Cognitive Impairment in the 3xTg-AD Mouse Model of Alzheimer’s Disease is Affected by Aβ-ImmunoTherapy and Cognitive Stimulation
Abstract
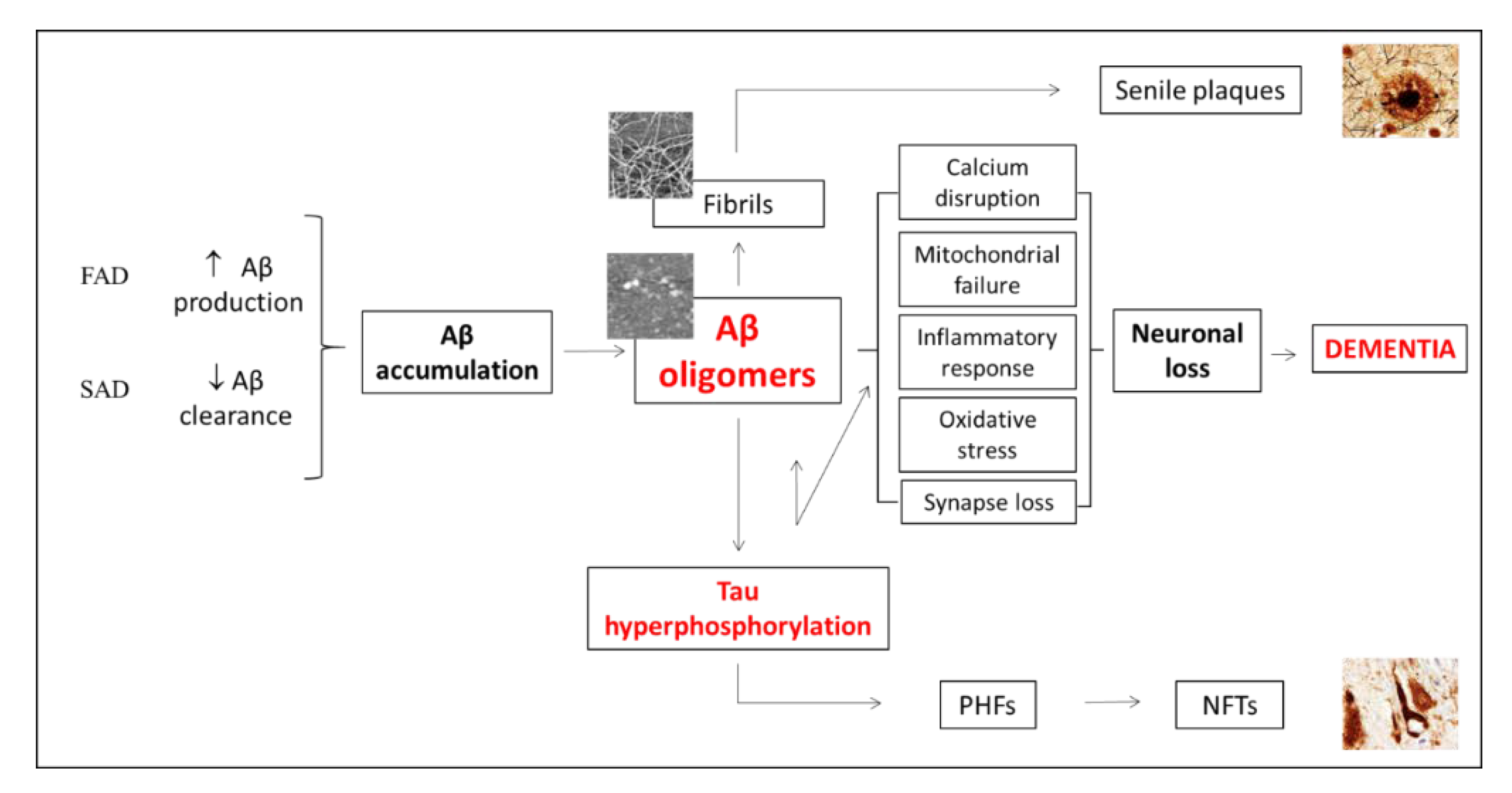
1. Introduction
2. Material and Methods
2.1. ScFv-h3D6 Production
2.2. Animals
2.3. Experimental Design
2.4. Sample Collection and Processing
2.5. Immunohistochemistry
2.6. Image Capture and Processing
2.7. Behavioral and Cognitive Tests
3. Results
3.1. Progression of BPSD-Like Symptoms
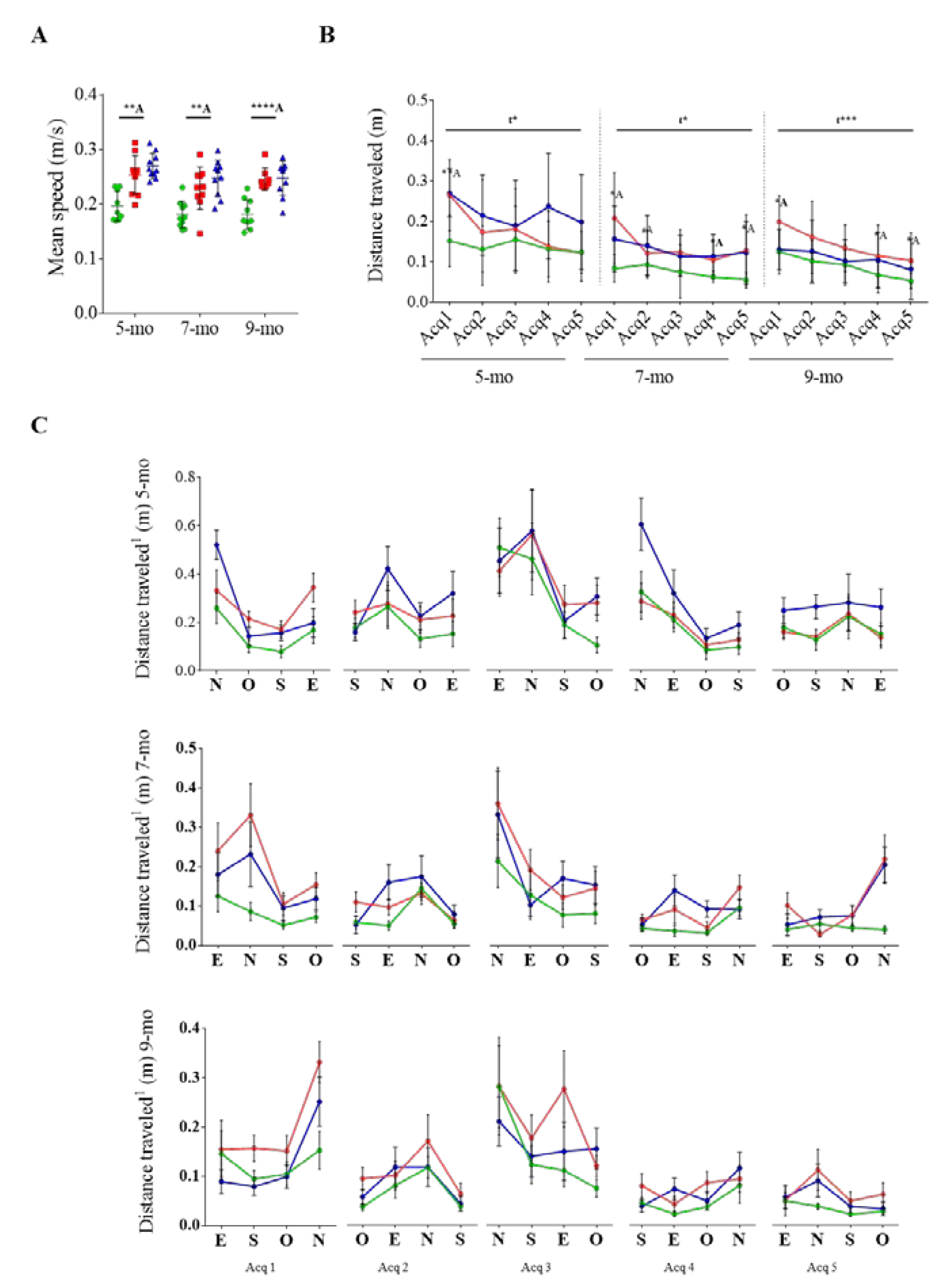
3.2. Effect of Cognitive Stimulation in Spatial Learning and Memory
3.3. Reversal Learning and Memory and Their Evolution
3.4. Effects of Chronic Immunotherapy in 3xTg-AD Mice
3.5. 3xTg-AD Mice Exhibit Evident Amyloid and Tau Pathologies at 9 Months of Age
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 3xTg-AD | triple-transgenic mouse model of AD |
| AD | Alzheimer’s disease |
| BPSD | behavioral and psychological symptoms of dementia |
| CT | corner test |
| MWM | Morris Water Maze |
| NTg | non-transgenic |
| OFT | open-field test |
References
- Christina, P. World Alzheimer Report 2018. Alzheimer’s Disease International. 2018. Available online: https://www.alz.co.uk/research/WorldAlzheimerReport2018.pdf (accessed on 20 September 2020).
- Mawuenyega, K.G.; Sigurdson, W.; Ovod, V.; Munsell, L.; Kasten, T.; Morris, J.C.; Yarasheski, K.E.; Bateman, R.J. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science 2010, 330, 1774. [Google Scholar] [CrossRef]
- Harrison, J.R.; Owen, M.J. Alzheimer’s disease: The amyloid hypothesis on trial. Br. J. Psychiatry 2016, 208, 1–3. [Google Scholar]
- Jack, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer Dement. 2018, 14, 535–562. [Google Scholar]
- Scheltens, P.; Blennow, K.; Breteler, M.M.B.; de Strooper, B.; Frisoni, G.B.; Salloway, S.; Van der Flier, W.M. Alzheimer’s disease. Lancet 2016, 388, 505–517. [Google Scholar] [CrossRef]
- Johnson, S.C.; Schmitz, T.W.; Moritz, C.H.; Meyerand, M.E.; Rowley, H.A.; Alexander, A.L.; Hansen, K.W.; Gleason, C.E.; Carlsson, C.M.; Ries, M.L.; et al. Activation of brain regions vulnerable to Alzheimer’s disease: The effect of mild cognitive impairment. Neurobiol. Aging 2006, 27, 1604–1612. [Google Scholar] [CrossRef]
- Sampath, D.; Sathyanesan, M.; Newton, S. Cognitive dysfunction in major depression and Alzheimer’s disease is associated with hippocampus–prefrontal cortex dysconnectivity. Neuropsychiatr. Dis. Treat. 2017, 13, 1509–1519. [Google Scholar] [CrossRef]
- Shimabukuro, J.; Awata, S.; Matsuoka, H. Behavioral and psychological symptoms of dementia characteristic of mild Alzheimer patients. Psychiatry Clin. Neurosci. 2005, 59, 274–279. [Google Scholar] [CrossRef]
- Lyketsos, C.G.; Carrillo, M.C.; Ryan, J.M.; Khachaturian, A.S.; Trzepacz, P.; Amatniek, J.; Cedarbaum, J.; Brashear, R.; Miller, D.S. Neuropsychiatric symptoms in Alzheimer’s disease. Alzheimer Dement. 2011, 7, 532–539. [Google Scholar] [CrossRef]
- Geda, Y.E.; Schneider, L.S.; Gitlin, L.N.; Miller, D.S.; Smith, G.S.; Bell, J.; Evans, J.; Lee, M.; Porsteinsson, A.; Lanctôt, K.L.; et al. Neuropsychiatric symptoms in Alzheimer’s disease: Past progress and anticipation of the future. Alzheimers Dement. 2013, 9, 602–608. [Google Scholar] [CrossRef]
