Neuronanomedicine: An Up-to-Date Overview
Abstract
:1. Introduction
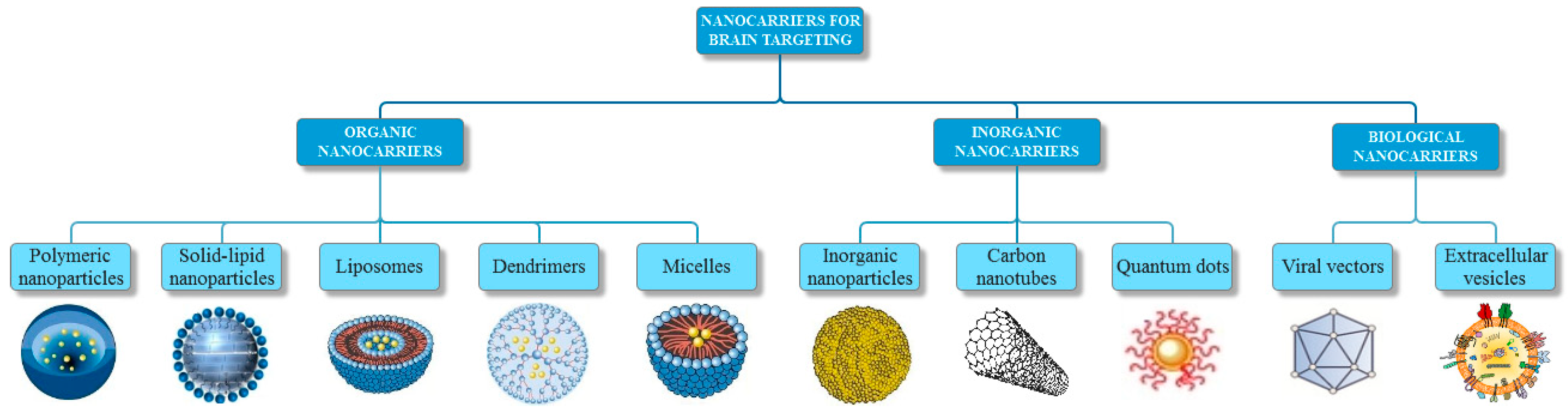
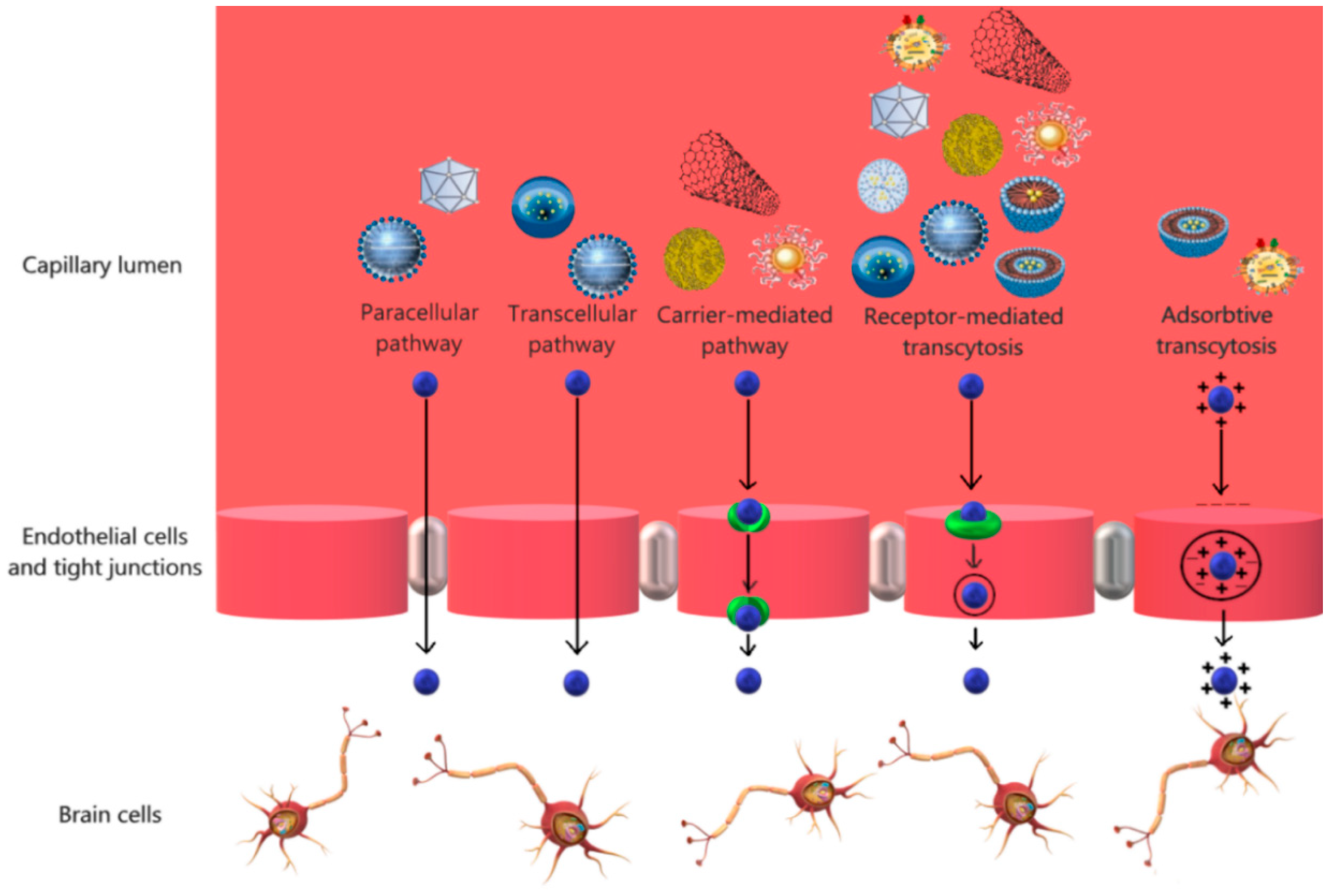
2. Nanocarriers for Brain Targeting
2.1. Organic Nanocarriers
2.1.1. Polymeric Nanoparticles
2.1.2. Solid-Lipid Nanoparticles
2.1.3. Liposomes
2.1.4. Dendrimers
2.1.5. Micelles
2.2. Inorganic Nanocarriers
2.2.1. Inorganic Nanoparticles
2.2.2. Carbon Nanotubes
2.2.3. Quantum Dots
2.3. Biological Vectors
2.3.1. Viral Vectors
2.3.2. Extracellular Vesicles
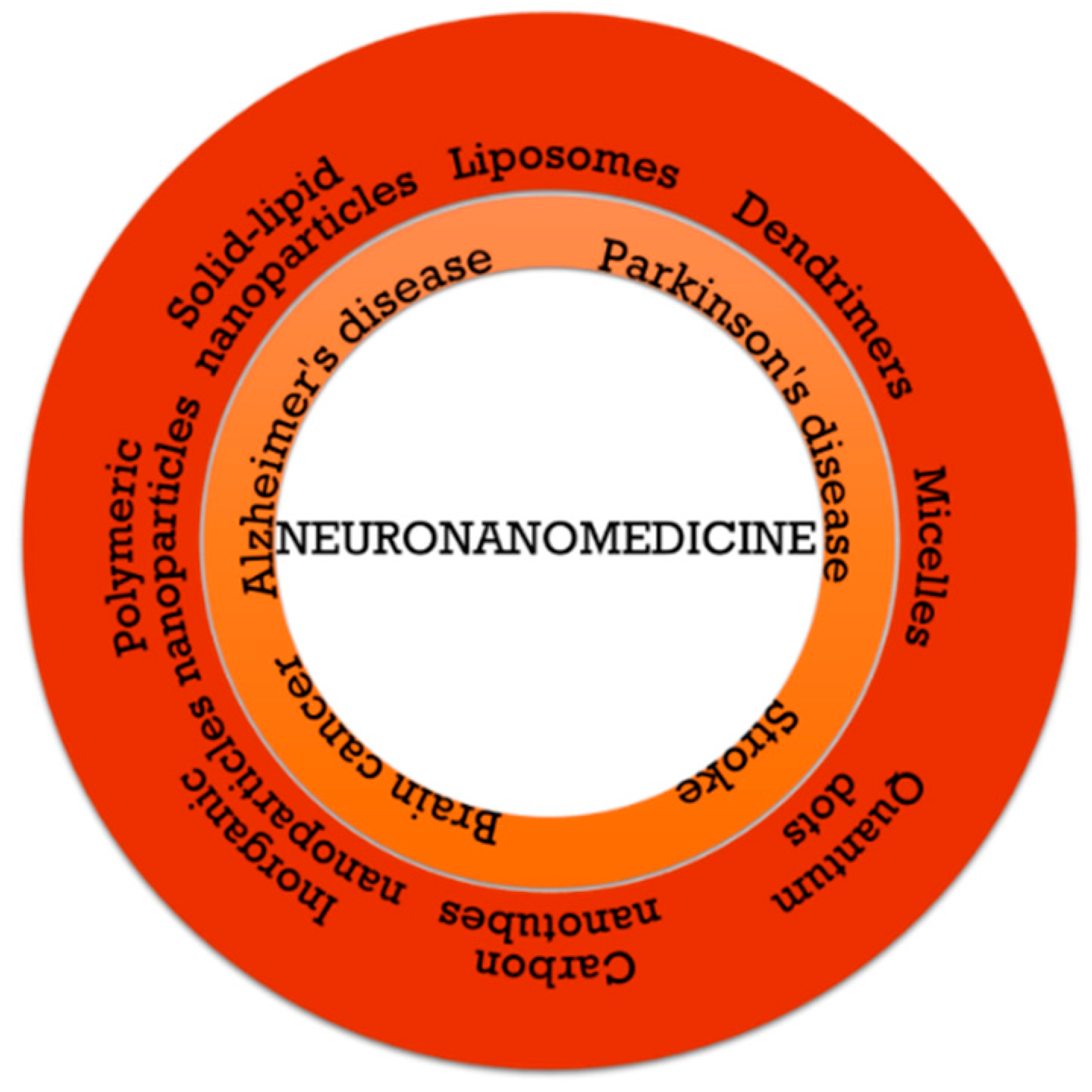
3. Nanomedicine in Central Nervous System Disorders
3.1. Brain Cancer
3.2. Neurodegenerative Diseases
3.3. Stroke
3.4. Clinical Applications
4. Challenges and Limitations
5. Conclusions and Perspectives
Author Contributions
Conflicts of Interest
References
- Kennedy, M.B. Biochemistry and neuroscience: The twain need to meet. Curr. Opin. Neurobiol. 2017, 43, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Rivnay, J.; Wang, H.; Fenno, L.; Deisseroth, K.; Malliaras, G.G. Next-generation probes, particles, and proteins for neural interfacing. Sci. Adv. 2017, 3, e1601649. [Google Scholar] [CrossRef] [PubMed]
- Mader, B.J.; Boulis, N.M. Chapter 9—Viral Vectors and Other Modulatory Biologics. In Innovative Neuromodulation; Arle, J., Shils, J., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 171–205. [Google Scholar]
- Crean, P.M.; Tirupathi, S. 24-Essentials of Neurology and Neuromuscular Disorders. In A Practice of Anesthesia for Infants and Children, 6th ed.; Coté, C.J., Lerman, J., Anderson, B.J., Eds.; Elsevier: Philadelphia, PA, USA, 2019; pp. 561.e4–580.e4. [Google Scholar]
- Barnabas, W. Drug targeting strategies into the brain for treating neurological diseases. J. Neurosci. Methods 2018, 311, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Di Luca, M.; Nutt, D.; Oertel, W.; Boyer, P.; Jaarsma, J.; Destrebecq, F.; Esposito, G.; Quoidbach, V. Towards earlier diagnosis and treatment of disorders of the brain. Bull. World Health Organ. 2018, 96, 298. [Google Scholar] [CrossRef] [PubMed]
- Komane, P.P.; Choonara, Y.E.; Toit, L.C.d.; Kumar, P.; Kondiah, P.P.D.; Modi, G.; Pillay, V. Diagnosis and Treatment of Neurological and Ischemic Disorders Employing Carbon Nanotube Technology. J. Nanomater. 2016, 2016, 34. [Google Scholar] [CrossRef]
- Dong, X. Current Strategies for Brain Drug Delivery. Theranostics 2018, 8, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- Siuly, S.; Zhang, Y. Medical Big Data: Neurological Diseases Diagnosis Through Medical Data Analysis. Data Sci. Eng. 2016, 1, 54–64. [Google Scholar] [CrossRef]
- Gupta, S.; Dhanda, S.; Sandhir, R. Anatomy and physiology of blood-brain barrier. In Brain Targeted Drug Delivery System; Gao, H., Gao, X., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 7–31. [Google Scholar]
- Alexander, J.J. Blood-brain barrier (BBB) and the complement landscape. Mol. Immunol. 2018, 102, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Tumani, H.; Huss, A.; Bachhuber, F. Chapter 2—The cerebrospinal fluid and barriers—Anatomic and physiologic considerations. In Handbook of Clinical Neurology; Deisenhammer, F., Teunissen, C.E., Tumani, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 21–32. [Google Scholar]
- Herrera, E.M.; Phillips-Farfán, B.V.; Ospina, G.G. Endothelial Cell Plasticity in the Normal and Injured Central Nervous System; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Edwards, C.A.; Kouzani, A.; Lee, K.H.; Ross, E.K. Neurostimulation Devices for the Treatment of Neurologic Disorders. Mayo Clin. Proc. 2017, 92, 1427–1444. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Singh, G.; Khanna, K.; Kaur, N. Nanotechnology: A Review, Second National Conference on Advances in Manufacturing Systems; S B S State Technical Campus: Ferozepur, India, 2015. [Google Scholar]
- Husain, Q. Carbon nanotubes mediated immobilized glucose oxidase biosensors as an effective and sensitive analytical tool. Biointerface Res. Appl. Chem. 2018, 8, 3060–3074. [Google Scholar]
- Kargozar, S.; Mozafari, M. Nanotechnology and Nanomedicine: Start small, think big. Mater. Today Proc. 2018, 5, 15492–15500. [Google Scholar] [CrossRef]
- Afchangi, L.; Fazli, M. Investigation of the nanotubes-H2O complex by molecular mechanics and semi empirical methods. Biointerface Res. Appl. Chem. 2018, 8, 3700–3704. [Google Scholar]
- Naskar, A.; Bera, S.; Bhattacharya, R.; Roy, S.S.; Jana, S. Solution based PEG and PVP capped maghemite–reduced graphene oxide nanocomposites: Cell viability study. Biointerface Res. Appl. Chem. 2018, 8, 3751–3757. [Google Scholar]
- Ramsden, J.J. Chapter 1—What is Nanotechnology? In Applied Nanotechnology, 3rd ed.; Ramsden, J.J., Ed.; William Andrew Publishing: Amsterdam, The Netherlands, 2018; pp. 3–13. [Google Scholar]
- Çetin, M.; Aytekin, E.; Yavuz, B.; Bozdağ-Pehlivan, S. Chapter 7—Nanoscience in Targeted Brain Drug Delivery. In Nanotechnology Methods for Neurological Diseases and Brain Tumors; Gürsoy-Özdemir, Y., Bozdağ-Pehlivan, S., Sekerdag, E., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 117–147. [Google Scholar]
- Rasoolzadeh, R.; Mehrnejad, F.; Taghdir, M.; Yaghmaei, P. Theoretical investigation of interaction energies between carbon and BN nanotubes with human hepcidin peptides: Insights into the semi empirical and Monte Carlo methods. Biointerface Res. Appl. Chem. 2018, 8, 3594–3601. [Google Scholar]
- Feldman, D. Polymer nanocomposites for tissue engineering, antimicrobials and drug delivery. Biointerface Res. Appl. Chem. 2018, 8, 3153–3160. [Google Scholar]
- Rahman, H.; Krishnamoorthy, B.; Tamilselvan, N.; Siram, K.; Karthik, S.; Hariprasad, R. Chapter 7—Nanomaterials in drug delivery: Existing scenario and potential scope. In Nanobiomaterials in Drug Delivery; Grumezescu, A.M., Ed.; William Andrew Publishing: Amsterdam, The Netherlands, 2016; pp. 197–228. [Google Scholar]
