Pharmacokinetic Profile of Kaurenoic Acid after Oral Administration of Araliae Continentalis Radix Extract Powder to Humans
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Herbal Extract
2.3. Content of KAU
2.4. Quantification of KAU in Biological Samples
2.5. Clinical Trials Design
2.6. Pharmacokinetic Analysis
2.7. Cell Culture
2.8. In Vitro Cytotoxicity Assay
2.9. Bidirectional Transport Assay
3. Results and Discussion
3.1. Content of Kaurenoic Acid
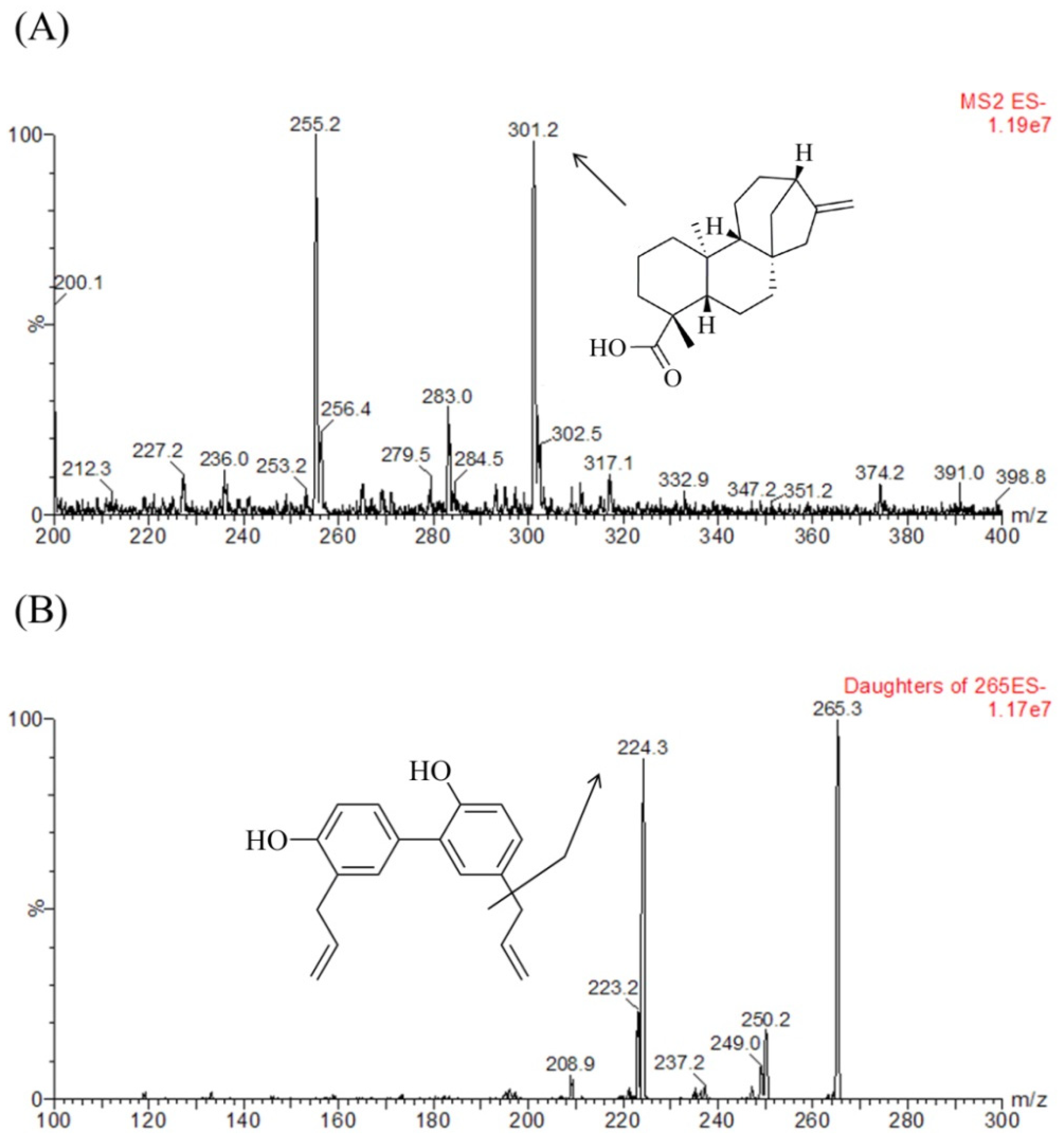
3.2. Quantification of KAU
3.3. Quantitative Method Validation
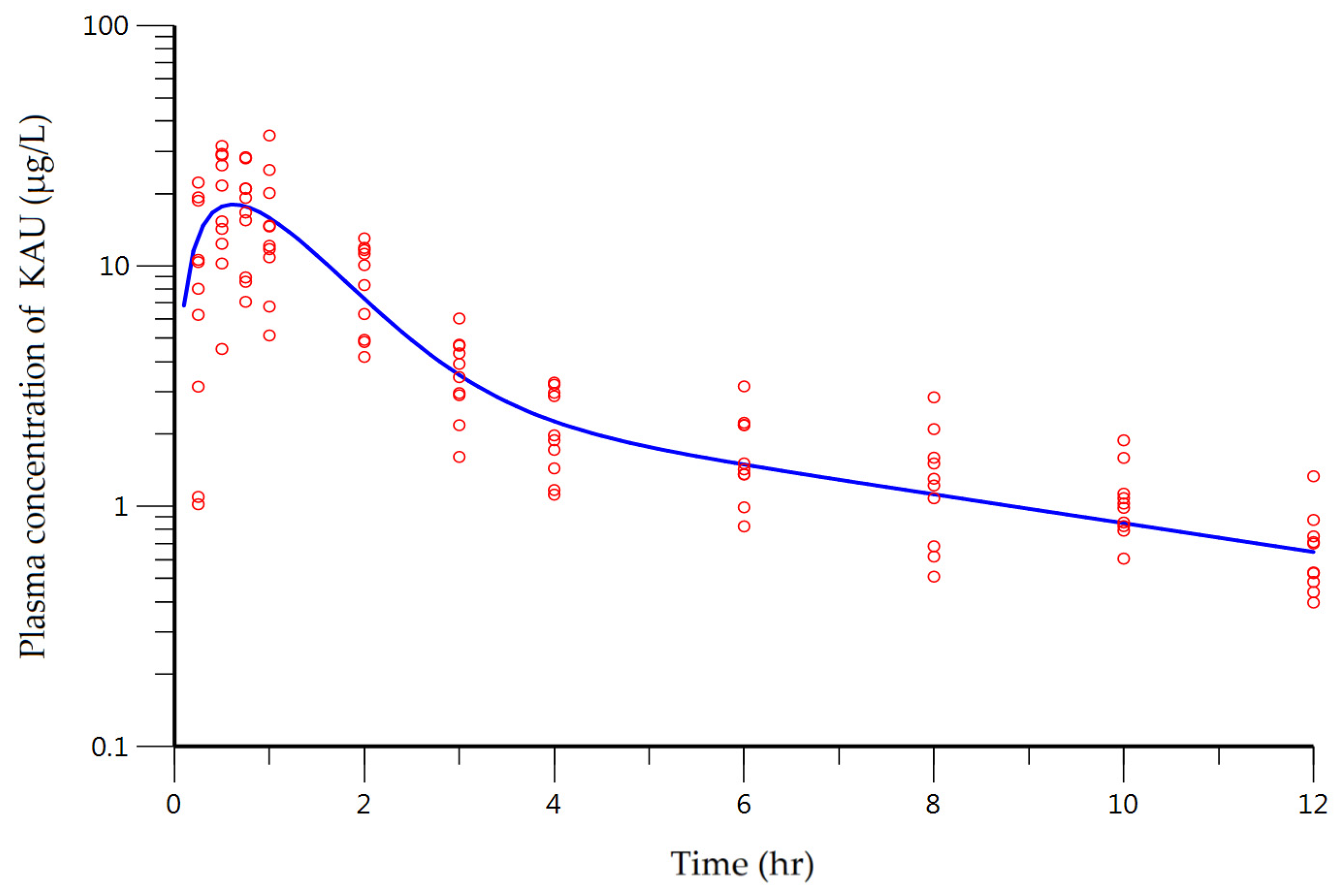
3.4.Clinical Pharmacokinetic Study
3.5. Cytotoxicity Assay of KAU
3.6. Bidirectional Transport Assay
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lim, H.; Jung, H.A.; Choi, J.S.; Kim, Y.S.; Kang, S.S.; Kim, H.P. Anti-inflammatory activity of the constituents of the roots of aralia continentalis. Arch. Pharm. Res. 2009, 32, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Hong, M.S.; Lee, J.S.; Leem, K.H.; Kim, C.J.; Kim, J.W.; Lim, S. Effects of aralia continentalis on hyperalgesia with peripheral inflammation. Phytother. Res. 2005, 19, 511–513. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.O.; Ban, J.Y.; Kim, J.Y.; Jeong, H.Y.; Lee, I.S.; Song, K.-S.; Bae, K.; Seong, Y.H. Aralia cordata protects against amyloid β protein (25–35)–induced neurotoxicity in cultured neurons and has antidementia activities in mice. J. Pharmacol. Sci. 2009, 111, 22–32. [Google Scholar] [CrossRef]
- Liu, X.; Hou, D.; Zhao, N.; Wang, B. Extraction and antioxidant activity of flavonoids from aralia cordata. J. Chin. Med. Mater. 2010, 33, 1484–1487. [Google Scholar]
- Cheng, W.L.; Lin, T.Y.; Tseng, Y.H.; Chu, F.H.; Chueh, P.J.; Kuo, Y.H.; Wang, S.Y. Inhibitory effect of human breast cancer cell proliferation via p21-mediated g1 cell cycle arrest by araliadiol isolated from aralia cordata thunb. Planta Med. 2011, 77, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.D.; Lee, J.Y.; Cho, B.J.; Park, T.W.; Kim, C.J. The analgesic and