Formulation and Characterization of Carvedilol Leciplex for Glaucoma Treatment: In-Vitro, Ex-Vivo and In-Vivo Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Formulation of Carvedilol Leciplex
2.3. Evaluation of Carvedilol Leciplex
2.3.1. Determination of Entrapment Efficiency (EE)
2.3.2. Particle Size, Distribution and Zeta Potential
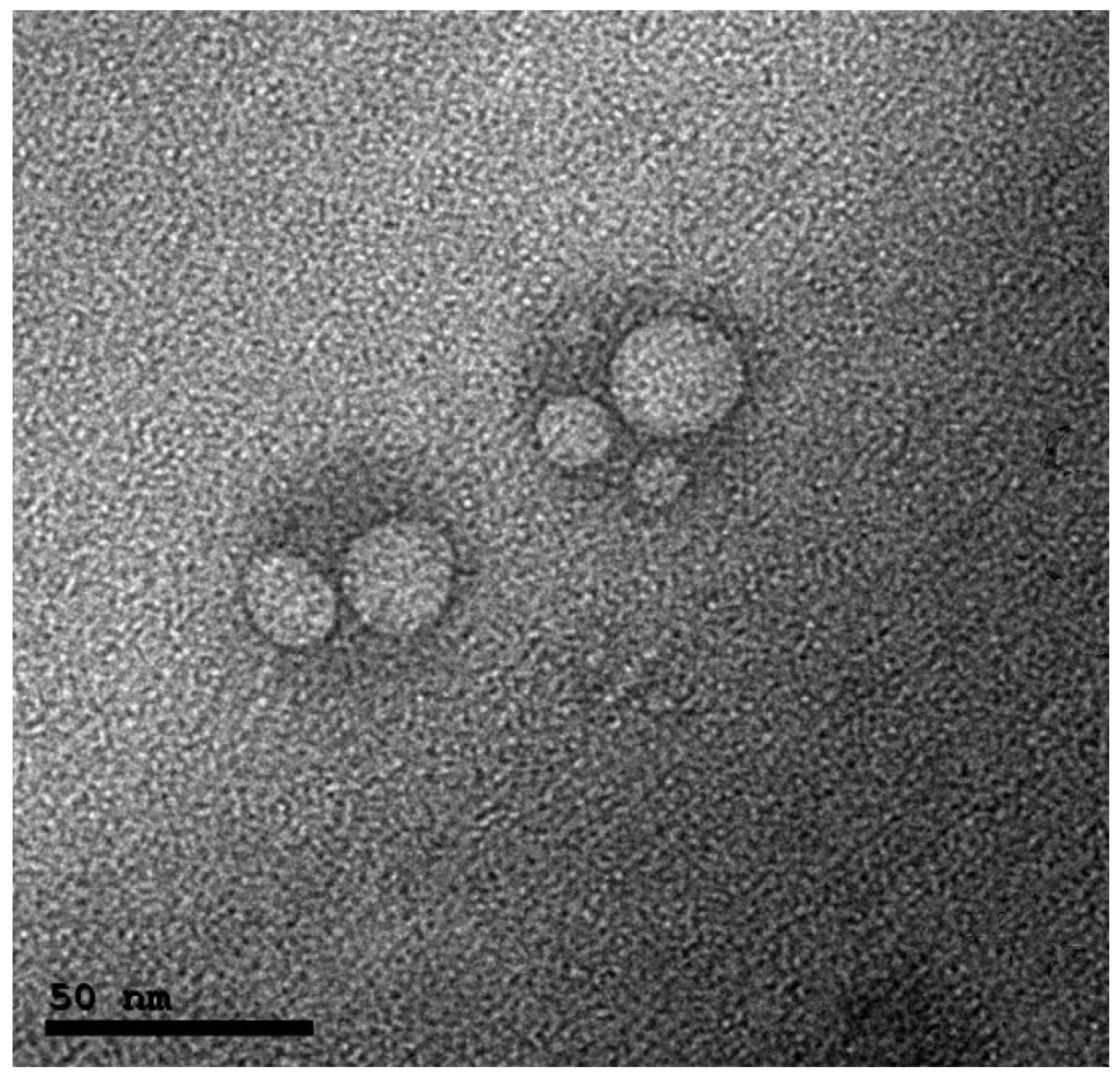
2.3.3. Morphology
2.4. Ex-Vivo Corneal Permeation Study
2.5. In-Vivo Evaluation of Carvedilol Leciplex
2.5.1. Pharmacokinetic Study
2.5.2. Pharmacodynamic Study
2.5.3. Histological Examination
2.6. Statistical Analysis of Data
3. Results and Discussion
3.1. In-Vitro Evaluation of Carvedilol Leciplex
3.1.1. Entrapment Efficiency
3.1.2. Particle Size, Distribution and Zeta Potential
3.1.3. Morphology
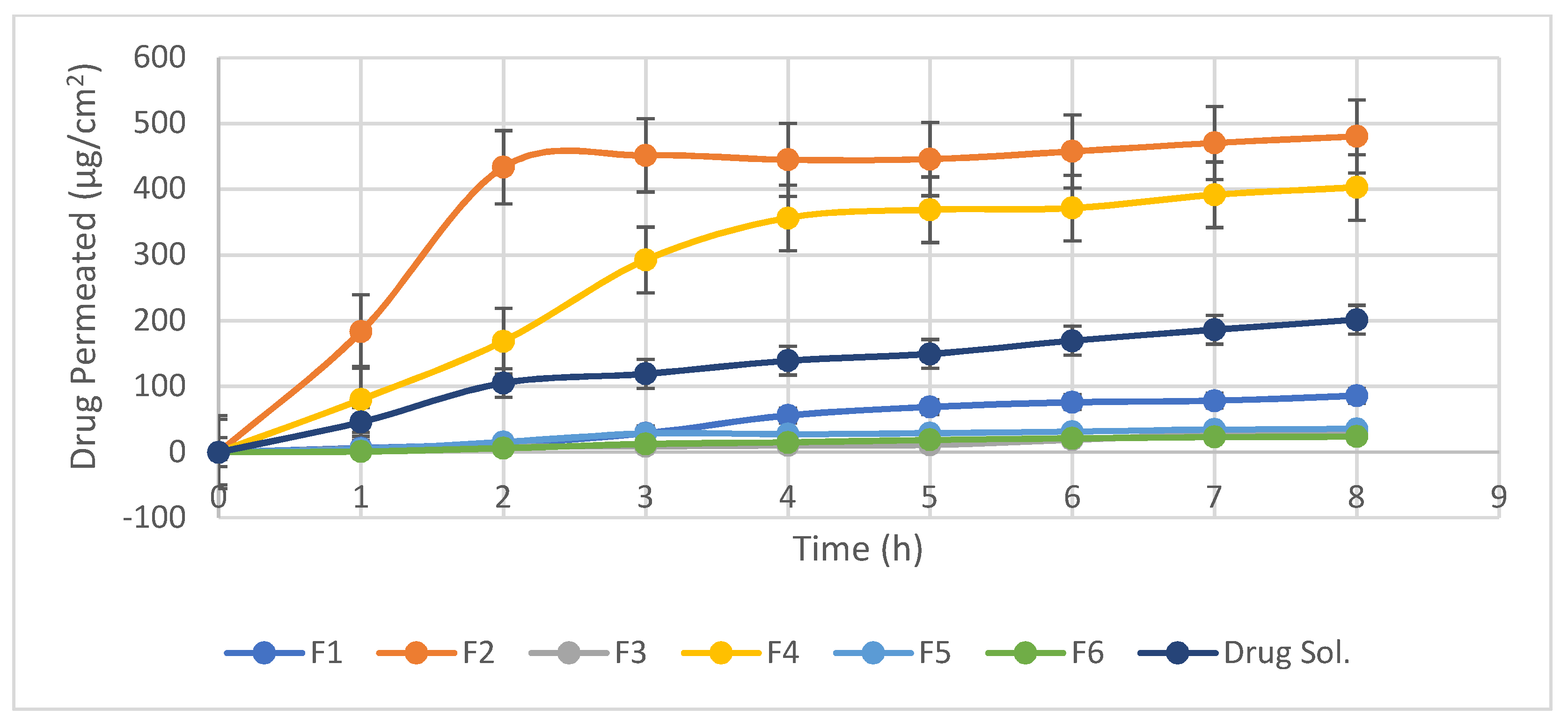
3.2. Ex-Vivo Corneal Permeation
3.3. In-Vivo Evaluation of Carvedilol Leciplex
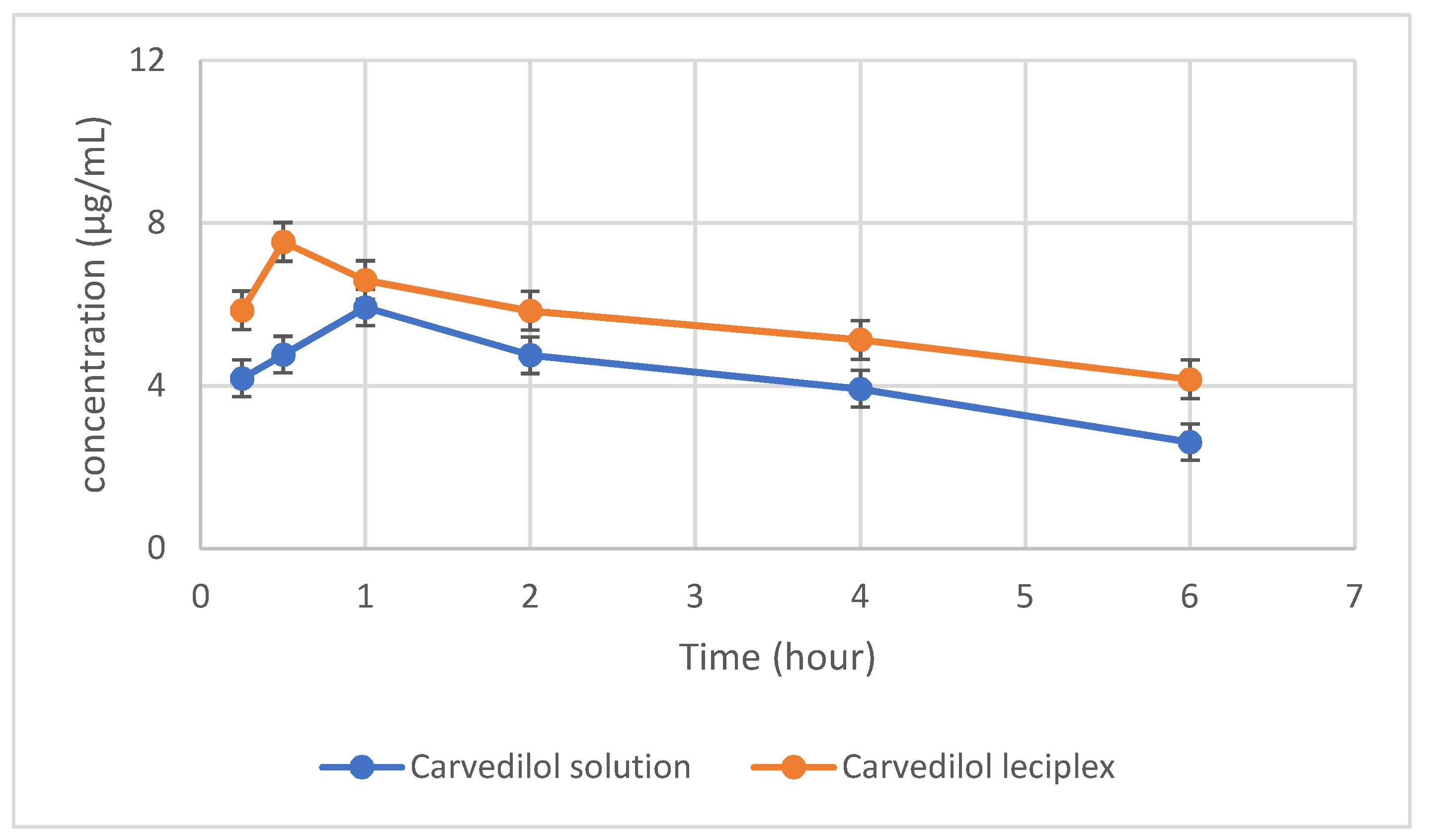
3.3.1. Pharmacokinetic Study
3.3.2. Pharmacodynamic Study
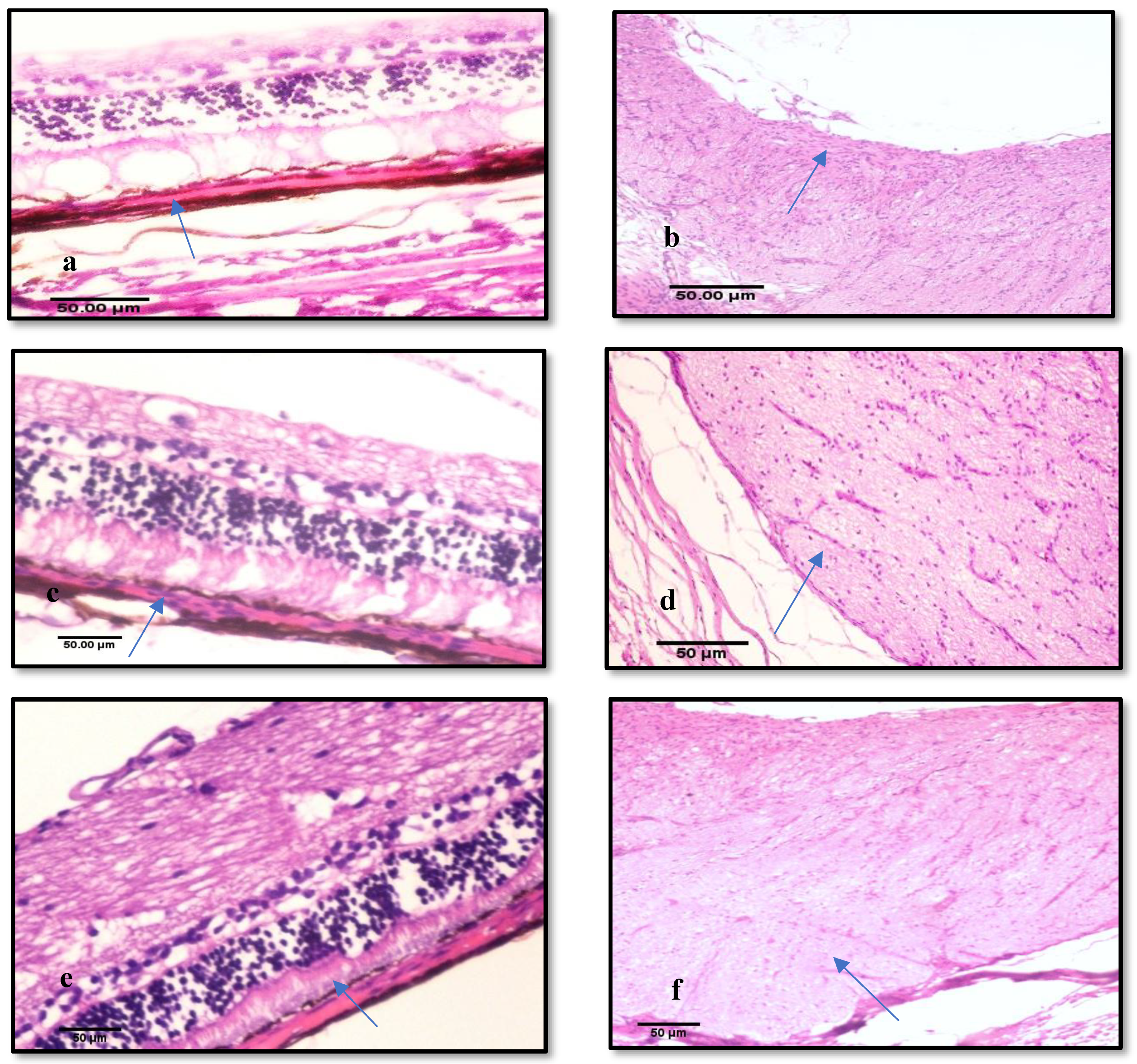
3.3.3. Histological Examination
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kundu, P.; Pani, N.R.; Barik, A.; Mishra, B.; Park, I.T.; Mouza, S.E.Z.; Bengal, W. Analysis of carvedilol and spironolactone in pharmaceutical dosage form and dissolution samples by simultaneous equation method and derivative method. Biopharm J. 2016, 2, 77–86. [Google Scholar]
