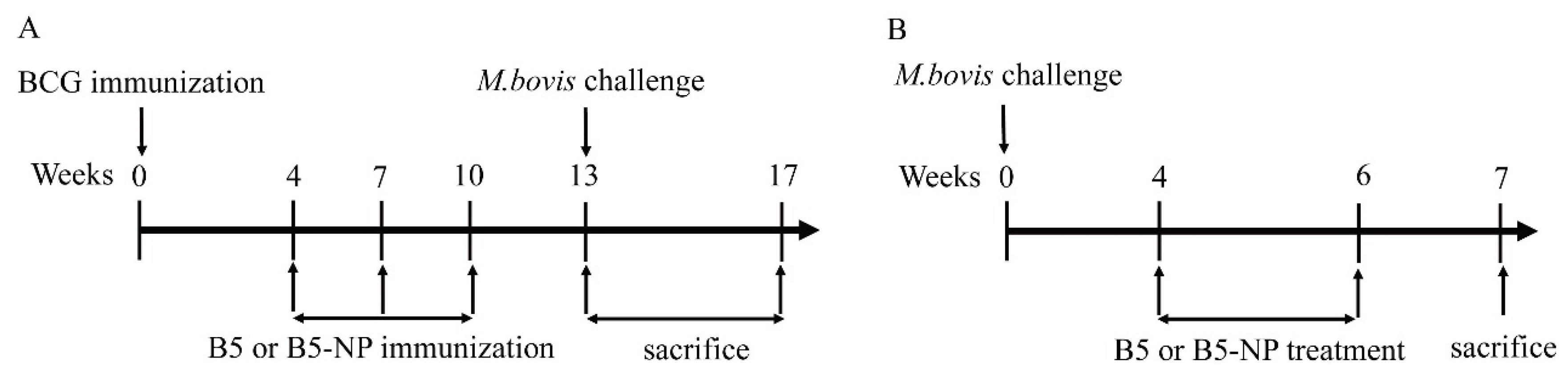
2.7. Mice Immunization and M. bovis Challenge
2.8. Enzyme-Linked Immunosorbent Assay (ELISA) Assay
2.9. Flow Cytometry
2.10. Colony-Forming Units (CFU) Enumeration
2.11. Lung Histology
2.12. Statistical Analysis
3. Results
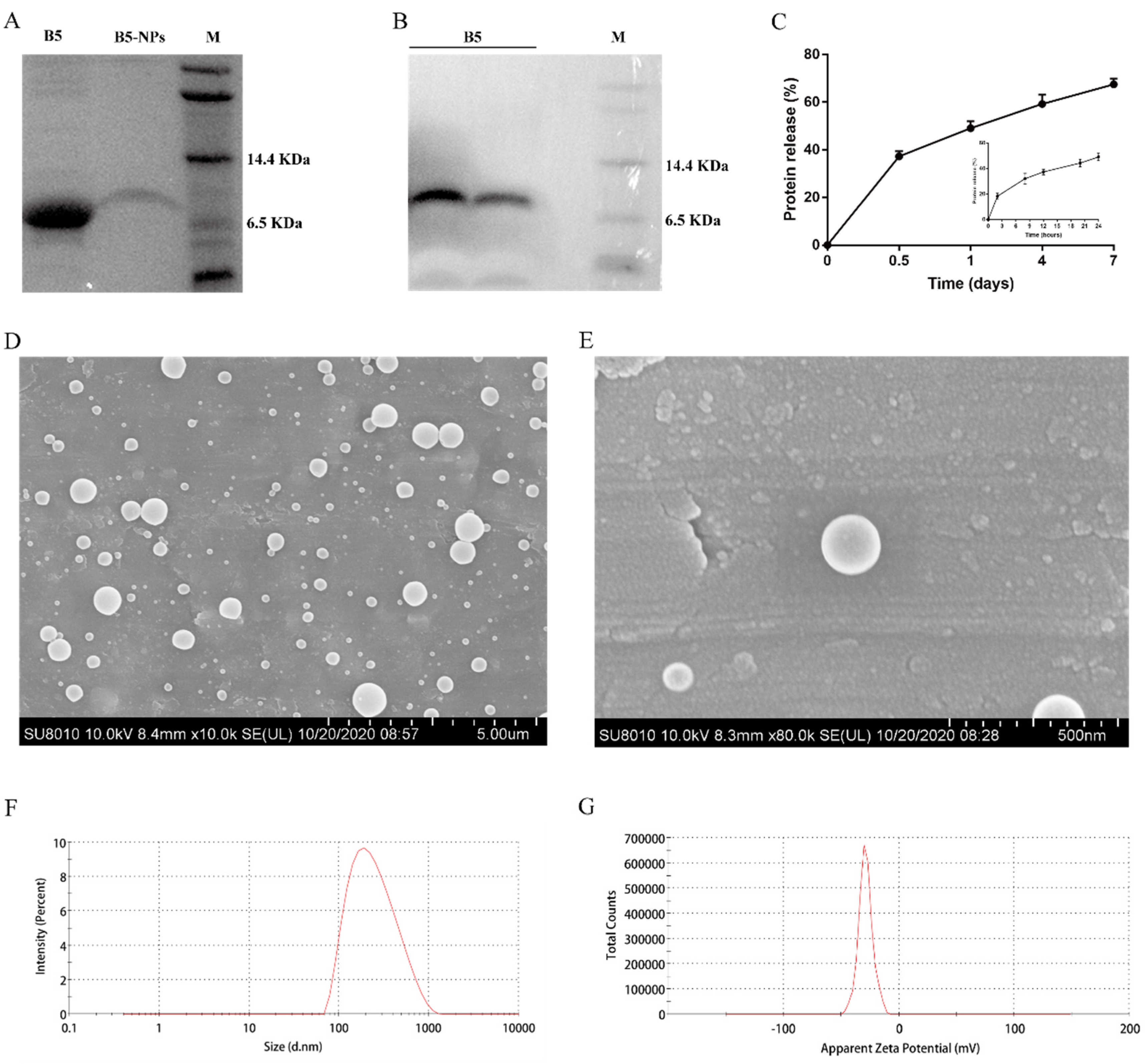
3.1. Physicochemical and Morphological Characterization of B5-NPs
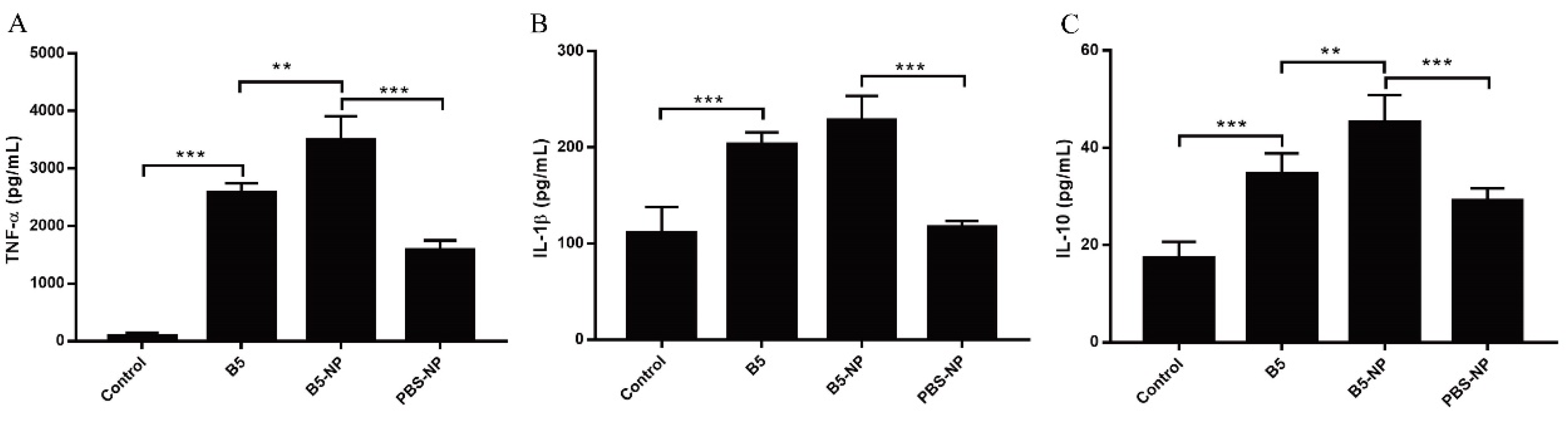
3.2. B5-NPs Enhance the Cytokine Secretion in J774A.1 Cells
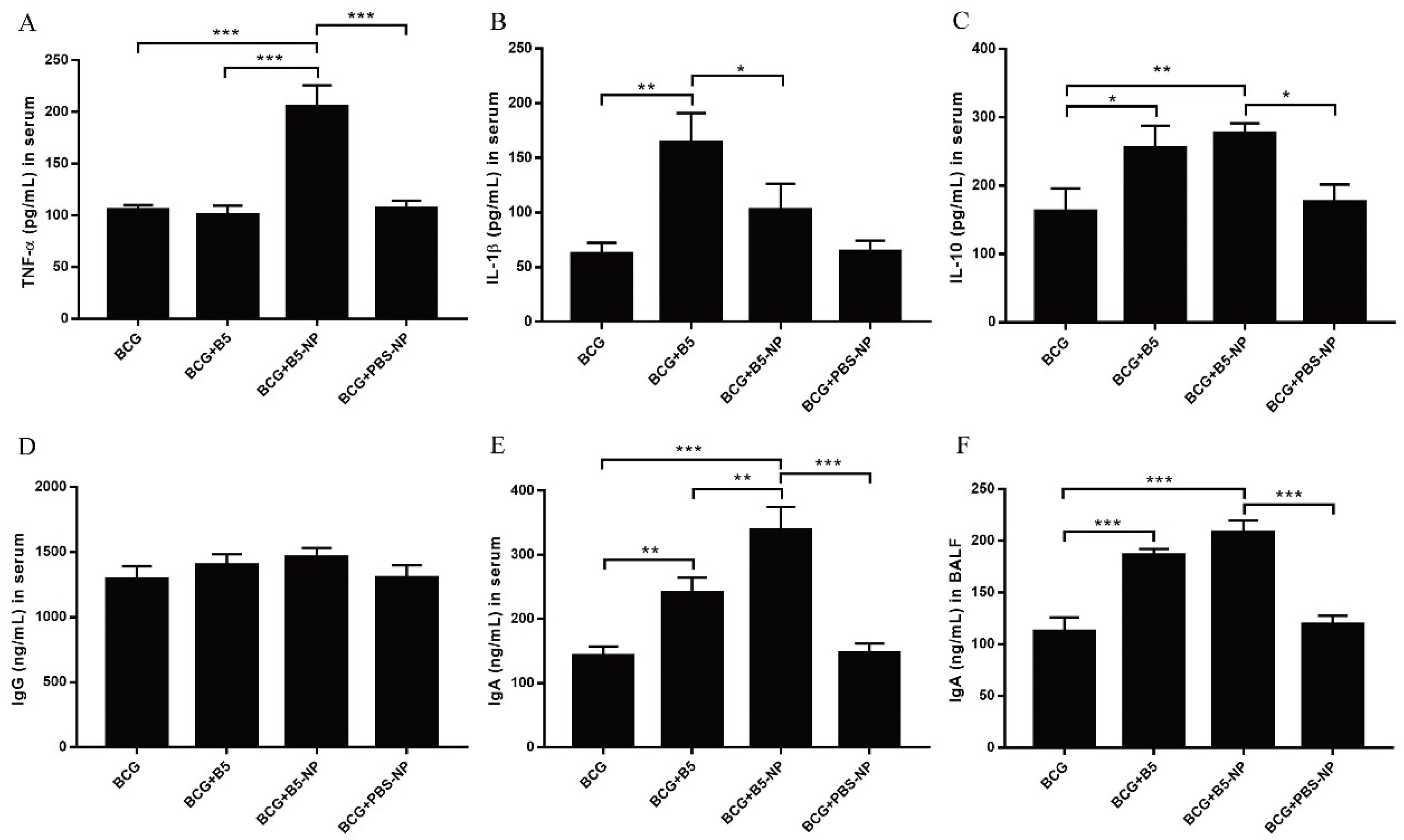
3.3. B5-NPs Promote TNF-α and IgA Production in Mice
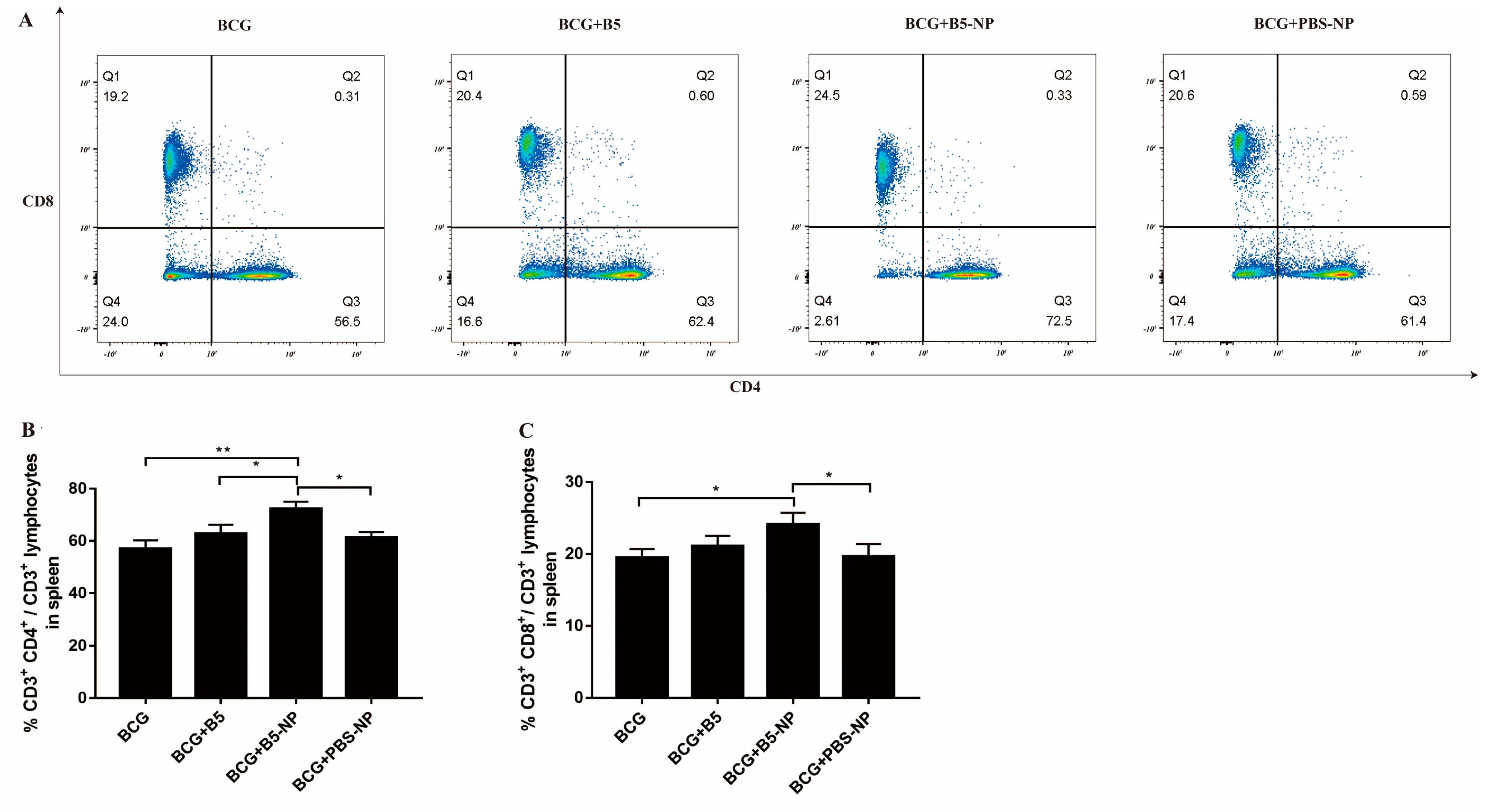
3.4. B5-NPs Increase the Population of CD4+ and CD8+ T Cells in Spleen
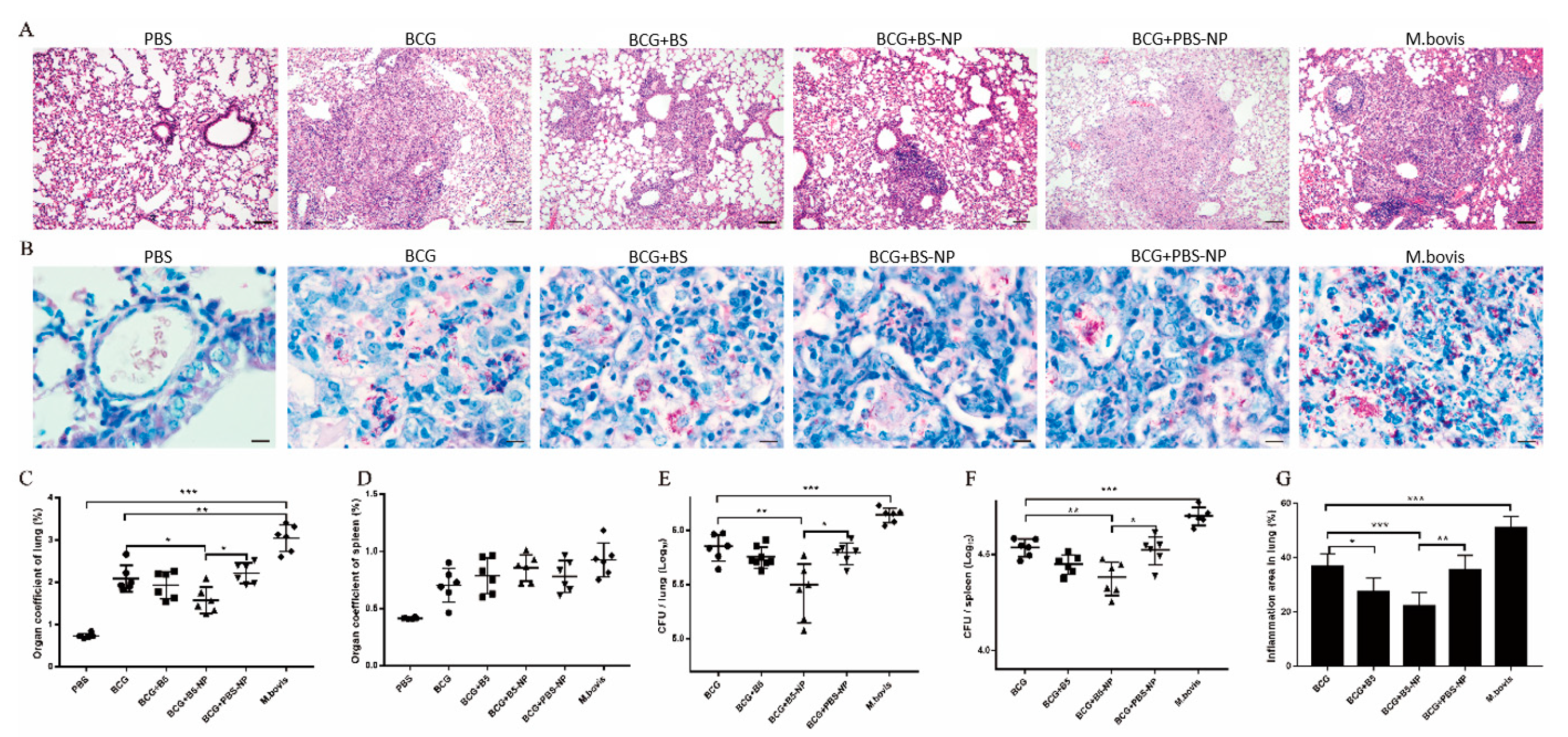
3.5. B5-NPs Enhance Protective Efficacy against M. bovis
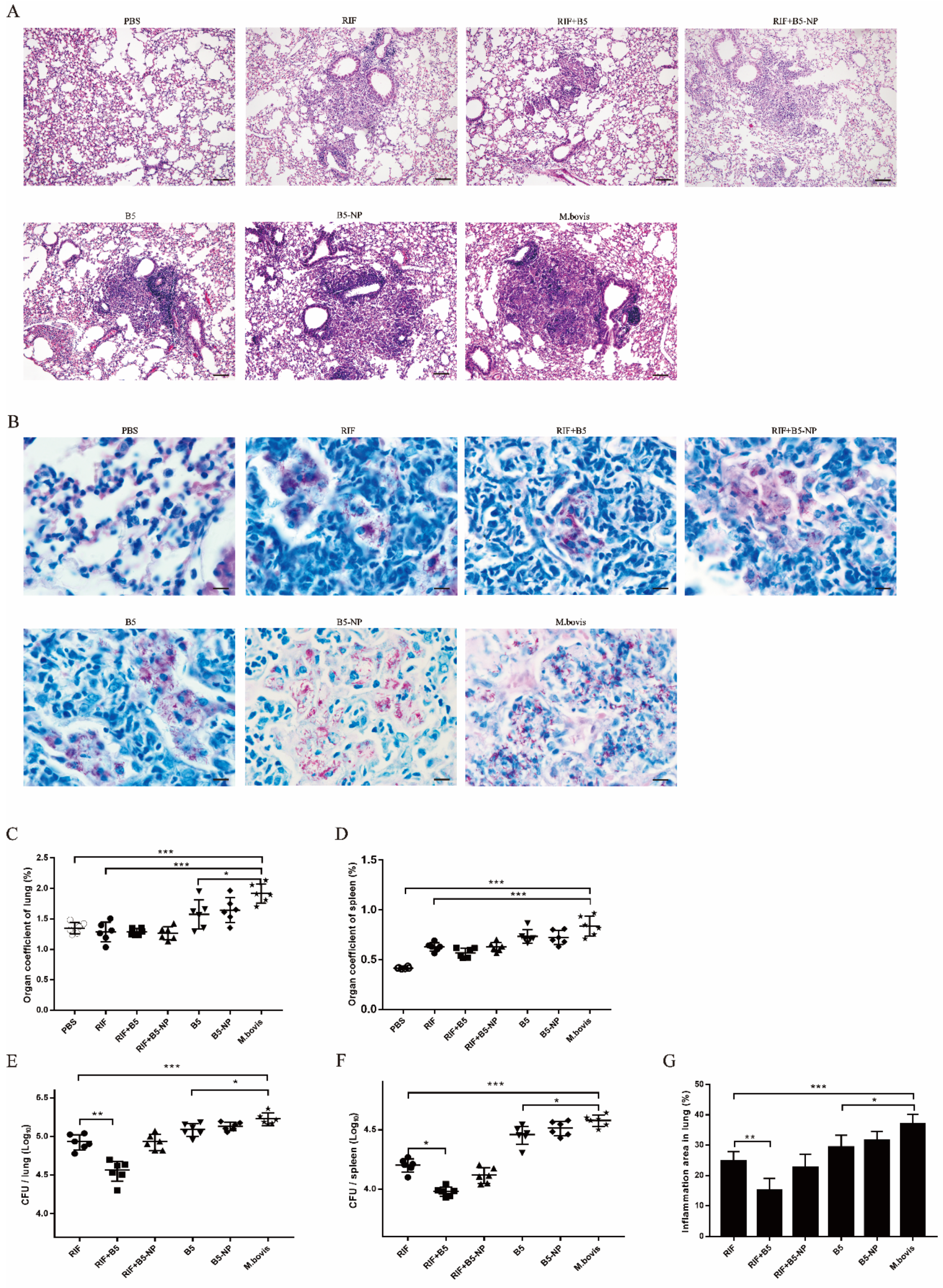
3.6. B5 Promotes the Therapeutic Effect of RIF against M. bovis
3.7. B5 and B5-NPs Reduce M. bovis-Induced TNF-α Hypersecretion
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization (WHO). Global Tuberculosis Report. 2019. Available online: www.who.int/tb (accessed on 30 November 2019).
- Waters, W.R.; Palmer, M.V.; Thacker, T.C.; Davis, W.C.; Sreevatsan, S.; Coussens, P.; Meade, K.G.; Hope, J.C.; Estes, D.M. Tuberculosis immunity: Opportunities from studies with cattle. Clin. Dev. Immunol. 2011, 2011, 768542. [Google Scholar] [CrossRef]
- Fend, R.; Geddes, R.; Lesellier, S.; Vordermeier, H.M.; Corner, L.A.; Gormley, E.; Costello, E.; Hewinson, R.G.; Marlin, D.J.; Woodman, A.C.; et al. Use of an electronic nose to diagnose Mycobacterium bovis infection in badgers and cattle. J. Clin. Microbiol. 2005, 43, 1745–1751. [Google Scholar] [CrossRef] [Green Version]
- Jiang, G.; Wang, G.; Chen, S.; Yu, X.; Wang, X.; Zhao, L.; Ma, Y.; Dong, L.; Huang, H. Pulmonary tuberculosis caused by Mycobacterium bovis in China. Sci. Rep. 2015, 5, 8538. [Google Scholar] [CrossRef] [PubMed]