anti-inflammatory effects of 7-oxosandaracopimaric acid isolated from the roots of aralia cordata. Arch. Pharm. Res. 2010, 33, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Kang, S. Chemistry and biological activity of the constituents from aralia species. Ann. Rep. Nat. Prod. Sci. 1997, 5, 1–26. [Google Scholar]
- Okoye, T.C.; Akah, P.A.; Omeje, E.O.; Okoye, F.B.; Nworu, C.S. Anticonvulsant effect of kaurenoic acid isolated from the root bark of Annona senegalensis. Pharmacol. Biochem. Behav. 2013, 109, 38–43. [Google Scholar] [CrossRef]
- Lizarte Neto, F.S.; Tirapelli, D.P.C.; Ambrosio, S.R.; Tirapelli, C.R.; Oliveira, F.M.; Novais, P.C.; Peria, F.M.; Oliveira, H.F.; Carlotti Junior, C.G.; Tirapelli, L.F. Kaurene diterpene induces apoptosis in u87 human malignant glioblastoma cells by suppression of anti-apoptotic signals and activation of cysteine proteases. Braz. J. Med. Biol. Res. 2013, 46, 71–80. [Google Scholar] [CrossRef]
- Cavalcanti, B.C.; Bezerra, D.P.; Magalhaes, H.I.; Moraes, M.O.; Lima, M.A.S.; Silveira, E.R.; Camara, C.A.; Rao, V.S.; Pessoa, C.; Costa-Lotufo, L.V. Kauren-19-oic acid induces DNA damage followed by apoptosis in human leukemia cells. J. Appl. Toxicol. 2009, 29, 560–568. [Google Scholar] [CrossRef]
- Velikova, M.; Bankova, V.; Tsvetkova, I.; Kujumgiev, A.; Marcucci, M.C. Antibacterial ent-kaurene from brazilian propolis of native stingless bees. Fitoterapia 2000, 71, 693–696. [Google Scholar] [CrossRef]
- Cotoras, M.; Folch, C.; Mendoza, L. Characterization of the antifungal activity on botrytis cinerea of the natural diterpenoids kaurenoic acid and 3β-hydroxy-kaurenoic acid. J. Agric. Food Chem. 2004, 52, 2821–2826. [Google Scholar] [CrossRef]
- Santos, A.O.; Izumi, E.; Ueda-Nakamura, T.; Dias-Filho, B.P.; Veiga-Junior, V.F.; Nakamura, C.V. Antileishmanial activity of diterpene acids in copaiba oil. Mem. Inst. Oswaldo Cruz 2013, 108, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Batista, R.; Garcia, P.A.; Castro, M.A.; Miguel Del Corral, J.M.; Speziali, N.L.; de P Varotti, F.; de Paula, R.C.; Garcia-Fernandez, L.F.; Francesch, A.; San Feliciano, A.; et al. Synthesis, cytotoxicity and antiplasmodial activity of novel ent-kaurane derivatives. Eur. J. Med. Chem. 2013, 62, 168–176. [Google Scholar] [CrossRef]
