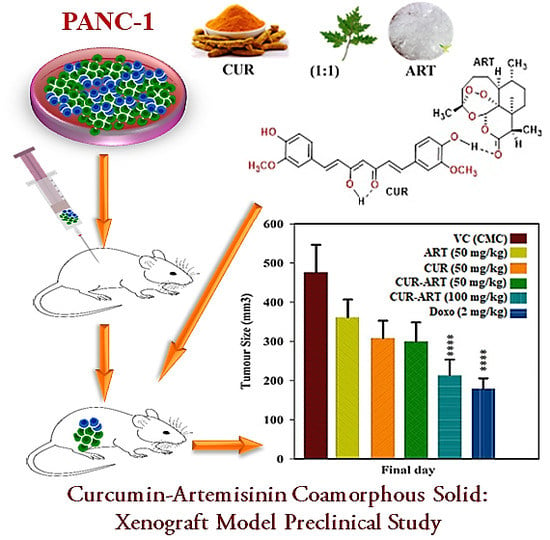
Curcumin-Artemisinin Coamorphous Solid: Xenograft Model Preclinical Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
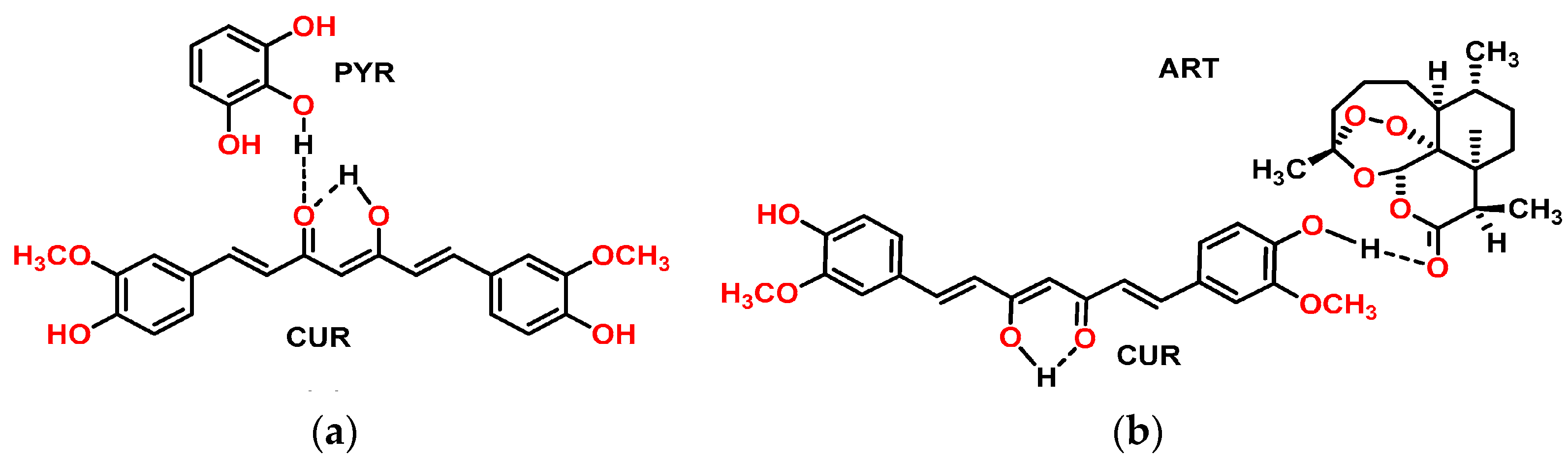
2.2.1. Curcumin Solids Preparation Method
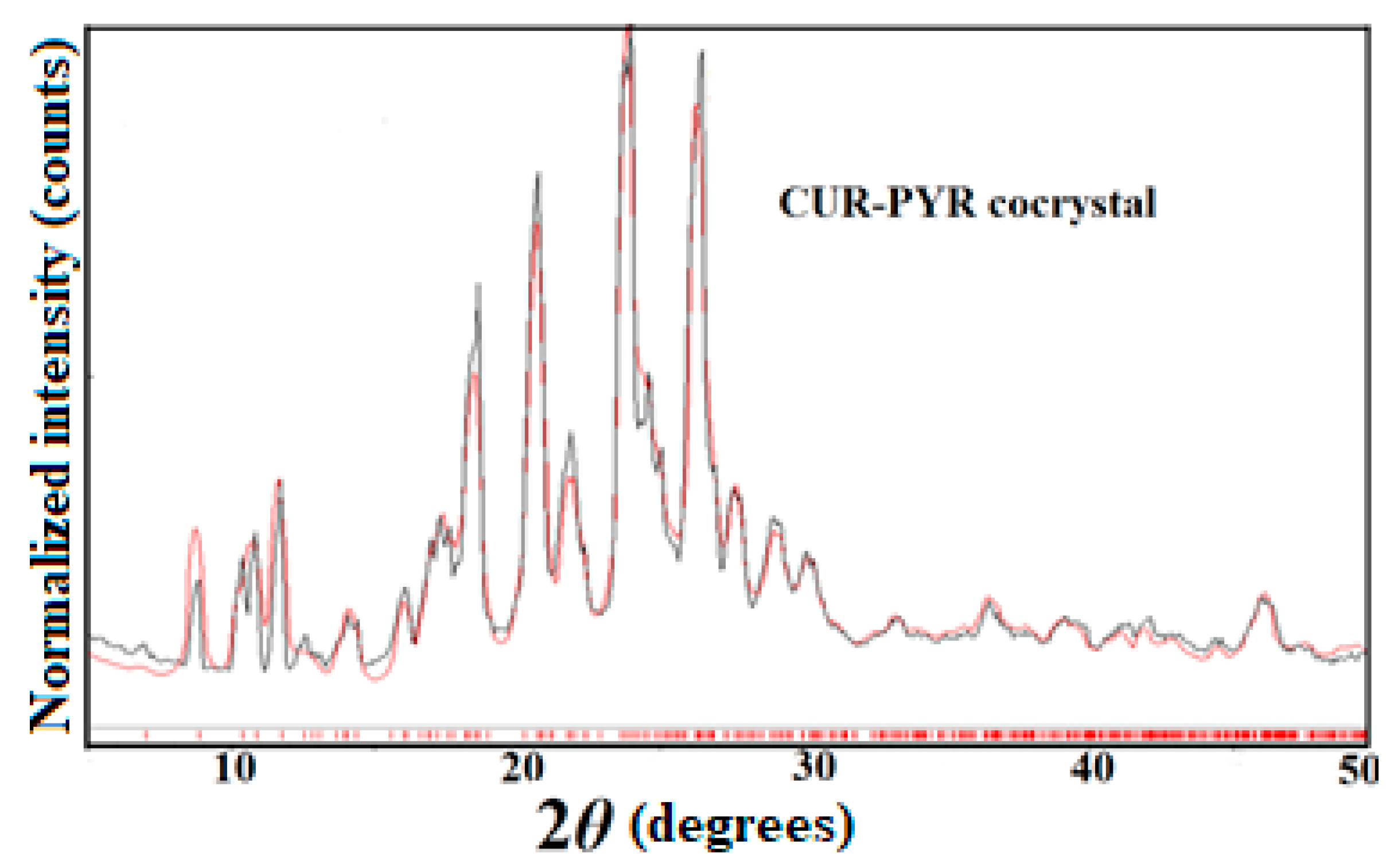
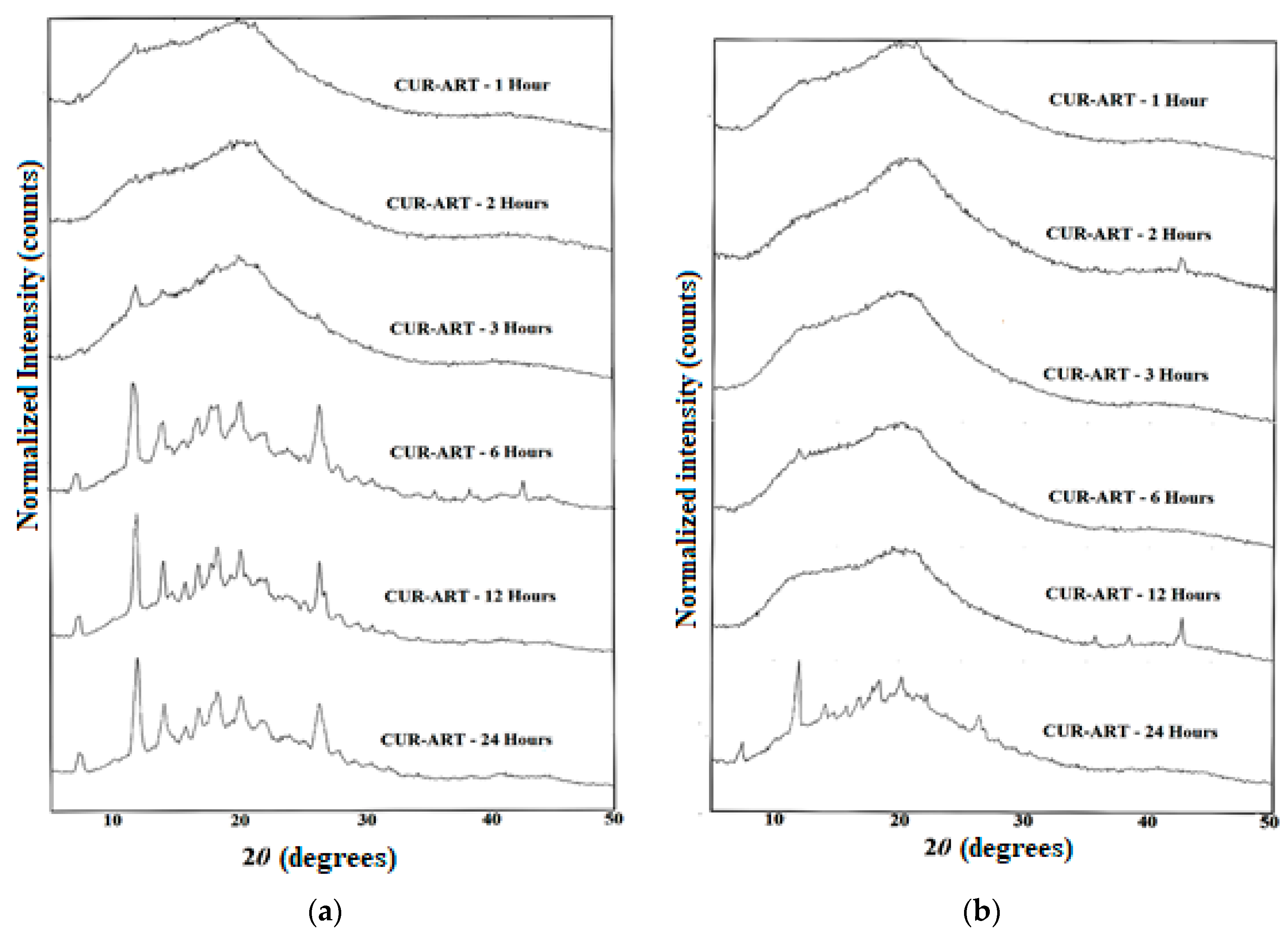
2.2.2. Characterization of Multicomponent Systems of Curcumin by PXRD
2.3. In Vivo Study Design and Drug Administration
2.3.1. Determination of Curcumin Plasma Concentration
2.3.2. Chromatographic Conditions
2.4. Preparation of SGF and SIF Media