- Comoglu, T.; Arisoy, S.; Akkus, Z.B. Nanocarriers for Effective Brain Drug Delivery. Curr. Top. Med. Chem. 2017, 17, 1490–1506. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Chen, J.; Gao, J. Nanocarriers as a powerful vehicle to overcome blood-brain barrier in treating neurodegenerative diseases: Focus on recent advances. Asian J. Pharm. Sci. 2018. [Google Scholar] [CrossRef]
- Claudio, P.; Reatul, K.; Brigitte, E.; Geraldine, P. Chapter 10—Drug-delivery nanocarriers to cross the blood–brain barrier. In Nanobiomaterials in Drug Delivery; Grumezescu, A.M., Ed.; William Andrew Publishing: Amsterdam, The Netherlands, 2016; pp. 333–370. [Google Scholar]
- Pund, S.; Joshi, A. Chapter 23—Nanoarchitectures for Neglected Tropical Protozoal Diseases: Challenges and State of the Art. In Nano- and Microscale Drug Delivery Systems; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 439–480. [Google Scholar]
- Jeevanandam, J.; Aing, Y.S.; Chan, Y.S.; Pan, S.; Danquah, M.K. Chapter 3—Nanoformulation and Application of Phytochemicals as Antimicrobial Agents. In Antimicrobial Nanoarchitectonics; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 61–82. [Google Scholar]
- Sengel-Turk, C.T.; Gumustas, M.; Uslu, B.; Ozkan, S.A. Chapter 10—Nanosized Drug Carriers for Oral Delivery of Anticancer Compounds and the Importance of the Chromatographic Techniques. In Nano- and Microscale Drug Delivery Systems; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 165–195. [Google Scholar]
- Singh, N.; Joshi, A.; Toor, A.P.; Verma, G. Chapter 27—Drug delivery: Advancements and challenges. In Nanostructures for Drug Delivery; Andronescu, E., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 865–886. [Google Scholar]
- El-Say, K.M.; El-Sawy, H.S. Polymeric nanoparticles: Promising platform for drug delivery. Int. J. Pharm. 2017, 528, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Kammari, R.; Das, N.G.; Das, S.K. Chapter 6—Nanoparticulate Systems for Therapeutic and Diagnostic Applications. In Emerging Nanotechnologies for Diagnostics, Drug Delivery and Medical Devices; Mitra, A.K., Cholkar, K., Mandal, A., Eds.; Elsevier: Boston, MA, USA, 2017; pp. 105–144. [Google Scholar]
- Chifiriuc, M.C.; Kamerzan, C.; Lazar, V. Chapter 12—Essential Oils and Nanoparticles: New Strategy to Prevent Microbial Biofilms. In Nanostructures for Antimicrobial Therapy; Ficai, A., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 279–291. [Google Scholar]
- Joseph, M.; Trinh, H.M.; Mitra, A.K. Chapter 7—Peptide and Protein-Based Therapeutic Agents. In Emerging Nanotechnologies for Diagnostics, Drug Delivery and Medical Devices; Mitra, A.K., Cholkar, K., Mandal, A., Eds.; Elsevier: Boston, MA, USA, 2017; pp. 145–167. [Google Scholar]
- Mihai, M.M.; Holban, A.M.; Călugăreanu, A.; Orzan, O.A. Chapter 11—Recent advances in diagnosis and therapy of skin cancers through nanotechnological approaches. In Nanostructures for Cancer Therapy; Ficai, A., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 285–306. [Google Scholar]
- Mandal, A.; Bisht, R.; Pal, D.; Mitra, A.K. Chapter 4—Diagnosis and Drug Delivery to the Brain: Novel Strategies. In Emerging Nanotechnologies for Diagnostics, Drug Delivery and Medical Devices; Mitra, A.K., Cholkar, K., Mandal, A., Eds.; Elsevier: Boston, MA, USA, 2017; pp. 59–83. [Google Scholar]
- Neves, A.R.; Queiroz, J.F.; Weksler, B.; Romero, I.A.; Couraud, P.O.; Reis, S. Solid lipid nanoparticles as a vehicle for brain-targeted drug delivery: Two new strategies of functionalization with apolipoprotein E. Nanotechnology 2015, 26, 495103. [Google Scholar] [CrossRef] [PubMed]
- Gastaldi, L.; Battaglia, L.; Peira, E.; Chirio, D.; Muntoni, E.; Solazzi, I.; Gallarate, M.; Dosio, F. Solid lipid nanoparticles as vehicles of drugs to the brain: Current state of the art. Eur. J. Pharm. Biopharm. 2014, 87, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.R.; Queiroz, J.F.; Lima, S.A.C.; Reis, S. Apo E-Functionalization of Solid Lipid Nanoparticles Enhances Brain Drug Delivery: Uptake Mechanism and Transport Pathways. Bioconjugate Chem. 2017, 28, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Magro, R.D.; Ornaghi, F.; Cambianica, I.; Beretta, S.; Re, F.; Musicanti, C.; Rigolio, R.; Donzelli, E.; Canta, A.; Ballarini, E.; et al. ApoE-modified solid lipid nanoparticles: A feasible strategy to cross the blood-brain barrier. J. Control. Release Off. J. Control. Release Soc. 2017, 249, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Abbina, S.; Parambath, A. 14—PEGylation and its alternatives: A summary. In Engineering of Biomaterials for Drug Delivery Systems; Parambath, A., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 363–376. [Google Scholar]
- Kulkarni, V.S.; Shaw, C. Chapter 4—Formulating Creams, Gels, Lotions, and Suspensions. In Essential Chemistry for Formulators of Semisolid and Liquid Dosages; Kulkarni, V.S., Shaw, C., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 29–41. [Google Scholar]
- Karandikar, S.; Mirani, A.; Waybhase, V.; Patravale, V.B.; Patankar, S. Chapter 10—Nanovaccines for oral delivery-formulation strategies and challenges. In Nanostructures for Oral Medicine; Andronescu, E., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 263–293. [Google Scholar]
- Rai, M.; Ingle, A.P.; Bansod, S.; Kon, K. Chapter 9—Tackling the Problem of Tuberculosis by Nanotechnology: Disease Diagnosis and Drug Delivery. In Nanotechnology in Diagnosis, Treatment and Prophylaxis of Infectious Diseases; Rai, M., Kon, K., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 133–149. [Google Scholar]