- Pereira, S.; Taleb-Contini, S.; Coppede, J.; Pereira, P.; Bertoni, B.; Franca, S.; Pereira, A.M. An ent-kaurane-type diterpene in croton antisyphiliticus mart. Molecules 2012, 17, 8851–8858. [Google Scholar] [CrossRef]
- De Andrade, B.B.; Moreira, M.R.; Ambrosio, S.R.; Furtado, N.; Cunha, W.R.; Heleno, V.; Silva, A.N.; Simao, M.R.; Da Rocha, E.; Martins, C. Evaluation of ent-kaurenoic acid derivatives for their anticariogenic activity. Nat. Prod. Commun. 2011, 6, 777–780. [Google Scholar] [PubMed]
- Vilegas, J.H.; de Marchi, E.; Lanças, F.M. Determination of coumarin and kaurenoic acid in mikania glomerata (“guaco”) leaves by capillary gas chromatography. Phytochem. Anal. 1997, 8, 74–77. [Google Scholar] [CrossRef]
- Costa-Lotufoa, L.V.; Cunha, G.M.; Fariasa, P.A.; Vianaa, G.S.; Cunhaa, K.M.; Pessoaa, C.; Moraes, M.O.; Silveirab, E.R.; Gramosab, N.V.; Rao, V.S. The cytotoxic and embryotoxic effects of kaurenoic acid, a diterpene isolated from copaifera langsdorffii oleo-resin. Toxicon 2002, 40, 1231–1234. [Google Scholar] [CrossRef]
- Mizokami, S.S.; Arakawa, N.S.; Ambrosio, S.R.; Zarpelon, A.C.; Casagrande, R.; Cunha, T.M.; Ferreira, S.H.; Cunha, F.Q.; Verri, W.A., Jr. Kaurenoic acid from sphagneticola trilobata inhibits inflammatory pain: Effect on cytokine production and activation of the no-cyclic gmp-protein kinase g-atp-sensitive potassium channel signaling pathway. J. Nat. Prod. 2012, 75, 896–904. [Google Scholar] [CrossRef]
- Cavalcanti, B.C.; Ferreira, J.R.; Moura, D.J.; Rosa, R.M.; Furtado, G.V.; Burbano, R.R.; Silveira, E.R.; Lima, M.A.; Camara, C.A.; Saffi, J.; et al. Structure-mutagenicity relationship of kaurenoic acid from xylopia sericeae (annonaceae). Mutat. Res. 2010, 701, 153–163. [Google Scholar] [CrossRef]
