Capsid-Targeted Viral Inactivation: A Novel Tactic for Inhibiting Replication in Viral Infections
Abstract
:1. Introduction
2. Mechanisms of CTVI
3. Application of CTVI for Different Viral Classes
3.1. Retroviruses
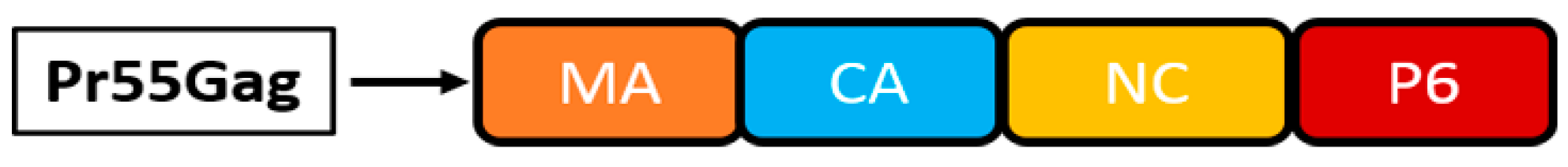
3.1.1. Murine Leukaemia Virus
3.1.2. Classical Swine Fever Virus
3.1.3. Human Immunodeficiency Virus-1
3.2. Flavivirus
3.2.1. Dengue 2 Virus
3.2.2. Japanese Encephalitis Virus
3.3. Hepadnaviruses
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Merchant, V.A. Intracellular immunization against viruses. J. Mich. Dent. Assoc. 2011, 93, 83–88. [Google Scholar]
- Wang, Z.H.; Qiu, H.; Chen, W. Capsid-targeted Viral Inactivation: A new antiviral strategy. China Biotechnol. 2007, 27, 88–92. [Google Scholar]
- Natsoulis, G.; Boeke, J.D. New antiviral strategy using capsid-nuclease fusion proteins. Nature 1991, 352, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Schumann, G.; Qin, L.; Rein, A.; Natsoulis, G.; Boeke, J.D. Therapeutic effect of Gag-nuclease fusion protein on retrovirus-infected cell cultures. J. Virol. 1996, 70, 4329–4337. [Google Scholar] [PubMed]
- Takashi, M.; Tomoaki, M.; Yasuhiro, A.; Takashi, S. Gene- and protein-delivered zinc finger-staphylococcal nuclease hybrid for inhibition of DNA replication of human papillomavirus. PLoS ONE 2013, 8, e56633. [Google Scholar] [CrossRef]
- Okui, N.; Kitamura, Y.; Kobayashi, N.; Sakuma, R.; Ishikawa, T.; Kitamura, T. Virion-targeted Viral Inactivation: New therapy against viral infection. Mol. Urol. 2001, 5, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Natsoulis, G.; Seshaiah, P.; Federspiel, M.J.; Rein, A.; Hughes, S.H.; Boeke, J.D. Targeting of a nuclease to murine leukemia virus capsids inhibits viral multiplication. Proc. Natl. Acad. Sci. USA 1995, 92, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Vanbrocklin, M.; Ferris, A.L.; Hughes, S.H.; Federspiel, M.J. Expression of a murine leukemia virus Gag-Escherichia coli RNase HI fusion polyprotein significantly inhibits virus spread. J. Virol. 1997, 71, 3312–3318. [Google Scholar] [PubMed]
- Wang, Y.-F.; Wang, Z.-H.; Li, Y.; Zhang, X.-J.; Sun, Y.; Li, M.; Qiu, H.-J. In vitro inhibition of the replication of classical swine fever virus by capsid-targeted virus inactivation. Antivir. Res. 2010, 85, 422–424. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Liu, K.; Wei, J.C.; Mao, X.; Chen, P.Y. Inhibition of replication of classical swine fever virus in a stable cell line by the viral capsid and Staphylococcus aureus nuclease fusion protein. J. Virol. Methods 2010, 167, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-M.; He, D.; Zhou, B.; Pang, R.; Liu, K.; Zhao, J.; Chen, P. In vitro inhibition of vesicular stomatitis virus replication by purified porcine Mx1 protein fused to HIV-1 Tat protein transduction domain (PTD). Antivir. Res. 2013, 99, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Pang, R.; He, D.N.; Zhou, B.; Liu, K.; Zhao, J.; Zhang, X.M.; Chen, P.Y. In vitro inhibition of Japanese encephalitis virus replication by capsid-targeted virus inactivation. Antivir. Res. 2013, 97, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, Y.H.; Xue, C.F.; Ding, J.; Gong, W.D.; Zhao, Y.; Huang, Y.X. Targeted ribonuclease can inhibit replication of hepatitis B virus. World J. Gastroenterol. 