- Scott, C.; Cavanaugh, J.S.; Pratt, R.; Silk, B.J.; LoBue, P.; Moonan, P.K. Human Tuberculosis Caused by Mycobacterium bovis in the United States, 2006–2013. Clin. Infect. Dis. 2016, 63, 594–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, B.; Dürr, S.; Alonso, S.; Hattendorf, J.; Laisse, C.J.; Parsons, S.D.; van Helden, P.D.; Zinsstag, J. Zoonotic Mycobacterium bovis-induced tuberculosis in humans. Emerg. Infect. Dis. 2013, 19, 899–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres-Gonzalez, P.; Cervera-Hernandez, M.E.; Martinez-Gamboa, A.; Garcia-Garcia, L.; Cruz-Hervert, L.P.; Bobadilla-Del Valle, M.; Ponce-de Leon, A.; Sifuentes-Osornio, J. Human tuberculosis caused by Mycobacterium bovis: A retrospective comparison with Mycobacterium tuberculosis in a Mexican tertiary care centre, 2000–2015. BMC Infect. Dis. 2016, 16, 657. [Google Scholar] [CrossRef] [Green Version]
- Buddle, B.M.; Wedlock, D.N.; Denis, M.; Vordermeier, H.M.; Hewinson, R.G. Update on vaccination of cattle and wildlife populations against tuberculosis. Vet. Microbiol. 2011, 151, 14–22. [Google Scholar] [CrossRef]
- Nugent, G.; Yockney, I.J.; Cross, M.L.; Buddle, B.M. Low-dose BCG vaccination protects free-ranging cattle against naturally-acquired bovine tuberculosis. Vaccine 2018, 36, 7338–7344. [Google Scholar] [CrossRef]
- Conlan, A.J.K.; Vordermeier, M.; de Jong, M.C.; Wood, J.L. The intractable challenge of evaluating cattle vaccination as a control for bovine Tuberculosis. Elife 2018, 7, e27694. [Google Scholar] [CrossRef]
- Mangtani, P.; Abubakar, I.; Ariti, C.; Beynon, R.; Pimpin, L.; Fine, P.E.; Rodrigues, L.C.; Smith, P.G.; Lipman, M.; Whiting, P.F.; et al. Protection by BCG vaccine against tuberculosis: A systematic review of randomized controlled trials. Clin Infect Dis. 2014, 58, 470–480. [Google Scholar] [CrossRef] [Green Version]
- AlMatar, M.; Makky, E.A.; Yakıcı, G.; Var, I.; Kayar, B.; Köksal, F. Antimicrobial peptides as an alternative to anti-tuberculosis drugs. Pharmacol. Res. 2018, 128, 288–305. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Saikia, U.N.; Sharma, S.; Verma, I. Activity of human beta defensin-1 and its motif against active and dormant Mycobacterium tuberculosis. Appl. Microbiol. Biotechnol. 2017, 101, 7239–7248. [Google Scholar] [CrossRef]