- Li, S.; Johnson, J.; Peck, A.; Xie, Q. Near infrared fluorescent imaging of brain tumor with IR780 dye incorporated phospholipid nanoparticles. J. Transl. Med. 2017, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, R.N. Liposomal Drug Delivery to the Central Nervous System. In Liposomes; Catala, A., Ed.; IntechOpen: London, UK, 2017. [Google Scholar]
- Zhou, Y.; Peng, Z.; Seven, E.S.; Leblanc, R. Crossing the blood-brain barrier with nanoparticles. J. Control. Release 2018, 270, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Priya, L.B.; Baskaran, R.; Padma, V.V. Chapter 21—Phytonanoconjugates in oral medicine. In Nanostructures for Oral Medicine; Andronescu, E., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 639–668. [Google Scholar]
- Verma, G.; Rajagopalan, M.D.; Valluru, R.; Sridhar, K.A. Chapter 7—Nanoparticles: A Novel Approach to Target Tumors. In Nano- and Microscale Drug Delivery Systems; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 113–129. [Google Scholar]
- Gumustas, M.; Sengel-Turk, C.T.; Gumustas, A.; Ozkan, S.A.; Uslu, B. Chapter 5—Effect of Polymer-Based Nanoparticles on the Assay of Antimicrobial Drug Delivery Systems. In Multifunctional Systems for Combined Delivery, Biosensing and Diagnostics; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 67–108. [Google Scholar]
- Acharya, G.; Mitra, A.K.; Cholkar, K. Chapter 10—Nanosystems for Diagnostic Imaging, Biodetectors, and Biosensors. In Emerging Nanotechnologies for Diagnostics, Drug Delivery and Medical Devices; Mitra, A.K., Cholkar, K., Mandal, A., Eds.; Elsevier: Boston, MA, USA, 2017; pp. 217–248. [Google Scholar]
- Srinageshwar, B.; Peruzzaro, S.; Andrews, M.; Johnson, K.; Hietpas, A.; Clark, B.; McGuire, C.; Petersen, E.; Kippe, J.; Stewart, A.; et al. PAMAM Dendrimers Cross the Blood-Brain Barrier When Administered through the Carotid Artery in C57BL/6J Mice. Int. J. Mol. Sci. 2017, 18, 628. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, H.; Wu, Y. Dendrimer advances for the central nervous system delivery of therapeutics. ACS Chem. Neurosci. 2014, 5, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Bhattacharjee, J.; Barick, K.C.; Verma, G.; Hassan, P.A.; Yakhmi, J.V. Chapter 7—Interfacial engineering of nanoparticles for cancer therapeutics. In Nanostructures for Cancer Therapy; Ficai, A., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 177–209. [Google Scholar]
- Wang, X.-J.; Gao, Y.-P.; Lu, N.-N.; Li, W.-S.; Xu, J.-F.; Ying, X.-Y.; Wu, G.; Liao, M.-H.; Tan, C.; Shao, L.-X.; et al. Endogenous Polysialic Acid Based Micelles for Calmodulin Antagonist Delivery against Vascular Dementia. ACS Appl. Mater. Interfaces 2016, 8, 35045–35058. [Google Scholar] [CrossRef] [PubMed]
- Atabaev, T.S. Chapter 8—Multimodal inorganic nanoparticles for biomedical applications. In Nanobiomaterials in Medical Imaging; Grumezescu, A.M., Ed.; William Andrew Publishing: Amsterdam, The Netherlands, 2016; pp. 253–278. [Google Scholar]
- Jiao, M.; Zhang, P.; Meng, J.; Li, Y.; Liu, C.; Luo, X.; Gao, M. Recent advancements in biocompatible inorganic nanoparticles towards biomedical applications. Biomater. Sci. 2018, 6, 726–745. [Google Scholar] [CrossRef] [PubMed]
- Tsou, Y.H.; Zhang, X.Q.; Zhu, H.; Syed, S.; Xu, X. Drug Delivery to the Brain across the Blood-Brain Barrier Using Nanomaterials. Small 2017, 13, 1701921. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, R.I. Blood-Brain Delivery Methods Using Nanotechnology. Pharmaceutics 2018, 10, 269. [Google Scholar] [CrossRef] [PubMed]
- Zarschler, K.; Rocks, L.; Licciardello, N.; Boselli, L.; Polo, E.; Garcia, K.P.; de Cola, L.; Stephan, H.; Dawson, K.A. Ultrasmall inorganic nanoparticles: State-of-the-art and perspectives for biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1663–1701. [Google Scholar] [CrossRef] [PubMed]
- Subramani, K.; Mehta, M. Chapter 19—Nanodiagnostics in microbiology and dentistry. In Emerging Nanotechnologies in Dentistry, 2nd ed.; Subramani, K., Ahmed, W., Eds.; William Andrew Publishing: Amsterdam, The Netherlands, 2018; pp. 391–419. [Google Scholar]
- Xue, Y. Chapter 11—Carbon Nanotubes for Biomedical Applications. In Industrial Applications of Carbon Nanotubes; Peng, H., Li, Q., Chen, T., Eds.; Elsevier: Boston, MA, USA, 2017; pp. 323–346. [Google Scholar]
- Ajitha, A.; Akhina, H.; Aswathi, M.; LovelyMathew, P.; Sabu, T. Carbon Nanotubes: An Ideal Candidate for Biomedical Applications. JSM Nanotechnol. Nanomed. 2018, 6, 1065. [Google Scholar]
- Alshehri, R.; Ilyas, A.M.; Hasan, A.; Arnaout, A.; Ahmed, F.; Memic, A. Carbon Nanotubes in Biomedical Applications: Factors, Mechanisms, and Remedies of Toxicity. J. Med. Chem. 2016, 59, 8149–8167. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Elhissi, A.; Dhanak, V.; Subramani, K. Chapter 18—Carbon nanotubes: Applications in cancer therapy and drug delivery research. In Emerging Nanotechnologies in Dentistry, 2nd ed.; Subramani, K., Ahmed, W., Eds.; William Andrew Publishing: Amsterdam, The Netherlands, 2018; pp. 371–389. [Google Scholar]
- Granada-Ramírez, D.A.; Arias-Cerón, J.S.; Rodriguez-Fragoso, P.; Vázquez-Hernández, F.; Luna-Arias, J.P.; Herrera-Perez, J.L.; Mendoza-Álvarez, J.G. 16—Quantum dots for biomedical applications. In Nanobiomaterials; Narayan, R., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 411–436. [Google Scholar]
- Aswathi, M.; Ajitha, A.; Akhina, H.; Lovely, M.; Thomas, S. Quantum Dots: A Promising Tool for Biomedical application. JSM Nanotechnol. Nanomed. 2018, 6, 1066. [Google Scholar]
