Solid Lipid Nanoparticles: Emerging Colloidal Nano Drug Delivery Systems
Abstract
:1. Introduction
1.1. Advantages of SLNs
- Provide high stability to incorporated drugs
- Feasibility of incorporating both hydrophilic and lipophilic drugs
- Improve bioavailability of poorly water soluble molecules
- Ease in sterilization and scale up
- Immobilizing drug molecules within solid lipids provides protection from photochemical, oxidative, and chemical degradation of sensitive drugs, with reduced chances of drug leakage
- Drying by lyophilization is achievable
- Provide opportunities for targeted and controlled release of drug
- Biocompatible and biodegradable compositional ingredients [4]
1.2. Disadvantages of SLNs
- Various factors affect the loading or encapsulation of drugs in SLNs, such as interaction of drug and lipid melt, nature or state of lipid matrix, drug miscibility with lipid matrix, and the drug being dispersed or dissolved in the lipid matrix
- The dispersions have a high (70–90%) water content [16]
1.3. Nanostructured Lipid Carriers
- Imperfect type NLCs are prepared by mixing of solid lipids with small amounts of oils (liquid lipids) and thus demonstrate high drug loading.
- In multiple type NLCs, the amount of oily lipids are higher, and therefore yields high drug solubility as compared to solid lipids-. The reason of this phenomenon is based on the fact that the solubility of lipophilic drugs in solid lipids are lower than the liquid lipids (oils).
- Amorphous type NLCs contain additional specific lipids e.g., isopropyl myristate, hydroxyl octacosanyl, hydroxyl stearate etc. to avoid crystallization of solid lipid upon cooling. Thus, expulsion of drug caused by crystallization of solid lipids could be prevented by amorphous type NLCs [16].
1.4. Lipid Drug Conjugates
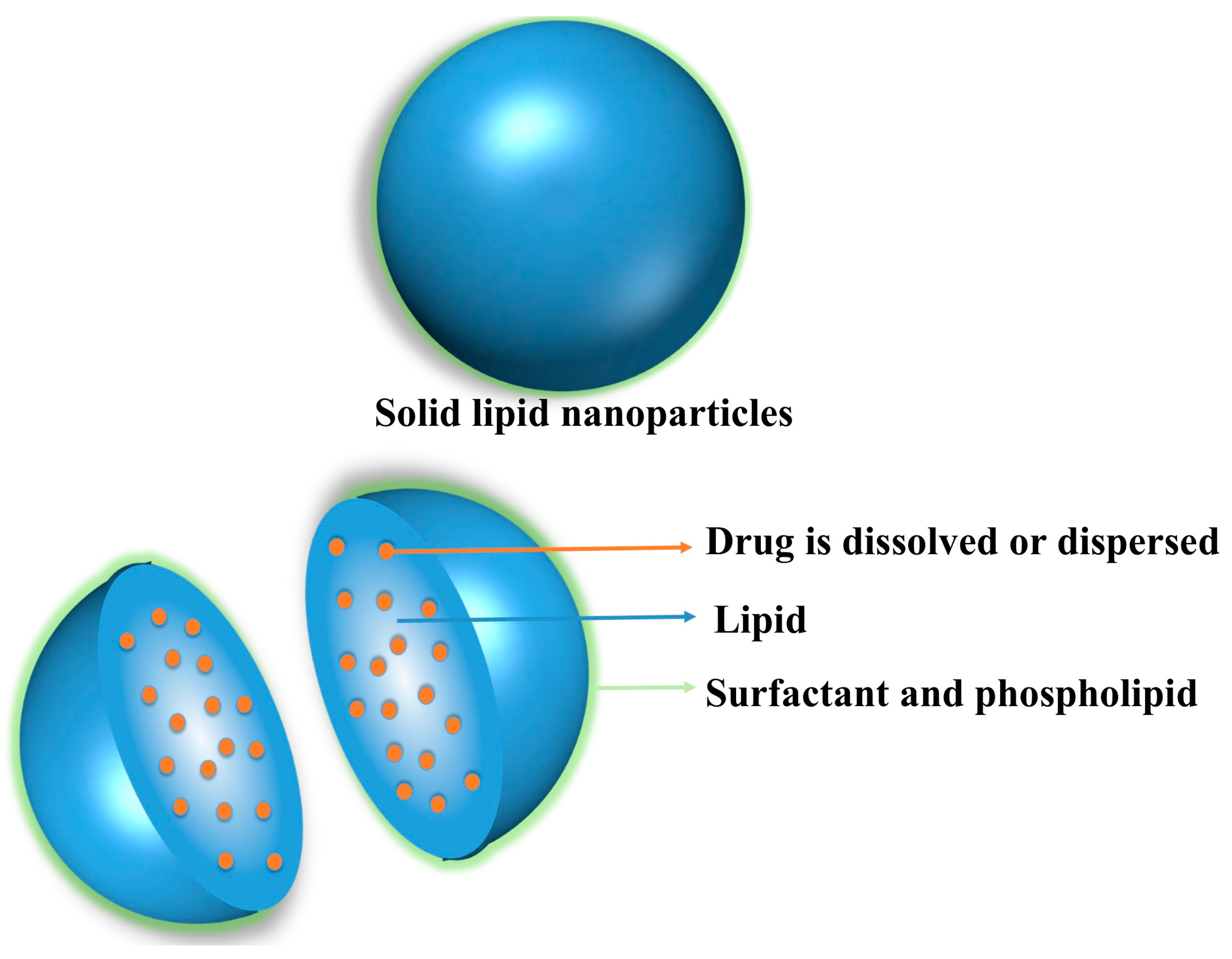