Supersaturation Study
2.5. Acute Toxicity Study
2.6. In Vitro Cell Culture
2.7. In Vivo PANC-1 Tumor Growth Xenograft Model
2.8. Statistical Analysis
2.8.1. Bioavailability Study
2.8.2. Xenograft Studies
3. Results and Discussion
3.1. Bioavailability Studies
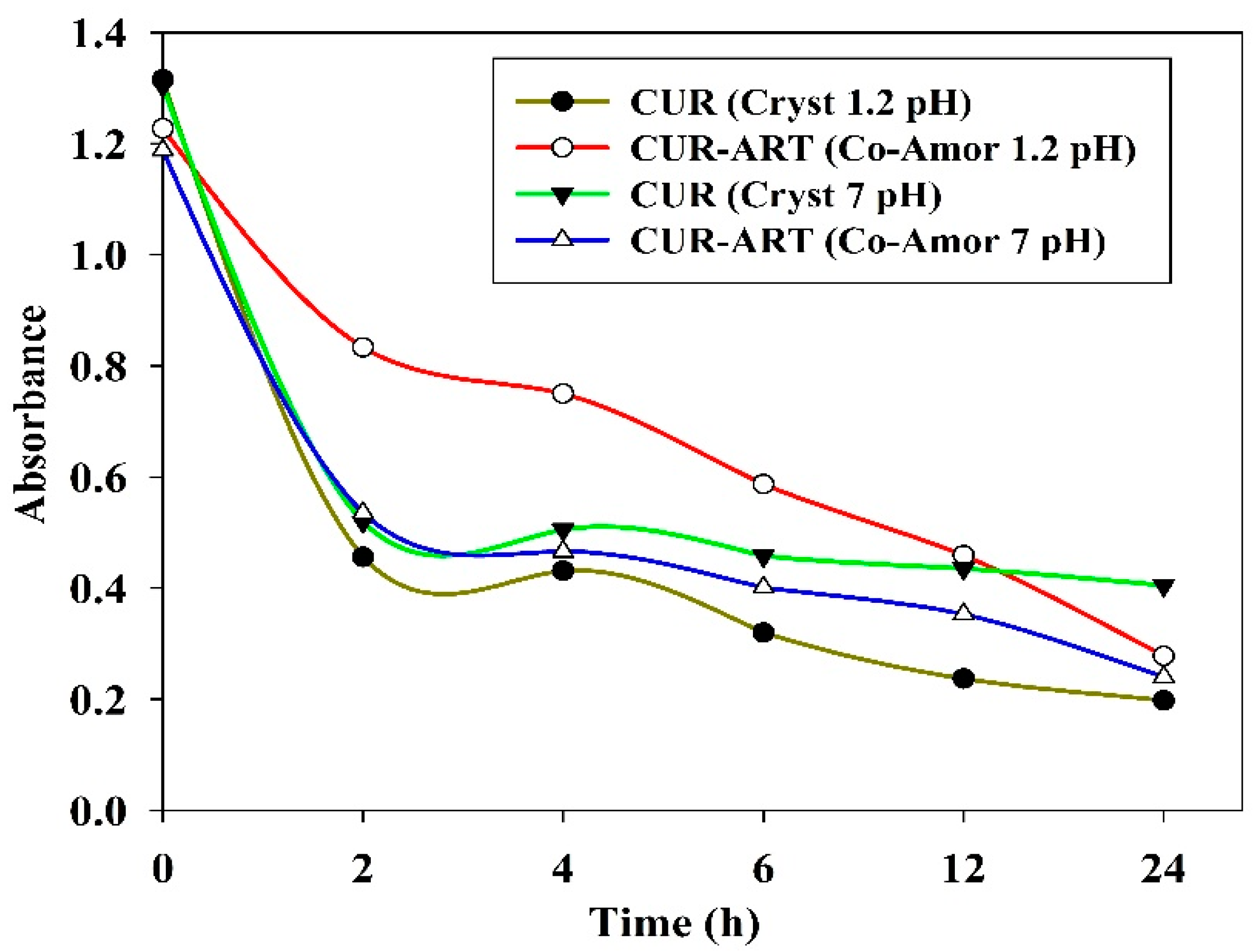
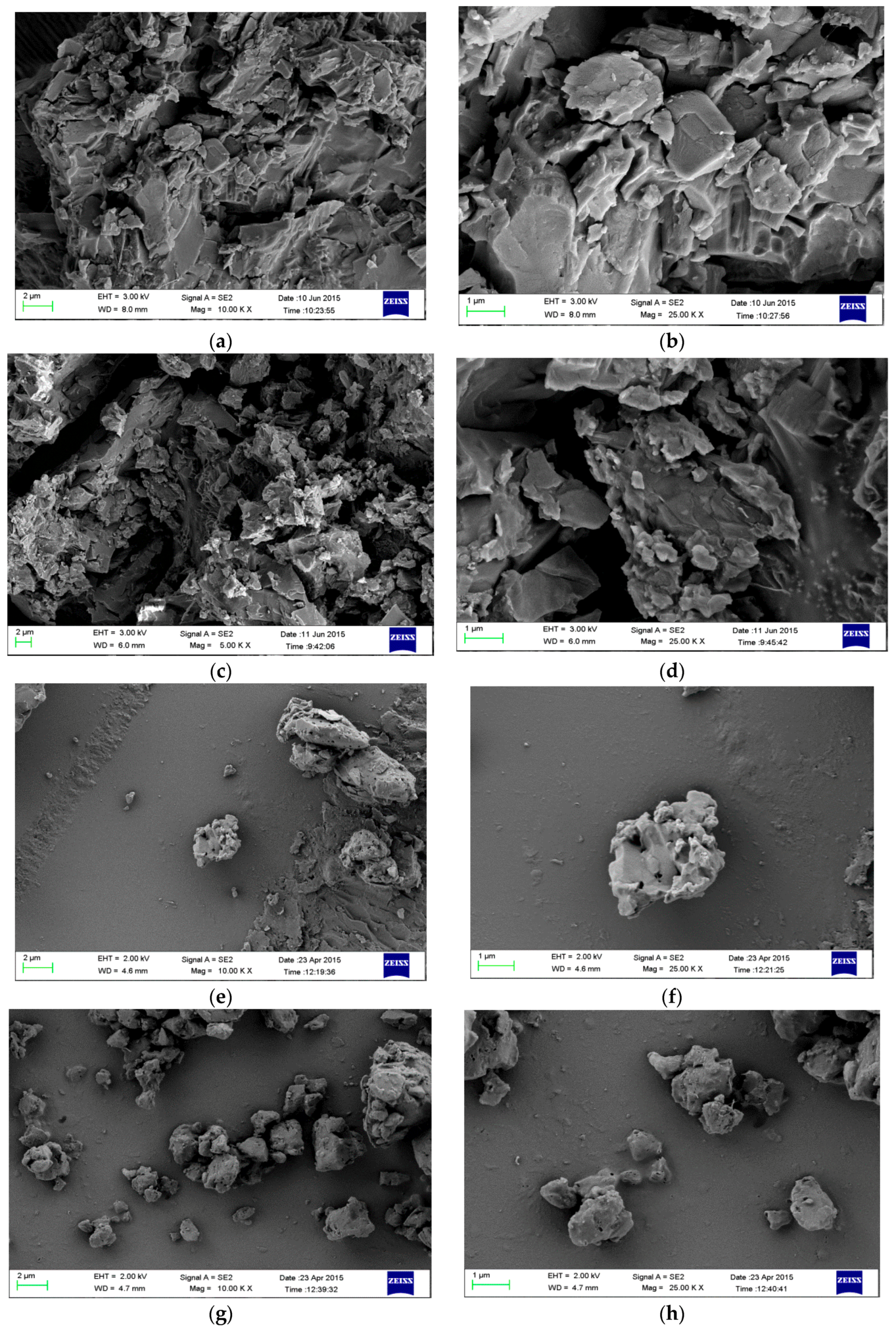
3.2. Phase Stability in SGF and SIF Media
3.3. Acute Toxicity Study
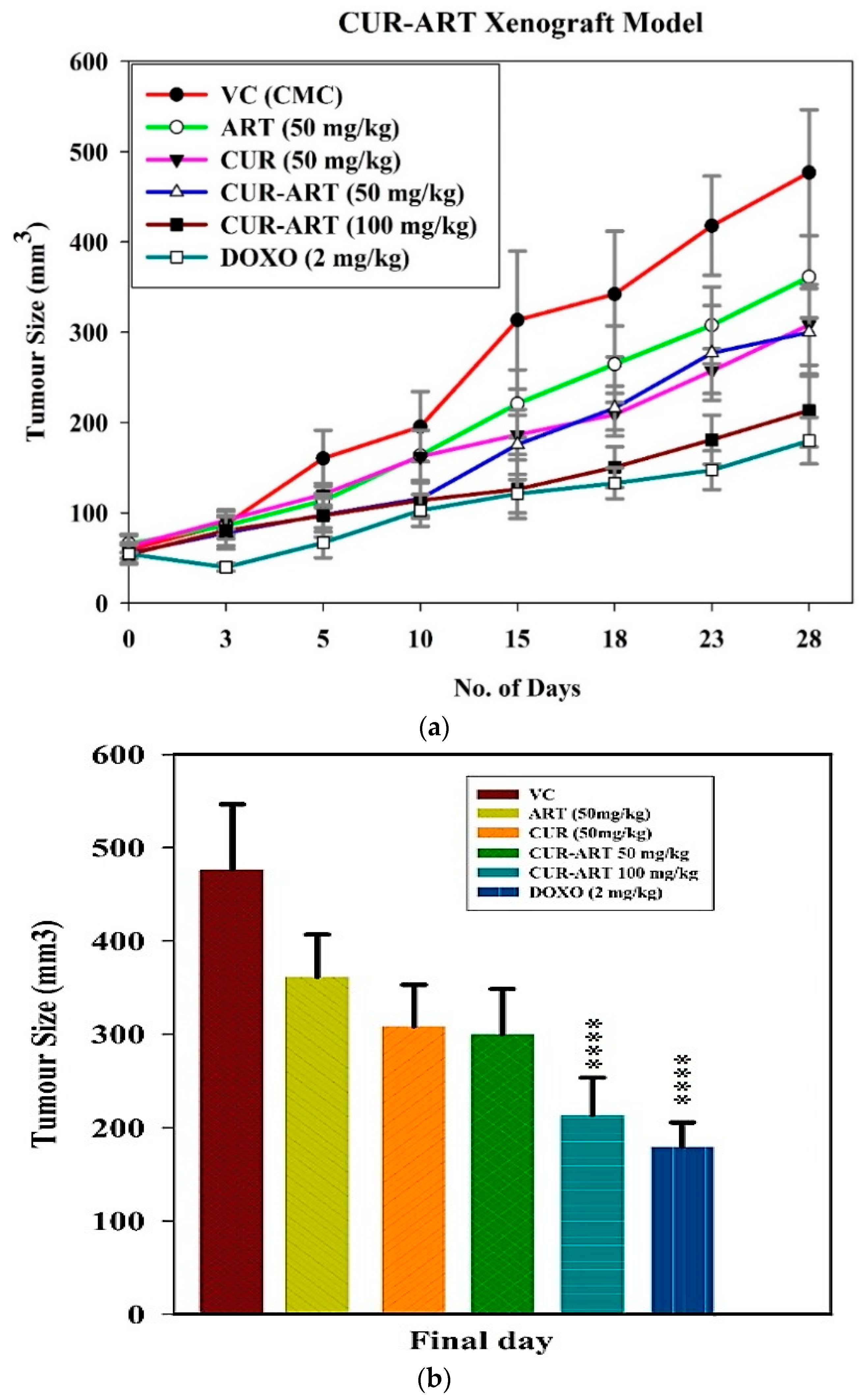
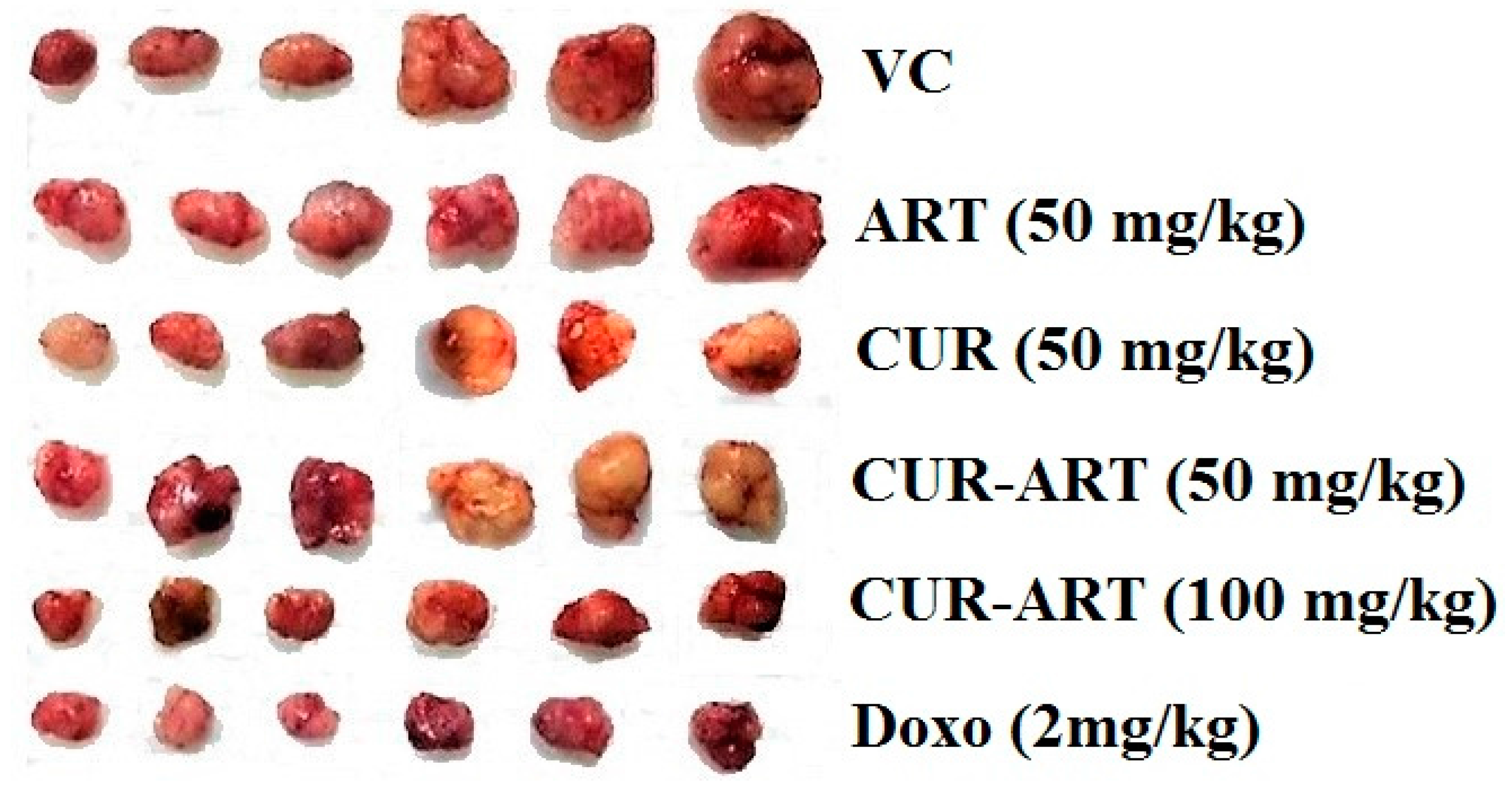
3.4. In Vivo Antitumor Effect of CUR-ART against Panc-1 Xenograft