- Hunter, J.E.; Ramos, L.; Wolfe, J.H. Viral Vectors in the CNS. In Reference Module in Neuroscience and Biobehavioral Psychology; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Choudhury, S.R.; Hudry, E.; Maguire, C.A.; Sena-Esteves, M.; Breakefield, X.O.; Grandi, P. Viral vectors for therapy of neurologic diseases. Neuropharmacology 2017, 120, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; McCarty, D.M. Crossing the blood-brain-barrier with viral vectors. Curr. Opin. Virol. 2016, 21, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Pulicherla, K.K.; Verma, M.K. Targeting therapeutics across the blood brain barrier (BBB), prerequisite towards thrombolytic therapy for cerebrovascular disorders-an overview and advancements. AAPS PharmSciTech 2015, 16, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, J.; Stewart, T.; Banks, W.A.; Zhang, J. The Transport Mechanism of Extracellular Vesicles at the Blood-Brain Barrier. Curr. Pharm. Des. 2017, 23, 6206–6214. [Google Scholar] [CrossRef] [PubMed]
- András, I.E.; Toborek, M. Extracellular Vesicles of the Blood-Brain Barrier. Tissue Barriers 2015. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, J.D.; Yang, C.; Lake, A.M. Systems biology in the central nervous system: A brief perspective on essential recent advancements. Curr. Opin. Syst. Biol. 2017, 3, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Soni, S.; Ruhela, R.K.; Medhi, B. Nanomedicine in Central Nervous System (CNS) Disorders: A Present and Future Prospective. Adv. Pharm. Bull. 2016, 6, 319–335. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, M.; Eslamifar, M.; Khezri, K.; Dizaj, S.M. Applications of nanotechnology in drug delivery to the central nervous system. Biomed. Pharm. 2019, 111, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Zhen, X.; Feng, X.; Xie, C.; Zheng, Y.; Pu, K. Surface engineering of semiconducting polymer nanoparticles for amplified photoacoustic imaging. Biomaterials 2017, 127, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Richard, S.; Boucher, M.; Lalatonne, Y.; Mériaux, S.; Motte, L. Iron oxide nanoparticle surface decorated with cRGD peptides for magnetic resonance imaging of brain tumors. Biochim. Et Biophys. Acta Gen. Subj. 2017, 1861, 1515–1520. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tu, M.; Tian, B.; Yi, Y.; Wei, Z.; Wei, F. Synthesis of tumor-targeted folate conjugated fluorescent magnetic albumin nanoparticles for enhanced intracellular dual-modal imaging into human brain tumor cells. Anal. Biochem. 2016, 512, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.-H.; Kim, A.R.; Kim, S.-H.; Lee, S.-J.; Chung, H.; Yoon, M.-Y. Development of a novel imaging agent using peptide-coated gold nanoparticles toward brain glioma stem cell marker CD133. Acta Biomater. 2017, 47, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Habib, A.A.; Zhao, D. Phosphatidylserine-targeted liposome for enhanced glioma-selective imaging. Oncotarget 2016, 7, 38693–38706. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, K.; Wang, Z.; Kokuryo, D.; Aoki, I.; Yokoyama, M. A polymeric micelle magnetic resonance imaging (MRI) contrast agent reveals blood–brain barrier (BBB) permeability for macromolecules in cerebral ischemia-reperfusion injury. J. Control. Release 2017, 253, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Cheng, S.; Zhang, X.; Tian, Q.; Pi, J.; Tang, J.; Huang, Q.; Wang, F.; Chen, J.; Xie, Z.; et al. Efficacy of NGR peptide-modified PEGylated quantum dots for crossing the blood–brain barrier and targeted fluorescence imaging of glioma and tumor vasculature. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Malinovskaya, Y.; Melnikov, P.; Baklaushev, V.; Gabashvili, A.; Osipova, N.; Mantrov, S.; Ermolenko, Y.; Maksimenko, O.; Gorshkova, M.; Balabanyan, V.; et al. Delivery of doxorubicin-loaded PLGA nanoparticles into U87 human glioblastoma cells. Int. J. Pharm. 2017, 524, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Mondal, J.; Patra, M.; Panigrahi, A.K.; Khuda-Bukhsh, A.R. Boldine-loaded PLGA nanoparticles have improved efficiency of drug carriage and protective potential against Cisplatin-induced toxicity. J. Ayurveda Integr. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.M.; Quijano, A.R.; Seo, Y.-E.; Jackson, C.; Josowitz, A.D.; Noorbakhsh, S.; Merlettini, A.; Sundaram, R.K.; Focarete, M.L.; Jiang, Z.; et al. Biodegradable PEG-poly(ω-pentadecalactone-co-p-dioxanone) nanoparticles for enhanced and sustained drug delivery to treat brain tumors. Biomaterials 2018, 178, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Zhang, X.; Mu, H.; Meng, Q.; Jiang, Y.; Wang, Y.; Lu, X.; Wang, A.; Liu, S.; Zhang, Y.; et al. RVG29-modified docetaxel-loaded nanoparticles for brain-targeted glioma therapy. Int. J. Pharm. 2018, 543, 179–189. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Cai, P.; Li, J.; Zhang, T.; Lin, L.; Abbasi, A.Z.; Henderson, J.T.; Rauth, A.M.; Wu, X.Y. Blood-brain barrier-penetrating amphiphilic polymer nanoparticles deliver docetaxel for the treatment of brain metastases of triple negative breast cancer. J. Control. Release 2017, 246, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Rip, J.; Gaillard, P.J.; de Lange, E.C.M.; Hammarlund-Udenaes, M. The Impact of Liposomal Formulations on the Release and Brain Delivery of Methotrexate: An In Vivo Microdialysis Study. J. Pharm. Sci. 2017, 106, 2606–2613. [Google Scholar] [CrossRef] [PubMed]