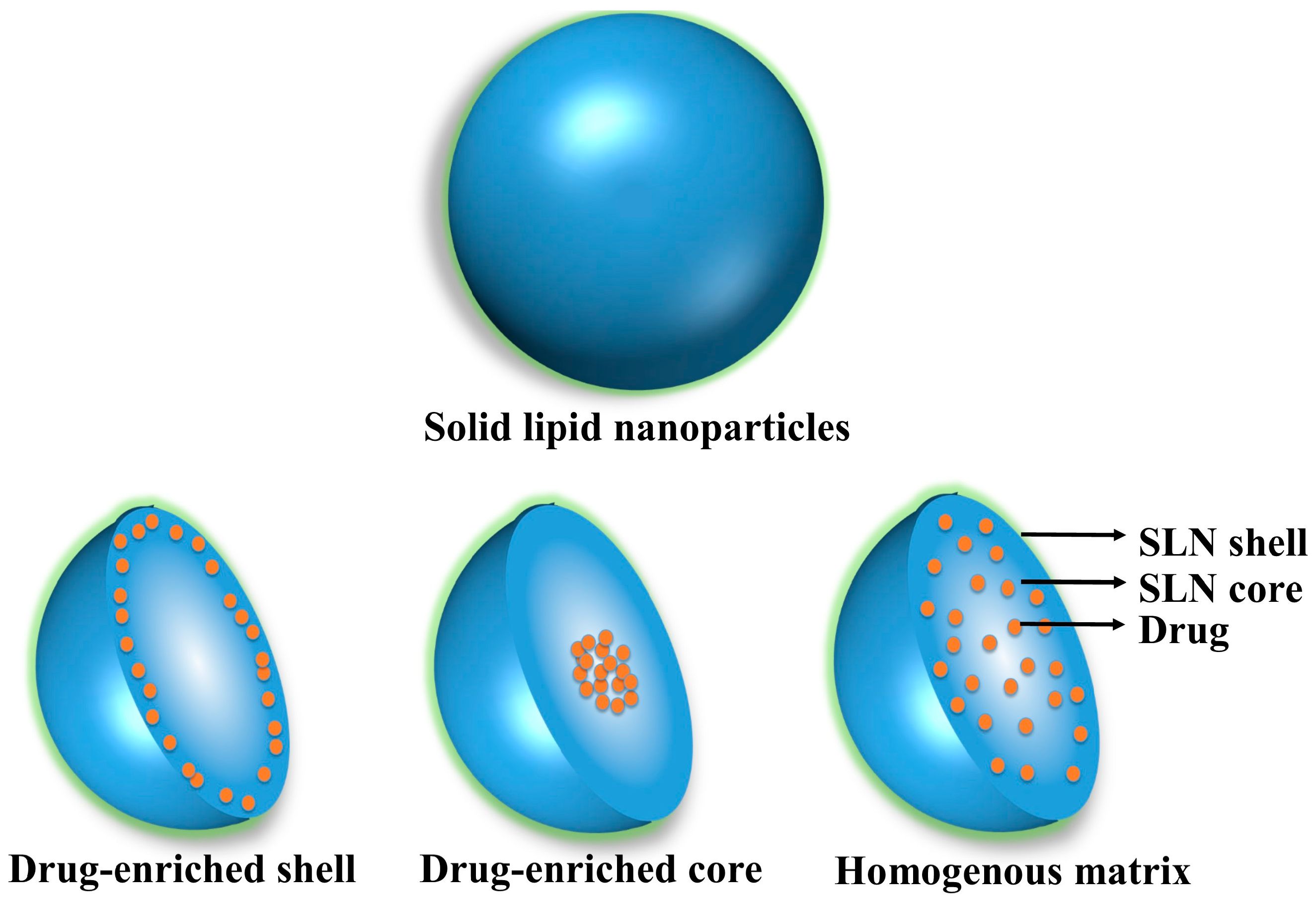
2. Compositional Profile of SLNs
3. Fabrication Techniques of SLNs
3.1. High Shear Homogenization
3.2. Ultrasonication or High Speed Homogenization
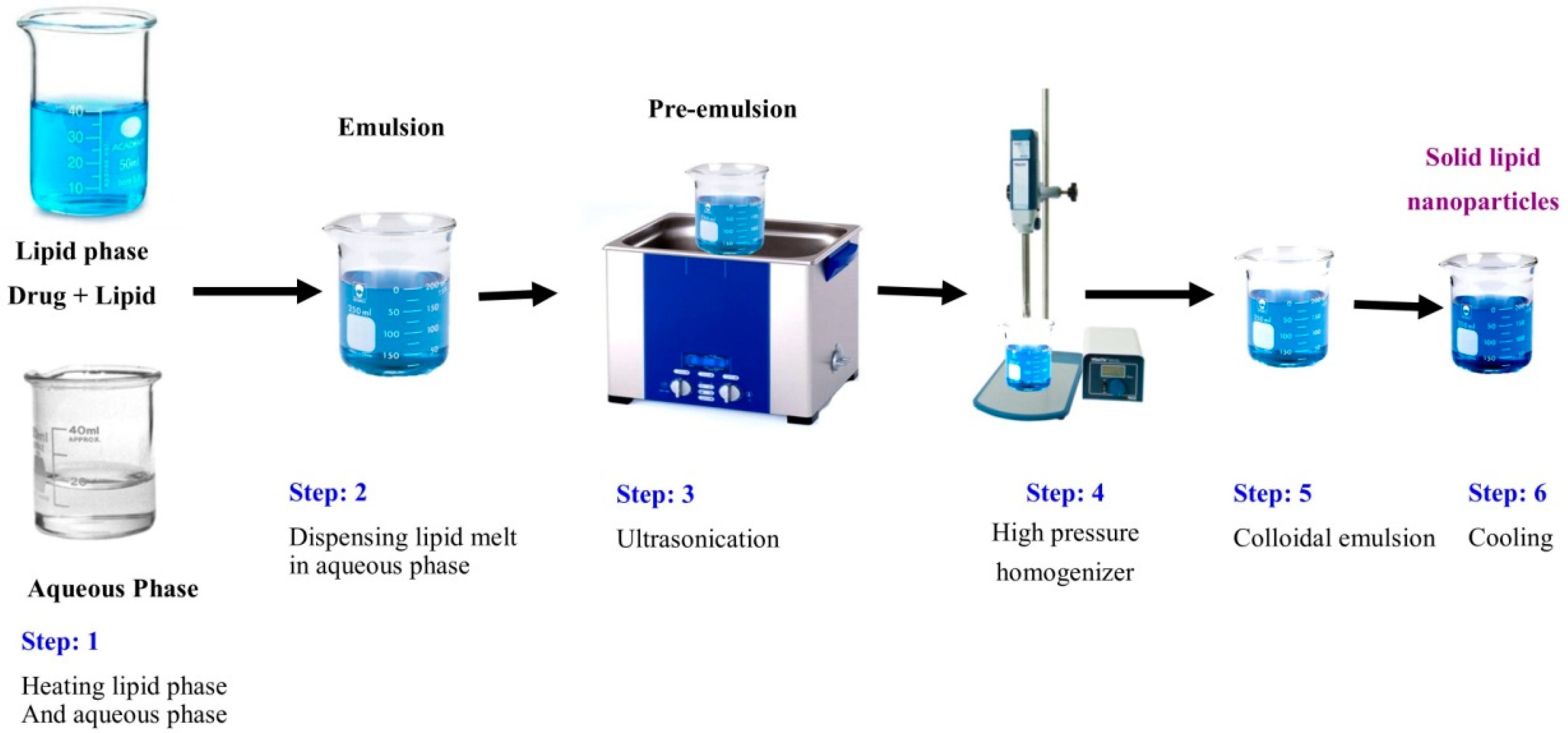
3.3. Hot Homogenization
3.4. Cold Homogenization
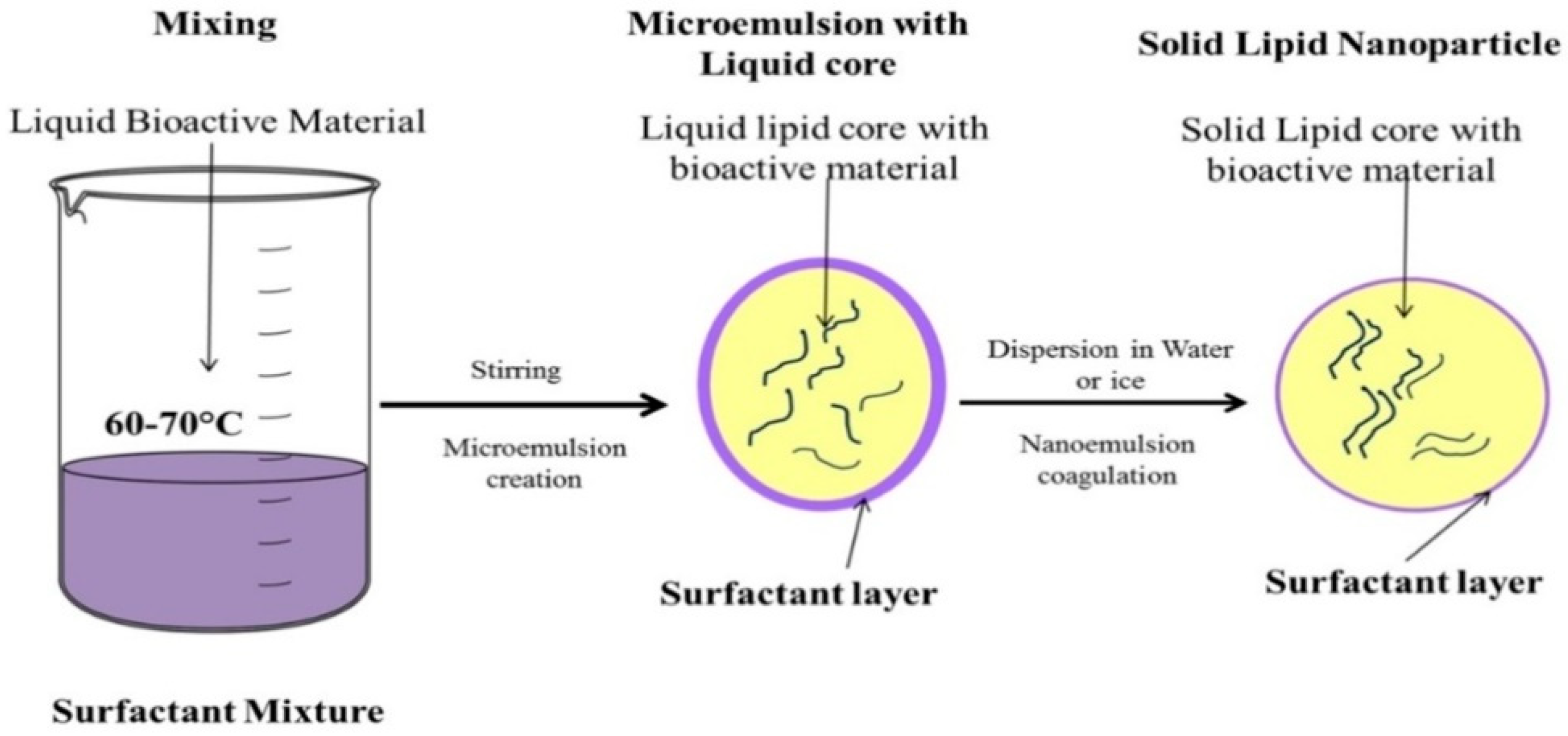
3.5. Microemulsion Based Method
3.6. Supercritical Fluid Based Method
3.7. Solvent Emulsification Evaporation Method
3.8. Double Emulsion Method
3.9. Spray Drying Method
4. Drying Techniques of SLNs
4.1. Spray Drying
4.2. Lyophilization
5. Characterization Techniques of SLNs
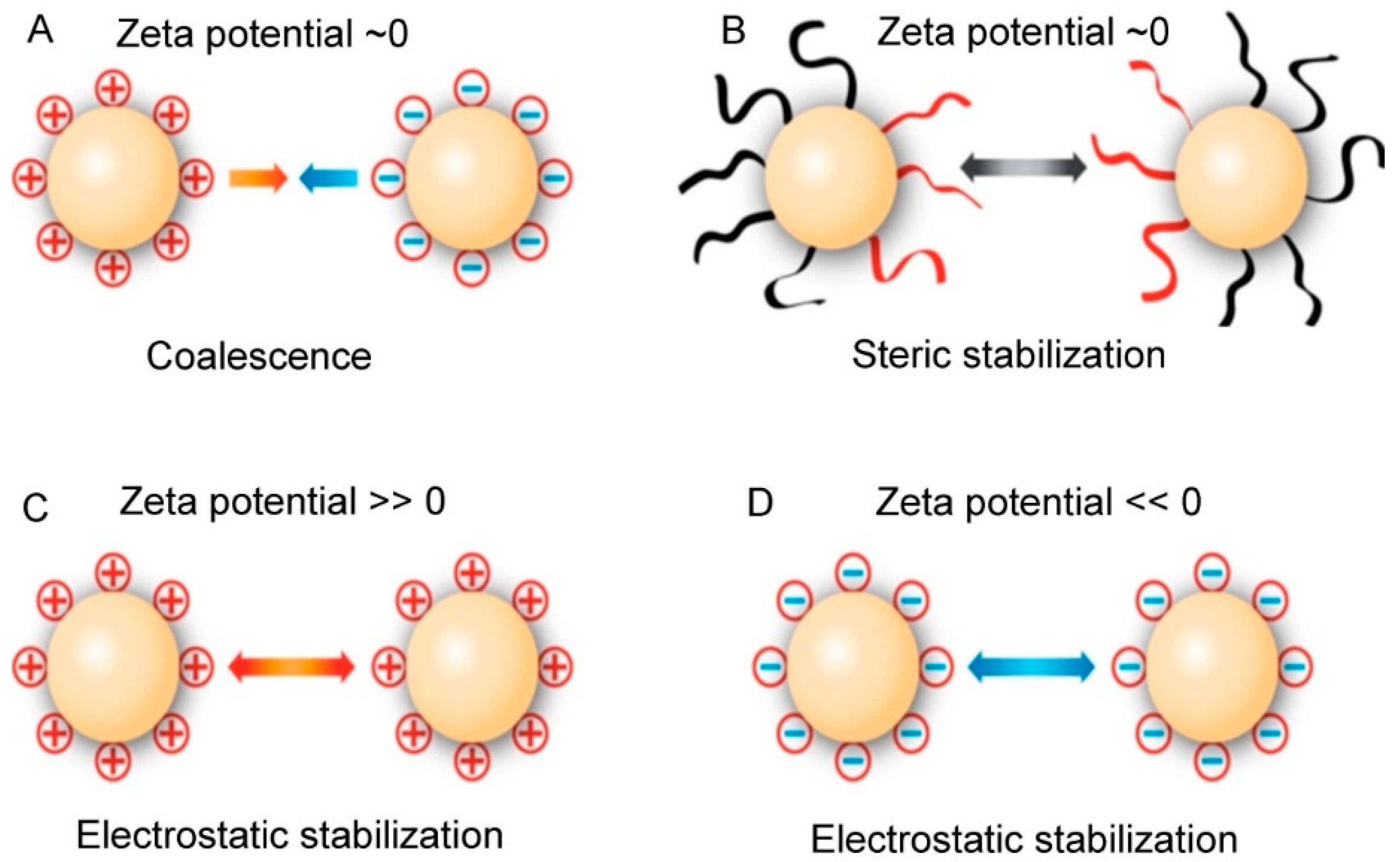
5.1. Particle Size and Zeta Potential
5.2. Surface Morphology
5.3. Degree of Crystallinity
5.4. Acoustic Methods
6. Scale-Up of SLNs Production
- Thermostated aluminum chamber (syringe) containing pneumatically functioned piston for delivering the microemulsion at a designated flux.