- Esquerda-Canals, G.; Montoliu-Gaya, L.; Güell-Bosch, J.; Villegas, S. Mouse Models of Alzheimer’s Disease. J. Alzheimer Dis. 2017, 57, 1171–1183. [Google Scholar] [CrossRef]
- Drummond, E.; Wisniewski, T. Alzheimer’s disease: Experimental models and reality. Acta Neuropathol. 2017, 133, 155–175. [Google Scholar] [CrossRef]
- Scheltens, N.M.E.; Tijms, B.M.; Koene, T.; Barkhof, F.; Teunissen, C.E.; Wolfsgruber, S.; Wagner, M.; Kornhuber, J.; Peters, O.; Cohn-Sheehy, B.I.; et al. Cognitive subtypes of probable Alzheimer’s disease robustly identified in four cohorts. Alzheimer Dement. 2017. [Google Scholar] [CrossRef]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef]
- Visser, P.J.; Vos, S.; van Rossum, I.; Scheltens, P. Comparison of International Working Group criteria and National Institute on Aging-Alzheimer’s Association criteria for Alzheimer’s disease. Alzheimers Dement. 2012, 8, 560–563. [Google Scholar] [CrossRef]
- Carrillo, M.C.; Dean, R.A.; Nicolas, F.; Miller, D.S.; Berman, R.; Khachaturian, Z.; Bain, L.J.; Schindler, R.; Knopman, D. Revisiting the framework of the National Institute on Aging-Alzheimer’s Association diagnostic criteria. Alzheimers Dement. 2013, 9, 594–601. [Google Scholar] [CrossRef]
- van der Staay, F.J.; Arndt, S.S.; Nordquist, R.E. Evaluation of animal models of neurobehavioral disorders. Behav. Brain Funct. 2009, 5, 11. [Google Scholar] [CrossRef]
- Neha Sodhi, R.K.; Jaggi, A.S.; Singh, N. Animal models of dementia and cognitive dysfunction. Life Sci. 2014, 109, 73–86. [Google Scholar] [CrossRef]
- Webster, S.J.; Bachstetter, A.D.; Nelson, P.T.; Schmitt, F.A.; Van Eldik, L.J. Using mice to model Alzheimer’s dementia: An overview of the clinical disease and the preclinical behavioral changes in 10 mouse models. Front. Genet. 2014, 5, 88. [Google Scholar] [CrossRef]
- Webster, S.J.; Bachstetter, A.D.; Van Eldik, L.J. Comprehensive behavioral characterization of an APP/PS-1 double knock-in mouse model of Alzheimer’s disease. Alzheimers Res. Ther. 2013, 5, 28. [Google Scholar] [CrossRef]
- Lalonde, R.; Fukuchi, K.-I.; Strazielle, C. Neurologic and motor dysfunctions in APP transgenic mice. Rev. Neurosci. 2012, 23, 363–379. [Google Scholar] [CrossRef]
- Kobayashi, D.T.; Chen, K.S. Behavioral phenotypes of amyloid-based genetically modified mouse models of Alzheimer’s disease. Genes. Brain. Behav. 2005, 4, 173–196. [Google Scholar] [CrossRef]
- Eriksen, J.L.; Janus, C.G. Plaques, Tangles, and Memory Loss in Mouse Models of Neurodegeneration. Behav. Genet. 2007, 37, 79–100. [Google Scholar] [CrossRef]
- Hall, A.M.; Roberson, E.D. Mouse models of Alzheimer’s disease. Brain Res. Bull. 2012, 88, 3–12. [Google Scholar] [CrossRef]
- Giménez-Llort, L.; Blázquez, G.; Cañete, T.; Johansson, B.; Oddo, S.; Tobeña, A.; LaFerla, F.M.; Fernández-Teruel, A. Modeling behavioral and neuronal symptoms of Alzheimer’s disease in mice: A role for intraneuronal amyloid. Neurosci. Biobehav. Rev. 2007, 31, 125–147. [Google Scholar] [CrossRef]
- Oddo, S.; Caccamo, A.; Shepherd, J.D.; Murphy, M.P.; Golde, T.E.; Kayed, R.; Metherate, R.; Mattson, M.P.; Akbari, Y.; LaFerla, F.M. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: Intracellular Abeta and synaptic dysfunction. Neuron 2003, 39, 409–421. [Google Scholar]