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Note
- Vikram, P.; Chiruvellam, K.K.; Ripain, I.H.; Arifullah, M. A recent review on phytochemical constituents and medicinal properties of kesum (Polygonum minus Huds.). Asian Pac. J. Trop. Biomed. 2014, 4, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Gurib-Fakim, A. Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol. Asp. Med. 2006, 27, 1–93. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J. Plants as a source of anti-cancer agents. J. Ethnopharm. 2005, 100, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Russell, J.M. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar]
- Isacchi, B.; Bergonzi, M.C.; Grazioso, M.; Righeschi, C.; Pietretti, A.; Severini, C.; Bilia, A.R. Artemisinin and artemisinin plus curcumin liposomal formulations: Enhanced antimalarial efficacy against Plasmodium berghei-infected mice. Eur. J. Pharm. Biopharm. 2012, 80, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Molecular bioavailability of curcumin: problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Rane, G.; Kanchi, M.M.; Arfuso, F.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Tan, B.K.H.; Kumar, A.P.; Sethi, G. The multifaceted role of curcumin in cancer prevention and treatment. Molecules 2015, 20, 2728–2769. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M. Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y. Artemisinin-a gift from traditional Chinese medicine to the world (Nobel Lecture). Angew. Chem. Int. Ed. 2016, 55, 10210–10226. [Google Scholar] [CrossRef] [PubMed]
- The National Leading Group Office for Malaria Control. Communication on Malaria Control Research, 5 November 1972.