- At the bottom of the aluminum chamber, there is a stainless steel support for a sterile membrane filter (0.22 µm), to assure the sterility of the product.
- The stainless steel support is connected with a needle by Lure Lock connection. This apparatus is placed in an electric thermostated jacket. SLNs are formed by dispersing the warm microemulsion into an ice-cooled capsule containing water. The water is stirred by a cylindrical magnetic bar at a fixed rate (300 rpm).
- The microemulsion drops from the needle in the center of the capsule (ice-cooled). The SLN dispersion is stirred for additional 15 min after the widespread microemulsion dripping.
7. Drug Loading and Release Aspects of SLNs
7.1. Drug Loading into SLNs
7.2. Drug Release from SLNs
8. Routes of Administration for SLNs
8.1. Topical Route
8.2. Pulmonary Route
8.3. Oral Route
8.4. Intravenous Administration
8.5. Ocular Delivery
9. Protection of Incorporated Bioactives from Environmental Degradation in SLNs
10. Surface Modifications of SLNs
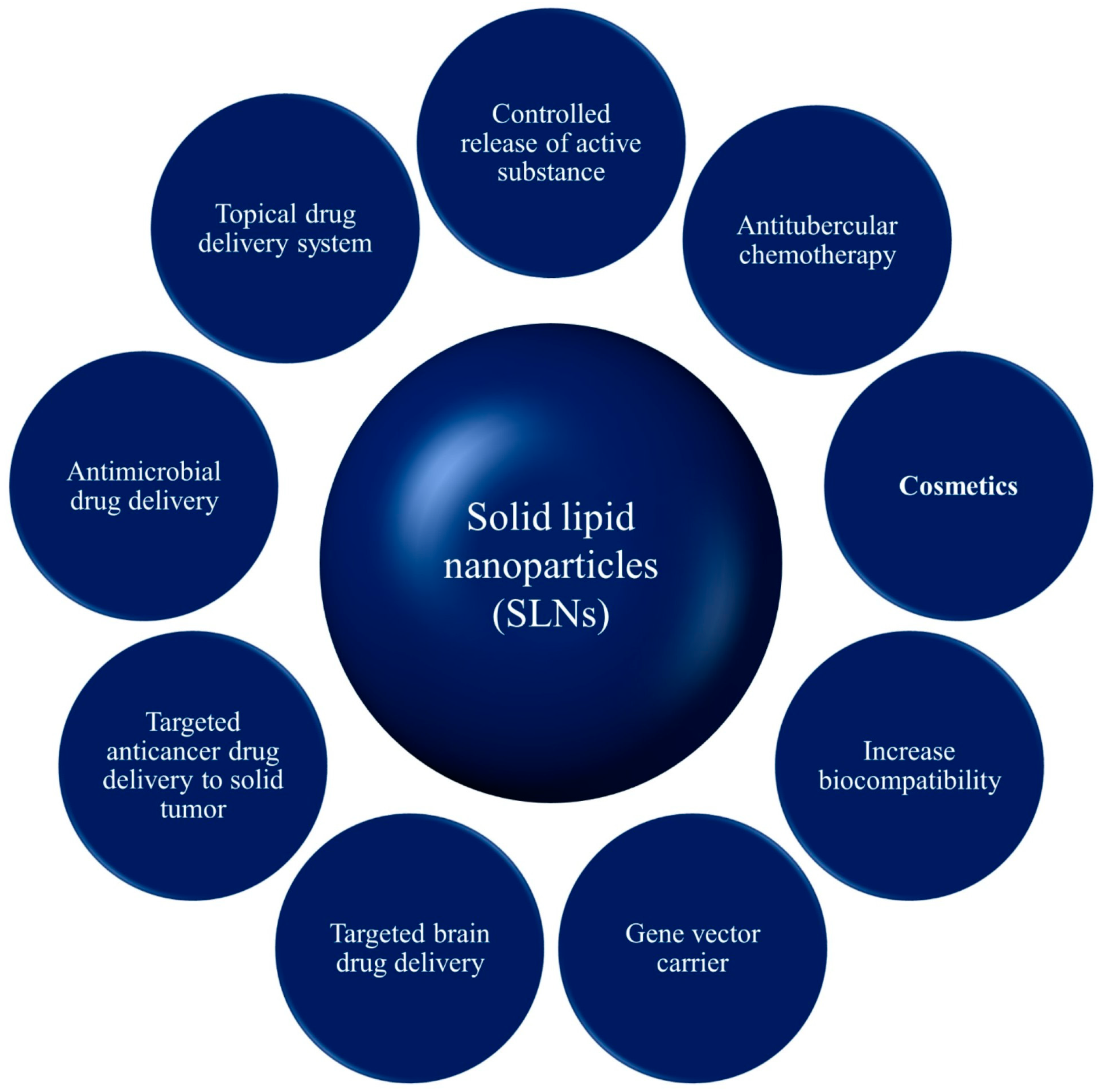
11. Applications of Solid Lipid Nanoparticles
11.1. Controlled Release of Drug
11.2. SLNs for Targeted Brain Drug Delivery
11.3. SLNs for Anticancer Drug Delivery
11.4. SLNs for Antimicrobial Drug Delivery
11.5. SLNs as Gene Carrier
11.6. SLNs for Topical Use
11.7. SLN in Cosmetics
11.8. SLNs as Adjuvant for Vaccines
11.9. SLNs in Antitubercular Chemotherapy
11.10. SLNs in Bioimaging
12. Toxicity Aspects of SLNs
12.1. Cytotoxicity of SLNs
12.1.1. Impact of Surface Charge
12.1.2. Effect of Composition on Cell Viability
12.2. Genotoxicity
12.3. Hemolytic Toxicity
13. Marketed Formulations of Solid Lipid Nanoparticles
14. Conclusions and Future Perspectives
Funding
Conflicts of Interest
References
- Geszke-Moritz, M.; Moritz, M. Solid lipid nanoparticles as attractive drug vehicles: Composition, properties and therapeutic strategies. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 982–994. [Google Scholar] [CrossRef] [PubMed]
- Adib, M.; Ghanbarzadeh, S.; Kouhsoltani, M.; Khosroshahi, A.Y.; Hamishehkar, H. The effect of particle size on the deposition of solid lipid nanoparticles in different skin layers: A histological study. Adv. Pharm. Bull. 2016, 6, 31–36. [Google Scholar] [CrossRef]
- Manjunath, K.; Reddy, J.S.; Venkateswarlu, V. Solid lipid nanoparticles as drug delivery systems. Methods Find Exp. Clin. Pharmacol. 2005, 27, 127–144. [Google Scholar] [CrossRef] [PubMed]
- Ekambaram, P.; Sathali, A.; Priyanka, K. Solid lipid nanoparticles: A review. Sci. Rev. Chem. Commun. 2012, 2, 80–102. [Google Scholar]
- Ramteke, K.H.; Joshi, S.A.; Dhole, S.N. Solid lipid nanoparticle: A Review. IOSR J. Pharm. 2012, 2, 34–44. [Google Scholar]
- Kushwaha, A.K.; Vuddanda, P.R.; Karunanidhi, P.; Singh, S.K.; Singh, S. Development and evaluation of solid lipid nanoparticles of raloxifene hydrochloride for enhanced bioavailability. BioMed. Res. Int. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.; Maranhão, R.C.; Tavares, E.R.; Carvalho, P.O.; Higuchi, M.L.; Mattos, F.R.; Serrano, C.V., Jr. Regression of atherosclerotic plaques of cholesterol-fed rabbits by combined chemotherapy with Paclitaxel and Methotrexate carried in lipid core nanoparticles. J. Cardiovasc. Pharmacol. Ther. 2018. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.P.; Parthiban, S.; Vikneswari, A.; Senthil kumar, G.P. A modern review on solid lipid nanoparticles as novel controlled drug delivery system. Int. J. Res. Pharm. Nano Sci. 2014, 3, 313–325. [Google Scholar]
- Garud, A.; Singh, D.; Garud, N. Solid lipid nanoparticle (SLN): Method, characterization and applications. Int. Curr. Pharm. J. 2012, 1, 384–393. [Google Scholar] [CrossRef]
- Sun, J.; Bi, C.; Chan, H.M.; Sun, S.; Zhang, Q.; Zheng, Y. Curcumin-loaded solid lipid nanoparticles have prolonged in vitro antitumour activity, cellular uptake and improved in vivo bioavailability. Colloids Surf. B 2013, 111, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, Y.J.; Peng, D.Y.; Li, Q.L.; Wang, X.S.; Wang, D.L.; Chen, W.D. Solid lipid nanoparticles as delivery systems for gambogenic acid. Colloids Surf. B 2013, 102, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Gupta, A.; Pawar, V.K.; Asthana, S.; Jaiswal, A.K.; Dube, A.; Chourasia, M.K. Chitosan-assisted immunotherapy for intervention of experimental leishmaniasis via amphotericin B-loaded solid lipid nanoparticles. Appl. Biochem. Biotechnol. 2014, 174, 1309–1330. [Google Scholar] [CrossRef] [PubMed]
- Rawat, M.K.; Jain, A.; Singh, S. Studies on binary lipid matrix based solid lipid nanoparticles of repaglinide: In vitro and in vivo evaluation. J. Pharm. Sci. 2011, 100, 2366–2378. [Google Scholar] [CrossRef] [PubMed]
- Westesen, K.; Bunjes, H.; Koch, M.H.J. Physicochemical characterization of lipid nanoparticles and evaluation of their drug loading capacity and sustained release potential. J. Control. Release 1997, 48, 223–236. [Google Scholar] [CrossRef]