- Oddo, S.; Caccamo, A.; Kitazawa, M.; Tseng, B.P.; LaFerla, F.M. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer’s disease. Neurobiol. Aging 2003, 24, 1063–1070. [Google Scholar]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- Panza, F.; Frisardi, V.; Imbimbo, B.P.; Seripa, D.; Solfrizzi, V.; Pilotto, A. Monoclonal antibodies against β-amyloid (Aβ) for the treatment of Alzheimer’s disease: The Aβ target at a crossroads. Expert Opin. Biol. Ther. 2011, 11, 679–686. [Google Scholar] [CrossRef]
- Salloway, S.; Sperling, R.; Fox, N.C.; Blennow, K.; Klunk, W.; Raskind, M.; Sabbagh, M.; Honig, L.S.; Porsteinsson, A.P.; Ferris, S.; et al. Two Phase 3 Trials of Bapineuzumab in Mild-to-Moderate Alzheimer’s Disease. N. Engl. J. Med. 2014, 370, 322–333. [Google Scholar] [CrossRef]
- Marín-Argany, M.; Rivera-Hernández, G.; Martí, J.; Villegas, S. An anti-Aβ (amyloid β) single-chain variable fragment prevents amyloid fibril formation and cytotoxicity by withdrawing Aβ oligomers from the amyloid pathway. Biochem. J. 2011, 437, 25–34. [Google Scholar] [CrossRef]
- Giménez-Llort, L.; Rivera-Hernández, G.; Marín-Argany, M.; Sánchez-Quesada, J.L.; Villegas, S. Early intervention in the 3xTg-AD mice with an amyloid β-antibody fragment ameliorates first hallmarks of Alzheimer disease. MAbs 2013, 5, 665–864. [Google Scholar] [CrossRef]
- Esquerda-Canals, G.; Marti, J.; Rivera-Hernández, G.; Giménez-Llort, L.; Villegas, S. Loss of deep cerebellar nuclei neurons in the 3xTg-AD mice and protection by an anti-amyloid β antibody fragment. MAbs 2013, 5, 660–664. [Google Scholar] [CrossRef]
- Montoliu-Gaya, L.; Esquerda-Canals, G.; Bronsoms, S.; Villegas, S. Production of an anti-Aβ antibody fragment in Pichia pastoris and in vitro and in vivo validation of its therapeutic effect. PLoS ONE 2017, 12, e0181480. [Google Scholar] [CrossRef]
- Esquerda-Canals, G.; Roda, A.R.; Martí-Clúa, J.; Montoliu-Gaya, L.; Rivera-Hernández, G.; Villegas, S. Treatment with scFv-h3D6 Prevented Neuronal Loss and Improved Spatial Memory in Young 3xTg-AD Mice by Reducing the Intracellular Amyloid-β Burden. J. Alzheimer Dis. 2019, 70, 1069–1091. [Google Scholar] [CrossRef]
- Güell-Bosch, J.; Lope-Piedrafita, S.; Esquerda-Canals, G.; Montoliu-Gaya, L.; Villegas, S. Progression of Alzheimer’s disease and effect of scFv-h3D6 immunotherapy in the 3xTg-AD mouse model: An in vivo longitudinal study using Magnetic Resonance Imaging and Spectroscopy. NMR Biomed. 2020. [Google Scholar] [CrossRef]
- Janus, C.; Westaway, D. Transgenic mouse models of Alzheimer’s disease. Physiol. Behav. 2001, 73, 873–886. [Google Scholar]
- Guell-Bosch, J.; Esquerda-Canals, G.; Montoliu-Gaya, L.; Villegas, S. (Eds.) Prospective Therapies for Alzheimer Disease: Biomarkers, Clinical Trials and Preclinical Research. In Frontiers in Clinical Drug Research—CNS and Neurological Disorders; Bentham Science Publishers: Sharjah, UAE, 2016; pp. 114–191. [Google Scholar]
- Yeung, S.T.; Martinez-Coria, H.; Ager, R.R.; Rodriguez-Ortiz, C.J.; Baglietto-Vargas, D.; LaFerla, F.M. Repeated cognitive stimulation alleviates memory impairments in an Alzheimer’s disease mouse model. Brain Res. Bull. 2015, 117, 10–15. [Google Scholar] [CrossRef]
- The Jackson Laboratory B6;129-Tg(APPSwe, tauP301L)1Lfa Psen1/Mmjax. Available online: https://www.jax.org/strain/004807 (accessed on 26 October 2018).
