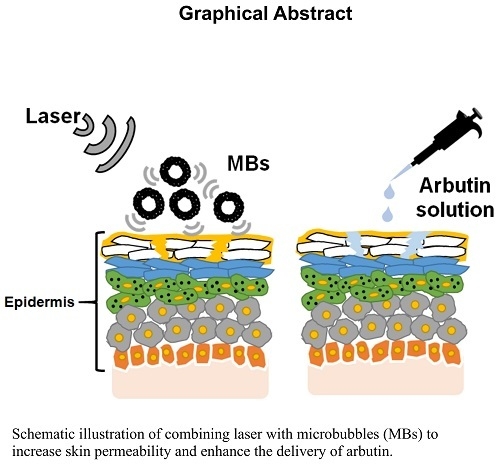
Combining Microbubble Contrast Agent with Pulsed-Laser Irradiation for Transdermal Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production of Albumin-Shelled MBs
2.2. Laser-Induced MB Disruption
2.3. Penetration Depth in Pigskin
2.4. In Vitro Skin Penetration by β-arbutin Solution
2.5. HPLC Analysis of β-arbutin
2.6. Animal Treatments
2.7. Histological Study
2.8. Statistical Analysis
3. Results
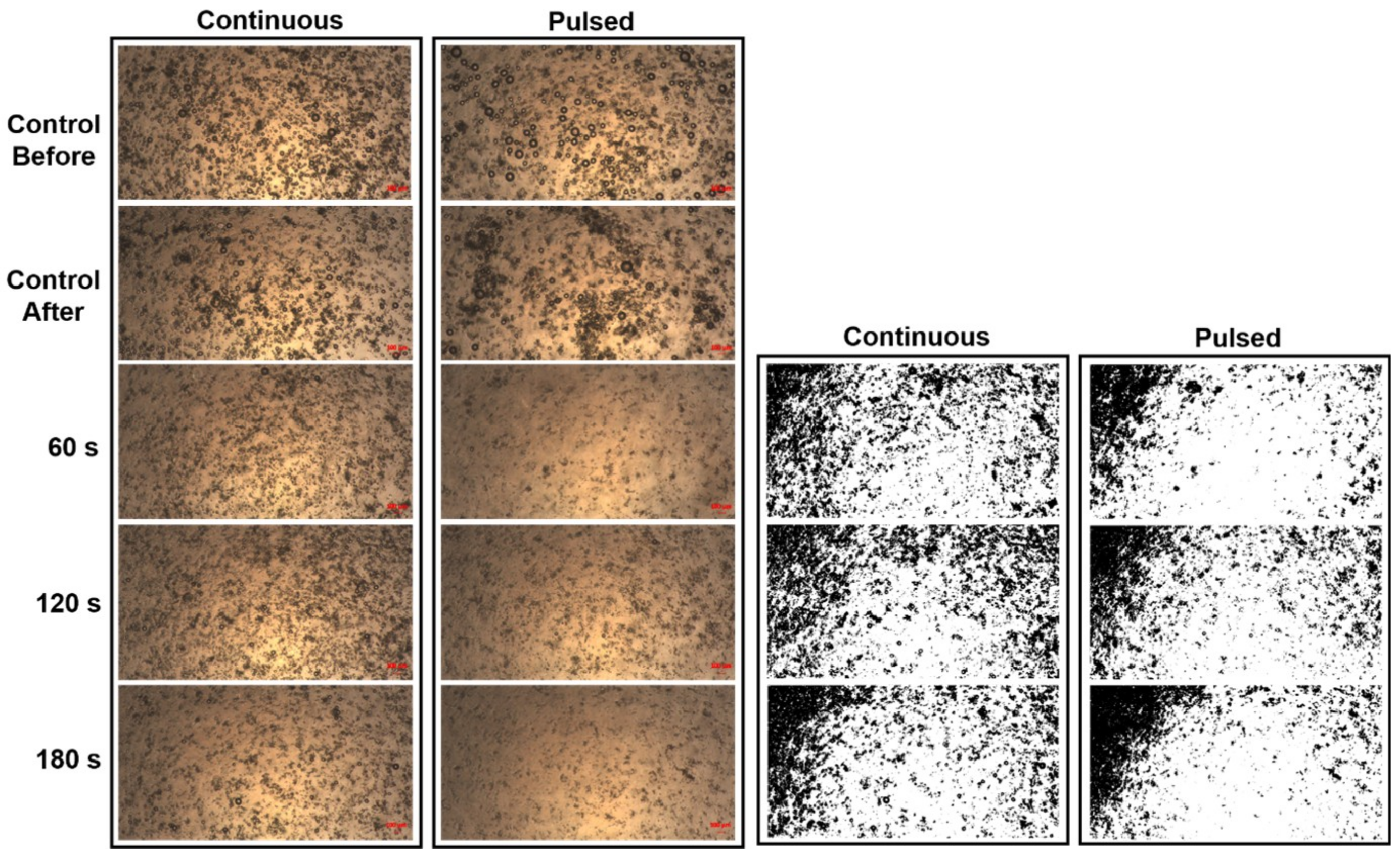
3.1. Laser-Induced MB Disruption
3.2. Penetration Depth in Pigskin
3.3. In Vitro Skin Penetration by the β-arbutin Solution
3.4. Animal Treatments
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tzanakis, I.; Lebon, G.S.; Eskin, D.G.; Pericleous, K.A. Characterizing the cavitation development and acoustic spectrum in various liquids. Ultrason. Sonochem. 2017, 34, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Dalecki, D. Biological Effects of Microbubble-Based Ultrasound Contrast Agents. In Contrast Media in Ultrasonography: Basic Principles and Clinical Applications; Emilio, Q., Ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 2005; pp. 77–85. [Google Scholar]
- Rota, C.; Raeman, C.H.; Child, S.Z.; Dalecki, D. Detection of acoustic cavitation in the heart with microbubble contrast agents in vivo: A mechanism for ultrasound-induced arrhythmias. J. Acoust. Soc. Am. 2006, 120, 2958–2964. [Google Scholar] [CrossRef] [PubMed]
- Van der Wouw, P.A.; Brauns, A.C.; Bailey, S.E.; Powers, J.E.; Wilde, A.A. Premature ventricular contractions during triggered imaging with ultrasound contrast. J. Am. Soc. Echocardiogr. 2000, 13, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Cao, L.Q.; Dou, C.Y.; Armstrong, W.F.; Miller, D. Impact of myocardial contrast echocardiography on vascular permeability: An in vivo dose response study of delivery mode, pressure amplitude and contrast dose. Ultrasound Med. Biol. 2003, 29, 1341–1349. [Google Scholar] [CrossRef]
- Liao, A.H.; Lu, Y.J.; Hung, C.R.; Yang, M.Y. Efficacy of transdermal magnesium ascorbyl phosphate delivery after ultrasound treatment with microbubbles in gel-type surrounding medium in mice. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Liao, A.H.; Ma, W.C.; Wang, C.H.; Yeh, M.K. Penetration depth, concentration and efficiency of transdermal α-arbutin delivery after ultrasound treatment with albumin-shelled microbubbles in mice. Drug Deliv. 2016, 23, 2173–2182. [Google Scholar] [CrossRef] [PubMed]
