Designing of the Anticancer Nanocomposite with Sustained Release Properties by Using Graphene Oxide Nanocarrier with Phenethyl Isothiocyanate as Anticancer Agent
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture and Maintenance
2.3. Synthesis of Graphene Oxide
2.4. Drug loading on GO
2.5. Physicochemical Characterization
2.6. Determination of Anticancer Activity
Cytotoxicity Assay
3. Results
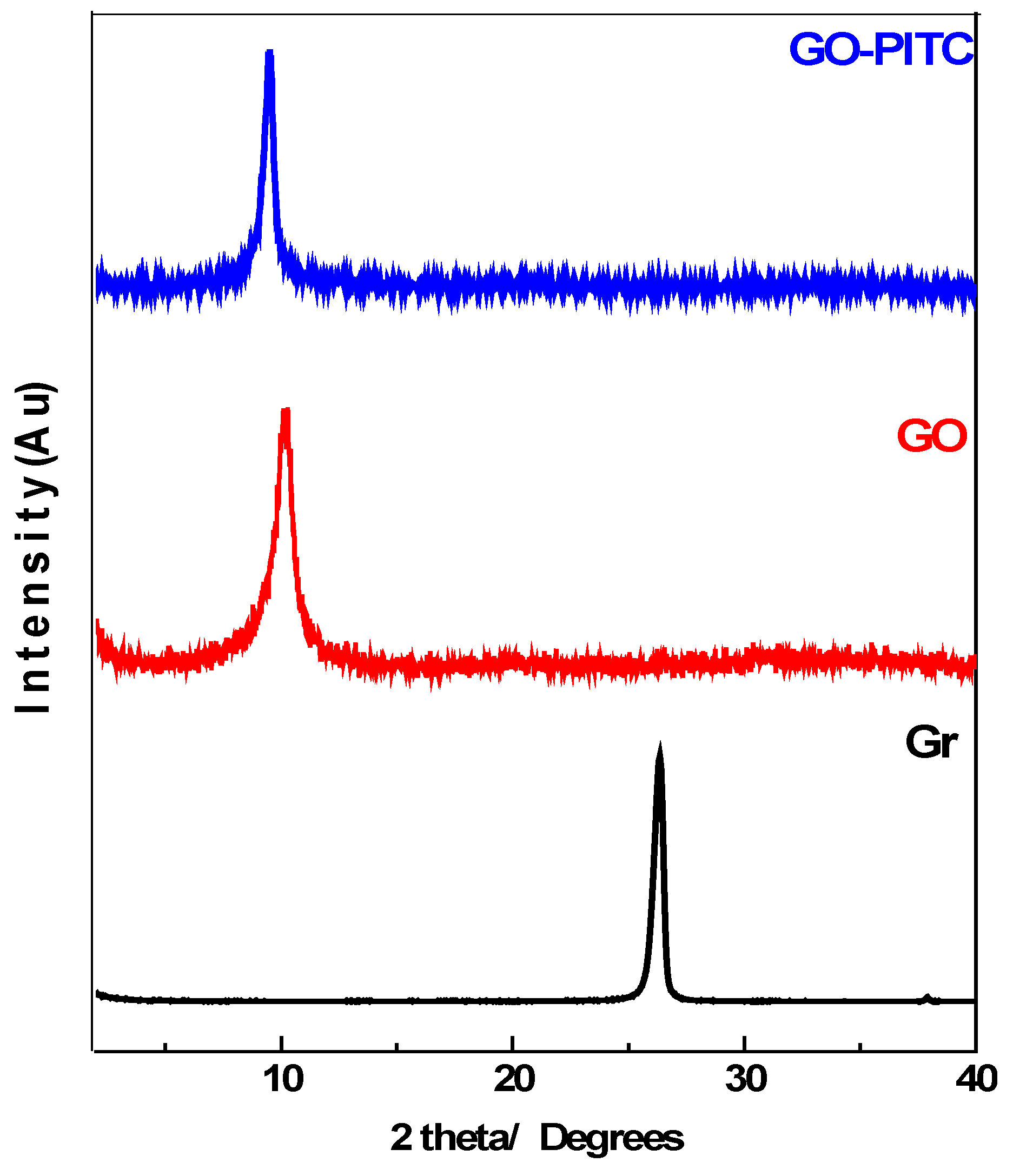
3.1. Powder X-ray Diffraction (PXRD)
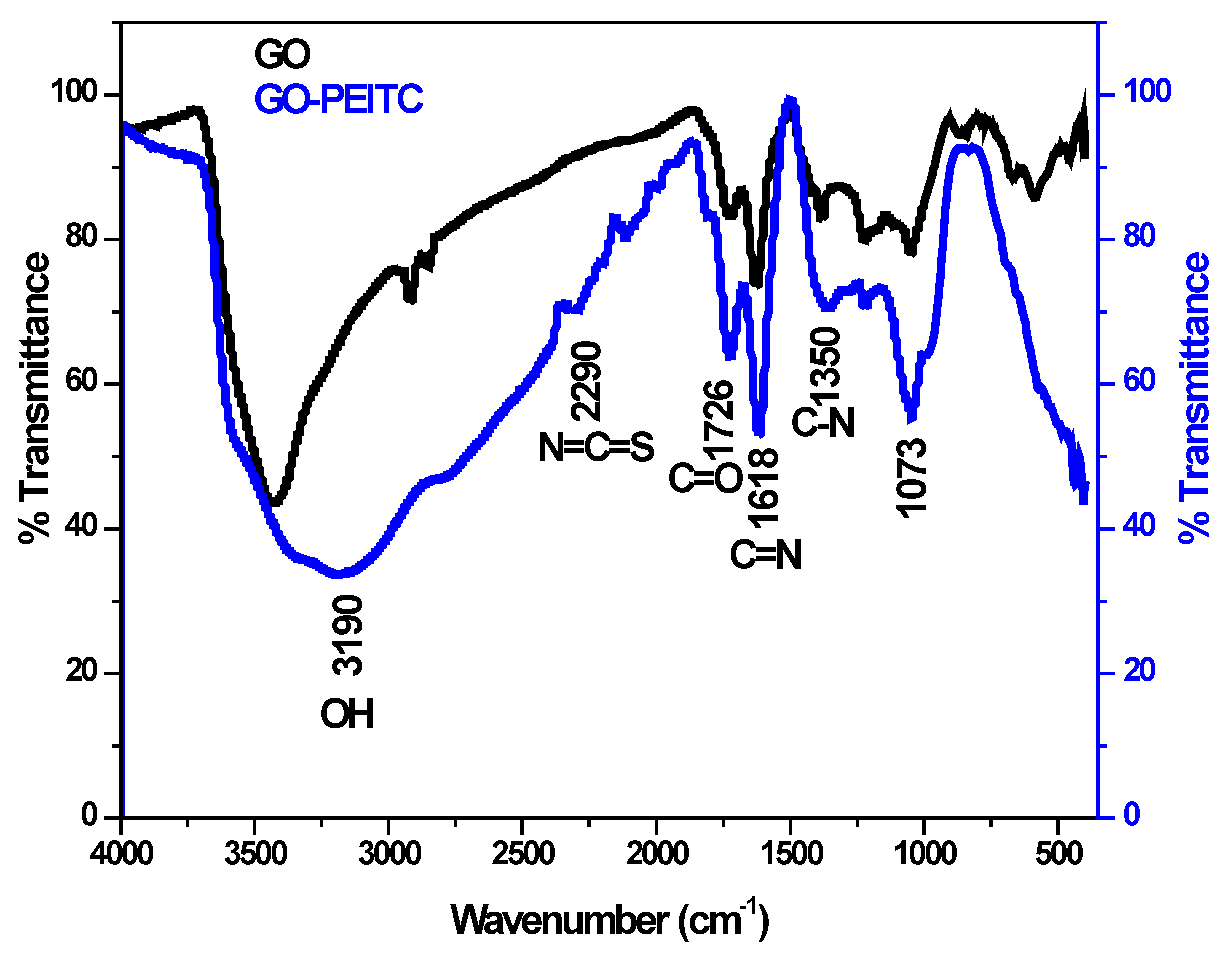
3.2. Infrared Spectroscopy
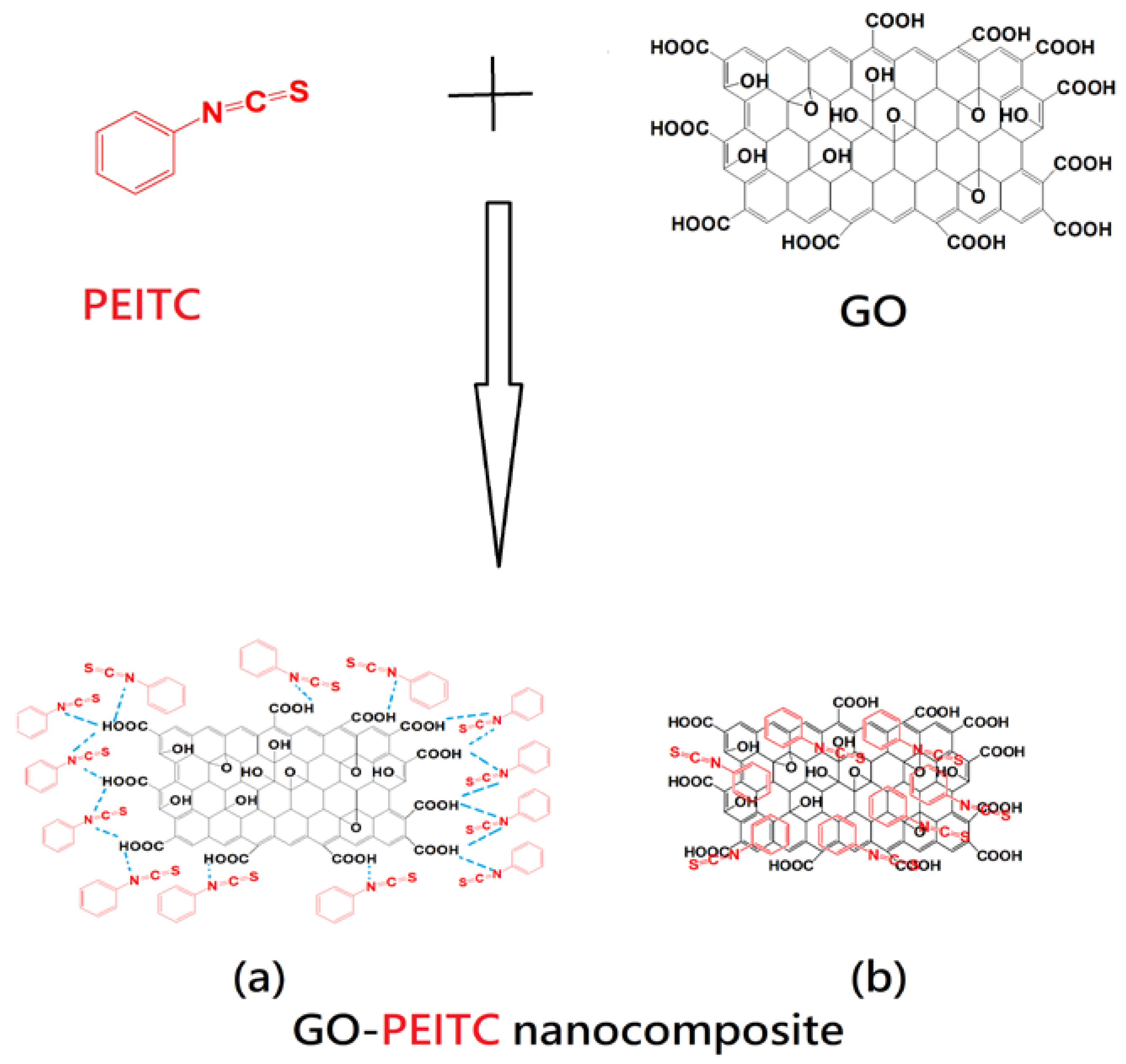
3.3. Schematic Representation of the Structures
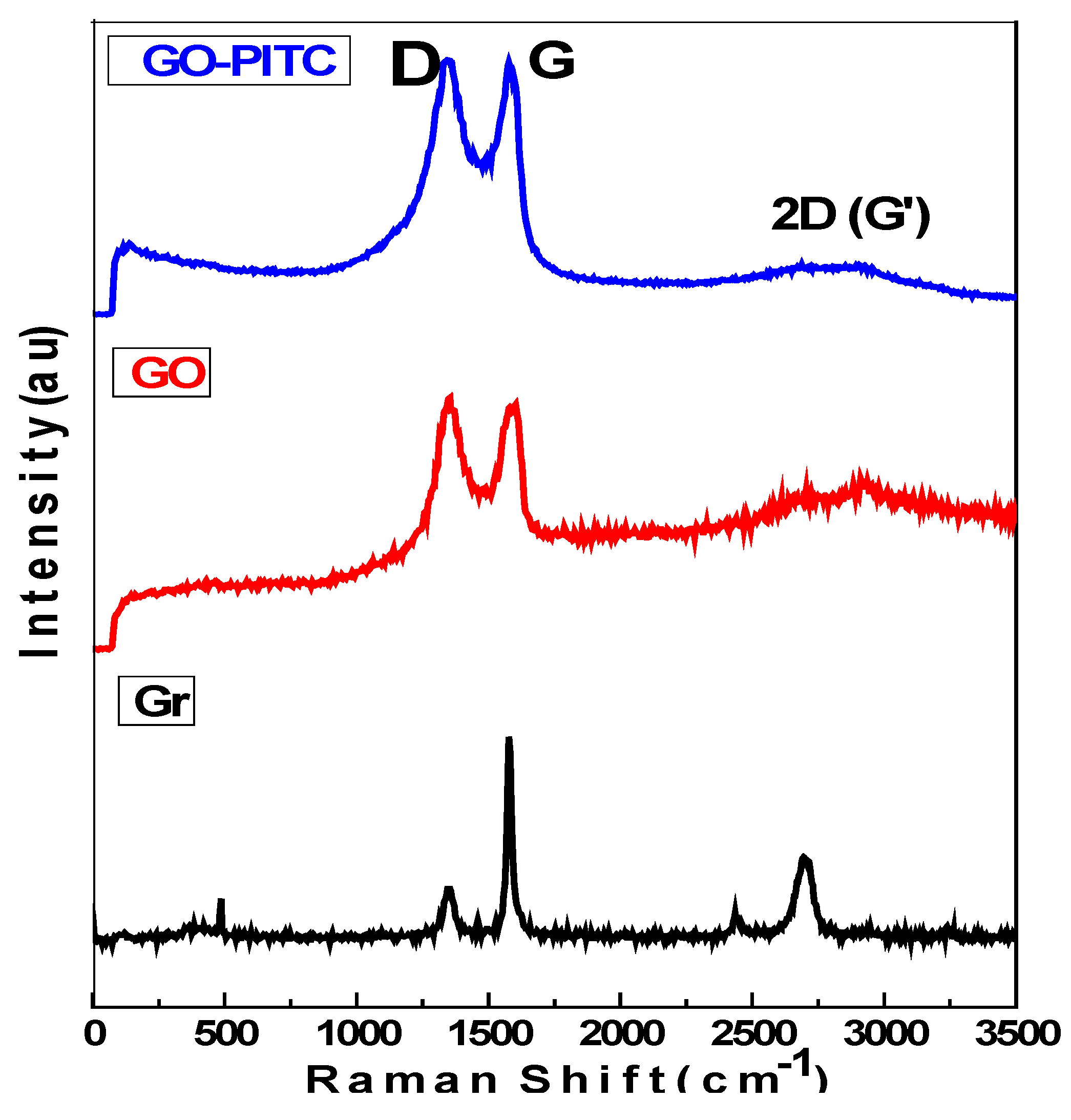
3.4. Raman Spectroscopic Analysis
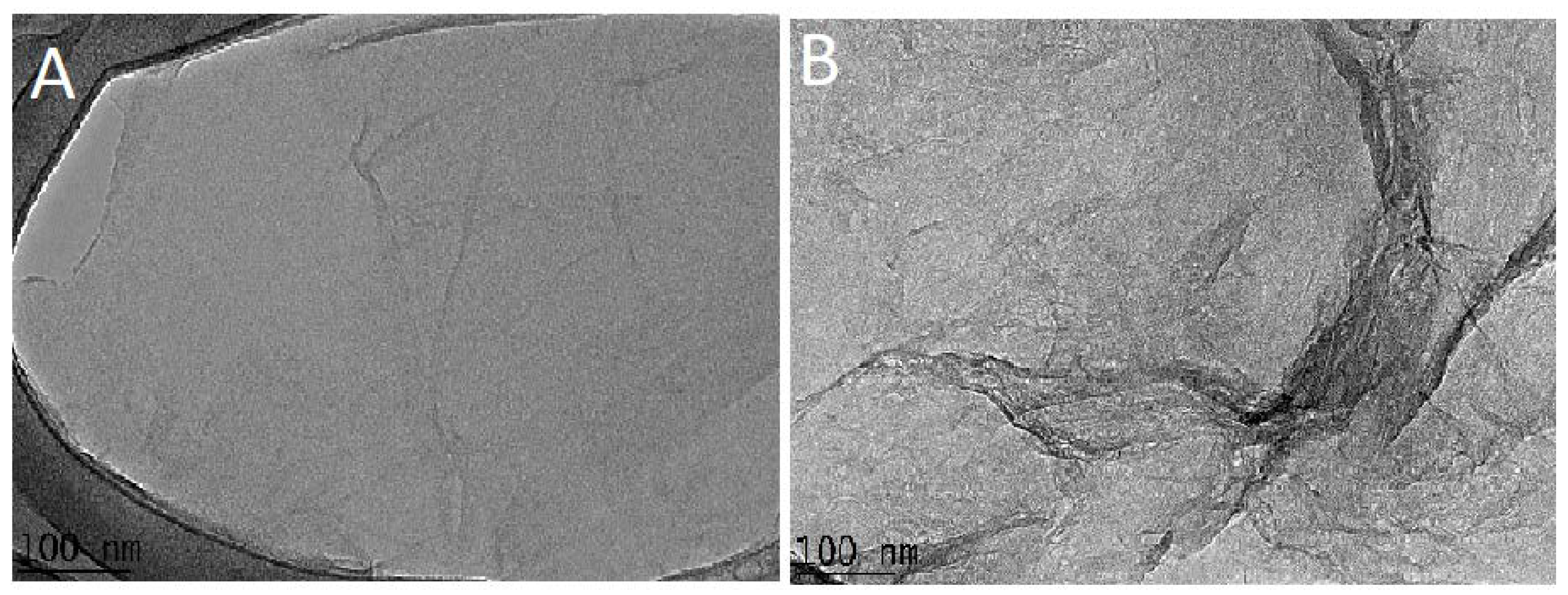
3.5. Transmission Electronic Microscopic (TEM) Analysis
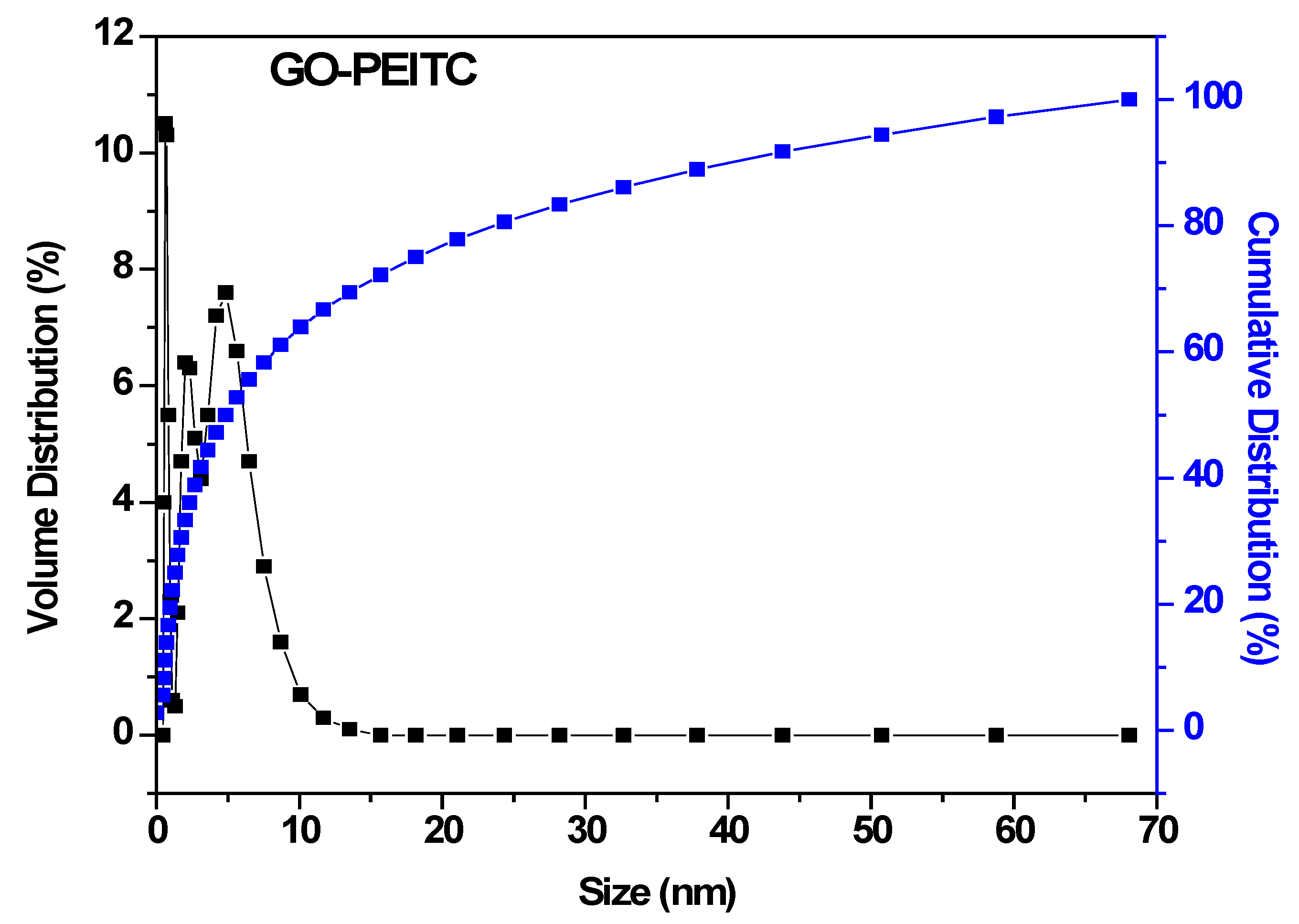
3.6. Particle Size Analysis
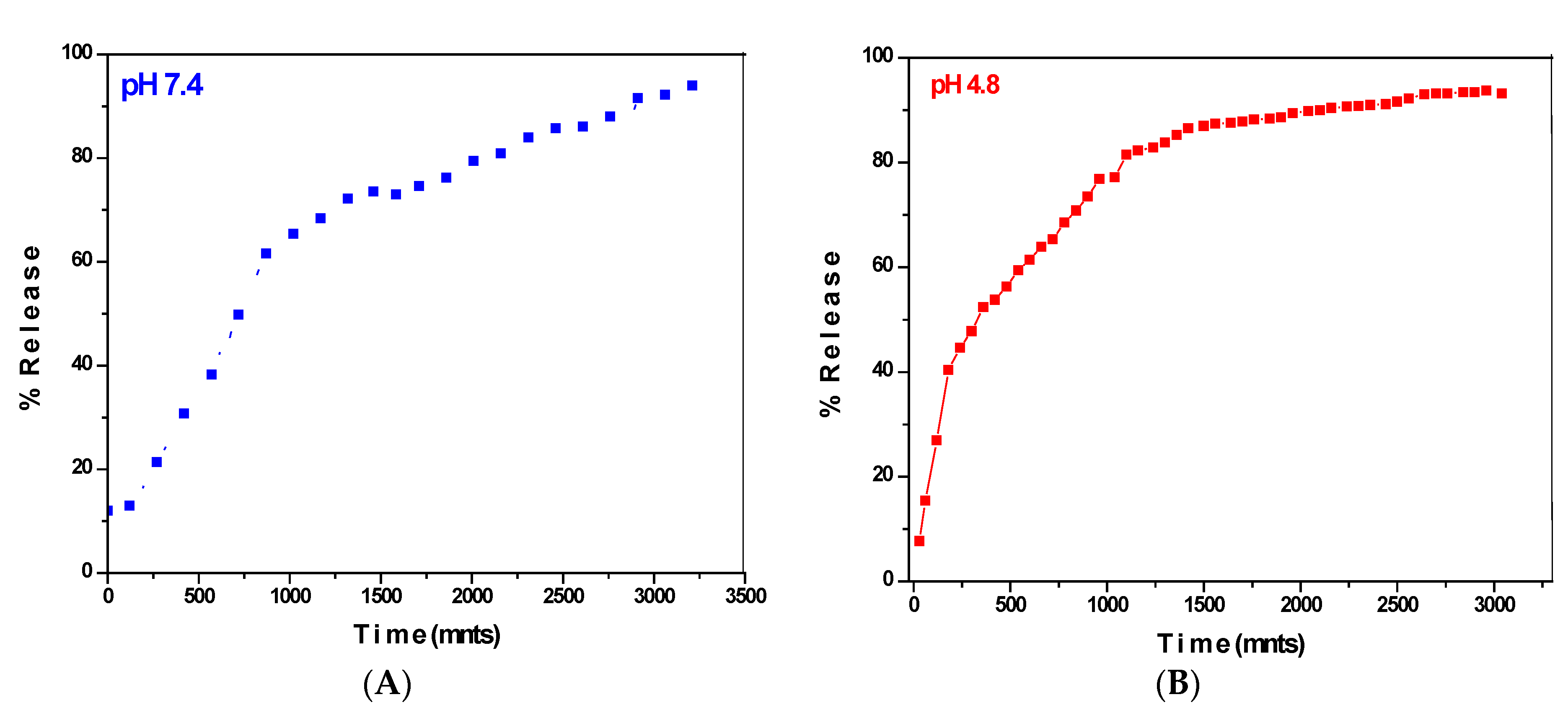
3.7. Release Studies
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Nanomedicine: Current status and future prospects. FASEB J. 2005, 19, 311–330. [Google Scholar] [PubMed]
- Kawasaki, E.S.; Player, A. Nanotechnology, nanomedicine, and the development of new, effective therapies for cancer. Nanomedicine 2005, 1, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [PubMed]
- Mirza, A.Z.; Siddiqui, F.A. Nanomedicine and drug delivery: A mini review. Int. Nano Lett. 2014, 4, 94. [Google Scholar]