- Carroll, J.C.; Rosario, E.R.; Kreimer, S.; Villamagna, A.; Gentzschein, E.; Stanczyk, F.Z.; Pike, C.J. Sex differences in β-amyloid accumulation in 3xTg-AD mice: Role of neonatal sex steroid hormone exposure. Brain Res. 2010, 1366, 233–245. [Google Scholar] [CrossRef]
- Amrhein, V.; Greenland, S.; McShane, B. Scientists rise up against statistical significance. Nature 2019, 567, 305–307. [Google Scholar] [CrossRef]
- Esquerda-Canals, G.; Martí-Clúa, J.; Villegas, S. Pharmacokinetic parameters and mechanism of action of an efficient anti-Aβ single chain antibody fragment. PLoS ONE 2019, 14, e0217793. [Google Scholar] [CrossRef]
- Franklin, K.B.J.; Paxinos, G. Paxinos and Franklin’s The Mouse Brain in Stereotaxic Coordinates, 4th ed.; Academic Press: Cambridge, MA, USA, 2019; ISBN 9780123910578. [Google Scholar]
- Syvänen, S.; Hultqvist, G.; Gustavsson, T.; Gumucio, A.; Laudon, H.; Söderberg, L.; Ingelsson, M.; Lannfelt, L.; Sehlin, D. Efficient clearance of Aβ protofibrils in AβPP-transgenic mice treated with a brain-penetrating bifunctional antibody. Alzheimers Res. Ther. 2018, 10, 49. [Google Scholar] [CrossRef]
- Zetterberg, H.; Zetterberg, H.; Rinne, J.O.; Salloway, S.; Wei, J.; Black, R.; Grundman, M.; Liu, E. Investigators, for the A-001 201/202 Effect of Immunotherapy With Bapineuzumab on Cerebrospinal Fluid Biomarker Levels in Patients With Mild to Moderate Alzheimer Disease. Arch. Neurol. 2012, 69, 1002. [Google Scholar] [CrossRef]
- Roda, A.R.; Montoliu-Gaya, L.; Serra-Mir, G.; Villegas, S. Both Amyloid-beta Peptide and Tau Protein are affected by an Anti-amyloid-beta Antibody Fragment in Ederly 3xTg-AD Mice. Int. J. Mol. Sci. 2020, in press. [Google Scholar]
- Billings, L.M.; Oddo, S.; Green, K.N.; McGaugh, J.L.; LaFerla, F.M. Intraneuronal Aβ Causes the Onset of Early Alzheimer’s Disease-Related Cognitive Deficits in Transgenic Mice. Neuron 2005, 45, 675–688. [Google Scholar] [CrossRef]
- Oddo, S.; Billings, L.; Kesslak, J.P.; Cribbs, D.H.; LaFerla, F.M. Aβ immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron 2004, 43, 321–332. [Google Scholar] [CrossRef]
- Prut, L.; Belzung, C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur. J. Pharmacol. 2003, 463, 3–33. [Google Scholar]
- Nunez, J. Morris Water Maze Experiment. J. Vis. Exp. 2008. [Google Scholar] [CrossRef]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef]