- Oberli, M.A.; Schoellhammer, C.M.; Langer, R.; Blankschtein, D. Ultrasound-enhanced transdermal delivery: Recent advances and future challenges. Ther. Deliv. 2014, 5, 843–857. [Google Scholar] [CrossRef] [PubMed]
- Paltauf, G.; Schmidt-Kloiber, H. Microcavity dynamics during laser-induced spallation of liquids and gels. Appl. Phys. A 1996, 62, 303–311. [Google Scholar] [CrossRef]
- Vogel, A.; Noack, J.; Nahen, K.; Theisen, D.; Busch, S.; Parlitz, U.; Hammer, D.X.; Noojin, G.D.; Rockwell, B.A.; Birngruber, R. Energy balance or optical breakdown in water at nanosecond to femtosecond time scales. Appl. Phys. B 1999, 68, 271–280. [Google Scholar] [CrossRef]
- Goldberg, D.J.; Cutler, K.B. Nonablative treatment of rhytides with intense pulsed light. Lasers Surg. Med. 2000, 26, 196–200. [Google Scholar] [CrossRef]
- Jang, J.U.; Kim, S.Y.; Yoon, E.S.; Kim, W.K.; Park, S.H.; Lee, B.I.; Kim, D.W. Comparison of the effectiveness of ablative and non-ablative fractional laser treatments for early stage thyroidectomy scars. Arch. Plast. Surg. 2016, 43, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Metelitsa, A.I.; Alster, T.S. Fractionated laser skin resurfacing treatment complications: A review. Dermatol. Surg. 2010, 36, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.M.; Nelson, J.S.; Lask, G.P.; Geronemus, R.G.; Bernstein, L.J. Cryogen spray cooling in combination with nonablative laser treatment of facial rhytides. Arch. Dermatol. 1999, 135, 691–694. [Google Scholar] [CrossRef] [PubMed]
- Liao, A.H.; Lu, Y.J.; Lin, Y.C.; Chen, H.K.; Sytwu, H.K.; Wang, C.H. Effectiveness of a layer-by-layer microbubbles-based delivery system for applying minoxidil to enhance hair growth. Theranostics 2016, 6, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Liao, A.H.; Hung, C.R.; Chen, H.K.; Chiang, C.P. Ultrasound-mediated EGF-coated-microbubble cavitation in dressings for wound-healing applications. Sci. Rep. 2018, 8, 8327. [Google Scholar] [CrossRef] [PubMed]