- Westesen, K.; Siekmann, B.; Koch, M.H.J. Investigations on the physical state of lipid nanoparticles by synchrotron radiation X-ray diffraction. Int. J. Pharm. 1993, 93, 189–199. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ray, S.; Thakur, R.S. Solid lipid nanoparticles (SLN): A modern formulation approach in drug delivery system. Indian J. Pharm. Sci. 2009, 71, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Chaudhury, A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech 2011, 12, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Olbrich, C.; Gessner, A.; Kayser, O.; Muller, R.H. Lipid-drug conjugate (LDC) nanoparticles as novel carrier system for the hydrophilic antitrypanosomal drug diminazenediaceturate. J. Drug Target. 2002, 10, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, H.A.; Javadzadeh, Y.; Hamidi, M.; Jalali, M.B. Repaglinide-loaded solid lipid nanoparticles: Effect of using different surfactants/stabilizers on physicochemical properties of nanoparticles. DARU J. Pharm. Sci. 2015, 23, 46. [Google Scholar] [CrossRef] [PubMed]
- Kamble, V.A.; Jagdale, D.M.; Kadam, V.R.J. Solid lipid nanoparticles as drug delivery system. Int. J. Pharm. Biol. Sci. 2010, 1, 1–9. [Google Scholar]
- Patwekar, S.; Gattani, S.; Giri, R.; Bade, A.; Sangewar, B.; Raut, V. Review on nanoparticles used in cosmetics and dermal products. World J. Pharm. Pharm. Sci. 2014, 3, 1407–1421. [Google Scholar]
- Ganesan, P.; Narayanasamy, D. Lipid nanoparticles: Different preparation techniques, characterization, hurdles, and strategies for the production of solid lipid nanoparticles and nanostructured lipid carriers for oral drug delivery. Sustain. Chem. Pharm. 2017, 6, 37–56. [Google Scholar] [CrossRef]
- Jenning, V.; Thunemann, A.F.; Gohla, S.H. Characterization of a novel solid lipid nanoparticles carrier system based on binary mixtures of liquid and solid lipids. Int. J. Pharm. 2000, 199, 167–177. [Google Scholar] [CrossRef]
- Byrappa, K.; Ohara, S.; Adschiri, T. Nanoparticles synthesis using supercritical fluid technology–towards biomedical applications. Adv. Drug Deliv. Rev. 2008, 60, 299–327. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Eldridge, D.; Palombo, E.; Harding, I. Lipid Nanoparticles: Production, Characterization and Stability; Springer International Publishing: New York, NY, USA, 2015. [Google Scholar]
- Pooja, D.; Tunki, L.; Kulhari, H.; Reddy, B.; Sistla, R.K. Optimization of solid lipid nanoparticles prepared by a single emulsification solvent evaporation method. Data Brief. 2016, 6, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Heurtault, B.; Saulnier, P.; Benoit, J.P.; Proust, J.E.; Pech, B.; Richard, J. Lipid Nanocapsules, Preparation Process and Use as Medicine. U.S. Patent No. 8,057,823, 15 November 2011. [Google Scholar]
- Svilenov, H.; Tzachev, C. Solid lipid nanoparticles—A promising drug delivery system. In Nanomedicine; Seifalian, A., de Mel, A., Kalaskar, D.M., Eds.; One Central Press: London, UK, 2014; pp. 187–237. [Google Scholar]
- Jose, J.; Netto, G. Role of solid lipid nanoparticles as photoprotective agents in cosmetics. J. Cosmet. Dermatol. 2018, 1, 18. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2012, 64, 83–101. [Google Scholar] [CrossRef]
- Obeida, W.M.; Schwabe, K.; Muller, R.H. Preservation of nanostructured lipid carriers (NLC). Eur. J. Pharm. Biopharm. 2010, 76, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Charan, V.R.; Teja, V.; Chowdary, H.; Prasanna, Y.; Raju, N.; Surendra, R.V.; Vardhan, B.; Reddy, K.K. A glimpse on solid lipid nanoparticles as drug delivery systems. J. Glob. Trends Pharm. Sci. 2014, 5, 1649–1657. [Google Scholar]
- Saupe, A.; Gordon, K.C.; Rades., T. Structural investigations on nanoemulsions, solid lipid nanoparticles and nanostructured lipid carriers by cryo-field emission scanning electron microscopy and Raman spectroscopy. Int. J. Pharm. 2016, 314, 56–62. [Google Scholar] [CrossRef]
- Marengo, E.; Cavalli, R.; Caputo, O.; Rodriguez, L.; Gasco, M.R. Scale-up of the preparation process of lipid solid nanospheres: Part I. Int. J. Pharm. 2000, 205, 3–13. [Google Scholar] [CrossRef]
- Gohla, S.H.; Dingler, A. Scaling up feasibility of the production of solid lipid nanoparticles (SLNTM). Pharmazie 2001, 56, 61–63. [Google Scholar] [PubMed]
- Pardeshi, C.; Rajput, P.; Belgamwar, V.; Tekade, A.; Patil, G.; Chaudhary, K.; Sonje, A. Solid lipid based nanocarriers: An overview. Acta Pharm. 2012, 62, 433–472. [Google Scholar] [CrossRef] [PubMed]
- Muller, R.H.; Mader, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery—A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Uner, M.; Yener, G. Importance of solid lipid nanoparticles (SLN) in various administration routes and future perspectives. Int. J. Nanomed. 2007, 2, 289–300. [Google Scholar]
- Esposito, E.; Pecorelli, A.; Sguizzato, M.; Drechsler, M.; Mariani, P.; Carducci, F.; Valacchi, G. Production and characterization of nanoparticle based hyaluronate gel containing retinyl palmitate for wound healing. Curr. Drug Deliv. 2018. [Google Scholar] [CrossRef] [PubMed]
- Rabinarayan, P.; Padilama, S. Production of solid lipid nanoparticles-drug loading and release mechanism. J. Chem. Pharm. Res. 2010, 2, 211–227. [Google Scholar]
- Venkateswarlu, V.; Manjunath, K. Preparation, characterization and in vitro release kinetics of clozapine solid lipid nanoparticles. J. Control. Release 2004, 95, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.; Ihsan, A.; Madni, A.; Bajwa, S.Z.; Shi, D.; Webster, T.J.; Khan, W.S. Solid lipid nanoparticles for thermoresponsive targeting: Evidence from spectrophotometry, electrochemical, and cytotoxicity studies. Int. J. Nanomed. 2017, 12, 8325–8336. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.; Huang, W.C.; Chiang, W.H.; Liu, T.I.; Shen, M.Y.; Hsu, Y.H.; Lin, S.C.; Chiu, H.C. pH-Responsive therapeutic solid lipid nanoparticles for reducing P-glycoprotein-mediated drug efflux of multidrug resistant cancer cells. Int. J. Nanomed. 2015, 10, 5035–5048. [Google Scholar]