2003, 9, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Beterams, G.; Nassal, M. Significant interference with hepatitis B virus replication by a core-nuclease fusion protein. J. Biol. Chem. 2001, 276, 8875–8883. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.F.; Qin, E.D. Capsid-targeted viral inactivation can destroy dengue 2 virus from within in vitro. Arch. Virol. 2006, 151, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.F.; Qin, E.D. Development of cell lines stably expressing staphylococcal nuclease fused to dengue 2 virus capsid protein for CTVI. Acta Biochim. Biophys. Sin. 2004, 36, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.F.; Qin, E.; Yu, M.; Chen, S.-P.; Jiang, T.; Deng, Y.-Q.; Duan, H.; Zhao, H. Therapeutic effects of dengue 2 virus capsid protein and staphylococcal nuclease fusion protein on dengue-infected cell cultures. Arch. Virol. 2005, 150, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Mcnally, M.M.; Wahlin, K.J.; Canto-Soler, M.V. Endogenous expression of ASLV viral proteins in specific pathogen free chicken embryos: Relevance for the developmental biology research field. BMC Dev. Biol. 2010, 10, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Liu, K.; Chen, P.Y. Establishment and identification of classical swine fever virus(CSFV) capsid targeted nuclease expression system. Chin. J. Virol. 2008, 24, 451–455. [Google Scholar]
- Okui, N.; Kobayashi, N.; Kitamura, Y. Production of uninfectious human immunodeficiency virus type 1 containing viral protein R fused to a single-chain antibody against viral integrase. J. Virol. 1998, 72, 124–129. [Google Scholar]
- Kobinger, G.P.; Borsetti, A.; Nie, Z.; Mercier, J.; Daniel, N.; GöTtlinger, H.G.; Cohen, A. Virion-targeted viral inactivation of human immunodeficiency virus type 1 by using Vpr fusion proteins. J. Virol. 1998, 72, 5441–5448. [Google Scholar] [PubMed]
- Wu, X.; Liu, H.; Xiao, H.; Kim, J.; Seshaiah, P.; Natsoulis, G.; Boeke, J.D.; Hahn, B.H.; Kappes, J.C. Targeting foreign proteins to human immunodeficiency virus particles via fusion with Vpr and Vpx. J. Virol. 1995, 69, 3389–3398. [Google Scholar] [PubMed]
- Yu, Z.; Chen, P.; Cao, R.; Gu, J. Mutation of putative N-Linked glycosylation sites in Japanese encephalitis Virus Premembrane and Envelope proteins enhances humoral immunity in BALB/C mice after DNA vaccination. Virol. J. 2011, 8, 1–7. [Google Scholar]
- Vanbrocklin, M.; Federspiel, M.J. Capsid-targeted Viral Inactivation can eliminate the production of infectious murine leukemia virus in vitro. Virology 2000, 267, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Beterams, G.; Böttcher, B.; Nassal, M. Packaging of up to 240 subunits of a 17 kDa nuclease into the interior of recombinant hepatitis B virus capsids. FEBS Lett. 2000, 481, 169–176. [Google Scholar] [CrossRef]
- Wang, S.; Tate, M.W.; Gruner, S.M. Protein crowding impedes pressure-induced unfolding of staphylococcal nuclease. Biochim. Biophys. Acta 2012, 1820, 957–961. [Google Scholar] [CrossRef] [PubMed]
- Emilio, G.B.; Moschou, P.N.; Smertenko, A.P.; Bozhkov, P.V. Tudor staphylococcal nuclease links formation of stress granules and processing bodies with mRNA catabolism in Arabidopsis. Plant Cell 2015, 27, 926–943. [Google Scholar]
