Abstract
Advances in nanotechnology for drug delivery are fostering significant progress in medicine and diagnostics. The multidisciplinary nature of the nanotechnology field encouraged the development of innovative strategies and materials to treat a wide range of diseases in a highly specific way, which allows reducing the drug dosage and, consequently, improving the patient’s compliance. Due to their good biocompatibility, easy synthesis, and high versatility, inorganic frameworks represent a valid tool to achieve this aim. In this context, Mesoporous Silica Nanoparticles (MSNs) are emerging in the biomedical field. For their ordered porosity and high functionalizable surface, achievable with an inexpensive synthesis process and being non-hazardous to biological tissues, MSNs offer ideal solutions to host, protect, and transport drugs to specific target sites. Extensive literature exists on the use of MSNs as targeted vehicles for systemic (chemo) therapy and for imaging/diagnostic purposes. However, the aim of this review is to give an overview of the last updates on the potential applications of the MSNs for Topical Drug Delivery (TDD) and as drug delivery systems into the brain, discussing their performances and advantages in dealing with these intriguing biological barriers.
1. Introduction
Nanotechnology emerged as one of the most promising technologies in the 21st century, representing a multi-disciplinary approach applying engineering and biology principles at the molecular level. Nanotechnologies refer to the design of small but very smart and flexible structures, whose application covers different fields, ranging from electronics to information technology, energy, environmental science, transportation, biomedical research, food industry, and home and personal care. In particular, nanobiotechnology holds great potential in life science and it is increasingly attracting the attention of scientists all over the world. For instance, in the pharmaceutical field, smart nanostructures revolutionized how drugs are formulated and delivered (e.g., hosting old drugs in new packaging which are able to selectively recognize the target tissue), paving the way to nanomedicine, that marks an unparalleled opportunity to advance the treatment of various diseases, including cancer [1]. In this context, several nanostructured organic and inorganic functional materials are emerging as excellent candidates for delivery of a wide range of drugs [2]. Among them, Mesoporous Silica Nanoparticles (MSNs) have become a novel generation of inorganic framework for biomedical applications [3,4,5] due to their tunable size (2–50 nm), high surface area (700–1000 m2/g), well-ordered internal mesopores (typically 2–6 nm), large pore volume (0.6–1 cm3/g), high drug loading, good biocompatibility, and low production costs [6]. In addition, it is possible to chemically modify MSNs’ surface properties through covalent grafting of various organic functional groups to the free silanol groups. These advantageous features make MSNs ideal platforms to design multifunctional nanosystems, boasting eligible qualities for high drug loading and gradual release [7]. Vallet-Regí M et al. were the first to develop ordered mesoporous materials carrying the drug ibuprofen as drug delivery systems (DDSs) [8]. Since then, MSNs have been tested for the delivery of a variety of drugs including nonsteroidal anti-inflammatories (e.g., aspirin), antibiotics (e.g., vancomycin), and chemotherapeutics (e.g., doxorubicin and methotrexate) [9,10,11,12].
The big advantage of these nanomaterials is that they can be tailored to continuous or triggered release of a wide range of molecules, depending on the application. For instance, Sanchez-Salcedo S. et al. recently proposed new multifunctional polyethylenimine (PEI) coated core-shell Fe3O4@SiO2 MSNs, with a zwitterionic 2-methacryloyloxyethyl phosphorylcholine (MPC) surface, aimed at reducing unwanted protein (corona proteins) adsorption on the MSNs surface once entered the bloodstream, thus minimizing opsonization and prolonging MSNs blood circulation time. The device also carries an anti-TWIST siRNA, which, knocking down TWIST expression, sensitizes ovarian cancer cells to the action of the other cargo molecule, daunorubicin, whose release is triggered by externally applied oscillating magnetic fields (OMF) [13].
Notably, drug release control by means of opportune gatekeepers (e.g., pH, magnetic fields, light, temperature, reducing agents) can be so efficiently designed to achieve “zero premature release” [14]. Moreover, surface functionalization with targeting ligands [15] is the most exciting way to deliver the drug to target cell, performing an extremely selective therapy [16]. In this context, Lopez V. and co-workers developed Janus MSNs asymmetrically decorated with two targeting functions, folic acid (FA) to selectively recognize the folate receptors (FR) on the cell membranes and triphenylphosphine (TPP) to specifically target mitochondrial membranes, with the aim of improving the therapeutic efficacy [17]. Particularly fascinating is also the totally innovative approach of using human Decidua Mesenchymal Stem Cells (DMSCs) as carriers for doxorubicin (DOXO) loaded MSNs to direct the delivery system exclusively to the tumor site, exploiting DMSCs migratory attitude towards tumors [18].
In addition, unlike organic nanocarriers (e.g., nanocapsules, liposomes, polymeric micelles and NPs, protein NPs), characterized by physico-chemical instability, low encapsulation efficiency, premature drug leakage, and lack of tunable triggers for drug release [19], and unlike certain inorganic nanomaterials that are not biodegradable and/or toxic, MSNs’ own important properties make them ideal platforms for biomedical applications. In particular, they exhibit excellent biochemical and physico-chemical stability, excellent drug delivery and endocytotic behaviors, and, most importantly, high biocompatibility and degradability [3]. In fact, MSNs are characterized by a hydrolytically unstable framework, that, once in the bloodstream, is rapidly converted by hydrolysis into water-soluble silicic acid (Si(OH)4), which, in turn, is safely excreted through the urines [20]. Moreover, it is well established that, due to wide range of synthesis procedures and the resulting physical characteristics, general assumptions about MSNs potential toxicity and organ accumulation cannot be drawn. In fact, MSNs degradability and toxicity is affected by different parameters such as size (MSNs of 30 to 100 nm diameters are able to induce inflammatory response in animal models [21]), morphology, porosity, surface charge (e.g., anionic surfaces are generally less toxic than cationic NPs, which can cause hemolysis [22]), and functionalization. Therefore, in order to develop safe silica nanoparticles carriers, a fine-tuning of these structural characteristics is mandatory.
For all these peculiarities, MSNs have found potential application in targeted therapy, both in cancer treatment and in diagnosis. Indeed, multifunctional MSNs are currently being applied in the bio-sensing field and as ideal cell tracing in the detection of analytes within individual cells both in vitro and in vivo [23]. Since nanoparticles (NPs) do not suffer from fluorescent self-quenching, and other diffusion-related problems, they can be functionalized with large quantities of cell-recognition or other site-directing compounds. So, fluorescence-traceable MSNs are useful tools for cell tracking via fluorescence microscopy [24]. Likewise, magnetic resonance imaging (MRI)-traceable MSNs are employed in both clinical- and research-based fields, due to their deep tissue imaging capabilities [25].
To date, MSNs have been explored for simultaneous imaging and therapy, making them a biomedical platform for theranostic applications. MSNs have emerged as suitable for long-term quantitative imaging at low doses, since they are safely cleared from the body after imaging is complete [26]. It is also noteworthy that MSNs exhibit low hemolytic activity, confirming their suitability for systemic delivery through the bloodstream [27]. Not so long ago, dye-doped silica NPs, called Cornell dots (C dots), have been approved from the FDA for the first Investigational New Drug (IND) application for targeted molecular imaging in the oncology field [28,29].
However, beyond these “conventional” uses, MSNs have also attracted great interest in the dermatological and neurological fields. Therefore, the purpose of this review is to offer a brief, but exhaustive, overview on MSNs’ applications in the topical and neurological treatments, focusing the attention on MSNs’ interactions with biological barriers, such as skin and the blood-brain barrier (BBB).
2. Drug Delivery Systems (DDSs)
Drug delivery is the approach of administering therapeutic agents in formulations, technologies, and systems in order to safely achieve the desired therapeutic effect by decreasing fluctuations in serum drug concentrations, which results in reduced toxicity, sustained efficacy, and less frequent dosing.
