Encapsulation of Babchi Oil in Cyclodextrin-Based Nanosponges: Physicochemical Characterization, Photodegradation, and In Vitro Cytotoxicity Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Gas Chromatography Mass Spectrometry of the Babchi Oil (BO)
2.3. Synthesis of Nanosponges
2.4. Solubilization Efficiency of Nanosponges
2.5. Preparation of Babchi Oil-Loaded β-Cyclodextrin Nanosponges
2.6. Evaluation of Nanosponges
2.6.1. Determination of the BO Content in the Nanosponges
2.6.2. Determination of Size, Polydispersity Index, and Zeta Potential of the Nanosponges
2.6.3. Fourier Transform Infrared Spectroscopy
2.6.4. Thermal Analysis
2.6.5. X-ray Powder Diffraction
2.6.6. Surface Morphology
Field Emission Scanning Electron Microscopy
Transmission Electron Microscopy
2.7. Cytotoxicity Studies Against the HaCaT Cell Line
2.8. Photodegradation Study
2.9. Antibacterial Activity
2.10. Statistical Analysis
3. Results and Discussion
3.1. Identification of Various Compounds in BO Using GC-MS
3.2. Nanosponge Fabrication
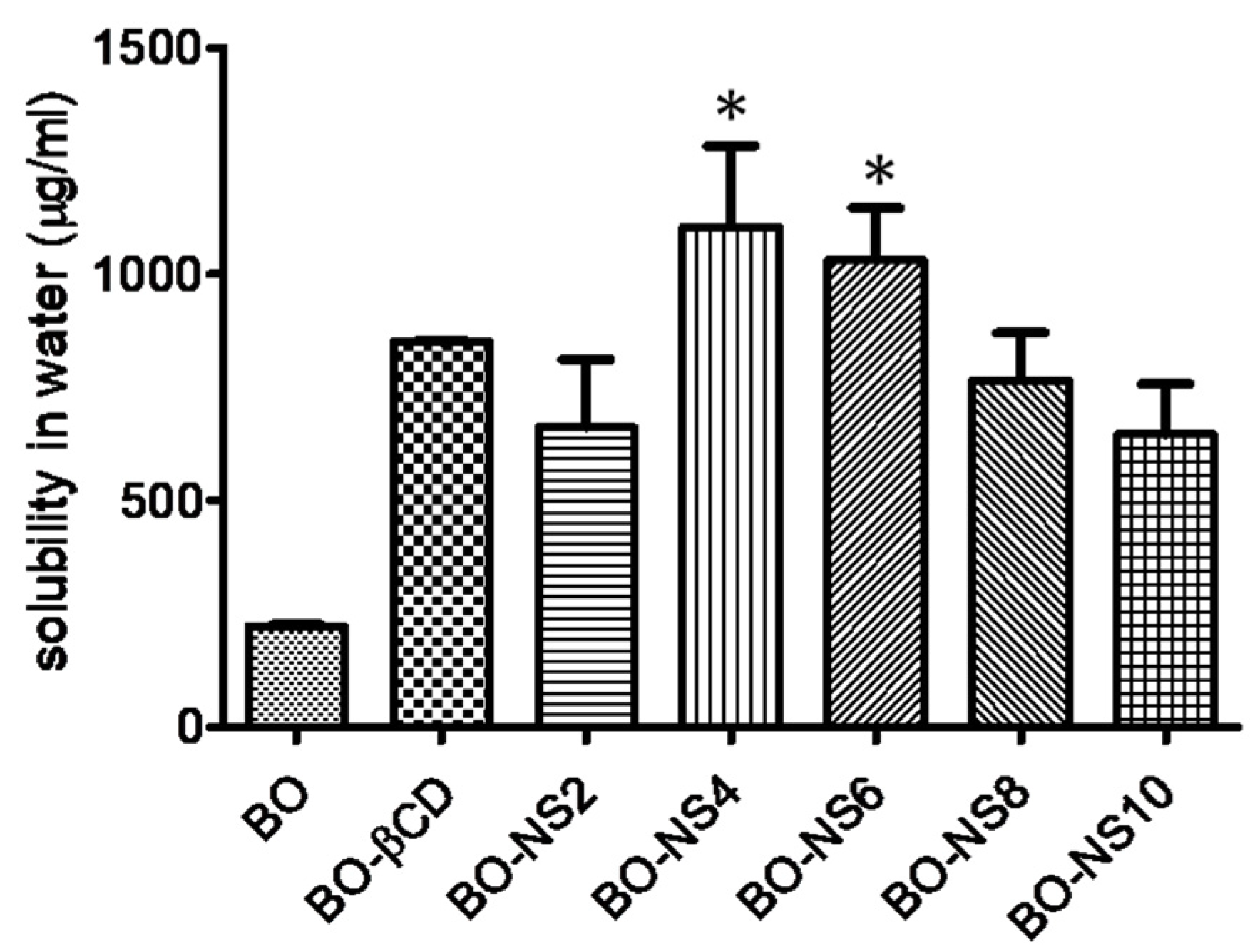
3.3. Solubilization Efficiency of Nanosponges
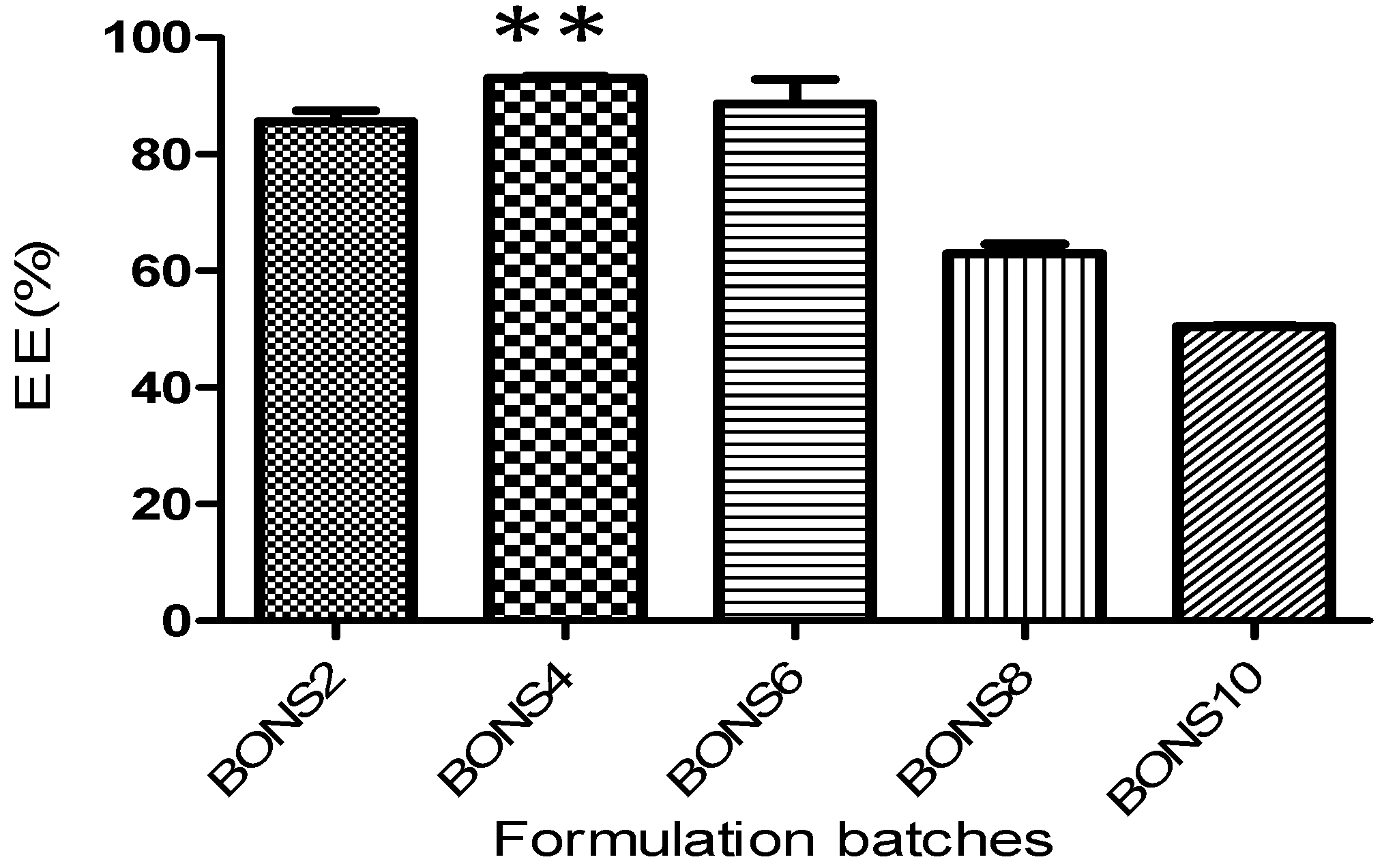
3.4. Loading and Encapsulation Efficiency
3.5. Particle Size, Polydispersity Index, and Zeta Potential Determination
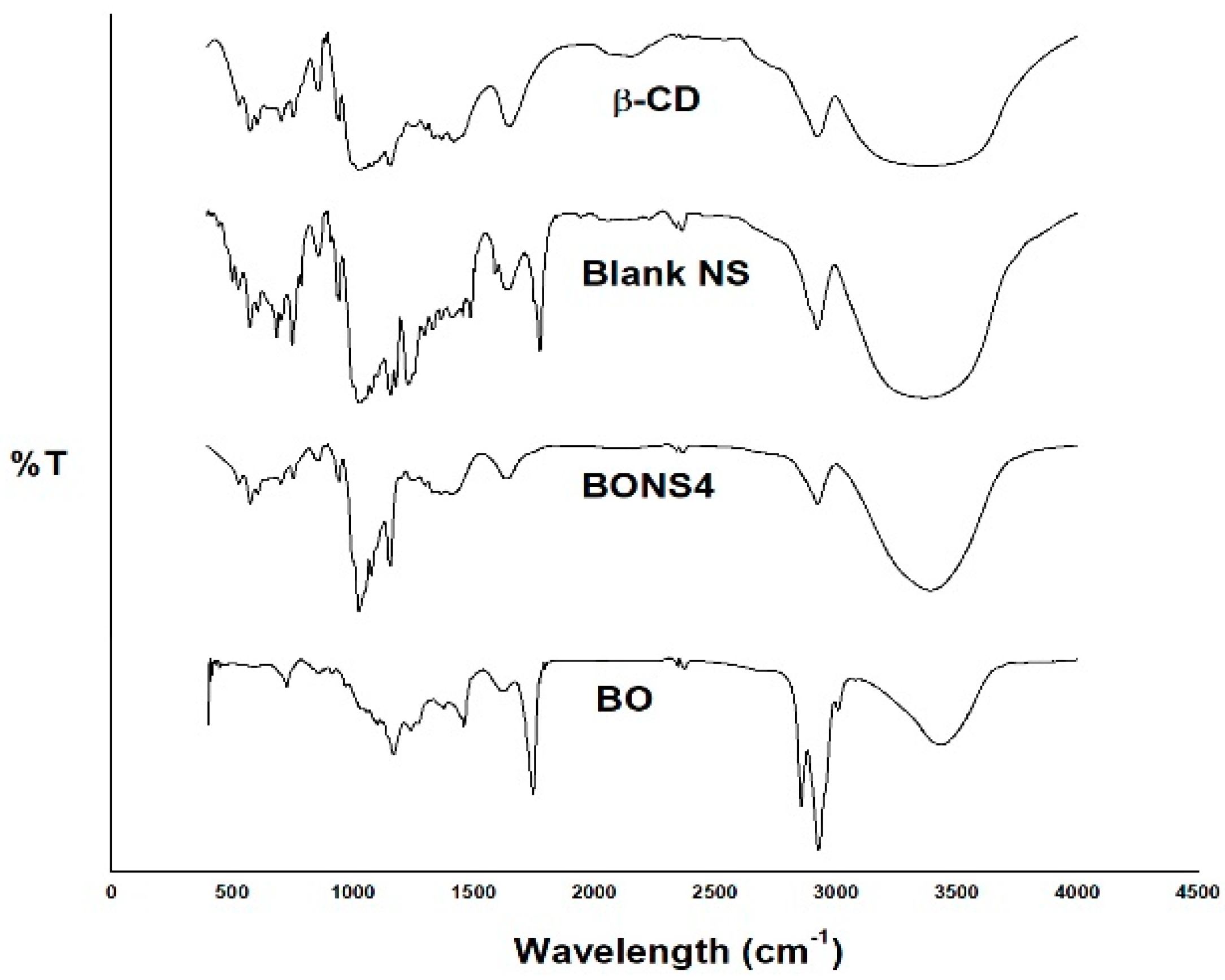
3.6. Fourier Transform Infrared Spectroscopy