- Su, F.; Wang, Y.; Liu, G.; Ru, K.; Liu, X.; Yu, Y.; Liu, J.; Wu, Y.; Quan, F.; Guo, Z.; et al. Generation of transgenic cattle expressing human β-defensin 3 as an approach to reducing susceptibility to Mycobacterium bovis infection. FEBS J. 2016, 283, 776–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldman, M.J.; Anderson, G.M.; Stolzenberg, E.D.; Kari, U.P.; Zasloff, M.; Wilson, J.M. Human beta-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell 1997, 88, 553–560. [Google Scholar] [CrossRef] [Green Version]
- Tecle, T.; Tripathi, S.; Hartshorn, K.L. Review: Defensins and cathelicidins in lung immunity. Innate Immun. 2010, 16, 151–159. [Google Scholar] [CrossRef]
- Hiemstra, P.S.; Amatngalim, G.D.; van der Does, A.M.; Taube, C. Antimicrobial Peptides and Innate Lung Defenses: Role in Infectious and Noninfectious Lung Diseases and Therapeutic Applications. Chest 2016, 149, 545–551. [Google Scholar] [CrossRef]
- Ryan, L.K.; Wu, J.; Schwartz, K.; Yim, S.; Diamond, G. β-Defensins Coordinate In Vivo to Inhibit Bacterial Infections of the Trachea. Vaccines 2018, 6, 57. [Google Scholar] [CrossRef] [Green Version]
- Sarfraz, M.; Suleman, M.; Tikoo, S.K.; Wheler, C.; Potter, A.A.; Gerdts, V.; Dar, A. Immune responses to in ovo vaccine formulations containing inactivated fowl adenovirus 8b with poly[di(sodium carboxylatoethylphenoxy)]phosphazene (PCEP) and avian beta defensin as adjuvants in chickens. Vaccine 2017, 35, 981–986. [Google Scholar] [CrossRef]
- Vemula, S.V.; Amen, O.; Katz, J.M.; Donis, R.; Sambhara, S.; Mittal, S.K. Beta-defensin 2 enhances immunogenicity and protection of an adenovirus-based H5N1 influenza vaccine at an early time. Virus Res. 2013, 178, 398–403. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Yang, Y.L.; Jang, S.H.; Jang, Y.S. Human β-defensin 2 plays a regulatory role in innate antiviral immunity and is capable of potentiating the induction of antigen-specific immunity. Virol. J. 2018, 15, 124. [Google Scholar] [CrossRef] [Green Version]