- Esquerda-Canals, G.; Martí-Clúa, J.; Roda, A.R.; Villegas, S. An Intracellular Amyloid-β/AβPP Epitope Correlates with Neurodegeneration in those Neuronal Populations Early Involved in Alzheimer’s Disease. J. Alzheimer Dis. 2017, 59, 1079–1096. [Google Scholar] [CrossRef]
- Nisbet, R.M.; Polanco, J.C.; Ittner, L.M.; Götz, J. Tau aggregation and its interplay with amyloid-β. Acta Neuropathol. 2015, 129, 207–220. [Google Scholar]
- Montoliu-Gaya, L.; Güell-Bosch, J.; Esquerda-Canals, G.; Roda, A.R.; Serra-Mir, G.; Lope-Piedrafita, S.; Sánchez-Quesada, J.L.; Villegas, S. Differential effects of apoE and apoJ mimetic peptides on the action of an anti-Aβ scFv in 3xTg-AD mice. Biochem. Pharmacol. 2018, 155, 380–392. [Google Scholar] [CrossRef]
- Roda, A.R.; Montoliu-Gaya, L.; Villegas, S. The Role of Apolipoprotein e Isoforms in Alzheimer’s Disease. J. Alzheimer Dis. 2019, 68, 459–471. [Google Scholar]
- Crous-Bou, M.; Minguillón, C.; Gramunt, N.; Molinuevo, J.L. Alzheimer’s disease prevention: From risk factors to early intervention. Alzheimer Res. Ther. 2017, 9, 71. [Google Scholar]
- Belfiore, R.; Rodin, A.; Ferreira, E.; Velazquez, R.; Branca, C.; Caccamo, A.; Oddo, S. Temporal and regional progression of Alzheimer’s disease-like pathology in 3xTg-AD mice. Aging Cell 2019, 18. [Google Scholar] [CrossRef]
- Busche, M.A.; Chen, X.; Henning, H.A.; Reichwald, J.; Staufenbiel, M.; Sakmann, B.; Konnerth, A. Critical role of soluble amyloid-β for early hippocampal hyperactivity in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2012, 109, 8740–8745. [Google Scholar] [CrossRef]
- Greene, S.J.; Killiany, R.J. Hippocampal Subregions are Differentially Affected in the Progression to Alzheimer’s Disease. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2012, 295, 132–140. [Google Scholar] [CrossRef]
- Su, L.; Hayes, L.; Soteriades, S.; Williams, G.; Brain, S.A.E.; Firbank, M.J.; Longoni, G.; Arnold, R.J.; Rowe, J.B.; O’Brien, J.T. Hippocampal Stratum Radiatum, Lacunosum, and Moleculare Sparing in Mild Cognitive Impairment. J. Alzheimer Dis. 2018, 61, 415–424. [Google Scholar] [CrossRef]
- Revilla, S.; Suñol, C.; García-Mesa, Y.; Giménez-Llort, L.; Sanfeliu, C.; Cristòfol, R. Physical exercise improves synaptic dysfunction and recovers the loss of survival factors in 3xTg-AD mouse brain. Neuropharmacology 2014, 81, 55–63. [Google Scholar] [CrossRef]