- Okui, N.; Sakuma, R.; Kobayashi, N.; Yoshikura, H.; Kitamura, T.; Chiba, J.; Kitamura, Y. Packageable antiviral therapeutics against human immunodeficiency virus type 1: Virion-targeted virus inactivation by incorporation of a single-chain antibody against viral integrase into progeny virions. Hum. Gene Ther. 2000, 11, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Hammond, S.M.; Bernstein, E.; Beach, D.; Hannon, G.J. An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature 2000, 404, 293–296. [Google Scholar] [PubMed]
- Li, S.M.; Bai, F.L.; Xu, W.J.; Yang, Y.B.; An, Y.; Li, T.H.; Yu, Y.H.; Li, D.S.; Wang, W.F. Removing residual DNA from Vero-cell culture-derived human rabies vaccine by using nuclease. Biol. J. Int. Assoc. Biol. Stand. 2014, 42, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Jariwala, N.; Rajasekaran, D.; Srivastava, J.; Gredler, R.; Akiel, M.A.; Robertson, C.L.; Emdad, L.; Fisher, P.B.; Sarkar, D. Role of the staphylococcal nuclease and tudor domain containing 1 in oncogenesis (Review). Int. J. Oncol. 2015, 46, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.N.; Kang, M.S.; Chen, H.C.; Shi, X.M.; Rui, Z.; Chen, J.; Du, Y.W. The staphylococcal nuclease prevents biofilm formation in Staphylococcus aureus and other biofilm-forming bacteria. Sci. China Life Sci. 2011, 54, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Meng, J.; Shi, C.; Hervin, K.; Fratamico, P.M.; Shi, X. Characterization and comparative analysis of a second thermonuclease from Staphylococcus aureus. Psychiatry Res. 2013, 168, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Tsong, T.Y.; Hsu, Y.-H.; Marszalek, P.E. Inhibitor binding increases the mechanical stability of staphylococcal nuclease. Biophys. J. 2011, 100, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- Spencer, D.; Bertrand, G.-M.E.; Stites, W.E. The pH dependence of staphylococcal nuclease stability is incompatible with a three-state denaturation model. Biophys. Chem. 2013, 180–181, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Sagheddu, C.; Boccaccio, A.; Dibattista, M.; Montani, G.; Tirindelli, R.; Menini, A. Calcium concentration jumps reveal dynamic ion selectivity of calcium-activated chloride currents in mouse olfactory sensory neurons and TMEM16b-transfected HEK 293T cells. J. Physiol. 2010, 588, 4189–4204. [Google Scholar] [CrossRef] [PubMed]
- Nizamutdinova, E.K.; Shirshikova, T.V.; Mardanova, A.M.; Sharipova, M.R.; Bogomol’Naya, L.M. Effect of mutations in extracellular nuclease on the characteristics of the pigmented and nonpigmented Serratia marcescens strains. Microbiology 2016, 85, 42–46. [Google Scholar] [CrossRef]
- Trifonova, E.A.; Saveleva, A.V.; Romanova, A.V.; Filipenko, E.A.; Sapotsky, M.V.; Malinovsky, V.I.; Kochetov, A.V.; Shumny, V.K. Transgenic expression of Serratia marcescens native and mutant nucleases modulates tobacco mosaic virus resistance in Nicotiana tabacum L. Genetika 2015, 51, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, Y.; Ishikawa, T.; Okui, N.; Kobayashi, N.; Kanda, T.; Shimada, T.; Miyake, K.; Yoshiike, K. Inhibition of replication of HIV-1 at both early and late stages of the viral life cycle by single-chain antibody against viral integrase. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1999, 20, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Elis, E.; Ehrlich, M.; Prizan-Ravid, A.; Laham-Karam, N.; Bacharach, E. p12 tethers the murine leukemia virus pre-integration complex to mitotic chromosomes. PLoS Pathog. 2012, 8, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.H.; Marc, L.; Marc, T.; Alamgir, A.S.M. Antigenic subclasses of polytropic murine leukemia virus (MLV) isolates reflect three distinct groups of endogenous polytropic MLV-related sequences in NFS/N mice. J. Virol. 2003, 77, 10327–10338. [Google Scholar] [CrossRef]
- Schumann, G.; Cannon, K.; Ma, W.P.; Crouch, R.J.; Boeke, J.D. Antiretroviral effect of a gag-RNase HI fusion gene. Gene Ther. 1997, 4, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Chae-Ryun, Y.; Naomi, R. Mutations affecting the MA portion of the v-Abl protein reveal a conserved role of Gag in Abelson murine leukemia virus (MLV) and Moloney MLV. J. Virol. 2008, 82, 5307–5315. [Google Scholar]
- Rekha, K.; Sivasubramanian, C.; Chung, I.M.; Thiruvengadam, M. Growth and replication of infectious bursal disease virus in the DF-1 cell line and chicken embryo fibroblasts. BioMed Res. Int. 2014, 2014, 494835–494835. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qi, X.; Gao, H.; Gao, Y.; Lin, H.; Song, X.; Pei, L.; Wang, X. Comparative study of the replication of infectious bursal disease virus in DF-1 cell line and chicken embryo fibroblasts evaluated by a new real-time RT-PCR. J. Virol. Methods 2009, 157, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Maas, R.; van Zoelen, D.; Oei, H.; Claassen, I. Replacement of primary chicken embryonic fibroblasts (CEF) by the DF-1 cell line for detection of avian leucosis viruses. Biologicals 2006, 34, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Lassiter, K.; Dridi, S.; Piekarski, A.; Greene, E.; Hargis, B.; Kong, B.W.; Bottje, W. Bioenergetics in chicken embryo fibroblast cells: Evidence of lower proton leak in spontaneously immortalized chicken embryo fibroblasts compared to young and senescent primary chicken embryo fibroblast cells. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2014, 175, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.W.; Lee, J.Y.; Bottje, W.G.; Lassiter, K.; Lee, J.; Foster, D.N. Genome-wide differential gene expression in immortalized DF-1 chicken embryo fibroblast cell line. BMC Genom. 2011, 12, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Minias, A.E.; Brzostek, A.M.; Koryckamachala, M.; Dziadek, B.; Minias, P.; Rajagopalan, M.; Madiraju, M.; Dziadek, J. RNase HI Is Essential for Survival of Mycobacterium smegmatis. PLoS ONE 2015, 10, e0126260. [Google Scholar] [CrossRef] [PubMed]
- Kita, M.; Nakamura, H.; Yu, T. Density functional study of the phosphate diester hydrolysis of RNA in RNA/DNA hybrid by RNase HI. Mol. Phys. 2014, 112, 355–364. [Google Scholar] [CrossRef]
- Loukachevitch, L.V.; Egli, M. Crystallization and preliminary X-ray analysis of Escherichia coli RNase HI–dsRNA complexes. Acta Crystallogr. 2007, 63, 84–88. [Google Scholar]