- Cervantes-Villagrana, A.R.; Hernández-Pando, R.; Biragyn, A.; Castañeda-Delgado, J.; Bodogai, M.; Martínez-Fierro, M.; Sada, E.; Trujillo, V.; Enciso-Moreno, A.; Rivas-Santiago, B. Prime-boost BCG vaccination with DNA vaccines based in β-defensin-2 and mycobacterial antigens ESAT6 or Ag85B improve protection in a tuberculosis experimental model. Vaccine 2013, 31, 676–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selsted, M.E.; Tang, Y.Q.; Morris, W.L.; McGuire, P.A.; Novotny, M.J.; Smith, W.; Henschen, A.H.; Cullor, J.S. Purification, primary structures, and antibacterial activities of beta-defensins, a new family of antimicrobial peptides from bovine neutrophils. J. Biol. Chem. 1993, 268, 6641–6648. [Google Scholar] [PubMed]
- Kang, J.; Zhao, D.; Lyu, Y.; Tian, L.; Yin, X.; Yang, L.; Teng, K.; Zhou, X. Antimycobacterial activity of Pichia pastoris-derived mature bovine neutrophil β-defensins 5. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1823–1834. [Google Scholar] [CrossRef] [PubMed]
- Pati, R.; Shevtsov, M.; Sonawane, A. Nanoparticle Vaccines against Infectious Diseases. Front. Immunol. 2018, 9, 2224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Z.; Gao, S.; Cui, X.; Sun, D.; Zhao, K. Adjuvants and delivery systems based on polymeric nanoparticles for mucosal vaccines. Int. J. Pharm. 2019, 572, 118731. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.; Ahmed, N.; Rehman, A.U. Recent applications of PLGA based nanostructures in drug delivery. Colloids Surf. B Biointerfaces 2017, 159, 217–231. [Google Scholar] [CrossRef]
- Cruz, L.J.; Tacken, P.J.; Eich, C.; Rueda, F.; Torensma, R.; Figdor, C.G. Controlled release of antigen and Toll-like receptor ligands from PLGA nanoparticles enhances immunogenicity. Nanomedicine 2017, 12, 491–510. [Google Scholar] [CrossRef] [Green Version]
- Malik, A.; Gupta, M.; Mani, R.; Bhatnagar, R. Single-dose Ag85B-ESAT6-loaded poly(lactic-co-glycolic acid) nanoparticles confer protective immunity against tuberculosis. Int. J. Nanomed. 2019, 14, 3129–3143. [Google Scholar] [CrossRef] [Green Version]
- Hafner, A.M.; Corthésy, B.; Textor, M.; Merkle, H.P. Surface-assembled poly(I:C) on PEGylated PLGA microspheres as vaccine adjuvant: APC activation and bystander cell stimulation. Int. J. Pharm. 2016, 514, 176–188. [Google Scholar] [CrossRef] [Green Version]