- Saifullah, B.; Hussein, M.Z.; Al Ali, S.H.H. Controlled-release approaches towards the chemotherapy of tuberculosis. Int. J. Nanomed. 2012, 7, 5451–5463. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Roy, I.; Yang, C.; Prasad, P.N. Nanochemistry and Nanomedicine for Nanoparticle-based Diagnostics and Therapy. Chem. Rev. 2016, 116, 2826–2885. [Google Scholar] [CrossRef] [PubMed]
- Taghdisi, S.M.; Danesh, N.M.; Lavaee, P.; Emrani, A.S.; Hassanabad, K.Y.; Ramezani, M.; Abnous, K. Double targeting, controlled release and reversible delivery of daunorubicin to cancer cells by polyvalent aptamers-modified gold nanoparticles. Mater. Sci. Eng. C 2016, 61, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New Developments in Liposomal Drug Delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef] [PubMed]
- Barahuie, F.; Hussein, M.Z.; Hussein-Al-Ali, S.H.; Arulselvan, P.; Fakurazi, S.; Zainal, Z. Preparation and controlled-release studies of a protocatechuic acid-magnesium/aluminum-layered double hydroxide nanocomposite. Int. J. Nanomed. 2013, 8, 1975–1987. [Google Scholar]
- Saifullah, B.; Arulselvan, P.; El Zowalaty, M.E.; Fakurazi, S.; Webster, T.J.; Geilich, B.M.; Hussein, M.Z. Development of a biocompatible nanodelivery system for tuberculosis drugs based on isoniazid-Mg/Al layered double hydroxide. Int. J. Nanomed. 2014, 9, 4749–4762. [Google Scholar]
- Xavier, A.C.; de Moraes, M.L.; Ferreira, M. Immobilization of aloin encapsulated into liposomes in Layer-by-layer films for transdermal drug delivery. Mater. Sci. Eng. C 2013, 33, 1193–1196. [Google Scholar] [CrossRef] [PubMed]
- Elgadir, M.A.; Uddin, M.S.; Ferdosh, S.; Adam, A.; Chowdhury, A.J.K.; Sarker, M.Z.I. Impact of chitosan composites and chitosan nanoparticle composites on various drug delivery systems. J. Food Drug Anal. 2015, 23, 619–629. [Google Scholar] [PubMed]