- Lakkadwala, S.; Singh, J. Co-delivery of doxorubicin and erlotinib through liposomal nanoparticles for glioblastoma tumor regression using an in vitro brain tumor model. Colloids Surf. B Biointerfaces 2019, 173, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Lakkadwala, S.; Singh, J. Dual Functionalized 5-Fluorouracil Liposomes as Highly Efficient Nanomedicine for Glioblastoma Treatment as Assessed in an In Vitro Brain Tumor Model. J. Pharm. Sci. 2018, 107, 2902–2913. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhao, Y.; Chen, Y.; Yang, Z.; Zhang, L.; Xiao, W.; Yang, J.; Guo, L.; Wu, Y. Dual-targeting for brain-specific liposomes drug delivery system: Synthesis and preliminary evaluation. Bioorg. Med. Chem. 2018, 26, 4677–4686. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Lv, L.; Shi, H.; Hua, Y.; Lv, W.; Wang, X.; Xin, H.; Xu, Q. PEGylated Polyamidoamine dendrimer conjugated with tumor homing peptide as a potential targeted delivery system for glioma. Colloids Surf. B Biointerfaces 2016, 147, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.K.; Gajbhiye, V.; Kesharwani, P.; Jain, N.K. Ligand anchored poly(propyleneimine) dendrimers for brain targeting: Comparative in vitro and in vivo assessment. J. Colloid Interface Sci. 2016, 482, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Asghar, S.; Xu, Y.; Chen, Z.; Zhang, J.; Ping, Q.; Xiao, Y. Tween 80-modified hyaluronic acid-ss-curcumin micelles for targeting glioma: Synthesis, characterization and their in vitro evaluation. Int. J. Biol. Macromol. 2018, 120, 2579–2588. [Google Scholar] [CrossRef] [PubMed]
- Kafa, H.; Wang, J.T.-W.; Rubio, N.; Klippstein, R.; Costa, P.M.; Hassan, H.A.F.M.; Sosabowski, J.K.; Bansal, S.S.; Preston, J.E.; Abbott, N.J.; et al. Translocation of LRP1 targeted carbon nanotubes of different diameters across the blood–brain barrier in vitro and in vivo. J. Control. Release 2016, 225, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Pang, R.T.-W.; Fatseas, G.; Koina, M.E.; Adamson, S.; Eamegdool, S.S.; Weible, M.W.; Pham, N.; Pham, B.T.T.; Hawkett, B.S.; Chan-Ling, T. Mechanism of Ultrasmall Superparamagnetic Iron Oxide Nanoparticles-Induced Glioblastoma Multiforme Cytotoxicity: Effects on Mitochondrial Function; Neuro Oncology and Rehabilitation, Brain Disord and Therapy: Brisbane, Australia, 2016; p. 56. [Google Scholar]
- Gitler, A.D.; Dhillon, P.; Shorter, J. Neurodegenerative disease: Models, mechanisms, and a new hope. Dis. Models Mech. 2017, 10, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Eraña, H.; López-Martínez, E.; Claes, N.; Martín, V.F.; Solís, D.M.; Bals, S.; Cortajarena, A.L.; Castilla, J.; Liz-Marzán, L.M. Detection of amyloid fibrils in Parkinson’s disease using plasmonic chirality. Proc. Natl. Acad. Sci. USA 2018, 115, 3225–3230. [Google Scholar] [CrossRef] [PubMed]
- Pansieri, J.; Gerstenmayer, M.; Lux, F.; Mériaux, S.; Tillement, O.; Forge, V.; Larrat, B.; Marquette, C. Magnetic Nanoparticles Applications for Amyloidosis Study and Detection: A Review. Nanomaterials 2018, 8, 740. [Google Scholar] [CrossRef] [PubMed]
- Plissonneau, M.; Pansieri, J.; Heinrich-Balard, L.; Morfin, J.-F.; Stransky-Heilkron, N.; Rivory, P.; Mowat, P.; Dumoulin, M.; Cohen, R.; Allémann, É.; et al. Gd-nanoparticles functionalization with specific peptides for ß-amyloid plaques targeting. J. Nanobiotechnol. 2016, 14, 60. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.M.; Wang, J.T.-W.; Morfin, J.-F.; Khanum, T.; To, W.; Sosabowski, J.; Tóth, E.; Al-Jamal, K.T. Functionalised Carbon Nanotubes Enhance Brain Delivery of Amyloid-Targeting Pittsburgh Compound B (PiB)-Derived Ligands. Nanotheranostics 2018, 2, 168–183. [Google Scholar] [CrossRef] [PubMed]
- Carradori, D.; Balducci, C.; Re, F.; Brambilla, D.; le Droumaguet, B.; Flores, O.; Gaudin, A.; Mura, S.; Forloni, G.; Ordoñez-Gutierrez, L.; et al. Antibody-functionalized polymer nanoparticle leading to memory recovery in Alzheimer’s disease-like transgenic mouse model. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, J.A.; Gomes, B.; Fricker, G.; Coelho, M.A.N.; Rocha, S.; Pereira, M.C. Cellular uptake of PLGA nanoparticles targeted with anti-amyloid and anti-transferrin receptor antibodies for Alzheimer’s disease treatment. Colloids Surf. B Biointerfaces 2016, 145, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhang, C.; Guo, Q.; Wan, X.; Shao, X.; Liu, Q.; Zhang, Q. Dual-functional nanoparticles for precise drug delivery to Alzheimer’s disease lesions: Targeting mechanisms, pharmacodynamics and safety. Int. J. Pharm. 2017, 525, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Barbara, R.; Belletti, D.; Pederzoli, F.; Masoni, M.; Keller, J.; Ballestrazzi, A.; Vandelli, M.A.; Tosi, G.; Grabrucker, A.M. Novel Curcumin loaded nanoparticles engineered for Blood-Brain Barrier crossing and able to disrupt Abeta aggregates. Int. J. Pharm. 2017, 526, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.; Ghate, M.V.; Mallik, S.B.; Lewis, S.A. Amino acid conjugated chitosan nanoparticles for the brain targeting of a model dipeptidyl peptidase-4 inhibitor. Int. J. Pharm. 2018, 547, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, V.; Gaud, R.; Bajaj, A.; Wairkar, S. Pharmacokinetics and pharmacodynamics of intranasally administered selegiline nanoparticles with improved brain delivery in Parkinson’s disease. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2609–2618. [Google Scholar] [CrossRef] [PubMed]
- Raj, R.; Wairkar, S.; Sridhar, V.; Gaud, R. Pramipexole dihydrochloride loaded chitosan nanoparticles for nose to brain delivery: Development, characterization and in vivo anti-Parkinson activity. Int. J. Biol. Macromol. 2018, 109, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-C.; Chen, I.Y.; Rajesh, R. Use of functionalized liposomes loaded with antioxidants to permeate the blood–brain barrier and inhibit β-amyloid-induced neurodegeneration in the brain. J. Taiwan Inst. Chem. Eng. 2018, 87, 1–14. [Google Scholar] [CrossRef]