- Contri, R.V.; Fiel, L.A.; Pohlmann, A.R.; Guterres, S.S.; Beck, R.C.R. Transport of substances and nanoparticles across the skin and in vitro models to evaluate skin permeation and/or penetration. In Nanocosmetics and Nanomedicines; Beck, R., Guterres, S., Pohlmann, A., Eds.; Springer: Berlin, Germany, 2011; pp. 3–35. [Google Scholar]
- Kelidari, H.R.; Saeedi, M.; Akbari, J.; Morteza-Semnani, K.; Gill, P.; Valizadeh, H.; Nokhodchi, A. Formulation optimization and in vitro skin penetration of spironolactone loaded solid lipid nanoparticles. Colloids Surf. B Biointerfaces 2015, 128, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Jenning, V.; Schafer-Korting, M.; Gohla, S. Vitamin A-loaded solid lipid nanoparticles for topical use: Drug release properties. J. Control. Release 2000, 66, 115–126. [Google Scholar] [CrossRef]
- Attama, A.A.; Muller-Goymann, C.C. Effects of beeswax modifications on the lipid matrix of solid lipid nanoparticles crystallinity. Colloids Surf. 2008, 315, 189–195. [Google Scholar] [CrossRef]
- Ozeki, T.; Mizoe, T.; Takashima, Y.; Yuasa, H.; Okada, H. Preparation of two-drug composite microparticles to improve the dissolution of insoluble drug in water for use with a 4-fluid nozzle spray drier. J. Control. Release 2005, 107, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Negi, J.S.; Chattopadhyay, P.; Sharma, A.K.; Ram, V. Development of solid lipid nanoparticles (SLNs) of lopinavir using hot self nano-emulsification (SNE) technique. Eur. J. Pharm. Sci. 2013, 48, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.; Kumar, A.; Wild, W.; Ferreira, D.; Santos, D.; Forbes, B. Long-term stability, biocompatibility and oral delivery potential of risperidone-loaded solid lipid nanoparticles. Int. J. Pharm. 2012, 436, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.R.; Kaur, I.P. Encapsulation of Rifampicin in a solid lipid nanoparticulate system to limit its degradation and interaction with Isoniazid at acidic pH. Int. J. Pharm. 2013, 446, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Dudhipala, N.; Janga, K.Y.; Gorre, T. Comparative study of nisoldipine-loaded nanostructured lipid carriers and solid lipid nanoparticles for oral delivery: Preparation, characterization, permeation and pharmacokinetic evaluation. Artif. Cells Nanomed. Biotechnol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.C.; Lu, L.F.; Cai, Y.; Zhu, J.B.; Liang, B.W.; Yang, C.Z. Body distribution in mice of intravenously injected camptothecin solid lipid nanoparticles and targeting effect on brain. J. Control. Release 1999, 59, 299–307. [Google Scholar] [CrossRef]
- Souto, E.B.; Müller, R.H. Lipid nanoparticles: Effect on bioavailability and pharmacokinetic changes. In Handbook of Experimental Pharmacology; Schäfer-Korting, M., Ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 2010; Volume 197, pp. 115–141. [Google Scholar]
- Rodríguez, P.; Delgado, D.; Gascón, A.R.; Solinís, M.A. Lipid nanoparticles as drug/gene delivery systems to the retina. J. Ocul. Pharmacol. Therap. 2013, 29, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Pignatello, R.; Puglisi, G. Nanotechnology in ophthalmic drug delivery: A survey of recent developments and patenting activity. Recent Patents Nanomed. 2011, 1, 42–54. [Google Scholar] [CrossRef]
- Li, J.; Guo, X.; Liu, Z.; Okeke, C.I.; Li, N.; Zhao, H.; Aggrey, M.O.; Pan, W.; Wu, T. Preparation and evaluation of charged solid lipid nanoparticles of tetrandrine for ocular drug delivery system: Pharmacokinetics, cytotoxicity and cellular uptake studies. Drug Dev. Ind. Pharm. 2014, 40, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Gokce, E.H.; Sandri, G.; Bonferoni, M.C.; Rossi, S.; Ferrari, F.; Guneri, T.; Caramella, C. Cyclosporine a loaded SLNs: Evaluation of cellular uptake and corneal cytotoxicity. Int. J. Pharm. 2008, 364, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.Y. Biocompatibility of chemically cross-linked gelatin hydrogels for ophthalmic use. J. Mater. Sci. Mater. Med. 2010, 21, 1899–1911. [Google Scholar] [CrossRef] [PubMed]
- Khurana, L.K.; Singh, R.; Singh, H.; Sharma, M. Systematic development and optimization of in-situ gelling system for Moxifloxacin ocular nanosuspension using high pressure homogenization with improved encapsulation efficiency. Curr. Pharm. Des. 2018. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.J.; Souto, E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Adv. Drug Deliv. Rev. 2007, 59, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, P.; Khatik, R.; Khandelwal, K.; Taneja, I.; Raju, K.S.R.; Paliwal, S.K.; Dwivedi, A.K.; Mishra, P.R. Pharmacokinetics study of arteether loaded solid lipid nanoparticles: An improved oral bioavailability in rats. Int. J. Pharm. 2014, 466, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Saljoughian, N.; Zahedifard, F.; Doroud, D.; Doustdari, F.; Vasel, M.; Papadopoulou, B.; Rafati, S. Cationic solid-lipid nanoparticles are as efficient as electroporation in DNA vaccination against visceral leishmaniasis in mice. Parasite Immunol. 