- Chung, C.; Kim, Y.K.; Shin, D.; Ryoo, S.R.; Hong, B.H.; Min, D.H. Biomedical applications of graphene and graphene oxide. Acc. Chem. Res. 2013, 46, 2211–2224. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, J.; Kim, S.; Min, D.H. Biosensors based on graphene oxide and its biomedical application. Adv. Drug Deliv. Rev. 2016, 105, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.S.; Modal, M.D.; Paik, P. Graphene Oxide for Biomedical Applications. J. Nanomed. Res. 2017, 5. [Google Scholar] [CrossRef]
- Zhou, L.; Jiang, H.; Wei, S.; Ge, X.; Zhou, J.; Shen, J. High-efficiency loading of hypocrellin B on graphene oxide for photodynamic therapy. Carbon 2012, 50, 5594–5604. [Google Scholar] [CrossRef]
- Yan, T.; Zhang, H.; Huang, D.; Feng, S.; Fujita, M.; Gao, X.D. Chitosan-Functionalized Graphene Oxide as a Potential Immunoadjuvant. Nanomaterials 2017, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Zhang, F.; Liu, L.; Zhang, Y.; Li, Y.; Li, H.; Xie, J. Surface modification of graphene oxide nanosheets by protamine sulfate/sodium alginate for anti-cancer drug delivery application. Appl. Surf. Sci. 2018, 440, 853–860. [Google Scholar] [CrossRef]
- Song, J.; Yang, X.; Jacobson, O.; Lin, L.; Huang, P.; Niu, G.; Ma, Q.; Chen, X. Sequential Drug Release and Enhanced Photothermal and Photoacoustic Effect of Hybrid Reduced Graphene Oxide-Loaded Ultrasmall Gold Nanorod Vesicles for Cancer Therapy. ACS Nano 2015, 9, 9199–9209. [Google Scholar] [PubMed]