- Guillope, R.; Escobar-Khondiker, M.; Guerineau, V.; Laprevote, O.; Hoglinger, G.U.; Champy, P. Kaurenoic acid from pulp of annona cherimolia in regard to annonaceae-induced parkinsonism. Phytother. Res. 2011, 25, 1861–1864. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.H.; Sant’Ana, A.E.; Bastos, D.Z. Determination of the diterpenoid, kaurenoic acid, in annona glabra by hplc. Phytochem. Anal. 2002, 13, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.A.; Humphrey, M.J.; Charuel, C. Design of toxicokinetic studies. Xenobiotica 1990, 20, 1187–1199. [Google Scholar] [CrossRef] [PubMed]
- Lan, K.; Xie, G.; Jia, W. Towards polypharmacokinetics: Pharmacokinetics of multicomponent drugs and herbal medicines using a metabolomics approach. Evid.-Based Complement. Altern. Med. 2013, 2013, 819147. [Google Scholar] [CrossRef]
- Gasparetto, J.C.; Peccinini, R.G.; de Francisco, T.M.; Cerqueira, L.B.; Campos, F.R.; Pontarolo, R. A kinetic study of the main guaco metabolites using syrup formulation and the identification of an alternative route of coumarin metabolism in humans. PLoS ONE 2015, 10, e0118922. [Google Scholar] [CrossRef] [PubMed]
- Matos, D.M.; Viana, M.R.; Alvim, M.C.O.; Carvalho, L.S.A.; Leite, L.H.R.; Da Silva Filho, A.A.; Nascimento, J.W.L. Pharmacokinetic profile and oral bioavailability of kaurenoic acid from Copaifera spp. In rats. Fitoterapia 2018, 128, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Leslie, E.M.; Deeley, R.G.; Cole, S.P. Multidrug resistance proteins: Role of p-glycoprotein, mrp1, mrp2, and bcrp (abcg2) in tissue defense. Toxicol. Appl. Pharmacol. 2005, 204, 216–237. [Google Scholar] [CrossRef]
- Madgula, V.L.; Avula, B.; Choi, Y.W.; Pullela, S.V.; Khan, I.A.; Walker, L.A.; Khan, S.I. Transport of schisandra chinensis extract and its biologically-active constituents across caco-2 cell monolayers—An in-vitro model of intestinal transport. J. Pharm. Pharmacol. 2008, 60, 363–370. [Google Scholar] [CrossRef]
- FDA. The Guidance for Industry: Bioanalytical Method Validation; Center for Drug Evaluation and Research (CDER), FDA: Silver Spring, MD, USA, 2013.