- Chen, Z.-L.; Huang, M.; Wang, X.-R.; Fu, J.; Han, M.; Shen, Y.-Q.; Xia, Z.; Gao, J.-Q. Transferrin-modified liposome promotes α-mangostin to penetrate the blood–brain barrier. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Igartúa, D.E.; Martinez, C.S.; Temprana, C.F.; Alonso, S.d.V.; Prieto, M.J. PAMAM dendrimers as a carbamazepine delivery system for neurodegenerative diseases: A biophysical and nanotoxicological characterization. Int. J. Pharm. 2018, 544, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Desai, P.P.; Patravale, V.B. Curcumin Cocrystal Micelles—Multifunctional Nanocomposites for Management of Neurodegenerative Ailments. J. Pharm. Sci. 2018, 107, 1143–1156. [Google Scholar] [CrossRef] [PubMed]
- Ruff, J.; Hüwel, S.; Kogan, M.J.; Simon, U.; Galla, H.-J. The effects of gold nanoparticles functionalized with ß-amyloid specific peptides on an in vitro model of blood–brain barrier. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- Lohan, S.; Raza, K.; Mehta, S.K.; Bhatti, G.K.; Saini, S.; Singh, B. Anti-Alzheimer’s potential of berberine using surface decorated multi-walled carbon nanotubes: A preclinical evidence. Int. J. Pharm. 2017, 530, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Carter, D.A.; Ong, Z.Y.; McGilvery, C.M.; Dunlop, I.E.; Dexter, D.T.; Porter, A.E. L-DOPA functionalized, multi-branched gold nanoparticles as brain-targeted nano-vehicles. Nanomed. Nanotechnol. Biol. Med. 2019, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.A.; Maklad, H.M.; Samy, D.M.; Abdelmonsif, D.A.; el Sabaa, B.M.; Elnozahy, F.Y. Cerium oxide nanoparticles could ameliorate behavioral and neurochemical impairments in 6-hydroxydopamine induced Parkinson’s disease in rats. Neurochem. Int. 2017, 108, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Lu, J.; Liu, W.-W.; Manaenko, A.; Hou, X.; Mei, Q.; Huang, J.-L.; Tang, J.; Zhang, J.H.; Yao, H.; et al. Advances in stroke pharmacology. Pharm. Ther. 2018, 191, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Barthels, D.; Das, H. Current advances in ischemic stroke research and therapies. Biochim. Et Biophys. Acta Mol. Basis Dis. 2018. [Google Scholar] [CrossRef]
- Sarmah, D.; Saraf, J.; Kaur, H.; Pravalika, K.; Tekade, R.K.; Borah, A.; Kalia, K.; Dave, K.R.; Bhattacharya, P. Stroke management: An emerging role of nanotechnology. Micromachines 2017, 8, 262. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Gao, X. The application of nanoparticles for neuroprotection in acute ischemic stroke. Ther. Deliv. 2017, 8, 915–928. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.S.; Chung, T.K.; Prout, B.S.; Nagahama, Y.; Raghavan, M.L.; Hasan, D.M. Iron nanoparticle contrast enhanced microwave imaging for emergent stroke: A pilot study. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2019, 59, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Cai, Q.; Tian, D.; Kong, D.K.; Gou, X.; Chen, Z.; Strittmatter, S.M.; Wang, Z.; Sheth, K.N.; Zhou, J. Targeted drug delivery to ischemic stroke via chlorotoxin-anchored, lexiscan-loaded nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1833–1842. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.D.; Xavier, M.; Leite, D.M.; Moreira, D.A.; Custódio, B.; Torrado, M.; Castro, R.; Leiro, V.; Rodrigues, J.; Tomás, H.; et al. PAMAM dendrimers: Blood-brain barrier transport and neuronal uptake after focal brain ischemia. J. Control. Release 2018, 291, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lin, G.; Luan, Y.; Ding, J.; Li, P.-C.; Zhao, Z.; Qian, C.; Liu, G.; Ju, S.; Teng, G.-J. HIF-prolyl hydroxylase 2 silencing using siRNA delivered by MRI-visible nanoparticles improves therapy efficacy of transplanted EPCs for ischemic stroke. Biomaterials 2018, 197, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Billinton, A.; Newton, P.; Lloyd, C.; Groves, M.; Welsh, F.; Bogstedt, A.; Eketjall, S.; McFarlane, M.; Perkinton, M.; Narwal, R.; et al. Preclinical Discovery and Development of MEDI1814, A Monoclonal Antibody Selectively Targeting Beta-Amyloid 42 (Aβ42). Alzheimer’s Dement. 2017, 13, P266. [Google Scholar] [CrossRef]
- Gao, Q.; Fang, Y.; Zhang, S.; Wong, H.S.H.; Chan, Y.E.; Wong, S.S.M.; Yung, K.K.L.; Lai, K.W.C. Dynamic effect of beta-amyloid 42 on cell mechanics. J. Biomech. 2019. [Google Scholar] [CrossRef] [PubMed]
- Catalan-Figueroa, J.; Palma-Florez, S.; Alvarez, G.; Fritz, H.F.; Jara, M.O.; Morales, J.O. Nanomedicine and nanotoxicology: The pros and cons for neurodegeneration and brain cancer. Nanomedicine 2016, 11, 171–187. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Nanda, R.; Sahoo, S. Nanotechnology in Healthcare: Applications and Challenges. Med. Chem. 2015, 5, 528–533. [Google Scholar] [CrossRef]
- Zhang, W. Nanoparticle aggregation: Principles and modeling. Adv. Exp. Med. Biol. 2014, 811, 19–43. [Google Scholar] [PubMed]
- Kermanizadeh, A.; Balharry, D.; Wallin, H.; Loft, S.; Moller, P. Nanomaterial translocation--the biokinetics, tissue accumulation, toxicity and fate of materials in secondary organs—A review. Crit. Rev. Toxicol. 2015, 45, 837–872. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Tang, M. Review of the effects of manufactured nanoparticles on mammalian target organs. J. Appl. Toxicol. 2018, 38, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Teleanu, R.I. Neurotoxicity of Nanomaterials: An Up-to-Date Overview. Nanomaterials 2019, 9, 96. [Google Scholar] [CrossRef] [PubMed]
- Shannahan, J. The biocorona: A challenge for the biomedical application of nanoparticles. Nanotechnol. Rev. 2017, 6, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Helms, H.C.; Abbott, N.J.; Burek, M.; Cecchelli, R.; Couraud, P.O.; Deli, M.A.; Forster, C.; Galla, H.J.; Romero, I.A.; Shusta, E.V.; et al. In vitro models of the blood-brain barrier: An overview of commonly used brain endothelial cell culture models and guidelines for their use. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2016, 36, 862–890. [Google Scholar] [CrossRef] [PubMed]
| Nanocarrier Type | Advantages | Disadvantages | Surface Functionalization Strategies |
|---|---|---|---|