- Padmanabam, G.; Nagaraj, V.A.; Rangarajan, P.N. Artemisinin-based combination with curcumin adds a new dimension to malaria therapy. Curr. Sci. 2012, 102, 704–711. [Google Scholar]
- Nandakumar, D.N.; Nagaraj, V.A.; Vathsala, P.G.; Rangarajan, P.; Padmanaban, G. Curcumin-artemisinin combination therapy for Malaria. Antimicrob. Agents Chemother. 2006, 50, 1859–1860. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Guidelines for the Treatment of Malaria, 3rd ed.; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Xu, Q.; Bauer, R.; Hendry, B.M.; Fan, T.P.; Zhao, Z.; Duez, P.; Simmonds, M.S.J.; Witt, C.M.; Lu, A.; Robinson, N.; et al. The quest for modernization of traditional Chinese medicine. BMC Complement. Altern. Med. 2013, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rapport, L.; Lockwood, B. Nutraceutical; Pharmaceutical Press: London, UK, 2002. [Google Scholar]
- Lockwood, B. Nutraceuticals; Pharmaceuticals Press: London, UK, 2007. [Google Scholar]
- Qureshi, S.; Shah, A.H.; Ageel, A.M. Toxicity studies on Alpinia galanga and Curcuma longa. Planta Med. 1992, 58, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, J.; Prasad, S.; Aggarwal, B.B. Curcumin and cancer cells: How many ways can curry kill tumor cells selectively? AAPS J. 2009, 11, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Epelbaum, R.; Schaffer, M.; Vizel, B.; Badmaev, V.; Bar-Sela, G. Curcumin and gemcitabine in patients with advanced pancreatic cancer. Nutr. Cancer 2010, 62, 1137–1141. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 2008, 14, 4491–4499. [Google Scholar] [CrossRef] [PubMed]
- Rachmawati, H.; Edityaningrum, C.A.; Mauludin, R. Molecular inclusion complex of curcumin-beta-cyclodextrin nanoparticle to enhance curcumin skin permeability from hydrophilic matrix gel. AAPS Pharm. Sci. Tech. 2013, 14, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Khalil, N.M.; Do, N.T.C.; Casa, D.M.; Dalmolin, L.F.; De, M.A.C.; Hoss, I. Pharmacokinetics of curcumin-loaded PLGA and PLGA-PEG blend nanoparticles after oral administration in rats. Colloids Surf. Biointerfaces 2013, 101, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, Y.; Su, T.; Feng, L.; Long, Y.; Chen, Z. Silica-coated flexible liposomes as a nanohybrid delivery system for enhanced oral bioavailability of curcumin. Int. J. Nanomed. 2012, 7, 5995–6002. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, Z.; Ou, C.; Chen, M.; Wang, L.; Yang, Z. The first supramolecular hydrogelator of curcumin. Chem. Commun. 2014, 50, 9413–9415. [Google Scholar] [CrossRef] [PubMed]