- Szumny, D.; Szela̧g, A. The influence of new beta-adrenolytics nebivolol and carvedilol on intraocular pressure and iris blood flow in rabbits. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 256, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, S.; Natarajan, J.V.; Howden, T.; Boey, F. Stable Liposomal Formulations for Ocular Drug Delivery. U.S. Patent US9956195B2, 1 May 2018. [Google Scholar]
- Kaur, I.P.; Rana, C.; Singh, M.; Bhushan, S.; Singh, H.; Kakkar, S. Development and evaluation of novel surfactant-based elastic vesicular system for ocular delivery of fluconazole. J. Ocul. Pharmacol. Ther. 2012, 28, 484–496. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Byrne, M.E. Challenges and solutions in topical ocular drug-delivery systems. Expert Rev. Clin. Pharmacol. 2008, 1, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular drug delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Trabado, J.; Diebold, Y.; Sanchez, A. Designing lipid nanoparticles for topical ocular drug delivery. Int. J. Pharm. 2017, 532, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhuang, C.; Wang, M.; Sun, X.; Nie, S.; Pan, W. Liposome coated with low molecular weight chitosan and its potential use in ocular drug delivery. Int. J. Pharm. 2009, 379, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Date, A.A.; Srivastava, D.; Nagarsenker, M.S.; Mulherkar, R.; Panicker, L.; Aswal, V.; Fahr, A. Lecithin-based novel cationic nanocarriers (LeciPlex) I: Fabrication, characterization and evaluation. Nanomedicine 2011, 6, 1309–1325. [Google Scholar] [CrossRef] [PubMed]