The main advantage of DDSs is that the drug is isolated from the bloodstream along its way, it does not interact with non-target cells, and it is released only when the desired site has been reached. This implies that the therapeutic efficacy is guaranteed by even lower doses of the encapsulated drug, thus reducing the side effects, which still represent the main limitation of the conventional formulations [2].
To fulfill this need, the delivery of drugs is often committed to smart NPs that are able to recognize the target organs, tissues, and cells, overcoming problems such as limited solubility, molecular aggregation, poor biodistribution, and lack of selectivity [30].
Different features make an efficient DDS: biocompatibility, high loading/encapsulation of desired drug molecules, absence of premature release, cell type or tissue specificity, and stimuli-responsivity (e.g., pH- or temperature-sensitive). The possibility of functionalizing the surface by conjugating target molecules (e.g., ligands) allows one to direct the loaded drug exclusively to the site of interest, exploiting the molecular recognition of target receptors [31,32].
MSNs possess all the characteristics of an efficient DDS [2], so they are emerging as good candidates to become reliable vehicles in a variety of applications [5,33,34].
3. MSNs in Topical Drug Delivery
The skin consists of four layers: the stratum corneum (SC), which is the outermost layer of the skin, the epidermis, the dermis, and the subcutaneous tissues (hypodermis). A number of appendages (i.e., hair follicles, sweat glands, and nails) are also associated to the skin. The SC represents the main barrier to drug permeation, consisting of a cornified cell envelope, composed of high density and low hydrated layers of flat and elongated corneocytes and a densely packed protein/lipid polymer structure right below the cornified cell envelope. The other layers and the appendages, although more permeable, offer several target sites for drug delivery [35].
Topical Drug Delivery (TDD) involves the use of drugs to be applied directly on the skin in a formulation that can be absorbed. To reach systemic circulation, the drug has to permeate the above described consecutive skin layers, i.e., once released from the TDD the drug penetrates into the SC, then passing to the more aqueous epidermis and, finally, absorbed by the capillaries in the dermis.
The use of TDD could potentially overcome the conventional routes of administration, oral and parenteral, still guaranteeing a comparable efficacy, but circumventing issues such as systemic side effect and needle phobia. Skin patches are among the first examples of topical drug delivery systems [36]. Therapeutic concentrations of topically delivered agents accumulate within the site of application, maintaining low serum levels, thus resulting in less organ toxicity [37].
In order to cross the multilayer barrier of the skin, it would be desirable to administer the chemical agents through carriers able to locally deliver the drug and to store it into the skin appendages. Among the different carriers proposed, silica nanoparticles gathered a wide consensus [38,39,40].
Chemicals can be absorbed by the skin only after having overcome its constituent layers, either via the intercellular route, with partitioning into the lipid matrix, or via the intracellular route, or through sweat glands or hair follicles [41]. In particular, some evidences suggest that the hair follicle may constitute a significant reservoir for topically applied molecules [42] and NPs [43,44]. In fact, their functional location could mediate the diffusion across the capillary walls of the stored molecules to the surrounding spaces. Therefore, these structures may represent a potential target for nanomaterial-based shuttles, as reported by Xuan et al., who showed accumulation of fluorescent MSNs in the hair follicles of porcine ear skin [44]. However, toxicological issues on transdermal delivery of nanomaterials are still under debate [44,45,46]. It is well established that size, shape, surface charge, z potential, and tendency to aggregate are crucial parameters affecting the interactions of NPs with human skin surface. Generally, only NPs below 1 nm are able to permeate the intact skin, while silica NPs with an average size lower than 25 nm can penetrate but not permeate the skin [44] and those with higher diameters (e.g., 55 ± 6 nm) are not able to cross the normal or perturbed mouse skin after one or five days of topical application [44]. Examples of therapeutic molecules topically delivered by means of silica NPs include corticosteroids, antifungals, antivirals, antibiotics, antiseptics, local anesthetics, antineoplastics, as well as antioxidant molecules included in cosmetic products (e.g., moisturizers, make-up, sunscreen etc.). Table 1 and Figure 1 summarize multiple uses of silica NPs in topical drug delivery.

Table 1.
Multifunctional Silica Nanoparticles (MSNs) as a versatile platform for Topical Drug Delivery.
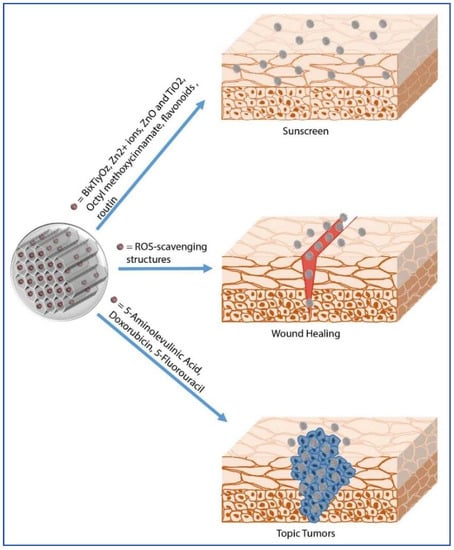
Figure 1.
MSNs as topical drug delivery systems.
4. MSNs in Cosmetics
Many substances currently proposed for topical drug delivery are being questioned for their adverse health and environmental effects, thus promoting the search for safer alternatives for human health. An example are the inorganic ingredients of sunscreens, used to preserve skin from UV radiation. In this context, silica NPs offer solutions which are highly compatible with human health and the environment [33].
Indeed, Sotiriou et al. increased the safety of sunscreen constituents (ZnO and TiO2) by coating the surface of these hazardous nanoparticles with silica layers [58]. Their toxicological data showed that the hermetic encapsulation of the ZnO nanorods in a thin silica shell minimized the bio-interactions of the core ZnO, in particular reducing the strong DNA damage otherwise observed with the pure uncoated ZnO nanorods [58]. The silica coating is not only useful to protect the skin against the toxicity of these UV filters, but also to improve their stability within the sunscreen formulation.
Knežević et al. [33] emphasized the high potential of functionalized silica-based nanomaterials (healthier and environmentally friendly) for application in skin protection from UV irradiation. Tolbert et al. have designed hybrid organic/inorganic silica particles, encapsulating organic sunscreens in silica NPs, in order to reduce their phototoxicity and degradation [59]. Similarly, the octyl methoxycinnamate, one of the most common UVB filters included in sunscreen formulations, when encapsulated in silica scaffold, improve its photostability [47], corroborating the usefulness of silica nanosystems in the cosmetic field.
Antioxidants like flavonoids are frequently used in sunscreen formulations to complement UV filter photoprotection [51]. MSNs have been proposed as an innovative topical carrier, able to preserve the physico-chemical and biological properties of labile active ingredients of dermocosmetic interest until their release on the skin. Ugazio et al. [48] demonstrated that the antioxidant efficacy of quercetin was maintained upon immobilization in the thermosensitive silica NPs. Furthermore, the association of quercetin to aminopropyl-functionalized silica NPs leads to 2-fold increase in skin penetration, compared to free quercetin, after 24 h of application [40,51,60]. The use of nanodosage forms of quercetin is promoted in various skin disorders, such as atopic dermatitis or psoriasis, as well as in sunscreen formulations. In fact, these innovative nanosized quercetin complexes show improved skin permeation compared to the free quercetin, whose topical penetration is hindered by its poor water solubility and low stability. In addition, the antiproliferative activity of quercetin-loaded (Q-loaded) MSNs have been evaluated in JR8 melanoma cells [51], confirming that the Q-nanosystems enhance the flavonoid cytotoxicity, compared to the free form, probably for an increase in the antioxidant release or in the cellular uptake of the nanosized system.
Other agents, such as routin, used in cosmetics for UV protection and for their antioxidant properties, have been incorporated in the MSNs cavities in order to improve their photostability and optimize their biological function [47,52].
These studies pave the way for an innovative employment of mesoporous silica materials in the skin care field and in topical products.