- Chen, L.; Zhong, X.; Yi, X.; Huang, M.; Ning, P.; Liu, T.; Ge, C.; Chai, Z.; Liu, Z.; Yang, K. Radionuclide (131)I labeled reduced graphene oxide for nuclear imaging guided combined radio- and photothermal therapy of cancer. Biomaterials 2015, 66, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Saikia, N.; Deka, R.C. Ab initio study on the noncovalent adsorption of camptothecin anticancer drug onto graphene, defect modified graphene and graphene oxide. J. Comput.-Aided Mol. Des. 2013, 27, 807–821. [Google Scholar] [PubMed]
- Karimzadeh, I.; Aghazadeh, M.; Doroudi, T.; Ganjali, M.R.; Kolivand, P.H. Superparamagnetic Iron Oxide (Fe3O4) Nanoparticles Coated with PEG/PEI for Biomedical Applications: A Facile and Scalable Preparation Route Based on the Cathodic Electrochemical Deposition Method. Adv. Phys. Chem. 2017, 2017, 7. [Google Scholar] [CrossRef]
- Dufour, V.; Stahl, M.; Baysse, C. The antibacterial properties of isothiocyanates. Microbiology 2015, 161, 229–243. [Google Scholar] [PubMed]
- Conaway, C.C.; Wang, C.X.; Pittman, B.; Yang, Y.M.; Schwartz, J.E.; Tian, D.; Mclntee, E.J.; Hecht, S.S.; Chung, F.L. Phenethyl Isothiocyanate and Sulforaphane and their N-acetylcysteine Conjugates Inhibit Malignant Progression of Lung Adenomas Induced by Tobacco Carcinogens in A/J Mice. Cancer Res. 2005, 65, 8548–8557. [Google Scholar] [CrossRef] [PubMed]
- Hong, E.; Kim, G.H. Anticancer and Antimicrobial Activities of β-Phenylethyl Isothiocyanate in Brassica rapa L. Food Sci. Technol. Res. 2008, 14, 377. [Google Scholar]
- Chve, H.L.T.; Glucosinolates, M.E. Toxic Constituents of Plant StuVs; Liener, I.E., Ed.; Academic Press: New York, NY, USA, 1980; Volume 43. [Google Scholar]