- Date, A.A.; Nagarsenker, M.S.; Patere, S.; Dhawan, V.; Gude, R.P.; Hassan, P.A.; Fahr, A. Lecithin-based novel cationic nanocarriers (Leciplex) II: Improving therapeutic efficacy of quercetin on oral administration. Mol. Pharm. 2011, 8, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.T.; Tsai, T.H.; Huang, Z.R.; Fang, J.Y. Effects of lipophilic emulsifiers on the oral administration of lovastatin from nanostructured lipid carriers: Physicochemical characterization and pharmacokinetics. Eur. J. Pharm. Biopharm. 2010, 74, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Elbaz, N.M.; Khalil, I.A.; Abd-Rabou, A.A.; El-Sherbiny, I.M. Chitosan-based nano-in-microparticle carriers for enhanced oral delivery and anticancer activity of propolis. Int. J. Biol. Macromol. 2016, 92, 254–269. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, J.D.S.; Turner, D.R. Theory and Practice of Histological Techniques, 7th ed.; Churchill Livingstone Elsevier: London, UK, 2013. [Google Scholar]
- Baba, K.; Tanaka, Y.; Kubota, A.; Kasai, H.; Yokokura, S.; Nakanishi, H. A method for enhancing the ocular penetration of eye drops using nanoparticles of hydrolyzable dye. J. Control Release 2011, 153, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Forrester, J.V.; Paul, G.M.; Lee, W.R. General and ocular pharmacology. In The Eye: Basic Sciences in Practice, 2nd ed.; WB Saunders: Philadelphia, PA, USA, 2002; p. 477. [Google Scholar]