- Sanphui, P.; Goud, N.R.; Khandavilli, U.B.R.; Bhanoth, S.; Nangia, A. New polymorphs of curcumin. Chem. Commun. 2011, 47, 5013–5015. [Google Scholar] [CrossRef] [PubMed]
- Sanphui, P.; Goud, N.R.; Khandavilli, U.B.R.; Nangia, A. Fast dissolving Curcumin cocrystals. Cryst. Growth Des. 2011, 11, 4135–4145. [Google Scholar] [CrossRef]
- Goud, N.R.; Suresh, K.; Sanphui, P.; Nangia, A. Fast dissolving eutectic compositions of curcumin. Int. J. Pharm. 2012, 439, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Suresh, K.; Mannava, M.K.C.; Nangia, A. A novel curcumin–artemisinin coamorphous solid: Physical properties and pharmacokinetic profile. RSC Adv. 2014, 4, 58357–58361. [Google Scholar] [CrossRef]
- Tong, W.Q.; Wen, H. Preformulation aspects of insoluble compounds. In Water-Insoluble Drug Formulation; CRC Press, Taylor and Francis: Boca Raton, FL, USA, 2008; Volume 63. [Google Scholar]
- Babu, N.J.; Nangia, A. Solubility advantage of amorphous drugs and pharmaceutical cocrystals. Cryst. Growth Des. 2011, 11, 2662–2679. [Google Scholar] [CrossRef]
- Sasaki, H.; Sunagawa, Y.; Takahashi, K.; Imaizumi, A.; Fukuda, H.; Hashimoto, T.; Wada, H.; Katanasaka, Y.; Kakeya, H.; Fujita, M.; et al. Innovative preparation of curcumin for improved oral bioavailability. Biol. Pharm. Bull. 2011, 34, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Marczylo, T.H.; Verschoyle, R.D.; Cooke, D.N.; Morazzoni, P.; Steward, W.P.; Gescher, A.J. Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemother. Pharmacol. 2007, 60, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Chaitrali, K.; Adriyan, K.; John, K.; Tim, G.; Anant, P. Mechanism for polymorphic transformation of artemisinin during high temperature extrusion. Cryst. Growth Des. 2013, 13, 5157–5161. [Google Scholar]