Recently, silica NPs have been proposed as a promising scaffold for the wound healing process due to the interactions occurring between the material surface and the tissue matrix, a nanobridging effect that promotes a rapid sealing of the wound [49]. Moreover, when the excellent tissue adhesive ability of MSNs is coupled to the delivery of active ingredients to damaged tissues the result is even better. In fact, the synergistic effect of nanobridging and ROS-scavenging structures, as in ceria nanocrystals decorated MSNs (MSN-Ceria), accelerates the wound healing process by reducing the oxidative stress of the microenvironment, thus improving the regeneration process of the injured skin and limiting scar formation [49]. These data were also confirmed in vivo, using a cutaneous wound rat model. MSN-Ceria nanostructures display the same adhesive strength as MSNs alone, but promote structure restoration of wounded skin due the ROS-scavenging activity. Treatment with this combination mended the wound area almost completely within 8 days, achieving the smooth appearance of the damaged skin area. Therefore, Ceria-MSN led to a significantly enhanced therapeutic effect and improved the quality of the healed skin [49].
The versatility of nanomodels like MSNs is also beneficial in the oral hygiene field. In fact, fluorescent MSNs can be uptaken, degraded, and/or excreted by the reconstructed human gingival epithelia (RHGE) [61], accumulating at the superficial corneum layer in a time-dependent manner and forming a “nanocoating-like barrier”. So, nanoparticle-based antimicrobial and anti-inflammatory agents could be employed for topical application in oral healthcare [61].
5. MSNs in the Topical Treatment of Cancer
Extensive literature is available on the use of targeted silica and silica-based NPs in cancer treatment, in order to overcome the well-known limitations of conventional chemotherapy due to high systemic exposure to anti-neoplastic agents that frequently results in dose-limiting toxicity [3,34].
The topical administration of anticancer drugs through nanoparticles-based delivery systems is an interesting alternative to the systemic skin cancer treatment for improving drug targeting and therapeutic benefits. Nanocarriers are not only useful to protect antitumor drugs against degradation, but also to enhance drug penetration into the deep layers of the epidermis. Furthermore, TDDSs significantly reduce antitumor drug side effects, as they do not directly enter into the bloodstream. Indeed, in vitro preliminary findings suggest that MSNs could represent a promising strategy and the most innovative technology in the topical application of anticancer therapies.
Anirudhan et al. [56] engineered a temperature- and ultrasound (US)-sensitive hybrid MSNs system (HMSN) for the delivery of 5-fluorouracil (5-FU), an anticancer drug. The NPs were developed by grafting the MSNs’ pores with a temperature and US sensitive copolymer of tetrahydropyranyl methacrylate (THPMA) and amino ethyl methacrylate (AEMA). Exposing the 5-FU loaded HMSNs to US frequency, the acetal bonds of THPMA moiety are cleaved with the consequent release of the encapsulated drug molecules. The US frequency also acts as a permeation enhancer, allowing controlled drug permeation across the skin barrier.
Methotrexate (MTX) is a chemotherapeutic agent employed in the treatment of different types of cancers and inflammatory processes, but also in bowel and Crohn’s diseases, psoriasis, and other skin disorders. Like any other chemotherapeutic agent, MTX activity associates to a series of unwanted effects, which can be overcome by the use of nanotechnology. Recently, Sapino et al. [38] have developed a series of formulations containing MTX-loaded (by absorption) MSNs for the topical administration of methotrexate (MTX). The MTX/MSN system increases the epidermal accumulation of the active molecule and allows delivering the drug to the deeper layers of the epidermis.
A promising drug delivery system that combines chemotherapy and photothermal therapy (PTT) has been proposed for the treatment of superficial tumors. The system (PVP@DOX/MSN@ICG) is composed of microneedle (MN) patches loaded with the chemotherapeutic drug Doxorubicin (DOX) and covalently conjugated to the photothermal indocyanine green (ICG), to enhance ICG stability and to maintain its photothermal efficiency. DOX and ICG are released in the tumor site at the same time by dissolution of the microneedles in the extracellular fluid [53].
The selectivity of the nanoparticles against the tumour cells is improved by decorating MSNs’ external surface with targeting molecules that specifically bind to receptors on the cell surface of interest to promote nanocarrier interaction and internalization. To date, only a few, but significant, examples of targeted MSNs have been developed for topical application in cancer treatment. Martìnez-Carmona et al. [55] proposed a novel nanocarrier based on mesoporous silica particles covered with a photosensitive protein shell, which is cleavable by light irradiation. Transferrin (Tf) molecules anchored through a UV-sensitive cross-linker on the surface of Doxorubicin (DOXO)-loaded MSNs, act as targeting agents and cleavable gatekeepers at the same time. The in vitro cytotoxicity of the MSN-Tf-DOXO system, evaluated on various tumor cell lines overexpressing transferrin receptors, showed excellent performance of the system. The developed carrier could be applied to the treatment of tumors easily accessible for direct light irradiation, such as skin cancer.
Photodynamic therapy (PDT) is a noninvasive cancer therapeutic technique that uses a photosensitizer and a particular type of light. The photosensitizer, upon exposure to a specific wavelength of light, converts surrounding oxygen into singlet oxygen, a form of reactive oxygen species that kills tumor cells. Ma et al. [54] realized a multifunctional MSN-based delivery system of 5-Aminolevulinic Acid (5-ALA), a precursor of the photosensitizer protoporphyrin IX (PphIX), for skin cancer therapy through the PDT process. The developed carrier consists of 5-ALA-loaded MSNPs externally functionalized with the targeting ligand folic acid (FA) that allows receptor-mediated endocytosis of the system into cancer cells and the polymer polyethylene glycol (PEG) that enhances its biocompatibility. PphIX formed from 5-ALA@HMSNP-PEG+FA upon light irradiation showed high photocytotoxicity to the cancer cells in vitro.
6. Drug Delivery as a Potential Approach to Cross the Blood-Brain Barrier (BBB)
The proper delivery of drugs to the central nervous system (CNS) is often hampered by the cunning nature of the blood-brain barrier (BBB) that serves as a critical barrier between the CNS and the peripheral circulation, maintaining the brain’s homeostasis and preserving its internal milieu [62]. Thus, although protecting the brain from noxious agents, BBB is also an obstacle to the delivery of beneficial drugs to treat CNS diseases [63,64]. Compared to other endothelial cells, the brain capillary endothelial cells (BCEC) show some features (e.g., the lack of fenestrations, the circumferential tight junctions complexes, minimal trancytosis, diminished pinocytosis and para- and transcellular barriers consisting of cell membranes, various enzymatic filters, and efflux transporters) which contribute to protecting the brain from toxic agents [65]. Around 100% of molecules over 500 Da and 98% of small molecules are not able to penetrate the brain after systemic administration and alterations in the BBB permeability occurring in pathological conditions such as Alzheimer’s (AD) and Parkinson’s (PD) diseases and stroke may limit the delivery of drugs into the CNS even more [66]. Therefore, new routes of drug administration able to transiently induce BBB disruption have been suggested, including intracerebral pathways, blood-to-brain delivery, and intranasal delivery coupled to biological, chemical, or physical stimuli such as zonula occludens toxin, mannitol, magnetic heating, and ultrasound. However, these approaches showed a series of disadvantages, such as being dangerous, expensive, and unsuitable for the treatment of most brain diseases and for the delivery of most drugs [67]. Therefore, the ideal method to transport drugs across the BBB should be controlled and not damage the barrier. Among the different approaches proposed, nanobiotechnology-based delivery methods provide the best prospects to achieve this ideal. The strategy of vector-mediated blood-to-brain delivery, aimed at increasing BBB permeability to the drug-carrier conjugate, can minimize potential injuries to the barrier. Being submicrometer objects that behave as a whole unit in terms of transport and chemical properties, nanomaterials are promising vehicles for direct drug transport across the intact BBB. This is due to their potential to enter the BCEC by means of normal endocytosis and transcytosis, thanks to their small size, as well as their high versatility, since they can be functionalized with multiple copies of the drug molecule and the targeting function of interest [63,64].