- Varghese, S.E.; Fariya, M.K.; Rajawat, G.S.; Steiniger, F.; Fahr, A.; Nagarsenker, M.S. Lecithin and PLGA-based self-assembled nanocomposite, Lecithmer: Preparation, characterization, and pharmacokinetic/pharmacodynamic evaluation. Drug Deliv. Transl. Res. 2016, 6, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Apaolaza, P.S.; Delgado, D.; Del Pozo-Rodriguez, A.; Gascon, A.R.; Solinis, M.A. A novel gene therapy vector based on hyaluronic acid and solid lipid nanoparticles for ocular diseases. Int. J. Pharm. 2014, 465, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.Y.; Randall, C.S.; Holl, W.W.; Constantinides, P.P.; Yue, T.L.; Feuerstein, G.Z. Carvedilol-liposome interaction: Evidence for strong association with the hydrophobic region of the lipid bilayers. BBA Biomembr. 1996, 1284, 20–28. [Google Scholar] [CrossRef]
- Rabinovich-Guilatt, L.; Couvreur, P.; Lambert, G.; Dubernet, C. Cationic vectors in ocular drug delivery. J. Drug Target. 2004, 12, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Lallemand, F.; Daull, P.; Benita, S.; Buggage, R.; Garrigue, J.S. Successfully improving ocular drug delivery using the cationic nanoemulsion, Novasorb. J. Drug Deliv. 2012, 2012, 604204. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.M.; Ashtikar, M.; Jain, A.S.; Makhija, D.T.; Nikam, Y.; Gude, R.P.; Steiniger, F.; Jagtap, A.A.; Nagarsenker, M.S.; Fahr, A. LeciPlex, invasomes, and liposomes: A skin penetration study. Int. J. Pharm. 2015, 490, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Peetla, C.; Labhasetwar, V. Effect of molecular structure of cationic surfactants on biophysical interactions of surfactant- modified nanoparticles with a model membrane and cellular uptake. Langmuir 2009, 25, 2369–2377. [Google Scholar] [CrossRef] [PubMed]