- Dengale, S.J.; Grohganz, H.; Rades, T.; Löbmann, K. Recent advances in co-amorphous drug formulations. Adv. Drug Deliv. Rev. 2016, 100, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Muranushi, N.; Sakuma, S.; Ida, Y.; Endoh, T.; Kadota, K.; Tozuka, Y. A Strategy for co-former selection to design stable co-amorphous formations based on physicochemical properties of non-steroidal inflammatory drugs. Pharm. Res. 2016, 33, 1018–1029. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, R.; Löbmann, K.; Grohganz, H.; Strachan, C.; Rades, T. Amino acids as co-amorphous excipients for simvastatin and glibenclamide: Physical properties and stability. Mol. Pharm. 2014, 11, 2381–2389. [Google Scholar] [CrossRef] [PubMed]
- Kasten, G.; Grohganz, H.; Rades, T.; Löbmann, K. Development of a screening method for co-amorphous formulations of drugs and amino acids. Eur. J. Pharm. Sci. 2016, 95, 28–35. [Google Scholar] [CrossRef] [PubMed]

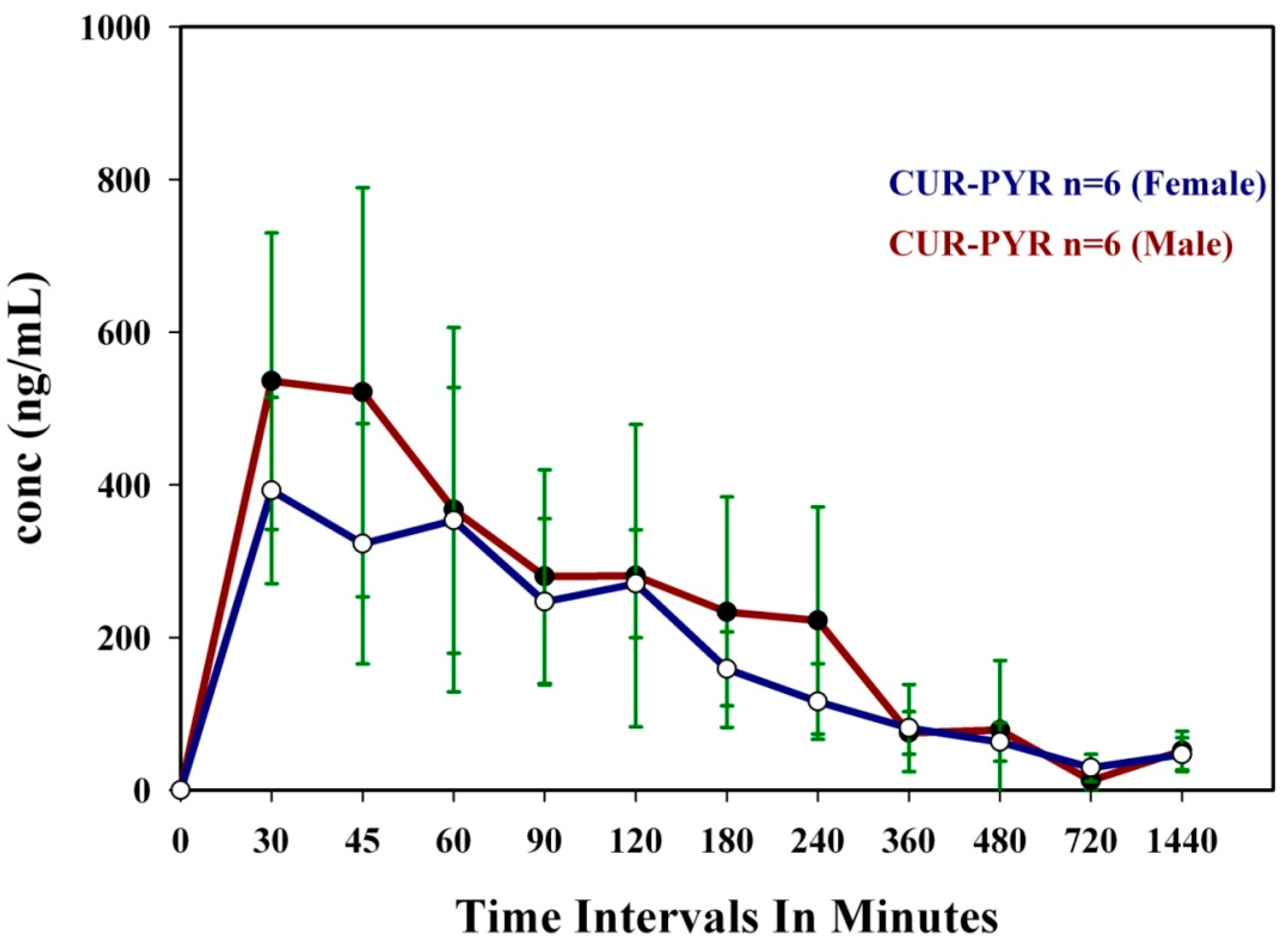
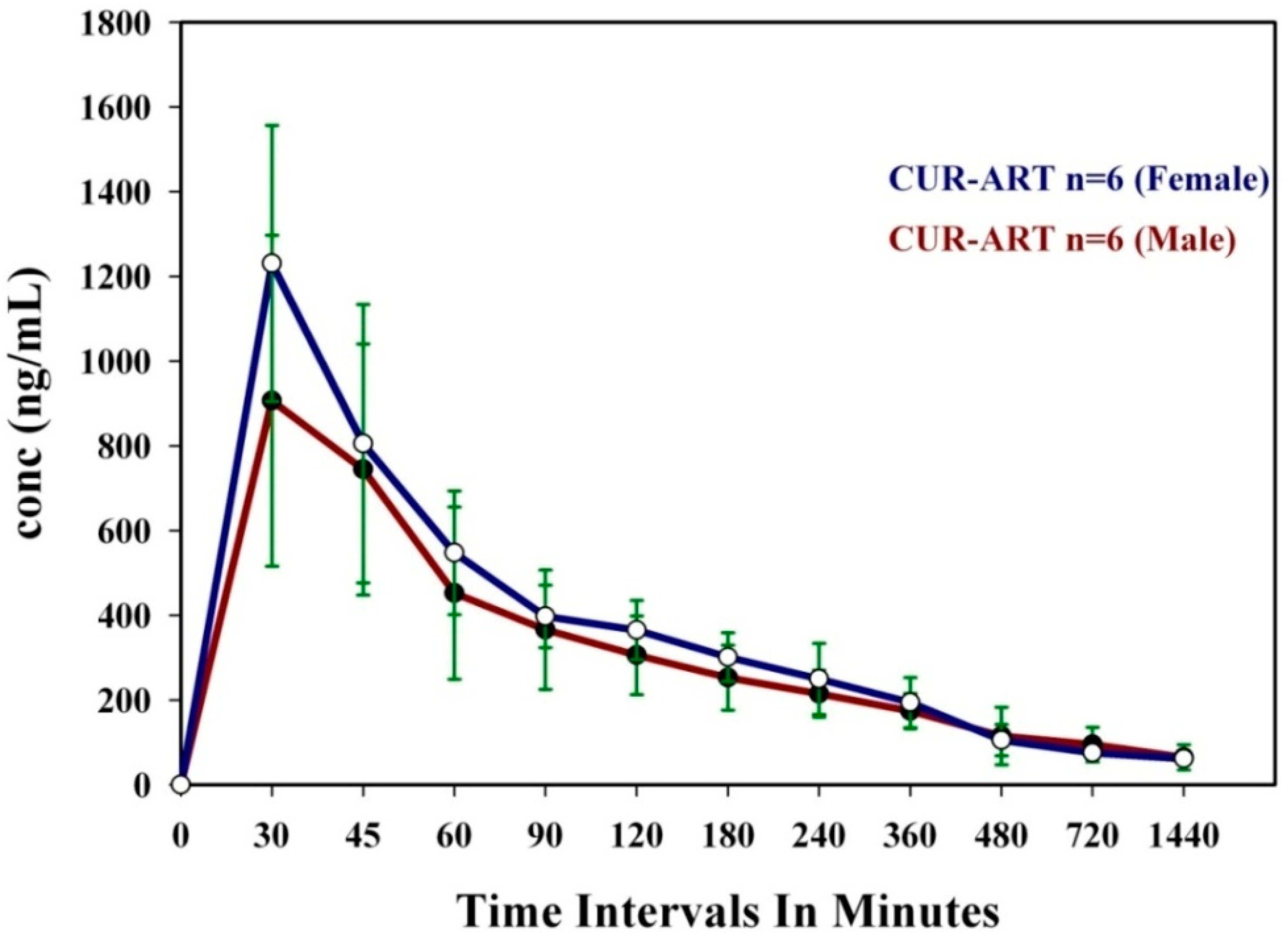


| Parameters | CUR-PYR (F) | CUR-ART (F) | CUR-PYR (M) | CUR-ART (M) | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | S.D. | Mean | S.D. | Mean | S.D. | Mean | S.D. | |
| Tmax (min) | 45.00 | 15.60 | 30.00 | 0.00 | 30.00 | 8.22 | 30.00 | 0.00 |
| Cmax (µg/mL) | 0.43 | 0.15 | 1.23 | 0.33 | 0.53 | 0.19 | 0.90 | 0.39 |
| T1/2 (h) | 7.70 | 3.27 | 6.70 | 0.68 | 6.00 | 2.78 | 7.00 | 1.66 |
| AUC(0–24) (µg·h/mL) | 1.91 | 0.25 | 3.69 | 0.69 | 2.18 | 0.59 | 3.45 | 0.83 |
| AUC(0–∞) (µg·h/mL) | 22.20 | 6.70 | 36.40 | 6.00 | 19.70 | 5.72 | 35.40 | 5.64 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mannava, M.K.C.; Suresh, K.; Kumar Bommaka, M.; Bhavani Konga, D.; Nangia, A. Curcumin-Artemisinin Coamorphous Solid: Xenograft Model Preclinical Study. Pharmaceutics 2018, 10, 7. https://doi.org/10.3390/pharmaceutics10010007
Mannava MKC, Suresh K, Kumar Bommaka M, Bhavani Konga D, Nangia A. Curcumin-Artemisinin Coamorphous Solid: Xenograft Model Preclinical Study. Pharmaceutics. 2018; 10(1):7. https://doi.org/10.3390/pharmaceutics10010007
Chicago/Turabian StyleMannava, M. K. Chaitanya, Kuthuru Suresh, Manish Kumar Bommaka, Durga Bhavani Konga, and Ashwini Nangia. 2018. "Curcumin-Artemisinin Coamorphous Solid: Xenograft Model Preclinical Study" Pharmaceutics 10, no. 1: 7. https://doi.org/10.3390/pharmaceutics10010007
APA StyleMannava, M. K. C., Suresh, K., Kumar Bommaka, M., Bhavani Konga, D., & Nangia, A. (2018). Curcumin-Artemisinin Coamorphous Solid: Xenograft Model Preclinical Study. Pharmaceutics, 10(1), 7. https://doi.org/10.3390/pharmaceutics10010007