Different types of nanocarriers have been extensively studied for drug delivery to the brain: liposomes, polymeric (synthetic and natural) and inorganic NPs (e.g., silica based, carbon nanotubes and gold NPs). Compared to synthetic polymeric NPs, the inorganic NPs offer a series of advantages, i.e., the control over shape and size as well as the ease of preparation and functionalization. Moreover, they can be easily detected by microscopy and analytic techniques (e.g., MRI, TEM, and ICP-MS). Although natural NPs (chitosan, alginate, poly(lysine), gelatin, and albumin) have the advantage of triggering biological signals by interacting with specific receptors/transporters expressed by BCEC, compared to inorganic NPs, they show lower versatility, higher batch-to-batch variability, and poor tracking capacity by imaging techniques. [66]. However, common issues in the use of inorganic NPs reside in their potential toxicity, organ accumulation, and degradation. In this context, although MSNs’ application as potential drug delivery systems have been largely exploited in the biomedical field, mainly for systemic administration, there is still no actual evidence on the toxic effects at the BBB level [67].
The main applications of silica NPs as drug delivery systems for brain related diseases are reported in Table 2 and Figure 2.

Table 2.
Recent advances on MSNs as brain drug delivery systems.
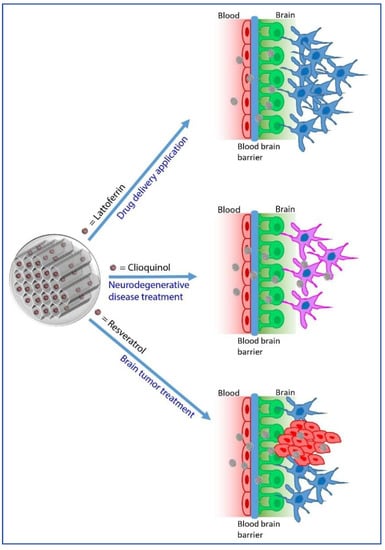
Figure 2.
Main applications of MSNs as drug delivery systems for brain related diseases.
7. MSNs as Drug Delivery Systems Targeting the BBB
In last two decades, a series of drug delivery systems targeting the BBB have been tested with various degrees of success. Recently, several studies demonstrated that NPs’ transcytosis across the BBB occurs through receptor-mediated transcytosis (RMT), a selective and non-invasive delivery mechanism. For example, in the vascular endothelial cells (VECs), RMT seems to occur by means of different receptors, including the transferrin receptor (TfR) and the low-density lipoprotein receptor (LRP). In addition, ligand-bearing liposomal or polymeric nanocarriers can also promote the delivery of drugs through the BBB [76,77,78,79,80]. However, these approaches did not translate to clinical practice for their poor delivery efficacy; therefore, developing alternative strategies is still mandatory.
To overcome the problem, Yang S. and colleagues designed a new drug carrier, consisting of silica NPs that exploit endogenous transcytosis pathway for effectively delivering therapeutics without disrupting the normal function of BBB [69].
In this work, the authors showed that fluorescence dye and polyethylene glycol (PEG) bearing MSNs grafted with the glycoprotein Lactoferrin (Lf) are able to cross the BBB (mimicked by in vitro co-cultures platforms) and that the efficiency of transcytosis increases as the particle size decreases [69]. Moreover, being highly expressed in human VECs of the BBB, Lactoferrin (Lf) ligands are more efficient and specific than those targeting the transferrin (Tf) receptor [68,81,82,83,84,85,86]. Besides, considering also their good biocompatibility and relatively low cost, Lf ligands represent promising candidates for BBB targeting [69].
8. MSNs-Based Therapy in Alzheimer’s Disease
Alzheimer’s disease is the most common form of dementia and its incidence is greatly increased as a result of the general aging of the population worldwide. The etiology of this pathology is currently based on two different hypotheses: (1) the aggregation and accumulation of amyloid-β (Aβ) peptides, typically leading to fibrils in the brains of patients and (2) the “tau hypothesis”, referring to the abnormal aggregation into masses of tau microtubule-associated proteins in conjunction with amyloid plaques [87].
Among various therapeutic NPs based prototypes, designed with the aim of inhibiting the Aβ aggregation occurring in the Alzheimer’s disease, MSNs offer the advantage, if opportunely functionalized, of selectively crossing the BBB, delivering relatively high payloads of cargo molecules into the brain [6].
Qu and co-workers observed that metal ions (e.g., Cu2+ ions) limited Aβ solubility by chelating histidine residues, promoting Aβ aggregation and inducing the formation of cytotoxic ROS (H2O2). Therefore, they engineered H2O2-responsive MSNs (MSN–Cu@IgG) coated with immunoglobulin G (IgG) in order to release the metal chelator clioquinol (CQ) upon redox actuation, thus inhibiting Aβ fibril formation [70]. In addition, the treatment with the nanovector increases cell viability, emphasizing its potential use in Alzheimer’s disease.
Yang et al., exploiting the metal chelating characteristics of CQ and the ability of gold NPs to inhibit Aβ aggregation, developed H2O2-responsive mesoporous silica-based vectors capped with gold nanoparticles and bearing CQ [71]. Ganji and collaborators designed MSNs for the delivery of rivastigmine hydrogen tartrate, a drug widely used in the treatment of Alzheimer’s disease, into human SY5Y neuroblastoma cells [72].
9. MSNs-Based Therapy to Cure Brain Tumors
Brain tumors affect approximately 5 to 10 people per 100,000 and are considered as one of the 10 main causes of death by cancer [88]. The standard treatment for glioma, a malignant tumor of nervous system tissue, typically involves surgical resection followed by a combination of radiation and chemotherapy, but this approach has not substantially improved the overall survival (the median survival period remains ~16 months, while the five-year survival rate is ~10%) [89].
In particular, the blood-brain barrier (BBB) and blood–tumor barrier (BTB) hamper the tumor penetration and uptake, which makes the treatment with most therapeutic agents for glioblastoma multiforme (GBM) particularly inefficient [90,91]. Therefore, cancer-targeted drug delivery systems with permeability of the blood–brain barrier (BBB) have become of great interest for the rational design of high-efficiency anticancer agents. Recently, You Y. and colleagues reported a strategy for the rational design of a tailored nanomedicine with enhanced BBB permeability to treat human brain glioma [75]. The authors designed and tested a new tailored MSNs nanosystem as a carrier for anticancer agents, using a novel organic selenium compound BSeC as a model molecule and the tripeptide RGD (arginine–glycine–aspartate) as targeting molecule, recognizing a subset of the integrins on tumor membranes [74]. The results showed that this nanosystem (BSeC@MSNs-RGD) exhibited high stability in human blood serum and could effectively transport across the BBB, thus enhancing the permeability of loaded drugs against BBB. In addition, this tailored MSNs also enhanced the cellular uptake of BSeC, thus significantly increasing its anticancer efficacy by a factor of hundreds of times by the induction of cell apoptosis. BSeC@MSNs-RGD selectively entered cancer cells, which express high levels of integrin molecules, compared to normal cells. The internalized BSeC@MSNs-RGD triggered mitochondrial dysfunction and intracellular ROS overproduction, which subsequently activated the p53 and MAPKs pathways to promote cell apoptosis. The nanosystem also showed excellent penetrating ability and inhibitory effects on human glioblastoma cell line U87 spheroids, supporting its in vivo anticancer potential. Furthermore, the tailored MSNs nanosystem significantly prolonged the blood circulation time of loaded drugs in vivo and was easily cleared by renal excretion, thus effectively reducing toxicity.