| Polymeric nanoparticles | biocompatibility, biodegradability, drug protection, ease of preparation, good tolerance controlled pharmacokinetics tunable physicochemical properties | neurotoxicity | polysorbate 80 RVG29 peptide anti-Aβ1-42 antibody monoclonal antibody (OX26) anti-Aβ (DE2B4) g7 ligand TGN peptides QSH peptides l-valine chlorotoxin |
| Solid-lipid nanoparticles | biocompatibility, high physical stability, bioavailability, drug protection, strict control of release, ease of preparation, good tolerance, and biodegradability without generating toxic by-products no neurotoxic effects reported hydrophobic drug entrapment efficiency lipophilicity possibility of passively cross the BBB | reduced hydrophilic drug entrapment efficiency sterilization difficulties | apolipoprotein E |
| Liposomes | possibility of entrapping both hydrophilic and hydrophobic compounds improved drug protection and targeting efficiency lipophilicity possibility of passively cross the BBB | neurotoxicity physicochemical instability tendency of fusion rapid clearance sterilization difficulties | phosphatidylserine-targeting antibody polyethylene glycol transferrin PFVYLI peptide penetratin peptide glucose-vitamin C complex phosphatidic acid apolipoprotein E |
| Dendrimers | possibility of entrapping both hydrophilic and hydrophobic compounds biodegradability stimuli-responsiveness enhanced targeting efficiency | neurotoxicity synthesis variability rapid clearance organ accumulation | polyethylene glycol glioma homing peptides sialic acid glucosamine concanavalin A |
| Micelles | no neurotoxic effects reported improved drug bioavailability physicochemical stability sustained and controlled release | use only for lipophilic drugs low drug loading capacity | Tween 80 |
| Inorganic nanoparticles | unique optical, electrical, and magnetic properties tunable size, shape, composition, structure, and porosity prolonged enhanced permeability and retention effect enhanced on-demand drug release by applying external stimuli (near-infrared radiation and magnetic field) | neurotoxicity high tendency of aggregation non-degradableorgan accumulation need further functionalization for BBB crossing | cyclo RGD peptides phosphonate polyethylene glycol bovine serum albumin folic acid CBP4 peptide KLVFF and LPFFD peptides CLPFFD peptides l-DOPA hif-prolyl hydroxylase 2 silencing |
| Carbon nanotubes | unique structure, exceptional electrical, mechanical, optical, and thermal properties, and high surface area | neurotoxicity need further functionalization for BBB crossing | Pittsburgh Compound B polysorbate and phospholipid coating |
| Quantum dots | exceptional optical and electrical properties | neurotoxicity need further functionalization for BBB crossing | polyethylene glycol asparagine-glycine-arginine peptides |
| Central Nervous System Disorder | Nanocarrier Type | Functionalization | Imaging Agent | Neuroimaging Technique | Study Model | Reference |
|---|---|---|---|---|---|---|
| Brain cancer | silica shells double coated with semiconducting polymer layers | cyclo RGD peptides | - | fluorescence and photoacoustic brightness imaging | in vitro—4T1 human breast cancer epithelial cells in vivo—tumor-bearing female mice | [79] |
| iron oxide nanoparticles | phosphonate polyethylene glycol and cyclo RGD peptides | - | magnetic resonance imaging | in vitro—U87-MG cells in vivo—tumor-bearing nude mice | [80] | |
| bovine serum albumin and tumor-specific folic acid | fluorescein isothiocyanate | magnetic resonance imaging | in vitro—U251 cells | [81] | ||
| gold nanoparticles | CBP4 peptide | fluorescein isothiocyanate | confocal microscopy | in vitro—U373 human glioma cells | [82] | |
| liposomes | - | heptamethine cyanine dye IR780 | near-infrared fluorescence imaging | in vitro—U87MG human glioma cells and T98G human glioblastoma cells in vivo—glioblastoma mouse models | [46] | |
| phosphatidylserine-targeting antibody | iron oxide nanoparticles and a near-infrared fluorescence dye | near-infrared fluorescence imaging and magnetic resonance imaging | in vitro—U87MG human glioma cells in vivo—tumor-bearing nude mice | [83] | ||
| micelles | - | gadolinium | magnetic resonance imaging | in vivo—Wistar male rats | [84] | |
| quantum dots | polyethylene glycol and asparagine–glycine–arginine peptides | - | IVIS imaging | in vitro—primary rat BCECs, astrocytes and C6 glioma cells in vivo—Sprague–Dawley male rats | [85] | |
| Neurodegenerative diseases | gadolinium-based nanoparticles | KLVFF and LPFFD peptides | - | fluorescence microscopy | in vivo—APPswe/PS1A246E/TTR mouse model | [103] |
| carbon nanotubes | Pittsburgh Compound B | gadolinium complexes | single photon emission computed tomography/computed tomography and γ-scintigraphy | in vivo—female C57BL/6 mice | [104] | |
| Stroke | Iron-oxide nanoparticles | - | - | microwave imaging | in vitro—gel brain phantom in vivo—New Zealand rabbits and a middle-aged human male | [124] |
| Central Nervous System Disorder | Nanocarrier Type | Functionalization | Active Compound | Study Model | Reference |
|---|---|---|---|---|---|
| Brain cancer | poly(lactide-co-glycolic) nanoparticles | poloxamer 188 | doxorubicin | in vitro—U-87 MG, ATCC cell line | [86] |
| - | cisplatin and boldine | in vivo – tumor-bearing swiss albino mice | [87] | ||
| polyethylene glycol and poly(ω-pentadecalactone-co-p-dioxanone) nanoparticles | - | VE822 | in vitro—RG2 cells in vivo —Tumor-bearing male Fischer 344 rats | [88] | |
| polyethylene glycol and poly(lactic-co-glycolic) acid nanoparticles | RVG29 peptide | docetaxel | in vitro—C6 cells in vivo—tumor-bearing adult Sprague–Dawley male rats | [89] | |