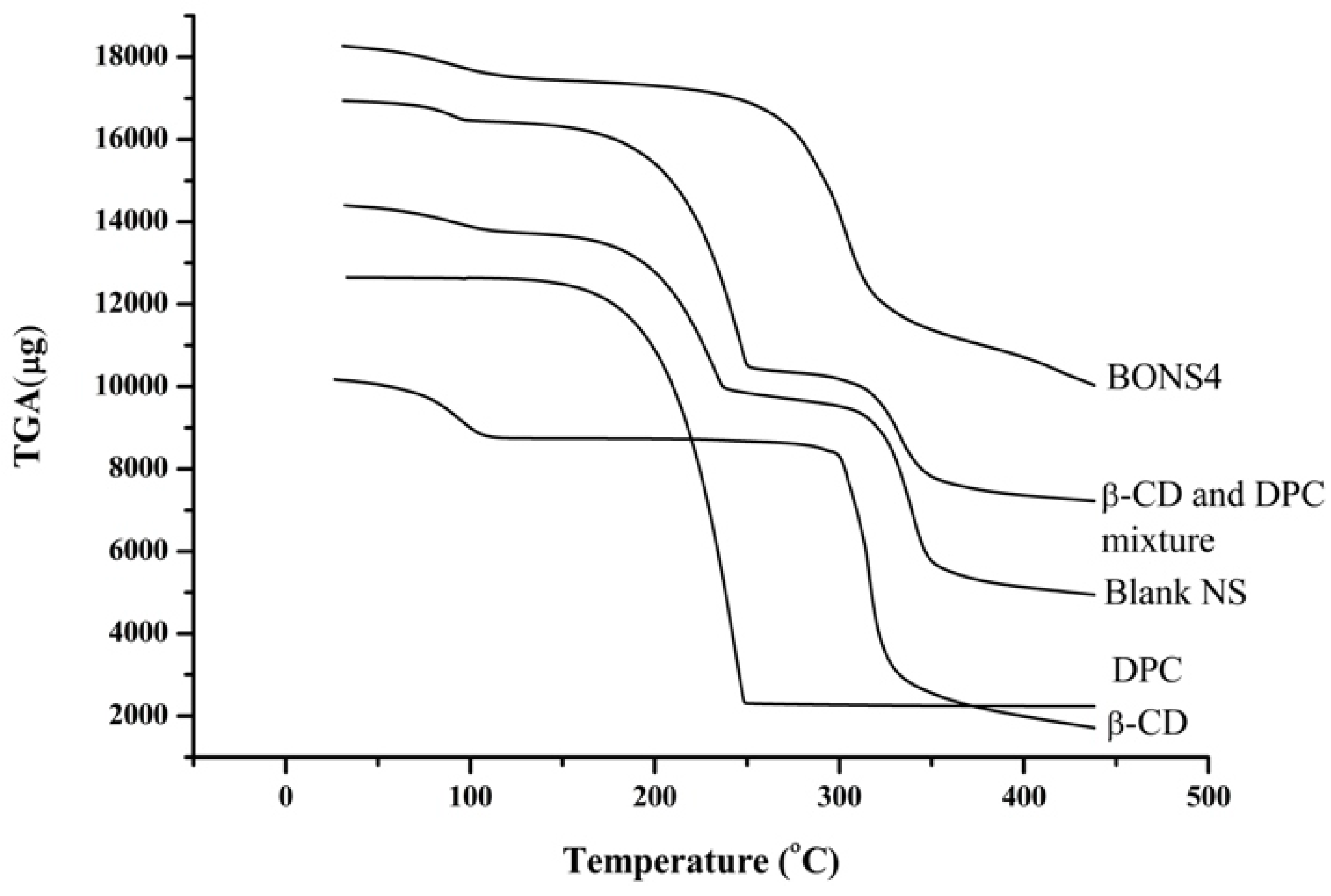
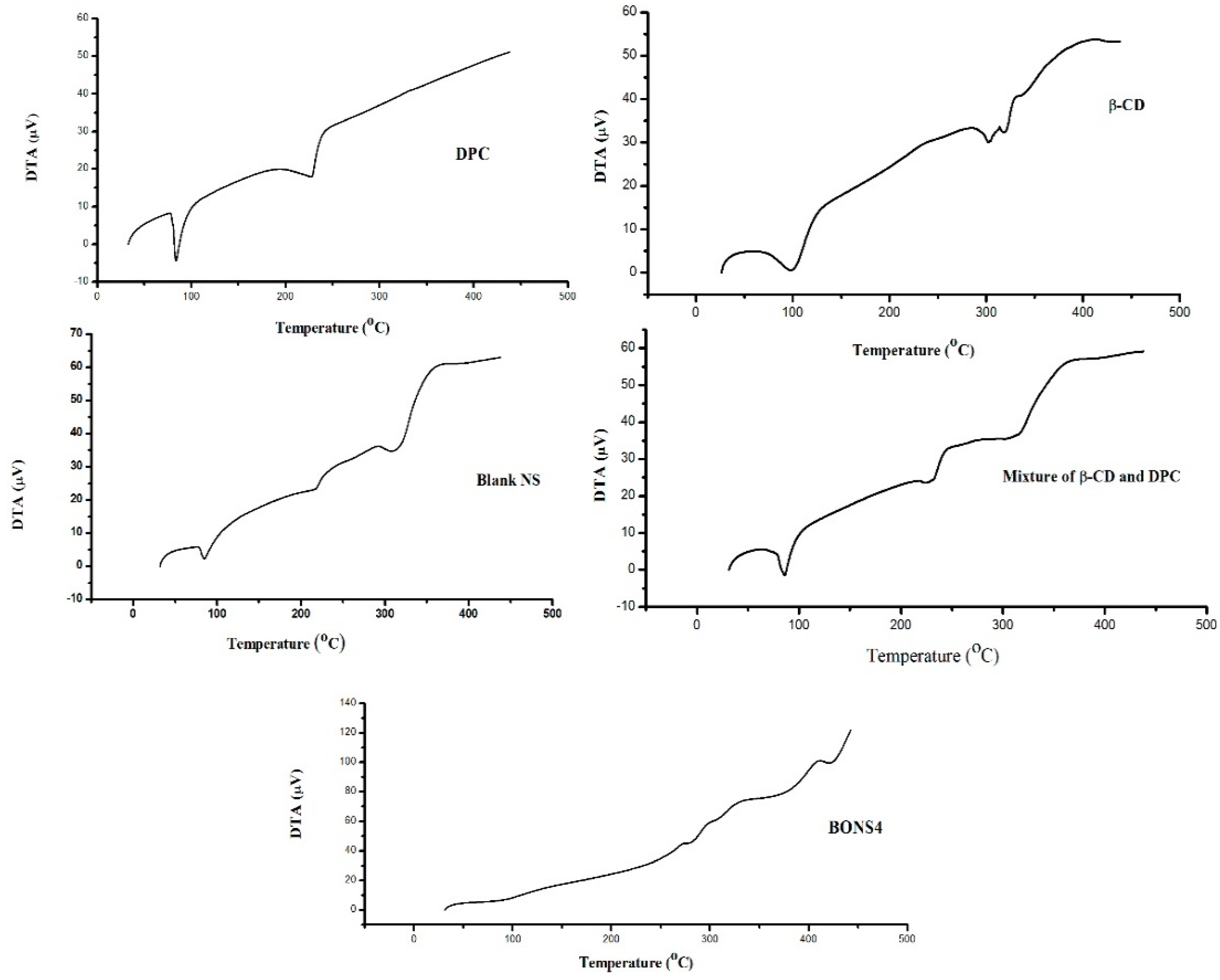
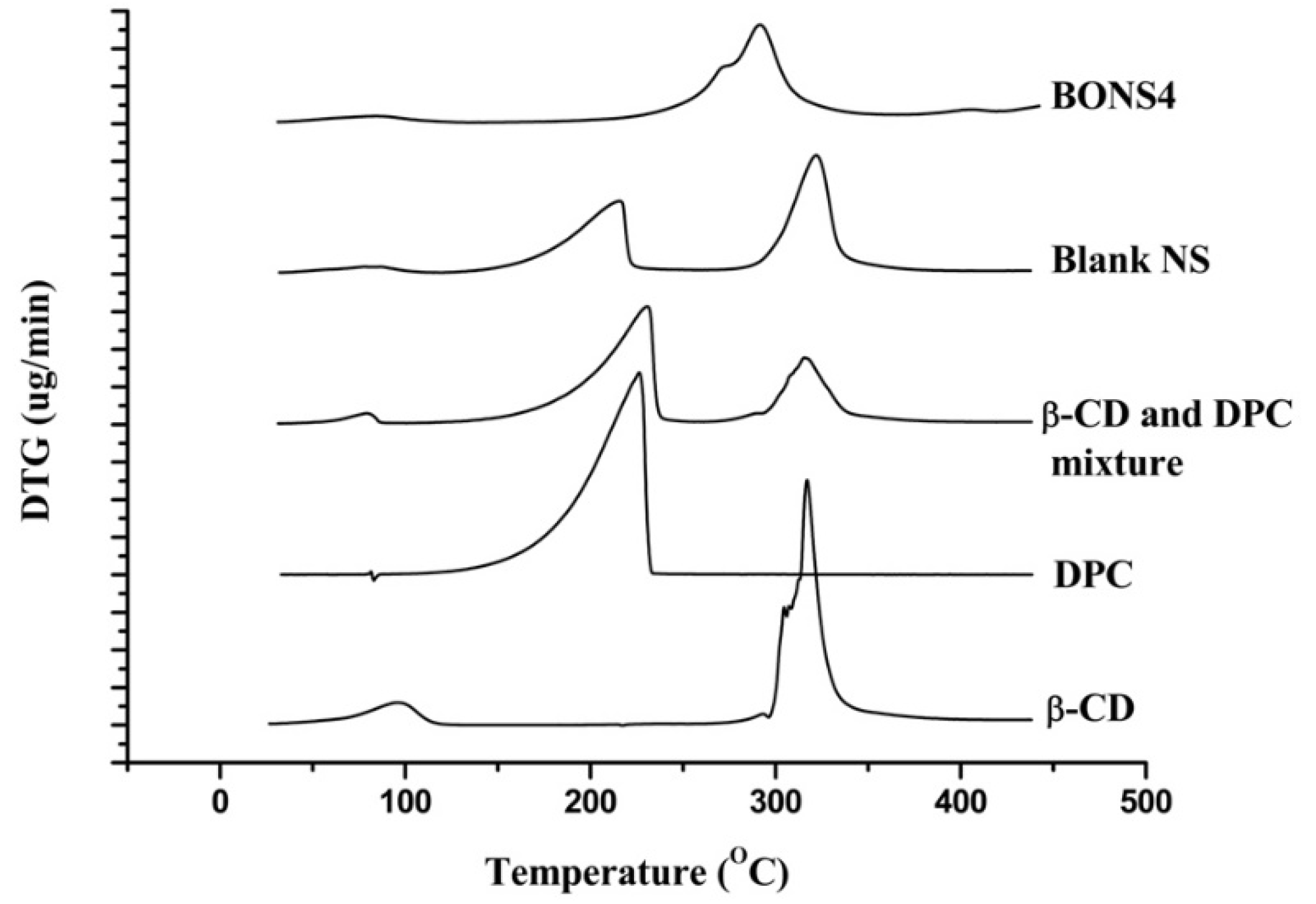
3.7. Thermal Analysis
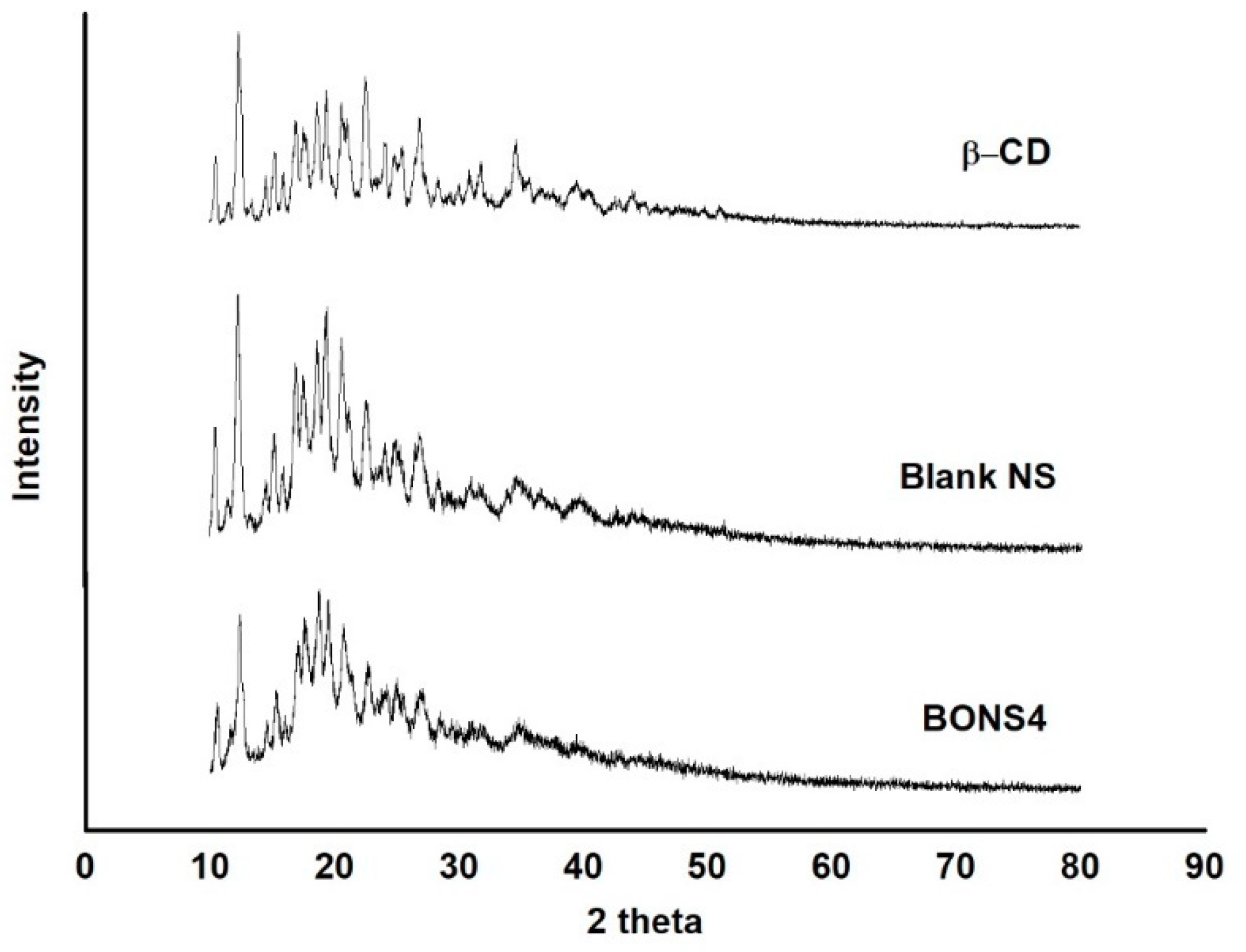
3.8. X-Ray Powder Diffraction
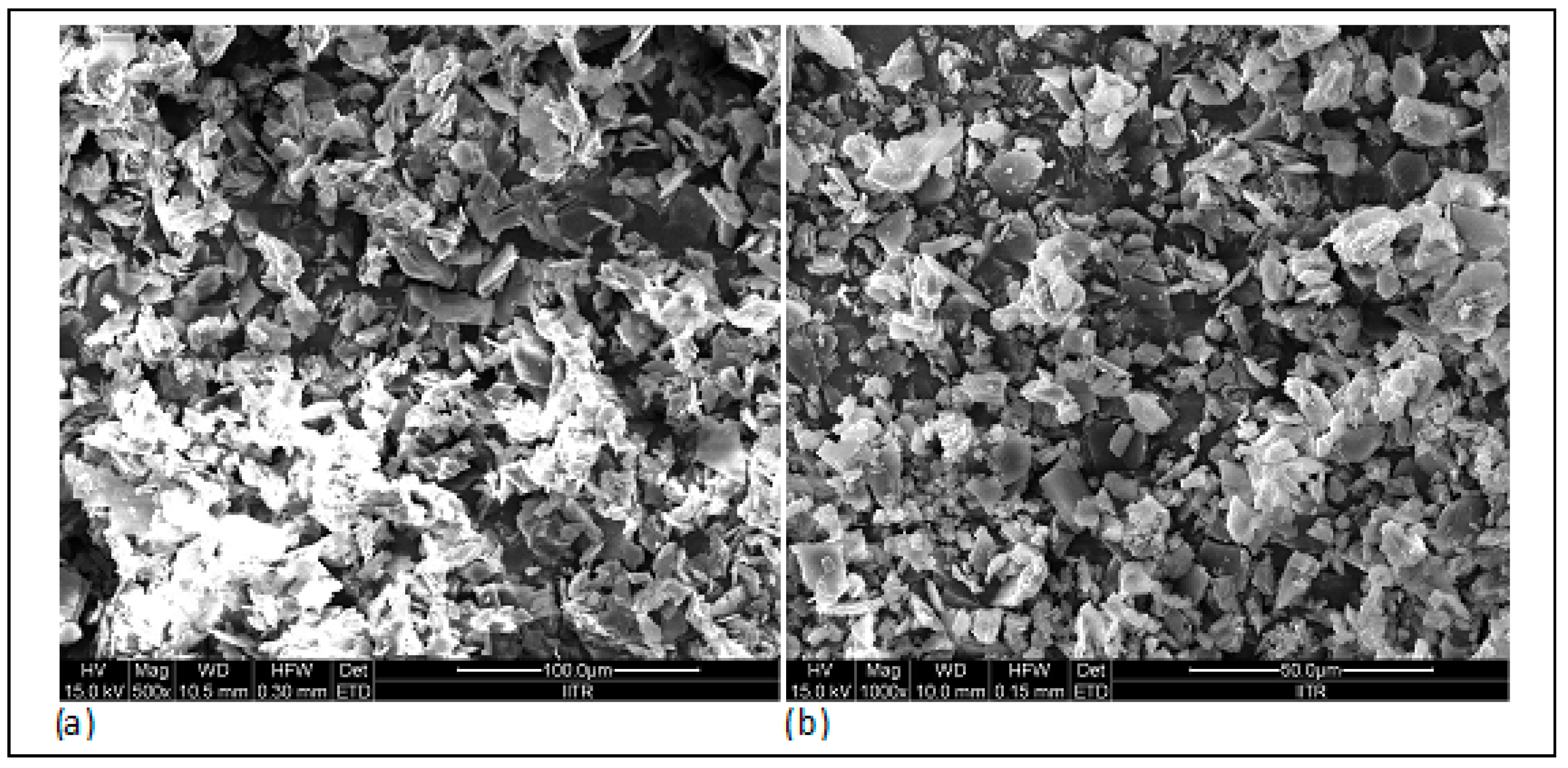
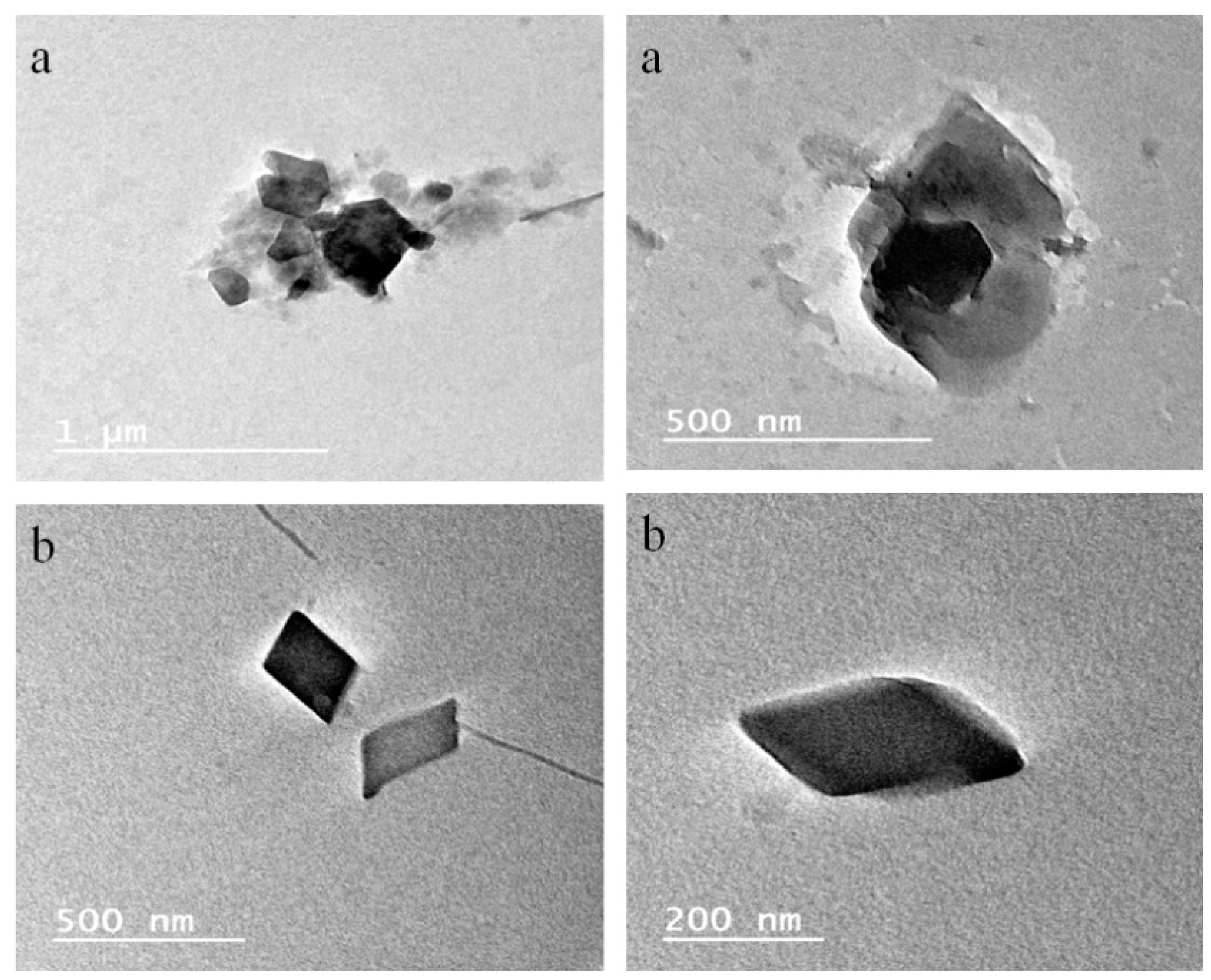
3.9. Surface Morphology
3.10. Cytotoxicity Studies against HaCaT Cell Lines
3.11. Photodegradation Study
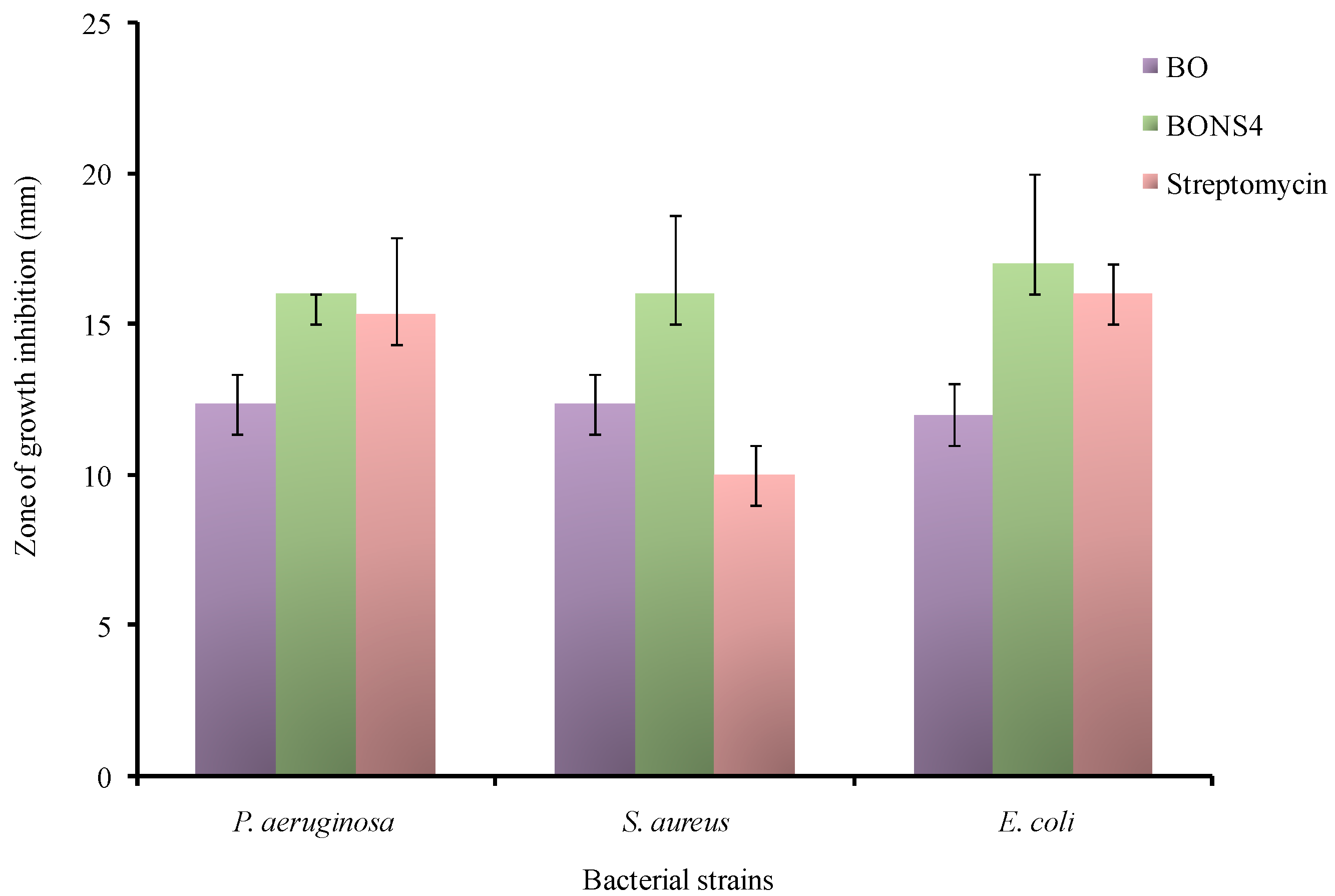
3.12. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Caramella, C. Characterization of chitosan hydrochloride–mucin interaction by means of viscosimetric and turbidimetric measurements. Eur. J. Pharm. Sci. 2000, 10, 251–257. [Google Scholar] [CrossRef]