Mo J. and co-workers tailored the size of MSNs loaded with the chemotherapeutic doxorubicin (DOX) and modified by the cancer targeting polymer PEI-cRGD (poly(ether imide)-cricoids Arg-Gly-Asp-Phe-Lys). The functionalized nanosystem (DOX@MSNs) selectively recognized overexpressing ανβ3 integrin U87 glioblastoma cells. Exposing the cells to MSNs ranging from 40 to 120 nm in diameter, the authors observed that the cellular uptake and the consequent inhibition of glioma cells were proportionally higher with the decrease of the particle size. In particular, the 40 nm DOX @ MSNs not only rapidly enter tumor cells, but also show a greater selectivity and antitumor activity compared to free Dox, inducing apoptosis in glioma cells through ROS overproduction. Moreover, the 40 nm DOX @ MSNs showed increased ability in overcoming the BBB, also interfering with the vasculogenic mimicry (VM) capacity of glioma cells by altering MMP-2, E-cadherin, and FAK proteins expression. This feature allows achieving a satisfactory antitumor efficacy, while avoiding unwanted toxicity to normal brain tissue [75].
10. Neurodegenerative Disease and MSNs Therapy
It has been well established that the accumulation of reactive oxygen and nitrogen species (RONS) are responsible for neuronal injury and inflammation, leading to various neurodegenerative disorders. A large number of studies evidenced that an antioxidant-based therapy can be effective to ameliorate the deleterious effects of RONS [92]. In this context, the antioxidant compound Resveratrol (RSV) is currently under investigation for its antioxidant properties, as it is able to remove the excess of RONS generated in the brain. However, since very low amount of RSV reach the CNS after systemic administration, Shen Y. et al. have recently proposed a new delivery system consisting of MSNs coated with polylactic acid (PLA) and conjugated with a ligand peptide recognizing the low-density lipoprotein receptor (LDLR). As conceived, the system strongly enhances RSV transcytosis across the BBB. The PLA coating prevented the RSV premature release, which occurred only in presence of high ROS levels that are able to accelerate PLA degradation. The RSV delivery across BBB was evaluated using a co-culture of rat brain microvascular endothelial cells (RBECs) and microglia cells, which mimic an in vitro BBB model. The conjugation of LDLR ligand peptide markedly enhanced the migration of MSNPs across the RBECs monolayer and RSV could be released and effectively reduce the phorbol-myristate-acetate or lipopolysaccharide mediated activation of the microglia cells [73].
Several phytochemicals are known to protect neurons from injuries occurring during neurodegenerative diseases [93,94,95,96,97,98,99]. Currently, drug delivery into the brain after systemic administration is limited by the poor permeability of the BBB. Thus, a promising alternative administration route to bypass the BBB is represented by the nose-to-brain delivery. Lungare S. and co-workers proposed MSNs bearing the poorly water-soluble phytochemicals chrysin and curcumin (MSNP) as potential drug delivery systems that exploit olfactory targeting for the treatment of neurodegenerative diseases. The authors show that small MSNP particles (below 500 nm) could be endocytosed by olfactory cells and that the slightly acidic pH of the nasal cavity can trigger the pH-dependent release of the phytochemicals. Overall, this drug delivery system targeting the BBB would represent an interesting alternative to the systemic administration, since a lower drug cargo is needed to achieve similar therapeutic effects [62].
11. Conclusions
Nanotechnologies have gained attention in all areas of science and, among the nanomaterials, MSNs boast considerable potential due to their unique properties. This review is an update on the advantages and the potential applications of MSNs as TDD and as drug vehicles opportunely tailored to overcome the BBB. Although a series of very interesting preclinical studies have been conducted, demonstrating MSNs’ ability in crossing both skin and the BBB, none of the proposed formulations have yet been tested in clinical trials. The main obstacle to reaching the clinic lies in MSNs’ main features (e.g., surface functionalization, charge, size, and shape) and on the difficulties in the reproducibility of a small-scale synthesis during scale-up processes under Good Manufacturing Practices (GMP) conditions. Moreover, although MSNs showed remarkably high biocompatibility in several in vivo studies, safety issues still have to be properly addressed, conducting crucial evaluations of MSNs pharmacokinetics, clearance, half-life, pharmacodynamics, immunogenicity, and organ accumulation. Therefore, MSNs’ future challenge is to overcome all these limitations in order to eventually start offering forefront and cost-effective solutions that satisfy many different clinical and cosmetic needs.
Funding
This study was supported by NANOCAL CUP J28C17000200006—POR Calabria FESR–FSE 2014–2020 Action 1.2.2—Grants for R&D Projects and by Associazione Italiana Ricerca sul Cancro (AIRC) Grant IG 15738/2014.
Conflicts of Interest
The authors declare no conflict of interest. L.P., A.L. and C.M. are scientific co-founders of NanoSiliCal Devices, a spin-off company of the University of Calabria. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Ma, Y.; Mou, Q.B.; Zhu, X.Y.; Yan, D.Y. Small molecule nanodrugs for cancer therapy. Mater. Today Chem. 2017, 4, 26–39. [Google Scholar] [CrossRef]
- Sayed, E.; Haj-Ahmad, R.; Ruparelia, K.; Arshad, M.S.; Chang, M.W.; Ahmad, Z. Porous Inorganic Drug Delivery Systems—A Review. AAPS PharmSciTech 2017, 18, 1507–1525. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Quan, G.; Wu, Q.; Zhang, X.; Niu, B.; Wu, B.; Huang, Y.; Pan, X.; Wu, C. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm. Sin. B 2018, 8, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Freitas, L.B.D.; Corgosinho, L.D.; Faria, J.A.Q.A.; dos Santos, V.M.; Resende, J.M.; Leal, A.S.; Gomes, D.A.; de Sousa, E.M.B. Multifunctional mesoporous silica nanoparticles for cancer-targeted, controlled drug delivery and imaging. Micropor. Mesopor. Mater. 2017, 242, 271–283. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Colilla, M.; Izquierdo-Barba, I.; Manzano, M. Mesoporous Silica Nanoparticles for Drug Delivery: Current Insights. Molecules 2017, 23, 47. [Google Scholar] [CrossRef] [PubMed]
- Croissant, J.G.; Fatieiev, Y.; Almalik, A.; Khashab, N.M. Mesoporous Silica and Organosilica Nanoparticles: Physical Chemistry, Biosafety, Delivery Strategies, and Biomedical Applications. Adv. Healthc. Mater. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.A.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regi, M.; Ramila, A.; del Real, R.P.; Perez-Pariente, J. A new property of MCM-41: Drug delivery system. Chem. Mater. 2001, 13, 308–311. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Balas, F.; Arcos, D. Mesoporous materials for drug delivery. Angew. Chem. Int. Ed. 2007, 46, 7548–7558. [Google Scholar] [CrossRef] [PubMed]