- Sabbagh, M.; Sadowsky, C.; Tousi, B.; Agronin, M.E.; Alva, G.; Armon, C.; Bernick, C.; Keegan, A.P.; Karantzoulis, S.; Baror, E.; et al. Effects of a combined transcranial magnetic stimulation (TMS) and cognitive training intervention in patients with Alzheimer’s disease. Alzheimer’s Dement. 2019. [Google Scholar] [CrossRef]
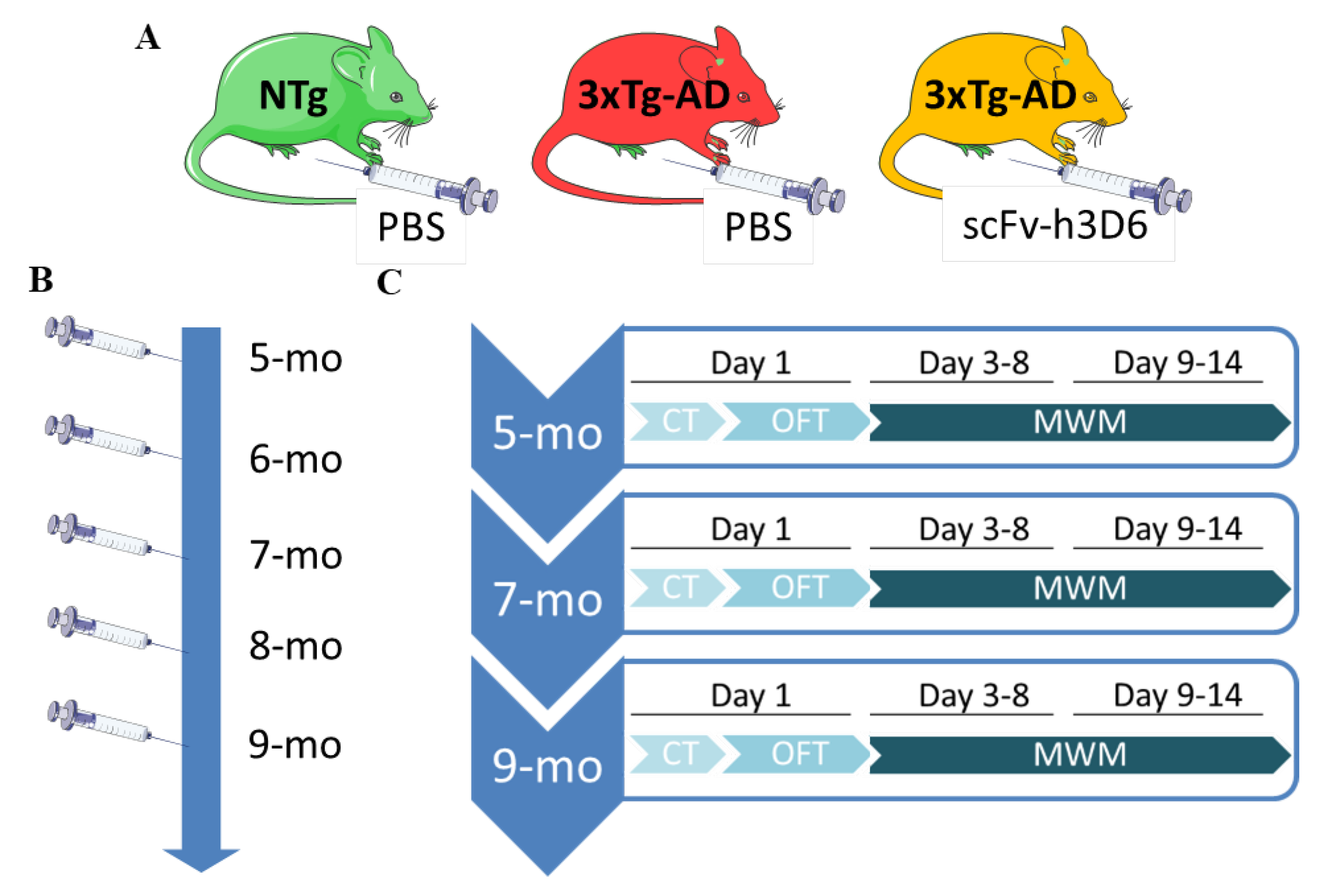
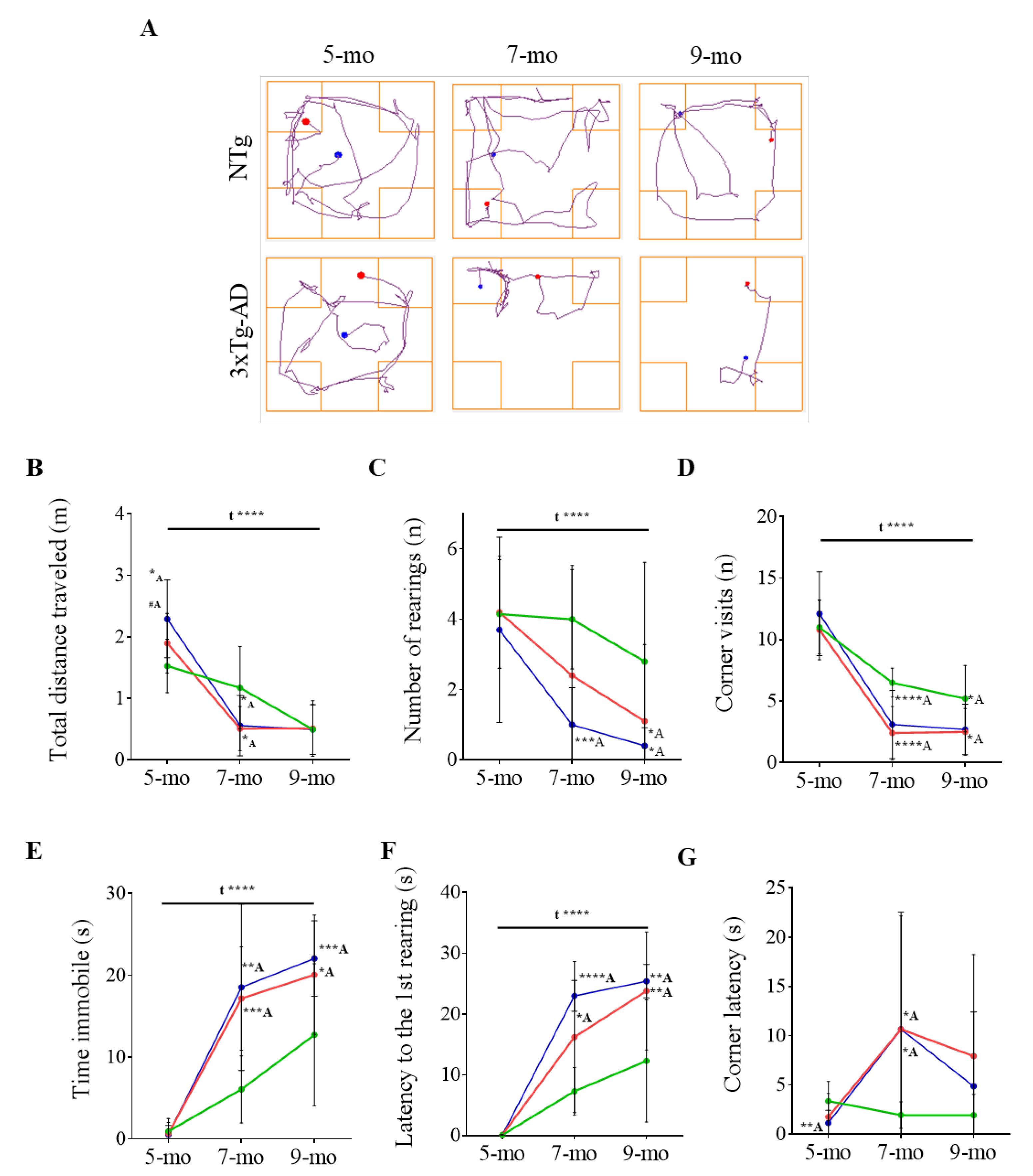
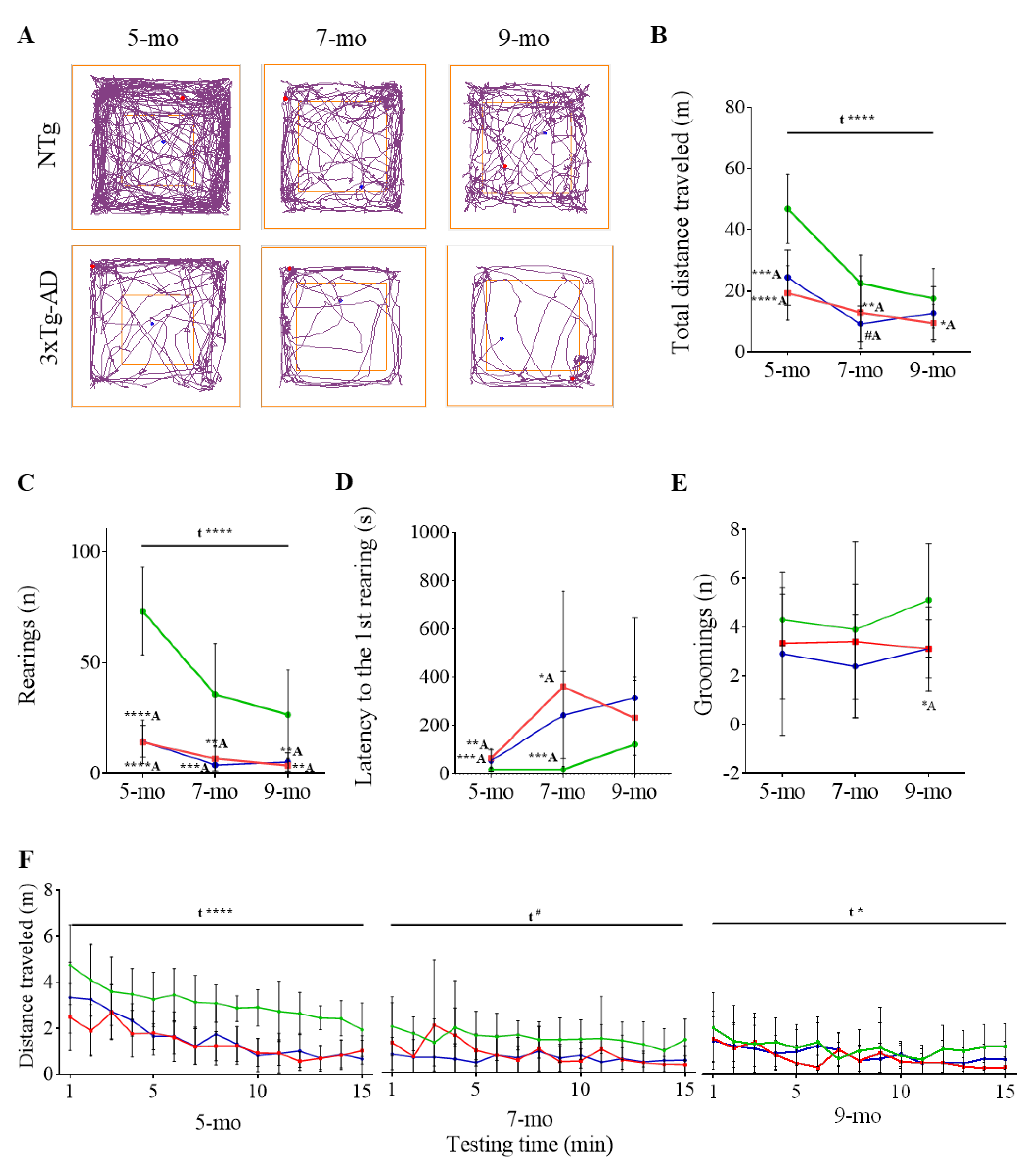
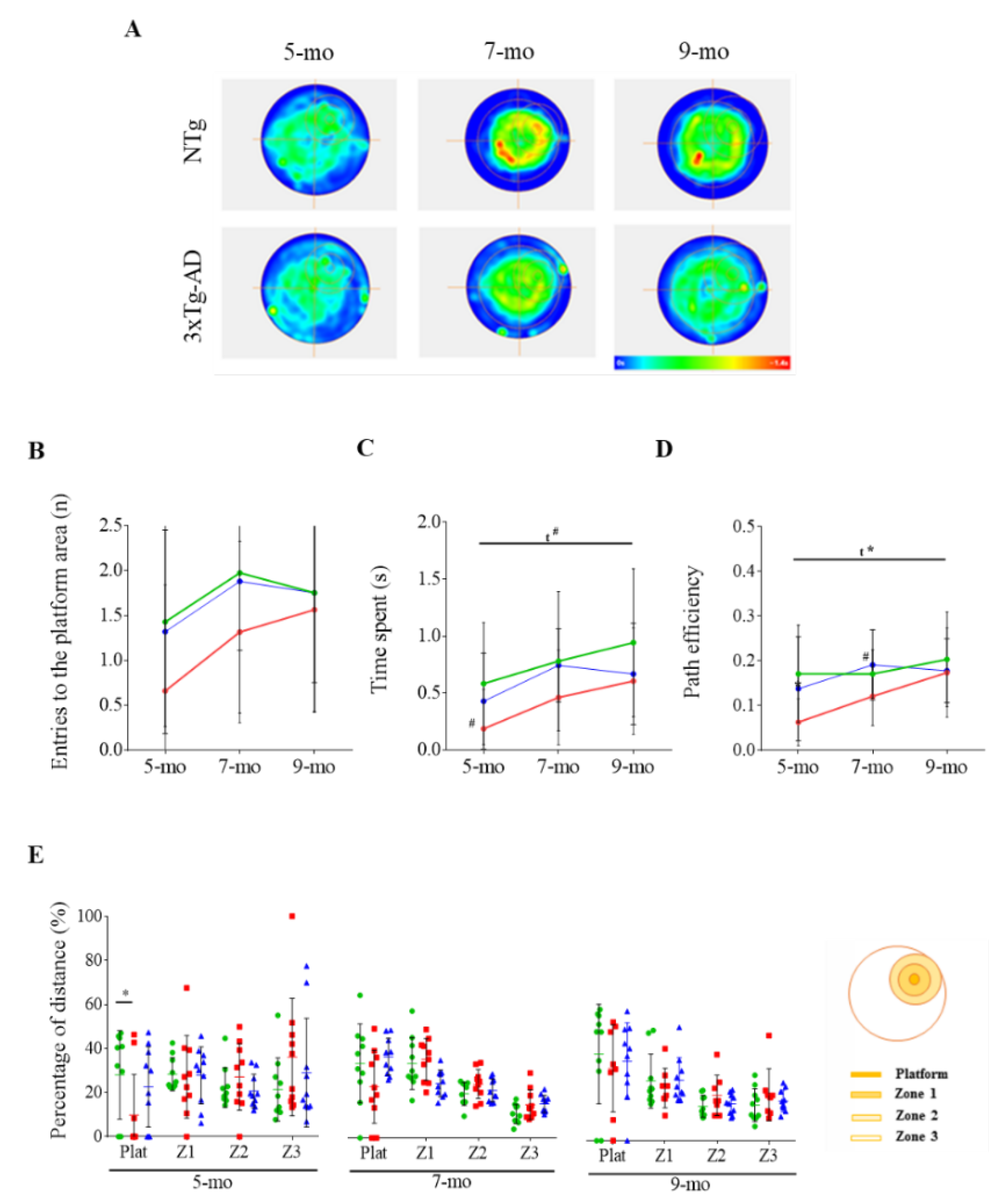


| Corner Test | NTg | PBS-Treated 3xTg-AD | ScFv-h3D6-Treated 3xTg-AD |
|---|---|---|---|
| Horizontal activity | |||
| Distance traveled (m) | |||
| 5-month-old | 1.53 ± 0.14 | 1.90 ± 0.15 #A | 2.29 ± 0.20 **A |
| 7-month-old | 0.96 ± 0.06 | 0.51 ± 0.11 **A | 0.56 ± 0.16 *A |
| 9-month-old | 0.50 ± 0.13 | 0.49 ± 0.16 | 0.49 ± 0.13 |
| Corner visits (n) | |||
| 5-month-old | 11.00 ± 0.68 | 10.80 ± 0.77 | 12.10 ± 1.08 |
| 7-month-old | 6.50 ± 0.37 | 2.4 ± 0.69 ****A | 3.10 ± 0.88 **A |
| 9-month-old | 5.20 ± 0.85 | 2.63 ± 0.58 *A | 2.70 ± 0.65 *A |
| Corner latency (s) | |||
| 5-month-old | 3.37 ± 0.63 | 1.76 ± 0.75 | 1.15 ± 0.41 **A |
| 7-month-old | 1.95 ± 0.43 | 10.67 ± 3.64 *A | 10.67 ± 3.77 *A |
| 9-month-old | 1.94 ± 0.67 | 5.50 ± 2.43 | 4.88 ± 2.39 |
| Time in the corner (s) | |||
| 5-month-old | 10.56 ± 0.66 | 10.25 ± 1.12 | 9.26 ± 1.17 |
| 7-month-old | 10.52 ± 1.08 | 5.99 ± 1.78 *A | 4.70 ± 1.43 **A |
| 9-month-old | 9.08 ± 1.77 | 8.20 ± 2.93 | 7.71 ± 1.86 |
| Vertical activity | |||
| Rearings (n) | |||
| 5-month-old | 4.15 ± 0.49 | 4.20 ± 0.51 | 3.70 ± 0.83 |
| 7-month-old | 4.00 ± 0.45 | 2.67 ± 1.01 | 1.00 ± 0.33 ****A |
| 9-month-old | 2.80 ± 0.89 | 0.38 ± 0.24 *A | 0.40 ± 0.16 *A |
| 1st rearing latency (s) | |||
| 5-month-old | 0.13 ± 0.02 | 0.13 ± 0.02 | 0.12 ±0.03 |
| 7-month-old | 7.30 ± 1.24 | 18.00 ± 3.72 *A | 23.00 ± 2.53 ****A |
| 9-month-old | 12.30 ± 3.18 | 25.88 ± 2.43 **A | 25.40 ± 2.76 **A |
| Other emotional behaviors | |||
| Immobility time (s) | |||
| 5-month-old | 0.92 ± 0.50 | 0.65 ± 0.43 | 0.53 ± 0.35 |
| 7-month-old | 6.06 ± 1.30 | 17.18 ± 2.00 ***A | 18.54 ± 3.21 **A |
| 9-month-old | 12.73 ± 2.75 | 20.59 ± 2.50 *A | 22.06 ± 1.46 **A |
| Defecations (boli) | |||
| 5-month-old | 0.40 ± 0.22 | 0.30 ± 0.21 | 0.20 ± 0.13 |