- Lei, R.; Hou, J.; Chen, Q.; Yuan, W.; Cheng, B.; Sun, Y.; Jin, Y.; Ge, L.; Ben-Sasson, S.A.; Chen, J.; et al. Self-Assembling Myristoylated Human α-Defensin 5 as a Next-Generation Nanobiotics Potentiates Therapeutic Efficacy in Bacterial Infection. ACS Nano. 2018, 12, 5284–5296. [Google Scholar] [CrossRef]
- Zhao, G.; Chen, Y.; He, Y.; Chen, F.; Gong, Y.; Chen, S.; Xu, Y.; Su, Y.; Wang, C.; Wang, J. Succinylated casein-coated peptide-mesoporous silica nanoparticles as an antibiotic against intestinal bacterial infection. Biomater. Sci. 2019, 7, 2440–2451. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Vaghasiya, K.; Gupta, P.; Singh, A.K.; Gupta, U.D.; Verma, R.K. Dynamic mucus penetrating microspheres for efficient pulmonary delivery and enhanced efficacy of host defence peptide (HDP) in experimental tuberculosis. J. Control. Release 2020, 324, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, E.; Sanz, J.; Dunn, J.L.; Khan, N.; Mendonca, L.E.; Pacis, A.; Tzelepis, F.; Pernet, E.; Dumaine, A.; Grenier, J.C.; et al. BCG Educates Hematopoietic Stem Cells to Generate Protective Innate Immunity against Tuberculosis. Cell 2018, 172, 176–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darrah, P.A.; Zeppa, J.J.; Maiello, P.; Hackney, J.A.; Wadsworth, M.H.; Hughes, T.K.; Pokkali, S.; Swanson, P.A.; Grant, N.L.; Rodgers, M.A.; et al. Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature 2020, 577, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Schaefers, M.M.; Duan, B.; Mizrahi, B.; Lu, R.; Reznor, G.; Kohane, D.S.; Priebe, G.P. PLGA-encapsulation of the Pseudomonas aeruginosa PopB vaccine antigen improves Th17 responses and confers protection against experimental acute pneumonia. Vaccine 2018, 36, 6926–6932. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lu, X.; Dong, X.; Luo, Y.; Wang, Q.; Liu, X.; Fu, J.; Zhang, Y.; Zhu, B.; Ma, X. Effects of hMASP-2 on the formation of BCG infection-induced granuloma in the lungs of BALB/c mice. Sci. Rep. 2017, 7, 2300. [Google Scholar] [CrossRef] [Green Version]
- Dorhoi, A.; Kaufmann, S.H. Tumor necrosis factor alpha in mycobacterial infection. Semin. Immunol. 2014, 26, 203–209. [Google Scholar] [CrossRef]