- Silveira, C.P.; Apolinario, L.M.; Favaro, W.J.; Paula, A.J.; Duran, N. Doxorubicin-Functionalized Silica Nanoparticles Incorporated into a Thermoreversible Hydrogel and Intraperitoneally Administered Result in High Prostate Antitumor Activity and Reduced Cardiotoxicity of Doxorubicin. ACS Biomater. Sci. Eng. 2016, 2, 1190–1199. [Google Scholar] [CrossRef]
- Cavallaro, G.; Pierro, P.; Palumbo, F.S.; Testa, F.; Pasqua, L.; Aiello, R. Drug delivery devices based on mesoporous silicate. Drug Deliv. 2004, 11, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Carino, I.S.; Pasqua, L.; Testa, F.; Aiello, R.; Puoci, F.; Iemma, F.; Picci, N. Silica-based mesoporous materials as drug delivery system for methotrexate release. Drug Deliv. 2007, 14, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Salcedo, S.; Vallet-Regi, M.; Shahin, S.A.; Glackin, C.A.; Zink, J.I. Mesoporous core-shell silica nanoparticles with anti-fouling properties for ovarian cancer therapy. Chem. Eng. J. 2018, 340, 114–124. [Google Scholar] [CrossRef]
- Cheng, Y.J.; Zhang, A.Q.; Hu, J.J.; He, F.; Zeng, X.; Zhang, X.Z. Multifunctional Peptide-Amphiphile End-Capped Mesoporous Silica Nanoparticles for Tumor Targeting Drug Delivery. ACS Appl. Mater. Interfaces 2017, 9, 2093–2103. [Google Scholar] [CrossRef] [PubMed]
- Pasqua, L.; Testa, F.; Aiello, R.; Cundari, S.; Nagy, J.B. Preparation of bifunctional hybrid mesoporous silica potentially useful for drug targeting. Microporous Microporous Mater. 2007, 103, 166–173. [Google Scholar] [CrossRef]
- Pasqua, L.; Leggio, A.; Sisci, D.; Ando, S.; Morelli, C. Mesoporous Silica Nanoparticles in Cancer Therapy: Relevance of the Targeting Function. Mini Rev. Med. Chem. 2016, 16, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Lopez, V.; Villegas, M.R.; Rodriguez, V.; Villaverde, G.; Lozano, D.; Baeza, A.; Vallet-Regi, M. Janus Mesoporous Silica Nanoparticles for Dual Targeting of Tumor Cells and Mitochondria. ACS Appl. Mater. Interfaces 2017, 9, 26697–26706. [Google Scholar] [CrossRef] [PubMed]
- Paris, J.L.; de la Torre, P.; Manzano, M.; Cabanas, M.V.; Flores, A.I.; Vallet-Regi, M. Decidua-derived mesenchymal stem cells as carriers of mesoporous silica nanoparticles. In vitro and in vivo evaluation on mammary tumors. Acta Biomater. 2016, 33, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Bamrungsap, S.; Zhao, Z.; Chen, T.; Wang, L.; Li, C.; Fu, T.; Tan, W. Nanotechnology in therapeutics: A focus on nanoparticles as a drug delivery system. Nanomedicine 2012, 7, 1253–1271. [Google Scholar] [CrossRef] [PubMed]
- Croissant, J.G.; Fatieiev, Y.; Khashab, N.M. Degradability and Clearance of Silicon, Organosilica, Silsesquioxane, Silica Mixed Oxide, and Mesoporous Silica Nanoparticles. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Frohlich, E. Value of phagocyte function screening for immunotoxicity of nanoparticles in vivo. Int. J. Nanomed. 2015, 10, 3761–3778. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles:applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef]
- Bergman, L.; Kankaanpaa, P.; Tiitta, S.; Duchanoy, A.; Li, L.; Heino, J.; Linden, M. Intracellular Degradation of Multilabeled Poly(Ethylene imine)-Mesoporous Silica-Silica Nanoparticles: Implications for Drug Release. Mol. Pharm. 2013, 10, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, Y.Q.; Fu, A.; Shi, W.; Yeo, D.; Luo, K.Q.; Ow, H.; Xu, C.J. Tracking mesenchymal stem cell tumor-homing using fluorescent silica nanoparticles. J. Mater. Chem. B 2015, 3, 1245–1253. [Google Scholar] [CrossRef]
- Nakamura, T.; Sugihara, F.; Matsushita, H.; Yoshioka, Y.; Mizukami, S.; Kikuchi, K. Mesoporous silica nanoparticles for F-19 magnetic resonance imaging, fluorescence imaging, and drug delivery. Chem. Sci. 2015, 6, 1986–1990. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wu, M.; Zhao, J.L.X.J. Recent development of silica nanoparticles as delivery vectors for cancer imaging and therapy. Nanomed. Nanotechnol. 2014, 10, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Haynes, C.L. Impacts of Mesoporous Silica Nanoparticle Size, Pore Ordering, and Pore Integrity on Hemolytic Activity. J. Am. Chem. Soc. 2010, 132, 4834–4842. [Google Scholar] [CrossRef] [PubMed]
- Benezra, M.; Penate-Medina, O.; Zanzonico, P.B.; Schaer, D.; Ow, H.; Burns, A.; DeStanchina, E.; Longo, V.; Herz, E.; Iyer, S.; et al. Multimodal silica nanoparticles are effective cancer-targeted probes in a model of human melanoma. J. Clin. Investig. 2011, 121, 2768–2780. [Google Scholar] [CrossRef] [PubMed]
- Phillips, E.; Penate-Medina, O.; Zanzonico, P.B.; Carvajal, R.D.; Mohan, P.; Ye, Y.; Humm, J.; Gonen, M.; Kalaigian, H.; Schoder, H.; et al. Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe. Sci. Transl. Med. 2014, 6, 260ra149. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.Y.; Ting, X.Z.L.; Zhu, J.J. The Research Progress of Targeted Drug Delivery Systems. IOP Conf. Ser. Mater. Sci. 2017, 207. [Google Scholar] [CrossRef]
- Morelli, C.; Maris, P.; Sisci, D.; Perrotta, E.; Brunelli, E.; Perrotta, I.; Panno, M.L.; Tagarelli, A.; Versace, C.; Casula, M.F.; et al. PEG-templated mesoporous silica nanoparticles exclusively target cancer cells. Nanoscale 2011, 3, 3198–3207. [Google Scholar] [CrossRef] [PubMed]
- Ceresa, C.; Nicolini, G.; Rigolio, R.; Bossi, M.; Pasqua, L.; Cavaletti, G. Functionalized mesoporous silica nanoparticles: A possible strategy to target cancer cells reducing peripheral nervous system uptake. Curr. Med. Chem. 2013, 20, 2589–2600. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, N.Z.; Ilic, N.; Dokić, V.; Petrovic, R.; Janackovic, D.O.E. Mesoporous Silica and Organosilica Nanomaterials as UV-Blocking Agents. ACS Appl. Mater. Interfaces 2018, 10, 20231–20236. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Luo, L.; Liang, S.; Long, M.; Xu, H. Amino-functionalized mesoporous silica nanoparticles as efficient carriers for anticancer drug delivery. J. Biomater. Appl. 2017, 32, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Vitorino, C.; Sousa, J.; Pais, A. Overcoming the skin permeation barrier: Challenges and opportunities. Curr. Pharm. Des. 2015, 21, 2698–2712. [Google Scholar] [CrossRef] [PubMed]
- Pastore, M.N.; Kalia, Y.N.; Horstmann, M.; Roberts, M.S. Transdermal patches: History, development and pharmacology. Br. J. Pharmacol. 2015, 172, 2179–2209. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, M.; Srivastava, S.; Singh, D.; Saraf, S.; Saraf, S.; Singh, M.R. Perspectives of Lipid-Based Drug Carrier Systems for Transdermal Delivery. Crit. Rev. Ther. Drug Carr. Syst. 2018, 35, 331–367. [Google Scholar] [CrossRef] [PubMed]
- Sapino, S.; Oliaro-Bosso, S.; Zonari, D.; Zattoni, A.; Ugazio, E. Mesoporous silica nanoparticles as a promising skin delivery system for methotrexate. Int. J. Pharm. 2017, 530, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Zaccariello, G.; Back, M.; Zanello, M.; Canton, P.; Cattaruzza, E.; Riello, P.; Alimonti, A.; Benedetti, A. Formation and Controlled Growth of Bismuth Titanate Phases into Mesoporous Silica Nanoparticles: An Efficient Self-Sealing Nanosystem for UV Filtering in Cosmetic Formulation. ACS Appl. Mater. Interfaces 2017, 9, 1913–1921. [Google Scholar] [CrossRef] [PubMed]