- Ban, J.; Zhang, Y.; Huang, X.; Deng, G.; Hou, D.; Chen, Y.; Lu, Z. Corneal permeation properties of a charged lipid nanoparticle carrier containing dexamethasone. Int. J. Nanomed. 2017, 12, 1329–1339. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Peng, T.; Li, Y.; Zhan, Z.; Zeng, Y.; Huang, Y.; Wu, C. Ocular cubosome drug delivery system for timolol maleate: Preparation, characterization, cytotoxicity, ex Vivo, and in Vivo evaluation. AAPS PharmSciTech 2017, 18, 1329–1339. [Google Scholar] [CrossRef] [PubMed]
- Rathod, S. Design and evaluation of liposomal formulation of pilocarpine nitrate. Indian J. Pharm. Sci. 2010, 72, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Hathout, R.M.; Mansour, S.; Mortada, N.D.; Guinedi, A.S. Liposomes as an ocular delivery system for acetazolamide: in vitro and in vivo studies. AAPS Pharmscitech. 2007, 8, E1–E12. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, A.; Bucolo, C.; Drago, F.; Salomone, S.; Pignatello, R. Cationic solid lipid nanoparticles enhance ocular hypotensive effect of melatonin in rabbit. Int. J. Pharm. 2015, 478, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Fangueiro, J.F.; Andreani, T.; Fernandes, L.; Garcia, M.L.; Egea, M.A.; Silva, A.M.; Souto, E.B. Physicochemical characterization of epigallocatechin gallate lipid nanoparticles (EGCG-LNs) for ocular instillation. Colloids Surf. B Biointerfaces 2014, 123, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Qiu, F.; Sloat, B.R. Lecithin-based cationic nanoparticles as a potential DNA delivery system. Int. J. Pharm. 2006, 313, 206–213. [Google Scholar] [CrossRef] [PubMed]