- Lazzaro, B.P.; Zasloff, M.; Rolff, J. Antimicrobial peptides: Application informed by evolution. Science 2020, 368, eaau5480. [Google Scholar] [CrossRef]
- Rončević, T.; Puizina, J.; Tossi, A. Antimicrobial Peptides as Anti-Infective Agents in Pre-Post-Antibiotic Era? Int. J. Mol. Sci. 2019, 20, 5713. [Google Scholar] [CrossRef] [Green Version]
- Water, J.J.; Smart, S.; Franzyk, H.; Foged, C.; Nielsen, H.M. Nanoparticle-mediated delivery of the antimicrobial peptide plectasin against Staphylococcus aureus in infected epithelial cells. Eur. J. Pharm. Biopharm. 2015, 92, 65–73. [Google Scholar] [CrossRef]
- Wang, Y.; Li, P.; Kong, L. Chitosan-modified PLGA nanoparticles with versatile surface for improved drug delivery. AAPS Pharmscitech 2013, 14, 585–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oyewumi, M.O.; Kumar, A.; Cui, Z. Nano-microparticles as immune adjuvants: Correlating particle sizes and the resultant immune responses. Expert Rev. Vaccines 2010, 9, 1095–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allahyari, M.; Mohit, E. Peptide/protein vaccine delivery system based on PLGA particles. Hum. Vaccines Immunother. 2015, 12, 806–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, C.; Chen, Y.; Jing, Q.Z.; Wang, X.G. Preparation and characterization of catalase-loaded solid lipid nanoparticles protecting enzyme against proteolysis. Int. J. Mol. Sci. 2011, 12, 4282–4293. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Mizumo, S.; Horai, R.; Iwakura, Y.; Sugawara, I. Protective role of interleukin-1 in mycobacterial infection in IL-1 alpha/beta double-knockout mice. Lab Investig. 2000, 80, 759–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreira-Teixeira, L.; Redford, P.S.; Stavropoulos, E.; Ghilardi, N.; Maynard, C.L.; Weaver, C.T.; Freitas do Rosário, A.P.; Wu, X.; Langhorne, J.; O’Garra, A. T Cell-Derived IL-10 Impairs Host Resistance to Mycobacterium tuberculosis Infection. J. Immunol. 2017, 199, 613–623. [Google Scholar] [CrossRef] [Green Version]
- Gideon, H.P.; Phuah, J.; Myers, A.J.; Bryson, B.D.; Rodgers, M.A.; Coleman, M.T.; Maiello, P.; Rutledge, T.; Marino, S.; Fortune, S.M.; et al. Variability in tuberculosis granuloma T cell responses exists, but a balance of pro- and anti-inflammatory cytokines is associated with sterilization. PLoS Pathog 2015, 11, e1004603. [Google Scholar] [CrossRef] [Green Version]