2013, 35, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, L.; Liu, B.; Chen, Z.; Li, C. Hyaluronic acid decorated pluronic P85 solid lipid nanoparticles as a potential carrier to overcome multidrug resistance in cervical and breast cancer. Biomed. Pharmacother. 2017, 86, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.S.; Cho, C.W. Surface modification of solid lipid nanoparticles for oral delivery of curcumin: Improvement of bioavailability through enhanced cellular uptake, and lymphatic uptake. Eur. J. Pharm. Biopharm. 2017, 117, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Wang, Y.; Meng, X. Chitosan nanolayered cisplatin-loaded lipid nanoparticles for enhanced anticancer efficacy in cervical cancer. Nanoscale Res. Lett. 2016, 11, 524. [Google Scholar] [CrossRef] [PubMed]
- Aldayela, A.M.; O’Marya, H.L.; Valdesa, S.A.; Lia, X.; Thakkara, S.G.; Mustafaa, B.E.; Cuia, Z. Lipid nanoparticles with minimum burst release of TNF-α siRNA show strong activity against rheumatoid arthritis unresponsive to methotrexate. J. Control. Release 2018, 283, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, R.; Bargoni, A.; Podio, V.; Muntoni, E.; Zara, G.P.; Gasco, M.R. Duodenal administration of solid lipid nanoparticles loaded with different percentages of tobramycin. J. Pharm. Sci. 2003, 92, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Khosa, A.; Reddi, S.; Saha, R.N. Nanostructured lipid carriers for site-specific drug delivery. Biomed. Pharmacother. 2018, 103, 598–613. [Google Scholar] [CrossRef]
- Jain, A.K.; Jain, A.; Garg, N.K.; Agarwal, A.; Jain, A.; Jain, S.A.; Tyagi, R.K.; Jain, R.K.; Agrawal, H.; Agrawal, G.P. Adapalene loaded solid lipid nanoparticles gel: An effective approach for acne treatment. Coll. Surf. 2014, 121, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Stébé, M.-J.; Blin, J.-L.; Pasc, A. pH-controlled delivery of curcumin from compartmentalized solid lipid nanoparticles@ mesostructured silica matrix. J. Mater. Chem. B 2014, 2, 7910–7917. [Google Scholar] [CrossRef]
- Abbas, H.; Refai, H.; El Sayed, N. Superparamagnetic iron oxide-loaded lipid nanocarriers incorporated in thermosensitive in situ gel for magnetic brain targeting of clonazepam. J. Pharm. Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Lakkadwala, S.; Nguyen, S.; Lawrence, J.; Nauli, S.M.; Nesamony, J. Physicochemical characterisation, cytotoxic activity, and biocompatibility studies of tamoxifen loaded solid lipid nanoparticles prepared via a temperature-modulated solidification method. J. Microencap. 2014, 31, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Xiong, S.B.; Yang, H.; Yin, X.D.; Chao, R.B. Solid lipid nanoparticles of mitoxantrone for local injection against breast cancer and its lymph node metastases. Eur. J. Pharm. Sci. 2006, 28, 86–95. [Google Scholar] [CrossRef]
- Chirio, D.; Peira, E.; Battaglia, L.; Ferrara, B.; Barge, A.; Sapino, S.; Gallarate, M. Lipophilic prodrug of floxuridine loaded into solid lipid nanoparticles: In vitro cytotoxicity studies on different human cancer cell lines. J. Nanosci. Nanotechnol. 2018, 18, 556–563. [Google Scholar] [CrossRef]
- Lakshminarayanan, R.; Ye, E.; Young, D.J.; Li, Z.; Loh, X.J. Recent advances in the development of antimicrobial nanoparticles for combating resistant pathogens. Adv. Healthc. Mater. 2018. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Pascual, M.; Albano, A.; Solinís, M.A.; Serpe, L.; Rodríguez-Gascón, A.; Foglietta, F.; Battaglia, L. Gene delivery in the cornea: In vitro and ex vivo evaluation of solid lipid nanoparticle-based vectors. Nanomedicine 2018. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Goindi, S.; Katare, O.P. Formulation, characterisation and in vivo evaluation of lipid-based nanocarrier for topical delivery of diflunisal. J. Microencapsul. 2016, 33, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Kaul, S.; Gulati, N.; Verma, D.; Mukherjee, S.; Nagaich, U. Role of Nanotechnology in cosmeceuticals: A review of recent advances. J. Pharm. 2018. [Google Scholar] [CrossRef] [PubMed]
- Gonçalez, M.L.; Rigon, R.B.; Pereira-da-Silva, M.A.; Chorilli, M. Curcumin-loaded cationic solid lipid nanoparticles as a potential platform for the treatment of skin disorders. Int. J. Pharm. 2017, 72, 721–727. [Google Scholar]
- Stelzner, J.; Behrens, M.; Behrens, S.E.; Mäder, K. Squalene containing solid lipid nanoparticles, a promising adjuvant system for yeast vaccines. Vaccine 2018, 36, 2314–2320. [Google Scholar] [CrossRef] [PubMed]
- Patil, K.; Bagade, S.; Bonde, S.; Sharma, S.; Saraogi, G. Recent therapeutic approaches for the management of tuberculosis: Challenges and opportunities. Biomed. Pharmacother. 2018, 99, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Castellani, S.; Trapani, A.; Spagnoletta, A.; Toma, L.; Magrone, T.; Gioia, S.; Conese, M. Nanoparticle delivery of grape seed-derived proanthocyanidins to airway epithelial cells dampens oxidative stress and inflammation. J. Transl. Med. 2018, 16, 140. [Google Scholar] [CrossRef] [PubMed]