- Thejass, P.; Kuttan, G. Inhibition of Endothelial Cell Differentiation and Proinflammatory Cytokine Production during Angiogenesis by Allyl Isothiocyanate and Phenyl Isothiocyanate. Integr. Cancer Ther. 2007, 6, 389–399. [Google Scholar] [PubMed]
- Thejass, P.; Kuttan, G. Allyl isothiocyanate (AITC) and phenyl isothiocyanate inhibit tumour-specific angiogenesis by downregulating nitric oxide (NO) and tumour necrosis factor-α (TNF-α) production. Nitric Oxide 2007, 16, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Manesh, C.; Kuttan, G. Effect of naturally occurring allyl and phenyl isothiocyanates in the inhibition of experimental pulmonary metastasis induced by B16F-10 melanoma cells. Fitoterapia 2003, 74, 355–363. [Google Scholar] [CrossRef]
- Telang, U.; Brazeau, D.A.; Morris, M.E. Comparison of the effects of phenethyl isothiocyanate and sulforaphane on gene expression in breast cancer and normal mammary epithelial cells. Exp. Biol. Med. 2009, 234, 287–295. [Google Scholar]
- Tseng, E.; Scott-Ramsay, E.A.; Morris, M.E. Dietary organic isothiocyanates are cytotoxic in human breast cancer MCF-7 and mammary epithelial MCF-12A cell lines. Exp. Biol. Med. 2004, 229, 835–842. [Google Scholar]
- Tang, N.Y.; Huang, Y.T.; Yu, C.S.; Ko, Y.C.; Wu, S.H.; Ji, B.C.; Yang, J.S.; Yang, J.L.; Hsia, T.C.; Chen, Y.Y.; et al. Phenethyl Isothiocyanate (PEITC) Promotes G2/M Phase Arrest via p53 Expression and Induces Apoptosis through Caspase- and Mitochondria-dependent Signaling Pathways in Human Prostate Cancer DU 145 Cells. Anticancer Res. 2011, 31, 1691–1702. [Google Scholar] [PubMed]