- Cyktor, J.C.; Carruthers, B.; Kominsky, R.A.; Beamer, G.L.; Stromberg, P.; Turner, J. IL-10 inhibits mature fibrotic granuloma formation during Mycobacterium tuberculosis infection. J. Immunol. 2013, 190, 2778–2790. [Google Scholar] [CrossRef] [Green Version]
- Wusiman, A.; He, J.; Zhu, T.; Liu, Z.; Gu, P.; Hu, Y.; Liu, J.; Wang, D. Macrophage immunomodulatory activity of the cationic polymer modified PLGA nanoparticles encapsulating Alhagi honey polysaccharide. Int. J. Biol. Macromol. 2019, 134, 730–739. [Google Scholar] [CrossRef]
- Alkie, T.N.; Taha-Abdelaziz, K.; Barjesteh, N.; Bavananthasivam, J.; Hodgins, D.C.; Sharif, S. Characterization of Innate Responses Induced by PLGA Encapsulated- and Soluble TLR Ligands In Vitro and In Vivo in Chickens. PLoS ONE 2017, 12, e0169154. [Google Scholar] [CrossRef] [Green Version]
- Jasenosky, L.D.; Scriba, T.J.; Hanekom, W.A.; Goldfeld, A.E. T cells and adaptive immunity to Mycobacterium tuberculosis in humans. Immunol Rev. 2015, 264, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Achkar, J.M.; Casadevall, A. Antibody-mediated immunity against tuberculosis: Implications for vaccine development. Cell Host Microbe 2013, 13, 250–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Wang, X.X.; Wang, B.; Fu, L.; Javid, B. Latently and uninfected healthcare workers exposed to TB make protective antibodies against Mycobacterium tuberculosis. PNAS 2017, 114, 5023–5028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dijkman, K.; Sombroek, C.C.; Vervenne, R.A.W.; Hofman, S.O.; Boot, C.; Remarque, E.J.; Kocken, C.H.M.; Ottenhoff, T.H.M.; Kondova, I.; Khayum, M.A.; et al. Prevention of tuberculosis infection and disease by local BCG in repeatedly exposed rhesus macaques. Nat. Med. 2019, 25, 255–262. [Google Scholar] [CrossRef]
- Chereddy, K.K.; Her, C.H.; Comune, M.; Moia, C.; Lopes, A.; Porporato, P.E.; Vanacker, J.; Lam, M.C.; Steinstraesser, L.; Sonveaux, P.; et al. PLGA nanoparticles loaded with host defense peptide LL37 promote wound healing. J. Control. Release 2014, 194, 138–147. [Google Scholar] [CrossRef]