| Formulation Code | Composition | |||
|---|---|---|---|---|
| SPC | CTAB | DDAB | Carvedilol | |
| mg/mL | mg/mL | mg/mL | mg/mL | |
| F1 | 18.6 | 8.7 | - | 5 |
| F2 | 18.6 | - | 11.1 | 5 |
| F3 | 31 | 2.9 | - | 5 |
| F4 | 31 | - | 3.7 | 5 |
| F5 | 18.6 | - | - | 5 |
| F6 | 31 | - | - | 5 |
| Formulation Code | Characteristics | |||
|---|---|---|---|---|
| PS | PDI | ZP | EE | |
| nm | mV | % | ||
| F1 | 91.48 ± 1.8 | 0.18 | 34.5 ± 0.58 | 95.10 ± 1.0 |
| F2 | 16.04 ± 1.2 | 0.16 | 53.9 ± 0.91 | 95.59 ± 0.83 |
| F3 | 706 ± 0.98 | 0. 47 | 31.6 ± 0.46 | 96.00 ± 0.98 |
| F4 | 523 ± 1.1 | 0.50 | 47.2 ± 0.72 | 96.93 ± 1.3 |
| F5 | 1094 ± 2.3 | 0.40 | 2.82 ± 0.54 | 95.27 ± 0.69 |
| F6 | 1867 ± 1.5 | 0.70 | 7.83 ± 0.63 | 95.90 ± 0.94 |
| Formulae | Jss (µg/cm2/h) | Papp (µg/cm) | Q8 (µg/cm2) |
|---|---|---|---|
| F1 | 13.40 ± 0.36 | 0.0134 ± 0.060 | 85.80 ± 0.56 |
| F2 | 115.74 ± 0.21 | 0.1157 ± 0.044 | 480.36 ± 0.58 |
| F3 | 2.47 ± 0.17 | 0.00247 ± 0.023 | 27.10 ± 1.1 |
| F4 | 92.49 ± 0.54 | 0.0924 ± 0.091 | 402.83 ± 0.97 |
| F5 | 7.82 ± 0.29 | 0.0078 ± 0.023 | 35.53 ± 0.87 |
| F6 | 4.15 ± 0.13 | 0.00415 ± 0.020 | 23.60 ± 0.80 |
| Drug sol. | 35.15 ± 0.19 | 0.03515 ± 0.01 | 201.49 ± 0.50 |
| Pharmacokinetic Parameters | Carvedilol Solution | Carvedilol Leciplex |
|---|---|---|
| T1/2 (h) | 4.48 ± 0.8 | 7.0 ± 0.67 |
| tmax (h) | 1 ± 0.19 | 0.5 ± 0.0 |
| Cmax (µg/mL) | 5.93 ± 0.42 | 7.53 ± 0.61 |
| AUC (0–6) (µg h/ML) | 41.79 ± 2.8 | 74.47 ± 4.3 |
| MRT (h) | 6.64 ± 0.50 | 10.30 ± 0.96 |