- FDA. Botanical Drug Development Guidance for Industry; FDA: Silver Spring, MD, USA, 2016.
- Jung, H.R.; Kim, S.J.; Ham, S.H.; Cho, J.H.; Lee, Y.B.; Cho, H.Y. Simultaneous determination of puerarin and its active metabolite in human plasma by uplc-ms/ms: Application to a pharmacokinetic study. J. Chromatogr. B 2014, 971, 64–71. [Google Scholar] [CrossRef]
- Donna, A.V.; Patrick, J.F.; Anthony, B.C.; Ebenezer, B.A.; Christopher, D.E.; Yu, L.X.; Ajaz, S.H. Classification of drug permeability with a caco-2 cell monolayer assay. Clin. Res. Regul. Aff. 2007, 24, 39–47. [Google Scholar]
- Hubatsch, I.; Ragnarsson, E.G.; Artursson, P. Determination of drug permeability and prediction of drug absorption in caco-2 monolayers. Nat. Protoc. 2007, 2, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- FDA. Waiver of In Vivo Bioavailability and Bioequivalence Studies for Immediate-Release Solid Oral Dosage forms Based on a Biopharmaceutics Classification System Guidance for Industry; FDA: Silver Spring, MD, USA, 2017.
- Davies, B.; Morris, T. Physiological parameters in laboratory animals and humans. Pharm. Res. 1993, 10, 1093–1095. [Google Scholar] [CrossRef] [PubMed]
- Savic, R.M.; Karlsson, M.O. Importance of shrinkage in empirical bayes estimates for diagnostics: Problems and solutions. AAPS J. 2009, 11, 558–569. [Google Scholar] [CrossRef] [PubMed]
- FDA. Guidance for Industry: Drug Interaction Studies; FDA: Silver Spring, MD, USA, 2012.
- Liang, X.L.; Zhao, L.J.; Liao, Z.G.; Zhao, G.W.; Zhang, J.; Chao, Y.C.; Yang, M.; Yin, R.L. Transport properties of puerarin and effect of radix angelicae dahuricae extract on the transport of puerarin in caco-2 cell model. J. Ethnopharmacol. 2012, 144, 677–682. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, H.; Sun, F.; Zhao, L.; Zhong, Y.; Chai, Y.; Zhang, G. Intestinal transport of sophocarpine across the caco-2 cell monolayer model and quantification by lc/ms. Biomed. Chromatogr. 2014, 28, 885–890. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples are available from authors. |




| Spiked (ng/mL) | Measured (Mean ± SD) | Precision (CV, %) | Accuracy (%) |
|---|---|---|---|
| Intra-batch (n = 5) | |||
| 0.2 | 0.195 ± 0.013 | 6.72 | 97.50 |
| 0.6 | 0.564 ± 0.021 | 3.67 | 94.07 |
| 16 | 16.052 ± 0.182 | 1.13 | 100.33 |
| 80 | 79.783 ± 5.366 | 6.73 | 99.73 |
| Inter-batch (n = 5) | |||
| 0.2 | 0.206 ± 0.022 | 10.55 | 102.90 |
| 0.6 | 0.577 ± 0.034 | 5.91 | 96.21 |
| 16 | 15.958 ± 0.558 | 3.49 | 99.74 |
| 80 | 78.082 ± 2.114 | 2.71 | 97.60 |
| Condition | Spiked Concentration (ng/mL) | Measured Concentration (Mean ± SD) | Deviation (%) |
|---|---|---|---|
| Freeze and thaw stability (3 cycles) | 0.6 | 0.54 ± 0.02 | −4.72 |
| 80 | 75.41 ± 2.15 | −6.15 | |
| Short-term stability (25 °C for 8 h) | 0.6 | 0.54 ± 0.05 | −3.83 |
| 80 | 75.08 ± 0.77 | −5.89 | |
| Long-term stability (−80 °C for 3 months) | 0.6 | 0.48 ± 0.03 | −14.65 |
| 80 | 69.20 ± 7.78 | −13.27 | |
| Post-preparative stability (10 °C for 6 h) | 0.6 | 0.51 ± 0.04 | −9.21 |