- Hatahet, T.; Morille, M.; Hommoss, A.; Devoisselle, J.M.; Muller, R.H.; Begu, S. Quercetin topical application, from conventional dosage forms to nanodosage forms. Eur. J. Pharm. Biopharm. 2016, 108, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Larese Filon, F.; Mauro, M.; Adami, G.; Bovenzi, M.; Crosera, M. Nanoparticles skin absorption: New aspects for a safety profile evaluation. Regul. Toxicol. Pharmacol. 2015, 72, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Lademann, J.; Knorr, F.; Richter, H.; Jung, S.; Meinke, M.C.; Ruhl, E.; Alexiev, U.; Calderon, M.; Patzelt, A. Hair follicles as a target structure for nanoparticles. J. Innov. Opt. Health Sci. 2015, 8. [Google Scholar] [CrossRef]
- Sahle, F.F.; Giulbudagian, M.; Bergueiro, J.; Lademann, J.; Calderon, M. Dendritic polyglycerol and N-isopropylacrylamide based thermoresponsive nanogels as smart carriers for controlled delivery of drugs through the hair follicle. Nanoscale 2017, 9, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Pang, K.Y.; Ng, T.W.; Leung, P.C.; Zhang, C.F.; Leung, K.C.; Jin, L. Cellular Interactions and Formation of an Epithelial “Nanocoating-Like Barrier” with Mesoporous Silica Nanoparticles. Nanomaterials 2016, 6, 192. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, M.; Mostaghaci, B.; Sitti, M. Recent Advances in Skin Penetration Enhancers for Transdermal Gene and Drug Delivery. Curr. Gene Ther. 2017, 17, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Mebert, A.M.; Baglole, C.J.; Desimone, M.F.; Maysinger, D. Nanoengineered silica: Properties, applications and toxicity. Food Chem. Toxicol. 2017, 109, 753–770. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.S.; Lee, Y.C.; Kuo, Y.C.; Lin, C.C. Development of Octyl Methoxy Cinnamates (OMC)/Silicon Dioxide (SiO(2)) Nanoparticles by Sol-Gel Emulsion Method. Nanomaterials 2017, 7, 434. [Google Scholar] [CrossRef] [PubMed]
- Ugazio, E.; Gastaldi, L.; Brunella, V.; Scalarone, D.; Jadhav, S.A.; Oliaro-Bosso, S.; Zonari, D.; Berlier, G.; Miletto, I.; Sapino, S. Thermoresponsive mesoporous silica nanoparticles as a carrier for skin delivery of quercetin. Int. J. Pharm. 2016, 511, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, F.; Wang, S.; Lu, J.; Li, J.; Du, Y.; Sun, X.; Chen, X.; Gao, J.; Ling, D. Ceria nanocrystals decorated mesoporous silica nanoparticle based ROS-scavenging tissue adhesive for highly efficient regenerative wound healing. Biomaterials 2018, 151, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, H.; Choi, Y.; Lee, D.S.; Kim, J.; Yi, G.R. Colloidal Mesoporous Silica Nanoparticles as Strong Adhesives for Hydrogels and Biological Tissues. ACS Appl. Mater. Interfaces 2017, 9, 31469–31477. [Google Scholar] [CrossRef] [PubMed]
- Sapino, S.; Ugazio, E.; Gastaldi, L.; Miletto, I.; Berlier, G.; Zonari, D.; Oliaro-Bosso, S. Mesoporous silica as topical nanocarriers for quercetin: Characterization and in vitro studies. Eur. J. Pharm. Biopharm. 2015, 89, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Ebabe Elle, R.; Rahmani, S.; Lauret, C.; Morena, M.; Bidel, L.P.; Boulahtouf, A.; Balaguer, P.; Cristol, J.P.; Durand, J.O.; Charnay, C.; et al. Functionalized Mesoporous Silica Nanoparticle with Antioxidants as a New Carrier That Generates Lower Oxidative Stress Impact on Cells. Mol. Pharm. 2016, 13, 2647–2660. [Google Scholar] [CrossRef] [PubMed]
- Pei, P.; Yang, F.; Liu, J.; Hu, H.; Du, X.; Hanagata, N.; Zhao, S.; Zhu, Y. Composite-dissolving microneedle patches for chemotherapy and photothermal therapy in superficial tumor treatment. Biomater. Sci. 2018, 6, 1414–1423. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Qu, Q.; Zhao, Y. Targeted delivery of 5-aminolevulinic acid by multifunctional hollow mesoporous silica nanoparticles for photodynamic skin cancer therapy. ACS Appl. Mater. Interfaces 2015, 7, 10671–10676. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Carmona, M.; Baeza, A.; Rodriguez-Milla, M.A.; Garcia-Castro, J.; Vallet-Regi, M. Mesoporous silica nanoparticles grafted with a light-responsive protein shell for highly cytotoxic antitumoral therapy. J. Mater. Chem. B 2015, 3, 5746–5752. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Nair, A.S. Temperature and ultrasound sensitive gatekeepers for the controlled release of chemotherapeutic drugs from mesoporous silica nanoparticles. J. Mater. Chem. B 2018, 6, 428–439. [Google Scholar] [CrossRef]
- Singh, P.; Singh, H.; Castro-Aceituno, V.; Ahn, S.; Kim, Y.J.; Farh, M.E.; Yang, D.C. Engineering of mesoporous silica nanoparticles for release of ginsenoside CK and Rh2 to enhance their anticancer and anti-inflammatory efficacy: In vitro studies. J. Nanopart. Res. 2017, 19. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Watson, C.; Murdaugh, K.M.; Darrah, T.H.; Pyrgiotakis, G.; Elder, A.; Brain, J.D.; Demokritou, P. Engineering safer-by-design, transparent, silica-coated ZnO nanorods with reduced DNA damage potential. Environ. Sci. Nano 2014, 1, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Tolbert, S.H.; McFadden, P.D.; Loy, D.A. New Hybrid Organic/Inorganic Polysilsesquioxane-Silica Particles as Sunscreens. ACS Appl. Mater. Interfaces 2016, 8, 3160–3174. [Google Scholar] [CrossRef] [PubMed]
- Bagde, A.; Mondal, A.; Singh, M. Drug delivery strategies for chemoprevention of UVB-induced skin cancer: A review. Photodermatol. Photoimmunol. Photomed. 2018, 34, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wong, C.H.; Ng, T.W.; Zhang, C.F.; Leung, K.C.; Jin, L. The spherical nanoparticle-encapsulated chlorhexidine enhances anti-biofilm efficiency through an effective releasing mode and close microbial interactions. Int. J. Nanomed. 2016, 11, 2471–2480. [Google Scholar] [CrossRef]
- Lungare, S.; Hallam, K.; Badhan, R.K. Phytochemical-loaded mesoporous silica nanoparticles for nose-to-brain olfactory drug delivery. Int. J. Pharm. 2016, 513, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.K. Nanobiotechnology-based strategies for crossing the blood-brain barrier. Nanomedicine 2012, 7, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, J.; Liang, J.; Liu, X.; Li, W.; Liu, Z.; Ding, Z.; Tuo, D. Towards Improvements for Penetrating the Blood-Brain Barrier-Recent Progress from a Material and Pharmaceutical Perspective. Cells 2018, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Baghirov, H.; Karaman, D.; Viitala, T.; Duchanoy, A.; Lou, Y.R.; Mamaeva, V.; Pryazhnikov, E.; Khiroug, L.; de Lange Davies, C.; Sahlgren, C.; et al. Feasibility Study of the Permeability and Uptake of Mesoporous Silica Nanoparticles across the Blood-Brain Barrier. PLoS ONE 2016, 11, e0160705. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, C.; Praca, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood-brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sui, B.; Sun, J. Blood-brain barrier dysfunction induced by silica NPs in vitro and in vivo: Involvement of oxidative stress and Rho-kinase/JNK signaling pathways. Biomaterials 2017, 121, 64–82. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Ke, W.; Han, L.; Liu, Y.; Shao, K.; Jiang, C.; Pei, Y. Lactoferrin-modified nanoparticles could mediate efficient gene delivery to the brain in vivo. Brain Res. Bull. 2010, 81, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Du, D.; Li, L.; Xu, J.; Dutta, P.; Lin, Y. In Vitro Study of Receptor-Mediated Silica Nanoparticles Delivery across Blood-Brain Barrier. ACS Appl. Mater. Interfaces 2017, 9, 20410–20416. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Li, M.; Wu, L.; Chen, C.; Qu, X. Mesoporous silica nanoparticle-based H2O2 responsive controlled-release system used for Alzheimer’s disease treatment. Adv. Healthc. Mater. 2012, 1, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yin, T.; Liu, Y.; Sun, J.; Zhou, Y.; Liu, J. Gold nanoparticle-capped mesoporous silica-based H2O2-responsive controlled release system for Alzheimer’s disease treatment. Acta Biomater. 2016, 46, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Karimzadeh, M.; Rashidi, L.; Ganji, F. Mesoporous silica nanoparticles for efficient rivastigmine hydrogen tartrate delivery into SY5Y cells. Drug Dev. Ind. Pharm. 2017, 43, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Cao, B.; Snyder, N.R.; Woeppel, K.M.; Eles, J.R.; Cui, X.T. ROS responsive resveratrol delivery from LDLR peptide conjugated PLA-coated mesoporous silica nanoparticles across the blood-brain barrier. J. Nanobiotechnol. 2018, 16, 13. [Google Scholar] [CrossRef] [PubMed]