| Time (h) | Mean IOP of GP1 (mmHg) | Mean IOP of GP2 (mmHg) |
|---|---|---|
| 0 | 40.9 ± 1.2 | 41.4 ± 1.5 |
| 0.25 | 38.00 ± 3.8 | 30.9 ± 2.1 |
| 0.5 | 29.21 ± 3.1 | 22.6 ± 2.10 |
| 1 | 22.5 ± 2.0 | 21.6 ± 1.0 |
| 2 | 22.5 ± 2.0 | 20.9 ± 0.00 |
| 4 | 22.5 ± 2.0 | 20.6 ± 0.73 |
| 6 | 24.4 ± 2.0 | 21.83 ± 2.3 |
| 8 | 27.7 ± 2.2 | 22.23 ± 2.6 |
| 10 | 30.4 ± 2.7 | 22.23 ± 2.6 |
| 12 | 30.4 ± 2.7 | 22.23 ± 2.6 |
| 24 | 37.0 ± 3.0 | 23.91 ± 3.71 |
| 48 | 41.7 ± 2.3 | 33.23 ± 1.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, D.H.; Abdelmonem, R.; Abdellatif, M.M. Formulation and Characterization of Carvedilol Leciplex for Glaucoma Treatment: In-Vitro, Ex-Vivo and In-Vivo Study. Pharmaceutics 2018, 10, 197. https://doi.org/10.3390/pharmaceutics10040197
Hassan DH, Abdelmonem R, Abdellatif MM. Formulation and Characterization of Carvedilol Leciplex for Glaucoma Treatment: In-Vitro, Ex-Vivo and In-Vivo Study. Pharmaceutics. 2018; 10(4):197. https://doi.org/10.3390/pharmaceutics10040197
Chicago/Turabian StyleHassan, Doaa H., Rehab Abdelmonem, and Menna M. Abdellatif. 2018. "Formulation and Characterization of Carvedilol Leciplex for Glaucoma Treatment: In-Vitro, Ex-Vivo and In-Vivo Study" Pharmaceutics 10, no. 4: 197. https://doi.org/10.3390/pharmaceutics10040197
APA StyleHassan, D. H., Abdelmonem, R., & Abdellatif, M. M. (2018). Formulation and Characterization of Carvedilol Leciplex for Glaucoma Treatment: In-Vitro, Ex-Vivo and In-Vivo Study. Pharmaceutics, 10(4), 197. https://doi.org/10.3390/pharmaceutics10040197