| 7-month-old | 1.00 ± 0.26 | 0.56 ± 0.28 | 0.50 ± 0.22 |
| 9-month-old | 0.80 ± 0.20 | 0.25 ± 0.15 #A | 0.00 ± 0.00 ***A,#B |
| Open-Field Test | NTg | PBS-Treated 3xTg-AD | ScFv-h3D6-Treated 3xTg-AD |
|---|---|---|---|
| Horizontal activity | |||
| Total distance traveled (m) | |||
| 5-month-old | 46.87 ± 3.54 | 19.36 ± 2.79 ****A | 24.30 ± 2.88 ***A |
| 7-month-old | 22.49 ± 2.88 | 12.94 ± 3.75 #A | 9.23 ± 1.82 **A |
| 9-month-old | 17.54 ± 3.07 | 10.43 ± 2.05 *A | 12.74 ± 2.75 |
| Entries to the center (n) | |||
| 5-month-old | 41.70 ± 5.32 | 23.50 ± 4.92 *A | 26.60 ± 5.73 #A |
| 7-month-old | 22.00 ± 2.77 | 13.20 ± 4.86 | 12.3 ± 3.81 #A |
| 9-month-old | 15.6 ± 2.76 | 15.25 ± 4.64 | 14.10 ± 4.29 |
| Distance in the center (m) | |||
| 5-month-old | 6.61 ± 0.58 | 3.68 ± 0.73 **A | 4.02 ± 0.88 *A |
| 7-month-old | 2.85 ± 0.45 | 2.10 ± 0.82 | 1.57 ± 0.52 #A |
| 9-month-old | 1.93 ± 0.31 | 1.58 ± 0.45 | 1.91 ± 0.58 |
| Distance in the periphery (m) | |||
| 5-month-old | 40.26 ± 3.08 | 15.49 ± 2.20 ****A | 20.29 ± 2.34 ****A |
| 7-month-old | 19.65 ± 2.52 | 10.83 ± 3.06 *A | 7.66 ± 1.32 **A |
| 9-month-old | 15.61 ± 2.82 | 8.86 ± 1.61 *A | 10.84 ± 2.22 |
| Ratio center/periphery (dist) | |||
| 5-month-old | 0.17 ± 0.01 | 0.24 ± 0.04 | 0.20 ± 0.03 |
| 7-month-old | 0.15 ± 0.01 | 0.23 ± 0.05 | 0.17 ± 0.03 |
| 9-month-old | 0.13 ± 0.02 | 0.15 ± 0.03 | 0.16 ± 0.03 |
| Time in the center (s) | |||
| 5-month-old | 76.75 ± 9.03 | 93.07 ± 23.87 | 75.20 ± 17.30 |
| 7-month-old | 50.66 ± 7.40 | 159.5 ± 46.4 *A | 52.46 ± 17.91 *B |
| 9-month-old | 38.99 ± 7.74 | 57.98 ± 25.95 | 68.30 ± 23.00 |
| Time in the periphery (s) | |||
| 5-month-old | 823.3 ± 9.04 | 723.2 ± 78.2 | 824.7 ± 17.3 |
| 7-month-old | 849.3 ± 7.39 | 740.2 ± 46.34 *A | 847.5 ± 17.9 *B |
| 9-month-old | 861.01 ± 7.73 | 841.98 ± 25.94 | 831.7 ± 23.0 |
| Ratio center/periphery (time) | |||
| 5-month-old | 0.09 ± 0.01 | 0.15 ± 0.04 | 0.10 ± 0.02 |
| 7-month-old | 0.06 ± 0.01 | 0.27 ± 0.10 #A | 0.07 ± 0.02 #B |
| 9-month-old | 0.05 ± 0.01 | 0.08 ± 0.04 | 0.09 ± 0.03 |
| Vertical activity | |||
| Rearings (n) | |||
| 5-month-old | 73.20 ± 6.28 | 14.78 ± 3.22 ****A | 14.50 ± 2.25 ****A |
| 7-month-old | 35.60 ± 7.26 | 6.60 ± 1.75 **A | 3.80 ± 0.89 ***A |
| 9-month-old | 26.50 ± 6.36 | 3.57 ± 1.09 **A | 5.10 ± 1.33 **A |