- Le, D.H.; Shim, J.H.; Choi, K.H.; Shin, J.A.; Choi, E.S.; Kim, H.S.; Lee, S.J.; Kim, S.J.; Cho, N.P.; Cho, S.D. Effect of β-Phenylethyl Isothiocyanate from Cruciferous Vegetables on Growth Inhibition and Apoptosis of Cervical Cancer Cells through the Induction of Death Receptors 4 and 5. J. Agric. Food Chem. 2011, 59, 8124–8131. [Google Scholar]
- Rose, P.; Whiteman, M.; Huang, S.H.; Halliwell, B.; Ong, C.N. beta-Phenylethyl isothiocyanate-mediated apoptosis in hepatoma HepG2 cells. Cell. Mol. Life Sci. 2003, 60, 1489–1503. [Google Scholar] [CrossRef] [PubMed]
- Bansal, P.; Medhe, S.; Ganesh, N.; Srivastava, M.M. In vitro anticancer activity of dietary bioagent (isothiocyanates) on HepG2 and B16F10 cell lines a comparative study. Ann. Plant Sci. 2013, 2, 234–237. [Google Scholar]
- Yang, Y.T.; Shi, Y.; Jay, M.; Pasqua, A.J.D. Enhanced Toxicity of Cisplatin with Chemosensitizer Phenethyl Isothiocyanate toward Non-Small Cell Lung Cancer Cells When Delivered in Liposomal Nanoparticles. Chem. Res. Toxicol. 2014, 27, 946–948. [Google Scholar] [PubMed]
- Ma, X.; Tao, H.; Yang, K. A Functionalized Graphene Oxide–Iron Oxide Nanocomposite for Magnetically Targeted Drug Delivery, Photothermal Therapy, and Magnetic Resonance Imaging. Nano Res. 2012, 5, 199–212. [Google Scholar]
- Khan, M.; Khan, M.; Al-Marri, A.; Al-Warthan, A.; Alkhathlan, H.Z.; Siddiqui, M.R.H.; Nayak, V.; Kamal, A.; Adil, S.F. Apoptosis inducing ability of silver decorated highly reduced graphene oxide nanocomposites in A549 lung cancer. Int. J. Nanomed. 2016, 11, 873–883. [Google Scholar]