- Wen, A.H.; Choi, M.K.; Kim, D.D. Formulation of liposome for topical delivery of arbutin. Arch. Pharm. Res. 2006, 29, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Kawase, I.; Ishii, F. Glycine inhibits melanogenesis in vitro and causes hypopigmentation in vivo. Biol. Pharm. Bull. 2006, 30, 2031–2036. [Google Scholar] [CrossRef]
- Tsai, Y.H.; Lee, K.F.; Huang, Y.B.; Huang, C.T.; Wu, P.C. In vitro permeation and in vivo whitening effect of topical hesperetin microemulsion delivery system. Int. J. Pharm. 2010, 388, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.Y.; Seo, Y.K.; Park, J.M.; Seo, M.J.; Park, J.K.; Kim, J.W.; Park, C.S. Fermented rice bran downregulates MITF expression and leads to inhibition of α-MSH-induced melanogenesis in B16F1 melanoma. Biosci. Biotechnol. Biochem. 2009, 73, 1704–1710. [Google Scholar] [CrossRef] [PubMed]
- Quinto-Su, P.A.; Venugopalan, V.; Ohl, C.D. Generation of laser-induced cavitation bubbles with a digital hologram. Opt. Express 2008, 16, 18964–18969. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-San-Juana, J.C.; Rodriguez-Aboytesa, E.; Korneeva, N.; Baldovinos-Pantaleona, O.; Chiu-Zarateb, R.; Gutiérrez-Juárezb, G.; Dominguez-Cruzc, R.; Ramos-Garciaa, R. Cavitation Induced by Continuous Wave Lasers. In Proceedings of the SPIE Optical Trapping and Optical Micromanipulation IV, San Diego, CA, USA, 5 September 2007; Volume 6644. [Google Scholar]
- Rastopov, S.F.; Sukhodolsky, A.T. Sound Generation by Thermocavitation Induced CW—Laser in Solutions. In Proceedings of the SPIE Optical Radiation Interaction with Matter, Leningrad, Russian, 1 December 1990; Volume 1440, pp. 127–134. [Google Scholar]
- Omi, T.; Numano, K. The role of the CO2 laser and fractional CO2 laser in dermatology. Laser Ther. 2014, 23, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Zaleski-Larsen, L.A.; Fabi, S.G. Laser-assisted drug delivery. Dermatol. Surg. 2016, 42, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Aljuffali, I.A.; Fang, J.Y. Lasers as an approach for promoting drug delivery via skin. Expert. Opin. Drug Deliv. 2014, 11, 599–614. [Google Scholar] [CrossRef] [PubMed]









| Laser Type | Argon Ion Laser | Supercontinuum Fiber | Nd:YAG | CO2 Fractional |