- Reed, S.G.; Orr, M.T.; Fox, C.B. Key roles of adjuvants in modern vaccines. Nat. Med. 2013, 19, 1597–1608. [Google Scholar] [CrossRef]
- Stewart, E.; Triccas, J.A.; Petrovsky, N. Adjuvant Strategies for More Effective Tuberculosis Vaccine Immunity. Microorganisms 2019, 7, 225. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, M.Y.; Steinbach-Rankins, J.M.; Demuth, D.R. Functional assessment of peptide-modified PLGA nanoparticles against oral biofilms in a murine model of periodontitis. J. Control. Release 2019, 297, 3–13. [Google Scholar] [CrossRef]

| PLGA-NPs | Size (nm) | PDI | Zeta Potential (mV) | EE (%) | LE (%) |
|---|---|---|---|---|---|
| PBS-NPs | 193.2 ± 22.0 | 0.16 ± 0.03 | −25.1 ± 1.5 | ||
| B5-NPs | 206.6 ± 26.6 | 0.16 ± 0.04 | −27.1 ± 1.5 | 85.5 ± 2.5 | 0.5 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Z.; Liu, Y.; Sun, X.; Lin, J.; Yao, J.; Song, Y.; Li, M.; Liu, T.; Zhou, X. Immunoregulatory and Antimicrobial Activity of Bovine Neutrophil β-Defensin-5-Loaded PLGA Nanoparticles against Mycobacterium bovis. Pharmaceutics 2020, 12, 1172. https://doi.org/10.3390/pharmaceutics12121172
Liang Z, Liu Y, Sun X, Lin J, Yao J, Song Y, Li M, Liu T, Zhou X. Immunoregulatory and Antimicrobial Activity of Bovine Neutrophil β-Defensin-5-Loaded PLGA Nanoparticles against Mycobacterium bovis. Pharmaceutics. 2020; 12(12):1172. https://doi.org/10.3390/pharmaceutics12121172
Chicago/Turabian StyleLiang, Zhengmin, Yiduo Liu, Xingya Sun, Jingjun Lin, Jiao Yao, Yinjuan Song, Miaoxuan Li, Tianlong Liu, and Xiangmei Zhou. 2020. "Immunoregulatory and Antimicrobial Activity of Bovine Neutrophil β-Defensin-5-Loaded PLGA Nanoparticles against Mycobacterium bovis" Pharmaceutics 12, no. 12: 1172. https://doi.org/10.3390/pharmaceutics12121172
APA StyleLiang, Z., Liu, Y., Sun, X., Lin, J., Yao, J., Song, Y., Li, M., Liu, T., & Zhou, X. (2020). Immunoregulatory and Antimicrobial Activity of Bovine Neutrophil β-Defensin-5-Loaded PLGA Nanoparticles against Mycobacterium bovis. Pharmaceutics, 12(12), 1172. https://doi.org/10.3390/pharmaceutics12121172