- Munin, A.; Edwards-Lévy, F. Encapsulation of natural polyphenolic compounds; a review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, G.; Kumar, S.; Chhabra, L.; Mahant, S.; Rao, R. Essential oil–cyclodextrin complexes: An updated review. J. Incl. Phenom. Macrocycl. Chem. 2017, 89, 39–58. [Google Scholar] [CrossRef]
- Szejtli, J. Cyclodextrin in drug formulations Part, I. Pharm. Technol. Int. 1991, 3, 15–22. [Google Scholar]
- Cavalli, R.; Trotta, F.; Tumiatti, W. Cyclodextrin-based nanosponges for drug delivery. J. Incl. Phenom. Macrocycl. Chem. 2006, 56, 209–213. [Google Scholar] [CrossRef]
- Trotta, F.; Zanetti, M.; Cavalli, R. Cyclodextrin-based nanosponges as drug carriers. Beilstein J. Org. Chem. 2012, 8, 2091–2099. [Google Scholar] [CrossRef] [PubMed]
- Trotta, F.; Dianzani, C.; Caldera, F.; Mognetti, B.; Cavalli, R. The application of nanosponges to cancer drug delivery. Expert Opin. Drug Deliv. 2014, 11, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, S.; Cavalli, R.; Trotta, F. Cyclodextrin-based nanosponges: A versatile platform for cancer nanotherapeutics development. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 579–601. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, K.; Tanwar, Y.S.; Shende, P.; Cavalli, R. Biomimetic estimation of glucose using non-molecular and molecular imprinted polymer nanosponges. Int. J. Pharm. 2015, 494, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Chopra, B.; Dhingra, A.K.; Dhar, K.L. Psoralea corylifolia, L. (Buguchi)—Folklore to modern evidence: Review. Fitoterapia 2013, 90, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Rao, R. Psoralen: A promising boon in topical manifestations. IJP 2016, 3, 375–383. [Google Scholar]
- Chang, L. Psoriasis Phototherapy: UVA Beats UVB. WebMD Medical News Daniel De Noon. 2006. Available online: https://www.webmd.com/skin-problems-and-treatments/psoriasis/news/20060718/ psoriasis-phototherapy-uva-beats-uvb (accessed on 21 August 2018).