| 1st rearing latency (s) | |||
| 5-month-old | 18.70 ± 3.10 | 64.89 ± 13.50 **A | 54.40 ± 14.48 ***A |
| 7-month-old | 18.20 ± 4.36 | 360.9 ± 124.8 *A | 243.3 ± 57.1 ****A |
| 9-month-old | 123.4 ± 87.7 | 269.1 ± 48.9 | 314.9 ± 105.1 |
| Other emotional behaviors | |||
| Immobility time (s) | |||
| 5-month-old | 116.8 ± 38.44 | 304.95 ± 84.19 #A | 215.27 ± 88.67 |
| 7-month-old | 229.41 ± 80.38 | 459.06 ± 107.27 | 310.07 ± 127.14 |
| 9-month-old | 364.31 ± 108.38 | 285.86 ± 125.45 | 525.6 ± 115.84 |
| Self-groomings (n) | |||
| 5-month-old | 4.30 ± 0.34 | 3.33 ± 0.73 | 2.90 ± 1.06 |
| 7-month-old | 3.90 ± 1.14 | 3.40 ± 0.75 | 2.40 ± 0.7 |
| 9-month-old | 5.10 ± 0.74 | 3.50 ± 0.54 | 3.10 ± 0.38 *A |
| Self-groomings latency (s) | |||
| 5-month-old | 148.4 ± 39.3 | 265.4 ± 31.8 *A | 343.0 ± 98.8 #A |
| 7-month-old | 276.5 ± 71.1 | 381.7 ± 77.9 | 402.3 ± 94.1 |
| 9-month-old | 80.20 ± 12.76 | 177.4 ± 35.2 *A | 201.9 ± 32.0 **A |
| Time on self-grooming (s) | |||
| 5-month-old | 7.20 ± 0.98 | 5.24 ± 0.95 | 6.07 ± 2.31 |
| 7-month-old | 7.35 ± 1.93 | 6.15 ± 1.63 | 5.10 ± 1.42 |
| 9-month-old | 8.77 ± 1.42 | 7.46 ± 0.94 | 7.05 ± 0.77 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roda, A.R.; Esquerda-Canals, G.; Martí-Clúa, J.; Villegas, S. Cognitive Impairment in the 3xTg-AD Mouse Model of Alzheimer’s Disease is Affected by Aβ-ImmunoTherapy and Cognitive Stimulation. Pharmaceutics 2020, 12, 944. https://doi.org/10.3390/pharmaceutics12100944
Roda AR, Esquerda-Canals G, Martí-Clúa J, Villegas S. Cognitive Impairment in the 3xTg-AD Mouse Model of Alzheimer’s Disease is Affected by Aβ-ImmunoTherapy and Cognitive Stimulation. Pharmaceutics. 2020; 12(10):944. https://doi.org/10.3390/pharmaceutics12100944
Chicago/Turabian StyleRoda, Alejandro R., Gisela Esquerda-Canals, Joaquim Martí-Clúa, and Sandra Villegas. 2020. "Cognitive Impairment in the 3xTg-AD Mouse Model of Alzheimer’s Disease is Affected by Aβ-ImmunoTherapy and Cognitive Stimulation" Pharmaceutics 12, no. 10: 944. https://doi.org/10.3390/pharmaceutics12100944
APA StyleRoda, A. R., Esquerda-Canals, G., Martí-Clúa, J., & Villegas, S. (2020). Cognitive Impairment in the 3xTg-AD Mouse Model of Alzheimer’s Disease is Affected by Aβ-ImmunoTherapy and Cognitive Stimulation. Pharmaceutics, 12(10), 944. https://doi.org/10.3390/pharmaceutics12100944