- Dorniani, D.; Saifullah, B.; Barahuie, F.; Arulselvan, P.; Hussein, M.Z.B.; Fakurazi, S.; Twyman, L.J. Graphene Oxide-Gallic Acid Nanodelivery System for Cancer Therapy. Nanoscale Res. Lett. 2016, 11, 491. [Google Scholar] [PubMed]
- Barahuie, F.; Saifullah, B.; Dorniani, D.; Fakurazi, S.; Karthivashan, G.; Hussein, M.Z.; Elfghi, F.M. Graphene oxide as a nanocarrier for controlled release and targeted delivery of an anticancer active agent, chlorogenic acid. Mater. Sci. Eng. C 2017, 74, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [PubMed]
- Tang, L.A.; Lee, W.C.; Shi, H.; Wong, E.Y.; Sadovoy, A.; Gorelik, S.; Hobley, J.; Lin, C.T.; Loh, K.P. Highly Wrinkled Cross-Linked Graphene Oxide Membranes for Biological and Charge-Storage Applications. Small 2012, 8, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Balachandrab, V.; Murali, M.K. FT-IR, FT raman and DFT structure, vibrational frequency analysis and mulliken charges of 2 chlorophenyl isothiocyonate. Indian J. Pure Appl. Phys. 2012, 50, 19–25. [Google Scholar]
- Murali, M.K.; Balachandran, V. FT-IR and FT-Raman spectral analysis of 3-(trifluromethyl) phenyl isothiocyanate. Elixir Vib. Spec. 2011, 40, 5105–5107. [Google Scholar]
- Kalbac, M.; Hsieh, Y.P.; Farhat, H.; Kavan, L.; Hofmann, M.; Kong, J.; Dresselhaus, M.S. Defects in Individual Semiconducting Single Wall Carbon Nanotubes: Raman Spectroscopic and in Situ Raman Spectroelectrochemical Study. Nano Lett. 2010, 10, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Dresselhaus, M.S.; Jorio, A.; Hofmann, M.; Dresselhaus, G.; Saito, R. Perspectives on Carbon Nanotubes and Graphene Raman Spectroscopy. Nano Lett. 2010, 10, 751–758. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaya Seema, D.M.; Saifullah, B.; Selvanayagam, M.; Gothai, S.; Hussein, M.Z.; Subbiah, S.K.; Mohd Esa, N.; Arulselvan, P. Designing of the Anticancer Nanocomposite with Sustained Release Properties by Using Graphene Oxide Nanocarrier with Phenethyl Isothiocyanate as Anticancer Agent. Pharmaceutics 2018, 10, 109. https://doi.org/10.3390/pharmaceutics10030109
Jaya Seema DM, Saifullah B, Selvanayagam M, Gothai S, Hussein MZ, Subbiah SK, Mohd Esa N, Arulselvan P. Designing of the Anticancer Nanocomposite with Sustained Release Properties by Using Graphene Oxide Nanocarrier with Phenethyl Isothiocyanate as Anticancer Agent. Pharmaceutics. 2018; 10(3):109. https://doi.org/10.3390/pharmaceutics10030109
Chicago/Turabian StyleJaya Seema, Dasan Mary, Bullo Saifullah, Mariadoss Selvanayagam, Sivapragasam Gothai, Mohd Zobir Hussein, Suresh Kumar Subbiah, Norhaizan Mohd Esa, and Palanisamy Arulselvan. 2018. "Designing of the Anticancer Nanocomposite with Sustained Release Properties by Using Graphene Oxide Nanocarrier with Phenethyl Isothiocyanate as Anticancer Agent" Pharmaceutics 10, no. 3: 109. https://doi.org/10.3390/pharmaceutics10030109
APA StyleJaya Seema, D. M., Saifullah, B., Selvanayagam, M., Gothai, S., Hussein, M. Z., Subbiah, S. K., Mohd Esa, N., & Arulselvan, P. (2018). Designing of the Anticancer Nanocomposite with Sustained Release Properties by Using Graphene Oxide Nanocarrier with Phenethyl Isothiocyanate as Anticancer Agent. Pharmaceutics, 10(3), 109. https://doi.org/10.3390/pharmaceutics10030109