- Musthaba, S.M.; Baboota, S.; Ahmed, S.; Ahuja, A.; Ali, J. Status of novel drug delivery technology for phytotherapeutics. Expert Opin. Drug Deliv. 2009, 6, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, M.; Singh, D.; Singh, M.R. Novel colloidal carriers for psoriasis: Current issues, mechanistic insight and novel delivery approaches. J. Control. Release 2013, 170, 380–395. [Google Scholar] [CrossRef] [PubMed]
- Mognetti, B.; Barberis, A.; Marino, S.; Berta, G.; De Francia, S.; Trotta, F.; Cavalli, R. In vitro enhancement of anticancer activity of paclitaxel by a Cremophor free cyclodextrin-based nanosponge formulation. J. Incl. Phenom. Macrocycl. Chem. 2012, 74, 201–210. [Google Scholar] [CrossRef]
- Tejashri, G.; Amrita, B.; Darshana, J. Cyclodextrin based nanosponges for pharmaceutical use: A review. Acta Pharm. 2013, 63, 335–358. [Google Scholar] [CrossRef] [PubMed]
- Sapino, S.; Carlotti, M.E.; Cavalli, R.; Ugazio, E.; Berlier, G.; Gastaldi, L.; Morel, S. Photochemical and antioxidant properties of gamma-oryzanol in beta-cyclodextrin-based nanosponges. J. Incl. Phenom. Macrocycl. Chem. 2013, 75, 69–76. [Google Scholar] [CrossRef]
- Anandam, S.; Selvamuthukumar, S. Fabrication of cyclodextrin nanosponges for quercetin delivery: Physicochemical characterization, photostability, and antioxidant effects. J. Mater. Sci. 2014, 49, 8140–8153. [Google Scholar] [CrossRef]
- Ansari, K.A.; Vavia, P.R.; Trotta, F.; Cavalli, R. Cyclodextrin-based nanosponges for delivery of resveratrol: In vitro characterisation, stability, cytotoxicity and permeation study. AAPS PharmSciTech 2011, 12, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Darandale, S.S.; Vavia, P.R. Cyclodextrin-based nanosponges of curcumin: Formulation and physicochemical characterization. J. Incl. Phenom. Macrocycl. Chem. 2013, 75, 315–322. [Google Scholar] [CrossRef]
- Ramírez-Ambrosi, M.; Caldera, F.; Trotta, F.; Berrueta, L.Á.; Gallo, B. Encapsulation of apple polyphenols in β-CD nanosponges. J. Incl. Phenom. Macrocycl. Chem. 2014, 80, 85–92. [Google Scholar] [CrossRef]
- Rao, M.; Bajaj, A.; Khole, I.; Munjapara, G.; Trotta, F. In vitro and in vivo evaluation of β-cyclodextrin-based nanosponges of telmisartan. J. Incl. Phenom. Macrocycl. Chem. 2013, 77, 135–145. [Google Scholar] [CrossRef]
- Rao, M.R.; Shirsath, C. Enhancement of Bioavailability of Non-nucleoside Reverse Transciptase Inhibitor Using Nanosponges. AAPS PharmSciTech 2017, 18, 1728–1738. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, S.; Vavia, P.R.; Trotta, F.; Cavalli, R.; Tumbiolo, S.; Bertinetti, L.; Coluccia, S. Structural evidence of differential forms of nanosponges of beta-cyclodextrin and its effect on solubilization of a model drug. J. Incl. Phenom. Macrocycl. Chem. 2013, 76, 201–211. [Google Scholar] [CrossRef]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Schürer, N.; Köhne, A.; Schliep, V.; Barlag, K.; Goerz, G. Lipid composition and synthesis of HaCaT cells, an immortalized human keratinocyte line, in comparison with normal human adult keratinocytes. Exp. Dermatol. 1993, 2, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Borate, A.; Khambhapati, A.; Udgire, M.; Paul, D.; Mathur, S. Preliminary phytochemical studies and evaluation of antibacterial activity of Psoraleacorylifolia seed extract. IJMRR 2014, 2, 95–101. [Google Scholar]
- Loftsson, T.; Brewster, M. Pharmaceutical applications of cyclodextrins: Drug solubilisation and stabilization. J. Pharm. Pharmacol. 1996, 85, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Trotta, F.; Tumiatti, V.; Cavalli, R.; Roggero, C.; Mognetti, B.; Berta, G. Cyclodextrin-Based Nanosponges as a Vehicle for Antitumoral Drugs. Patent No. WO2009/003656 A1, 8 January 2009. [Google Scholar]
- Available online: http://www.inchem.org/documents/sids/sids/102090.pdf (accessed on 7 December 2016).