|---|---|---|---|---|
| Wavelength (nm) | 515 | 1064 | 532 | 10,600 |
| Mode of operation | Continuous | Pulsed | Pulsed | Pulsed |
| Energy (mJ) | n/a | n/a | 380 | 1.3 |
| Output power (mW) | 10.8 | 10.8 | n/a | n/a |
| Repetition rate | n/a | 80 MHz | 5 Hz | Single |
| Radiation repetitions (in vivo) | n/a | n/a | n/a | Seven times |
| Radiation times/repetitions (in vitro) | 60, 120, or 180 s | 60, 120, or 180 s | 60, 120, or 180 s | One, three, or seven times |
| MB dilution | Fivefold | Fivefold | Five- or tenfold | Tenfold |
| Average temperature increase (°C) | 0.7 | 1.0 | 0.86 or 0.23 | 1.1 |
| Group | Skin Weight (mg) | Amount of β-arbutin Penetrated Across Skin (mg/mL) | Amount of β-arbutin Deposited on Skin (mg/mL) | Total Amount of β-arbutin Penetrated (mg/mL) |
|---|---|---|---|---|
| C | 18.88 ± 3.30 | 2.397 ± 0.07 | 2.291 ± 0.419 | 4.688 ± 0.491 |
| L | 23.43 ± 0.93 | 4.876 ± 0.017 | 2.559 ± 0.129 | 7.435 ± 0.146 |
| L + S | 18.75 ± 3.54 | 3.287 ± 0.01 | 3.355 ± 0.350 | 6.642 ± 0.451 |
| L + MBs | 22.47 ± 1.10 | 4.892 ± 1.14 | 3.292 ± 0.481 | 8.184 ± 1.628 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, A.-H.; Chuang, H.-C.; Chang, B.-Y.; Kuo, W.-C.; Wang, C.-H.; Gao, H.-W.; Chiang, C.-P. Combining Microbubble Contrast Agent with Pulsed-Laser Irradiation for Transdermal Drug Delivery. Pharmaceutics 2018, 10, 175. https://doi.org/10.3390/pharmaceutics10040175
Liao A-H, Chuang H-C, Chang B-Y, Kuo W-C, Wang C-H, Gao H-W, Chiang C-P. Combining Microbubble Contrast Agent with Pulsed-Laser Irradiation for Transdermal Drug Delivery. Pharmaceutics. 2018; 10(4):175. https://doi.org/10.3390/pharmaceutics10040175
Chicago/Turabian StyleLiao, Ai-Ho, Ho-Chiao Chuang, Bo-Ya Chang, Wen-Chuan Kuo, Chih-Hung Wang, Hong-Wei Gao, and Chien-Ping Chiang. 2018. "Combining Microbubble Contrast Agent with Pulsed-Laser Irradiation for Transdermal Drug Delivery" Pharmaceutics 10, no. 4: 175. https://doi.org/10.3390/pharmaceutics10040175
APA StyleLiao, A.-H., Chuang, H.-C., Chang, B.-Y., Kuo, W.-C., Wang, C.-H., Gao, H.-W., & Chiang, C.-P. (2018). Combining Microbubble Contrast Agent with Pulsed-Laser Irradiation for Transdermal Drug Delivery. Pharmaceutics, 10(4), 175. https://doi.org/10.3390/pharmaceutics10040175