| 80 | 71.46 ± 2.45 | −10.44 |
| Characteristic (Units) | Mean | SD | Median | Range |
|---|---|---|---|---|
| Age (years) | 25.8 | 4.3 | 24.5 | 21.0–33.0 |
| Body weight (kg) | 73.3 | 6.8 | 73.65 | 60.4–86.7 |
| Height (cm) | 172.5 | 6.5 | 173.5 | 157.0–183.0 |
| SCr (mg/dL) | 0.95 | 0.09 | 0.97 | 0.80–1.10 |
| CrCl (mL/min) | 115.0 | 11.8 | 115.7 | 98.0–139.1 |
| BUN (mg/dL) | 17.4 | 11.7 | 14.3 | 10.3–51.9 |
| Total protein (g/dL) | 8.0 | 0.2 | 8.1 | 7.6–8.3 |
| Total cholesterol (mg/dL) | 170.2 | 16.6 | 167.6 | 148.1–203.4 |
| Triglyceride (mg/dL) | 148.3 | 31.0 | 139.2 | 95.7–201.0 |
| Albumin (g/dL) | 5.01 | 0.12 | 4.98 | 4.83–5.20 |
| Alk (U/L) | 65.6 | 14.5 | 64.8 | 47.3–93 |
| ALT (U/L) | 20.2 | 5.0 | 19.5 | 12.3–32.1 |
| AST (U/L) | 24.7 | 6.0 | 24.5 | 16.8–39.0 |
| GGTP (U/L) | 21.4 | 5.0 | 20.2 | 15.2–34.4 |
| Parameter | Unit | Definition | Estimate | CV% |
|---|---|---|---|---|
| Primary parameters | ||||
| CL/F | L/h | Systemic clearance | 23.89 | 9.30 |
| CLD/F | L/h | Inter-compartmental clearance | 15.55 | 13.77 |
| Ka | h−1 | Rate constant for absorption | 1.72 | 28.79 |
| V/F | L | Volume of distribution of the central compartment | 24.44 | 29.16 |
| V2/F | L | Volume of distribution of the peripheral compartment | 64.05 | 21.05 |
| ε | - | Proportional residual error | 0.48 | 11.09 |
| Secondary parameters * | ||||
| Cmax | μg/L | Peak plasma concentration | 18.02 | - |
| tmax | h | Time to reach the peak plasma concentration | 0.6 | - |
| AUC | μg∙h/L | Area under the time-plasma curve | 47.89 | - |
| t1/2 | h | Plasma elimination half-life | 4.97 | - |
| KAU (µM) | Caco-2 Papp (× 10−6 cm/s) | Efflux Ratio | |
|---|---|---|---|
| AP→BL | BL→AP | ||
| 1 | 0.18 ± 0.03 | 0.34 ± 0.01 | 1.98 |
| 10 | 4.47 ± 0.64 | 7.40 ± 1.55 | 1.70 |
| 50 | 21.78 ± 5.93 | 44.3 ± 2.71 | 2.15 |
| 100 | 49.42 ± 18.38 | 107.26 ± 32.14 | 2.37 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, E.-J.; Choi, G.-W.; Yang, S.-J.; Lee, Y.-B.; Cho, H.-Y. Pharmacokinetic Profile of Kaurenoic Acid after Oral Administration of Araliae Continentalis Radix Extract Powder to Humans. Pharmaceutics 2018, 10, 253. https://doi.org/10.3390/pharmaceutics10040253
Choi E-J, Choi G-W, Yang S-J, Lee Y-B, Cho H-Y. Pharmacokinetic Profile of Kaurenoic Acid after Oral Administration of Araliae Continentalis Radix Extract Powder to Humans. Pharmaceutics. 2018; 10(4):253. https://doi.org/10.3390/pharmaceutics10040253
Chicago/Turabian StyleChoi, Eun-Jeong, Go-Wun Choi, Seung-Jeong Yang, Yong-Bok Lee, and Hea-Young Cho. 2018. "Pharmacokinetic Profile of Kaurenoic Acid after Oral Administration of Araliae Continentalis Radix Extract Powder to Humans" Pharmaceutics 10, no. 4: 253. https://doi.org/10.3390/pharmaceutics10040253
APA StyleChoi, E.-J., Choi, G.-W., Yang, S.-J., Lee, Y.-B., & Cho, H.-Y. (2018). Pharmacokinetic Profile of Kaurenoic Acid after Oral Administration of Araliae Continentalis Radix Extract Powder to Humans. Pharmaceutics, 10(4), 253. https://doi.org/10.3390/pharmaceutics10040253