- Sharma, R.; Pathak, K. Polymeric nanosponges as an alternative carrier for improved retention of econazole nitrate onto the skin through topical hydrogel formulation. Pharm. Dev. Technol. 2011, 16, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Anandam, S.; Selvamuthukumar, S. Optimization of microwave-assisted synthesis of cyclodextrin nanosponges using response surface methodology. J. Porous Mater. 2014, 21, 1015–1023. [Google Scholar] [CrossRef]
- Ferro, M.; Castiglione, F.; Punta, C.; Melone, L.; Panzeri, W.; Rossi, B.; Trotta, F.; Mele, A. Anomalous diffusion of Ibuprofen in cyclodextrin nanosponge hydrogels: An HRMAS NMR study. Beilstein J. Org. Chem. 2014, 10, 2715–2723. [Google Scholar] [CrossRef] [PubMed]
- Selvamuthukumar, S.; Anandam, S.; Krishnamoorthy, K.; Rajappan, M. Nanosponges: A novel class of drug delivery system-review. J. Pharm. Pharm. Sci. 2012, 15, 103–111. [Google Scholar]
- Aldawsari, H.M.; Badr-Eldin, S.M.; Labib, G.S.; El-Kamel, A.H. Design and formulation of a topical hydrogel integrating lemongrass-loaded nanosponges with an enhanced antifungal effect: In vitro/in vivo evaluation. Int. J. Nanomed. 2015, 10, 893–902. [Google Scholar]
- Olteanu, A.A.; Aramă, C.C.; Radu, C.; Mihăescu, C.; Monciu, C.M. Effect of β-cyclodextrins based nanosponges on the solubility of lipophilic pharmacological active substances (repaglinide). J. Incl. Phenom. Macrocycl. Chem. 2014, 80, 17–24. [Google Scholar] [CrossRef]
- Carlotti, M.E.; Sapino, S.; Vione, D.; Pelizzetti, E.; Trotta, M. Photostability of Trolox in Water/Ethanol, Water, and Oramix CG 110 in the Absence and in the Presence of TiO2. J. Dispers. Sci. Technol. 2004, 25, 193–207. [Google Scholar] [CrossRef]
- Carlotti, M.E.; Sapino, S.; Vione, D.; Minero, C.; Peira, E.; Trotta, M. Study on the photodegradation of salicylic acid in different vehicles in the absence and in the presence of TiO2. J. Dispers. Sci. Technol. 2007, 28, 805–818. [Google Scholar] [CrossRef]
| Sr. No. | Nanosponge Type | Molar Ratio β-CD: DPC | Amount of β-CD (g) | Amount of DPC (g) | Practical Yield (g) ± SD |
|---|---|---|---|---|---|
| 1 | NS2 | 1:2 | 4.548 | 1.712 | 3.544 ± 0.220 |
| 2 | NS4 | 1:4 | 4.548 | 3.424 | 4.559 ± 0.199 |
| 3 | NS6 | 1:6 | 4.548 | 5.136 | 5.8966 ± 0.197 |
| 4 | NS8 | 1:8 | 4.548 | 6.848 | 6.434 ± 0.197 |
| 5 | NS10 | 1:10 | 4.548 | 8.56 | 6.735 ± 0.296 * |
| Sr. No. | Formulation | Particle Size (nm ± SD) | Zeta Potential (mV ± SD) | Poly Dispersity Index ± SD | % Encapsulation Efficiency ± SD |
|---|---|---|---|---|---|
| 1. | BONS1:2 | 261.6 ± 14.79 | −17.8 ± 2.52 | 0.312 ± 0.098 | 85.61 ± 1.848 |
| 2. | BONS1:4 | 360.9 ± 11.55 | −16.0 ± 1.15 | 0.311 ± 0.059 | 93.05 ± 0.283 ** |
| 3. | BONS1:6 | 234.3 ± 15.37 | −15.5 ± 1.17 | 0.188 ± 0.064 | 88.61 ± 4.286 |
| 4. | BONS1:8 | 484.2 ± 19.89 | −15.6 ± 2.39 | 0.509 ± 0.236 | 62.98 ± 1.669 |
| 5. | BONS1:10 | 243.3 ± 12.95 | −22.0 ± 2.47 | 0.361 ± 0.113 | 50.43 ± 0.173 |
| Formulating Materials and NS | Derivative Thermo Gravimetric Parameters | ||
|---|---|---|---|
| Temperature (°C) | Type of Peak | Derivative of Thermo Gravimetry (mg/min) | |
| β-CD | 98 | Exothermic | 0.30 |
| 304 | Exothermic | 1.54 | |
| 317 | Exothermic | 3.26 | |
| DPC | 226 | Sharp exothermic | 2.69 |
| β-CD and DPC mixture | 80 | Exothermic | 0.14 |
| 231 | Sharp exothermic | 1.57 | |
| 315 | Exothermic | 0.88 | |
| Blank NS | 217 | Exothermic | 0.96 |
| 323 | Exothermic | 1.57 | |
| BONS4 | 272 | Exothermic | 0.76 |
| 291 | Exothermic | 1.31 | |
| Formulating Materials and NS | Parameters of Differential Thermal Analysis | |||
|---|---|---|---|---|
| Temperature (°C) | Type of Peak | Change in Enthalpy (∆H) mJ/mg | DTA Sensitivity (μV) | |
| β-CD | 98 | Endothermic | 224 | 0.6 |
| 302 | Endothermic | 110 | 30 | |
| 320 | Endothermic | 110 | 32.51 | |
| DPC | 83 | Endothermic | 96.2 | −4.30 |
| 228 | Endothermic | 146 | 17.88 | |
| β-CD and DPC mixture | 86 | Endothermic | 108 | −1.50 |
| 231 | Endothermic | 49.5 | 24.3 | |
| 317 | Endothermic | 160 | 37 | |
| Blank NS | 85 | Endothermic | 36.6 | 2.3 |
| 218 | Endothermic | 35.3 | 23.4 | |
| 314 | Endothermic | 189 | 35.3 | |
| BONS4 | - | - | - | - |
| Sample | Concentration µg/mL | % Inhibition ± Standard Deviation (n = 3) | IC50 |
|---|---|---|---|
| Control | 0 | 0.00 ± 0.00 | 172.3 µg/mL |
| Babchi oil (BO) | 10 | 4.41 ± 1.21 | |
| 20 | 7.48 ± 1.47 | ||
| 40 | 16.84 ± 1.40 | ||
| 80 | 23.45 ± 1.20 | ||
| 160 | 38.61 ± 2.16 | ||
| 320 | 51.16 ± 2.23 | ||
| Babchi oil loaded nanosponges (BONS) | 10 | 4.26 ± 1.96 | 191.4 µg/mL |
| 20 | 9.60 ± 1.63 | ||
| 40 | 16.39 ± 1.74 | ||
| 80 | 25.86 ± 2.29 | ||
| 160 | 48.73 ± 2.09 | ||
| 320 | 61.27 ± 1.06 |
| Sr. No. | Oil and Its Formulation | Correlation Coefficient (r2) | Rate Constant (k) (1 × 10−3 min−1) |
|---|---|---|---|
| 1. | BO | 0.953 ± 0.14 | 6.909 ± 0.63 |
| 2. | BONS4 | 0.405 ± 0.10 | 2.303 ± 0.47 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, S.; Pooja; Trotta, F.; Rao, R. Encapsulation of Babchi Oil in Cyclodextrin-Based Nanosponges: Physicochemical Characterization, Photodegradation, and In Vitro Cytotoxicity Studies. Pharmaceutics 2018, 10, 169. https://doi.org/10.3390/pharmaceutics10040169
Kumar S, Pooja, Trotta F, Rao R. Encapsulation of Babchi Oil in Cyclodextrin-Based Nanosponges: Physicochemical Characterization, Photodegradation, and In Vitro Cytotoxicity Studies. Pharmaceutics. 2018; 10(4):169. https://doi.org/10.3390/pharmaceutics10040169
Chicago/Turabian StyleKumar, Sunil, Pooja, Francesco Trotta, and Rekha Rao. 2018. "Encapsulation of Babchi Oil in Cyclodextrin-Based Nanosponges: Physicochemical Characterization, Photodegradation, and In Vitro Cytotoxicity Studies" Pharmaceutics 10, no. 4: 169. https://doi.org/10.3390/pharmaceutics10040169
APA StyleKumar, S., Pooja, Trotta, F., & Rao, R. (2018). Encapsulation of Babchi Oil in Cyclodextrin-Based Nanosponges: Physicochemical Characterization, Photodegradation, and In Vitro Cytotoxicity Studies. Pharmaceutics, 10(4), 169. https://doi.org/10.3390/pharmaceutics10040169