- You, Y.Y.; Yang, L.Y.; He, L.Z.; Chen, T.F. Tailored mesoporous silica nanosystem with enhanced permeability of the blood-brain barrier to antagonize glioblastoma. J. Mater. Chem. B 2016, 4, 5980–5990. [Google Scholar] [CrossRef]
- Mo, J.; He, L.; Ma, B.; Chen, T. Tailoring Particle Size of Mesoporous Silica Nanosystem To Antagonize Glioblastoma and Overcome Blood-Brain Barrier. ACS Appl. Mater. Interfaces 2016, 8, 6811–6825. [Google Scholar] [CrossRef] [PubMed]
- Orive, G.; Ali, O.A.; Anitua, E.; Pedraz, J.L.; Emerich, D.F. Biomaterial-based technologies for brain anti-cancer therapeutics and imaging. Biochim. Biophys. Acta 2010, 1806, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Lockman, P.R.; Koziara, J.M.; Mumper, R.J.; Allen, D.D. Nanoparticle surface charges alter blood-brain barrier integrity and permeability. J. Drug Target. 2004, 12, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, U.; Sommerfeld, P.; Ulrich, S.; Sabel, B.A. Nanoparticle technology for delivery of drugs across the blood-brain barrier. J. Pharm. Sci. 1998, 87, 1305–1307. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Sakaeda, T.; Sugawara, T.; Hirano, K.; Stella, V.J. A novel chemical delivery system for brain targeting. Adv. Drug Deliv. Rev. 1999, 36, 255–275. [Google Scholar] [CrossRef]
- Gidwani, M.; Singh, A.V. Nanoparticle enabled drug delivery across the blood brain barrier: In vivo and in vitro models, opportunities and challenges. Curr. Pharm. Biotechnol. 2014, 14, 1201–1212. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Ke, W.; Liu, Y.; Jiang, C.; Pei, Y. The use of lactoferrin as a ligand for targeting the polyamidoamine-based gene delivery system to the brain. Biomaterials 2008, 29, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Fillebeen, C.; Descamps, L.; Dehouck, M.P.; Fenart, L.; Benaissa, M.; Spik, G.; Cecchelli, R.; Pierce, A. Receptor-mediated transcytosis of lactoferrin through the blood-brain barrier. J. Biol. Chem. 1999, 274, 7011–7017. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Jia, Q.; Huwel, S.; Xia, R.; Liu, T.; Gao, F.; Galla, H.J.; Gao, M. Receptor-mediated delivery of magnetic nanoparticles across the blood-brain barrier. ACS Nano 2012, 6, 3304–3310. [Google Scholar] [CrossRef] [PubMed]
- Hanada, S.; Fujioka, K.; Inoue, Y.; Kanaya, F.; Manome, Y.; Yamamoto, K. Cell-based in vitro blood-brain barrier model can rapidly evaluate nanoparticles’ brain permeability in association with particle size and surface modification. Int. J. Mol. Sci. 2014, 15, 1812–1825. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Maeda, J.; Higuchi, M.; Inoue, K.; Akita, H.; Harashima, H.; Suhara, T. Pharmacokinetics and brain uptake of lactoferrin in rats. Life Sci. 2006, 78, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.Y.; Chen, W.J.; Lee, W.Y.; Lo, S.T.; Lee, T.W.; Lo, J.M. In vitro and in vivo evaluation of lactoferrin-conjugated liposomes as a novel carrier to improve the brain delivery. Int. J. Mol. Sci. 2013, 14, 2862–2874. [Google Scholar] [CrossRef] [PubMed]
- Maccioni, R.B.; Farias, G.; Morales, I.; Navarrete, L. The revitalized tau hypothesis on Alzheimer’s disease. Arch. Med. Res. 2010, 41, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.; Wen, H.; Lu, W.L.; Du, J.; Guo, J.; Tian, W.; Men, Y.; Zhang, Y.; Li, R.J.; Yang, T.Y.; et al. Dual-targeting daunorubicin liposomes improve the therapeutic efficacy of brain glioma in animals. J. Controll. Release 2010, 141, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Vredenburgh, J.J.; Desjardins, A.; Reardon, D.A.; Peters, K.B.; Herndon, J.E., 2nd; Marcello, J.; Kirkpatrick, J.P.; Sampson, J.H.; Bailey, L.; Threatt, S.; et al. The addition of bevacizumab to standard radiation therapy and temozolomide followed by bevacizumab, temozolomide, and irinotecan for newly diagnosed glioblastoma. Clin. Cancer Res. 2011, 17, 4119–4124. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Jiang, M.; Jiang, D.; Feng, X.; Yao, J.; Song, Q.; Chen, H.; Gao, X.; Chen, J. Enhancing Glioblastoma-Specific Penetration by Functionalization of Nanoparticles with an Iron-Mimic Peptide Targeting Transferrin/Transferrin Receptor Complex. Mol. Pharm. 2015, 12, 2947–2961. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Chiang, C.F.; Wu, S.K.; Chen, L.F.; Hsieh, W.Y.; Lin, W.L. Targeting microbubbles-carrying TGFbeta1 inhibitor combined with ultrasound sonication induce BBB/BTB disruption to enhance nanomedicine treatment for brain tumors. J. Controll. Release. 2015, 211, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Niedzielska, E.; Smaga, I.; Gawlik, M.; Moniczewski, A.; Stankowicz, P.; Pera, J.; Filip, M. Oxidative Stress in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 4094–4125. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.; Spencer, J.P. Flavonoids, cognition, and dementia: Actions, mechanisms, and potential therapeutic utility for Alzheimer disease. Free Radic. Biol. Med. 2012, 52, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Solanki, I.; Parihar, P.; Mansuri, M.L.; Parihar, M.S. Flavonoid-based therapies in the early management of neurodegenerative diseases. Adv. Nutr. 2015, 6, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Shukitt-Hale, B. Blueberries and neuronal aging. Gerontology 2012, 58, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Mecocci, P.; Tinarelli, C.; Schulz, R.J.; Polidori, M.C. Nutraceuticals in cognitive impairment and Alzheimer’s disease. Front. Pharmacol. 2014, 5, 147. [Google Scholar] [CrossRef] [PubMed]
- Macready, A.L.; Kennedy, O.B.; Ellis, J.A.; Williams, C.M.; Spencer, J.P.; Butler, L.T. Flavonoids and cognitive function: A review of human randomized controlled trial studies and recommendations for future studies. Genes Nutr. 2009, 4, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Cherniack, E.P. A berry thought-provoking idea: The potential role of plant polyphenols in the treatment of age-related cognitive disorders. Br. J. Nutr. 2012, 108, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, J.B.; Ding, E.L.; Dixon, R.; Pasinetti, G.M.; Villarreal, F. The science of cocoa flavanols: Bioavailability, emerging evidence, and proposed mechanisms. Adv. Nutr. 2014, 5, 547–549. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).