- Zambrano-Zaragoza, M.L.; González-Reza, R.; Mendoza-Muñoz, N.; Miranda-Linares, V.; Bernal-Couoh, T.F.; Mendoza-Elvira, S.; Quintanar-Guerrero, D. Nanosystems in edible coatings: A novel strategy for food preservation. Int. J. Mol. Sci. 2018, 19, 705. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, J.; Moura, C.C.; Sarmento, B.; Reis, S. Solid lipid nanoparticles: A potential multifunctional approach towards rheumatoid arthritis theranostics. Molecules 2015, 20, 11103–11118. [Google Scholar] [CrossRef] [PubMed]
- Winter, E.; Pizzol, C.D.; Locatelli, C.; Crezkynski-Pasa, T.B. Development and evaluation of lipid nanoparticles for drug delivery: Study of toxicity in vitro and in vivo. J. Nanosci. Nanotechnol. 2016, 16, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Roghayeh, A.; Aref, S.; Rasedee, A. Cytotoxicity effect of solid lipid nanoparticles on human breast cancer cell lines. Biotechnology 2011, 10, 528–533. [Google Scholar]
- Bechinger, B.; Lohner, K. Detergent-like actions of linear amphipathic cationic antimicrobial peptides. Biochim. Biophys. Acta. 2006, 1758, 1529–1539. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.Y.; Liu, S.; Wong, H.L. Nanotoxicity: A key obstacle to clinical translation of siRNA-based nanomedicine. Nanomedicine 2014, 9, 295–312. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug. Deliv. Rev. 2002, 54, 131–155. [Google Scholar] [CrossRef]
- Weyenberg, F.P.; Van den Plas, D.; Vandervoort, J.; De Smet, K.; Sollie, P.; Ludwig, A. Cytotoxicity of submicron emulsions and solid lipid nanoparticles for dermal application. Int. J. Pharm. 2007, 337, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Dolatabadi, J.E.N.; Hamishehkar, H.; Eskandani, M.; Valizadeh, H. Formulation, characterization and cytotoxicity studies of alendronate sodium-loaded solid lipid nanoparticles. Colloids Surf. B Biointerfaces 2014, 117, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, K.V.; Pal, H.C.; Mondhe, D.M.; Kaur, I.P. The augmented anticancer potential of AP9-cd loaded solid lipid nanoparticles in human leukemia Molt-4 cells and experimental tumor. Chem.-Biol. Interact. 2016, 244, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Eskandani, N.H. Self-reporter shikonin-act-loaded solid lipid nanoparticle: Formulation, physicochemical characterization and geno/cytotoxicity evaluation. Eur. J. Pharma. Sci. 2014, 59, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Bansal, K.K.; Gupta, J.; Rosling, A.; Rosenholm, J.M. Renewable poly(δ-decalactone) based block copolymer micelles as drug delivery vehicle: In vitro and in vivo evaluation. Saudi Pharm. J. 2018, 26, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Negi, L.M.; Talegaonkar, S.; Jaggi, M.; Verma, A.K.; Verma, R.; Dobhal, S.; Kumar, V. Surface engineered nanostructured lipid carriers for targeting MDR tumor: Part II. In vivo biodistribution, pharmacodynamic and hematological toxicity studies. Colloids Surf. B Biointerfaces 2014, 123, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Kesharwani, P.; Garg, N.K.; Jain, A.; Jain, S.A.; Jain, A.K.; Nirbhavane, P.; Ghanghoria, R.; Tyagi, R.K.; Katare, O.P. Galactose engineered solid lipid nanoparticles for targeted delivery of doxorubicin. Colloids Surf. B Biointerfaces 2015, 134, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Padhye, S.G.; Nagarsenker, M.S. Simvastatin solid lipid nanoparticles for oral delivery: Formulation development and in vivo evaluation. Indian J. Pharm. Sci. 2013, 75, 591–598. [Google Scholar] [PubMed]

| Ingredients | Examples |
|---|---|
| Lipid component | Beeswax, Stearic acid, Cholesterol, Caprylic/capric triglyceride, Cetylpalmitate, Glyceryl stearate (-mono, and -tri), Glyceryl trilaurate, Glyceryl trimyristate, Glyceryl behenate (Compritol), Glyceryl tripalmitate, Hardened fat (Witepsol E85, H5 and W35), Monostearate monocitrate, Solid paraffin, Behenic acid |
| Surfactant/Emulsifiers | Phosphatidyl choline, Soy and Egg lecithin, Poloxamer, Poloxamine, Polysorbate 80 |
| Co-surfactant | Sodium dodecyl sulphate, Tyloxopol, Sodium oleate, Taurocholate sodium salt, Sodium glycocholate, Butanol |
| Preservative | Thiomersal |
| Cryoprotectant | Gelatin, Glucose, Mannose, Maltose, Lactose, Sorbitol, Mannitol, Glycine, Polyvinyl alcohol, Polyvinyl pyrrolidone |
| Charge modifiers | Dipalmitoyl phosphatidyl choline, Stearylamine, Dicetylphosphate, Dimyristoyl phophatidyl glycerol |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, V.; Bansal, K.K.; Verma, A.; Yadav, N.; Thakur, S.; Sudhakar, K.; Rosenholm, J.M. Solid Lipid Nanoparticles: Emerging Colloidal Nano Drug Delivery Systems. Pharmaceutics 2018, 10, 191. https://doi.org/10.3390/pharmaceutics10040191
Mishra V, Bansal KK, Verma A, Yadav N, Thakur S, Sudhakar K, Rosenholm JM. Solid Lipid Nanoparticles: Emerging Colloidal Nano Drug Delivery Systems. Pharmaceutics. 2018; 10(4):191. https://doi.org/10.3390/pharmaceutics10040191
Chicago/Turabian StyleMishra, Vijay, Kuldeep K. Bansal, Asit Verma, Nishika Yadav, Sourav Thakur, Kalvatala Sudhakar, and Jessica M. Rosenholm. 2018. "Solid Lipid Nanoparticles: Emerging Colloidal Nano Drug Delivery Systems" Pharmaceutics 10, no. 4: 191. https://doi.org/10.3390/pharmaceutics10040191
APA StyleMishra, V., Bansal, K. K., Verma, A., Yadav, N., Thakur, S., Sudhakar, K., & Rosenholm, J. M. (2018). Solid Lipid Nanoparticles: Emerging Colloidal Nano Drug Delivery Systems. Pharmaceutics, 10(4), 191. https://doi.org/10.3390/pharmaceutics10040191