| amphiphilic polymer-lipid nanoparticles | polysorbate 80 | docetaxel | in vitro—MDA-MB-231 cells in vivo—tumor-bearing severe combined immune deficiency mice | [90] | |
| liposomes | polyethylene glycol | methotrexate | in vivo – male Sprague–Dawley rats | [91] | |
| transferrin and PFVYLI peptide | doxorubicin and erlotinib | in vitro—U87 tumor cells, brain endothelial cells, and glial cells | [92] | ||
| transferrin and penetratin peptide | 5-fluorouracil | in vitro—U87 tumor cells and brain endothelial cells | [93] | ||
| glucose-vitamin C complex | paclitaxel | in vitro—C6 cells in vivo—C6 glioma-bearing Kunming mice | [94] | ||
| dendrimers | polyethylene glycol and glioma homing peptides | - | in vitro—U87MG cells in vivo—U87MG tumor-bearing BALB/c nude mice | [95] | |
| sialic acid, glucosamine, and concanavalin A | paclitaxel | in vitro—U373MG human astrocytoma cell line in vivo—Sprague–Dawley rats | [96] | ||
| micelles | Tween 80 | curcumin | in vitro—G422 cells | [97] | |
| multi-walled carbon nanotubes | Angiopep-2 | - | in vitro—primary porcine brain endothelial cells and primary rat astrocytes in vivo—GL261 glioma-bearing female C57/Bl6 mice | [98] | |
| USPIONS | - | - | in vitro—rat CNS-1 cells | [99] | |
| Neurodegenerative diseases | polyethylene glycol nanoparticles | anti-Aβ1-42 antibody | - | in vivo—NIHS adult male mice | [105] |
| poly(lactic-co-glycolic) acid nanoparticles | monoclonal antibody (OX26) and anti-Aβ (DE2B4) | - | in vitro—porcine brain capillary endothelial cells | [106] | |
| poly(lactic-co-glycolic) acid nanoparticles | g7 ligand | curcumin | in vitro—primary hippocampal cultures from rat brains | [108] | |
| polyethylene glycol-polylactic acid nanoparticles | TGN peptides and QSH peptides | coumarin-6 and H102 | in vitro—brain endothelial cells in vivo—5XFAD transgenic mice | [107] | |
| chitosan nanoparticles | L-valine | saxagliptin | in vivo—female Wistar rats | [109] | |
| - | selegiline | ex vivo—male Sprague–Dawley rats | [110] | ||
| - | pramipexole dihydrochloride | ex vivo—goat nasal mucosa in vivo—male Sprague–Dawley rats | [111] | ||
| liposomes | phosphatidic acid and apolipoprotein E | quercetin and rosmarinic acid | in vitro—brain microvascular endothelial cells and Aβ1-42-insulted SK-N-MC cells | [112] | |
| transferrin | α-mangostin | in vitro—brain endothelial cells in vivo—Sprague–Dawley rats | [113] | ||
| polyamidoamine dendrimers | - | carbamazepine | ex vivo—human red blood cells in vitro—N2a cell linein vivo—zebrafish | [114] | |
| micelles | - | curcumin | in vitro—U87MG cell line in vivo—female Sprague–Dawley rats | [115] | |
| gold nanoparticles | CLPFFD peptides, neutral methoxy terminated polyethylene glycol ligands, and negatively-charged monosulfonated triphenylphosphine ligands | - | in vitro—porcine brain capillary endothelial | [116] | |
| L-DOPA | - | in vitro—human brain endothelial cell line hCMEC/D3, brain microvascular endothelial cells, and mouse microglia N9 cell line | [118] | ||
| multi-walled carbon nanotubes | polysorbate and phospholipid coating | berberine | in vitro—human red blood cells and SH-SY5Y cells in vivo—male Wistar rats | [117] | |
| cerium oxide nanoparticles | - | - | in vivo—adult male Wistar rats | [119] | |
| Stroke | poly(lactic-co-glycolic) acid nanoparticles | chlorotoxin | Lexiscan and Nogo-66 | in vivo—male C57BL/6 mice | [125] |
| polyamidoamine dendrimers | polyethylene glycol | - | in vitro—rat primary astrocytes and mouse brain endothelial cells in vivo—male C57BL/6 mice | [126] | |
| iron oxide nanoparticles | hif-prolyl hydroxylase 2 silencing | siRNA | in vivo—female BALB/c nude mice | [127] |
| Nanocarrier Type | Neurotoxic Effect |
|---|---|
| Polymeric nanoparticles | neuronal apoptosis; neuroinflammation; increased oxidative stress |
| Liposomes | necrosis; neuroinflammation; hemorrhage; macrophage infiltration |
| Dendrimers | cell proliferation and migration inhibition; abnormal mitochondrial activity; apoptosis; affected neuronal differentiation; increased oxidative stress; DNA damage; decreased locomotor function |
| Gold nanoparticles | increased oxidative stress; cognition defects; astrogliosis |
| Silver nanoparticles | increased oxidative stress; apoptosis; necrosis; neuroinflammation |
| Iron oxide nanoparticles | synaptic transmission and nerve conduction alterations; neuroinflammation; apoptosis; macrophage infiltration |
| Titanium oxide nanoparticles | increased oxidative stress; neuroinflammation; apoptosis; synaptic transmission alterations and plasticity; genotoxicity |
| Silica nanoparticles | cognitive dysfunctions and impairment; neurodegeneration; synaptic transmission alterations |
| Carbon nanotubes | neuroinflammation; cell proliferation inhibition; apoptosis; increased oxidative stress; mitochondrial membrane potential reduction; lipid peroxidization; astrocyte function reduction; neurobehavioral toxicity |
| Quantum dots | increased oxidative stress; cell function damage; neurobehavioral toxicity; cognitive impairment |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Teleanu, R.I. Neuronanomedicine: An Up-to-Date Overview. Pharmaceutics 2019, 11, 101. https://doi.org/10.3390/pharmaceutics11030101
Teleanu DM, Chircov C, Grumezescu AM, Teleanu RI. Neuronanomedicine: An Up-to-Date Overview. Pharmaceutics. 2019; 11(3):101. https://doi.org/10.3390/pharmaceutics11030101
Chicago/Turabian StyleTeleanu, Daniel Mihai, Cristina Chircov, Alexandru Mihai Grumezescu, and Raluca Ioana Teleanu. 2019. "Neuronanomedicine: An Up-to-Date Overview" Pharmaceutics 11, no. 3: 101. https://doi.org/10.3390/pharmaceutics11030101
APA StyleTeleanu, D. M., Chircov, C., Grumezescu, A. M., & Teleanu, R. I. (2019). Neuronanomedicine: An Up-to-Date Overview. Pharmaceutics, 11(3), 101. https://doi.org/10.3390/pharmaceutics11030101