- Mccarthy, F.M.; Gravel, J.L.; Corney, B. Genetic analysis of bovine viral diarrhoea viruses from Australia. Vet. Microbiol. 2005, 106, 1–6. [Google Scholar]
- Vilcek, S.; Durkovic, B.; Kolesarova, M.; Paton, D.J. Genetic diversity of BVDV: Consequences for classification and molecular epidemiology. Prev. Vet. Med. 2005, 72, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Ridpath, J.F.; Neill, J.D.; Vilcek, S.; Dubovi, E.J.; Carman, S. Multiple outbreaks of severe acute BVDV in North America occurring between 1993 and 1995 linked to the same BVDV2 strain. Vet. Microbiol. 2006, 114, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Mao, L.; Zhao, Y.; Sun, Y.; He, K.; Jiang, J. Detection of border disease virus (BDV) in goat herds suffering diarrhea in eastern China. Virol. J. 2013, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rosamilia, A.; Grattarola, C.; Caruso, C.; Peletto, S.; Gobbi, E.; Tarello, V.; Caroggio, P.; Dondo, A.; Masoero, L.; Acutis, P.L. Detection of border disease virus (BDV) genotype 3 in Italian goat herds. Vet. J. 2014, 199, 446–450. [Google Scholar] [CrossRef] [PubMed]
- KöNig, M.; Lengsfeld, T.; Pauly, T.; Stark, R.; Thiel, H.J. Classical swine fever virus: Independent induction of protective immunity by two structural glycoproteins. J. Virol. 1995, 69, 6479–6486. [Google Scholar] [PubMed]
- Rümenapf, T.; Meyers, G.; Thiel, H.J. Classical swine fever virus: Recovery of infectious viruses from cDNA constructs and generation of recombinant cytopathogenic defective interfering particles. J. Virol. 1996, 70, 1588–1595. [Google Scholar]
- He, D.N.; Zhang, X.M.; Liu, K.; Pang, R.; Zhao, J.; Zhou, B.; Chen, P.Y. In vitro inhibition of the replication of classical swine fever virus by porcine Mx1 protein. Antivir. Res. 2014, 104, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Wang, Z.Y.; Zhang, X.J.; Sun, Y.; Li, M.; Qiu, H.J. In vitro inhibition of the replication of classical swine fever virus by capsid-targeted virus inactivation. Antivir. Res. 2010, 85, 422–424. [Google Scholar] [CrossRef] [PubMed]
- Endsley, M.A.; Somasunderam, A.D.; Li, G.; Oezguen, N.; Thiviyanathan, V.; Murray, J.L.; Rubin, D.H.; Hodge, T.W.; O’Brien, W.A.; Lewis, B. Nuclear trafficking of the HIV-1 pre-integration complex depends on the ADAM10 intracellular domain. Virology 2014, 454–455, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, Z.; Aiken, C. HIV-1 uncoating: Connection to nuclear entry and regulation by host proteins. Virology 2014, 454–455, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Kotsopoulou, E.; Kim, V.N.; Kingsman, A.J.; Kingsman, S.M.; Mitrophanous, K.A. A Rev-independent human immunodeficiency virus type 1 (HIV-1)-based vector that exploits a codon-optimized HIV-1 gag-pol gene. J. Virol. 2000, 74, 4839–4852. [Google Scholar] [CrossRef] [PubMed]
- Esposito, D.; Craigie, R. HIV Integrase Structure and Function. Adv. Virus Res. 1999, 52, 319–324. [Google Scholar] [PubMed]
- Grigoriev, F.V.; Golovacheva, A.Y.; Romanov, A.N.; Kondakova, O.A.; Sulimov, A.V.; Smolov, M.A.; Gottikh, M.B.; Sulimov, V.B.; Bogolyubov, A.A.; Kuznetsov, Y.V. Stability of HIV1 integrase–ligand complexes: The role of coordinating bonds. Struct. Chem. 2012, 23, 185–195. [Google Scholar] [CrossRef]
- Supachai, R.N.; Punnee, P.; Sorachai, N.; Jaranit, K.; Joseph, C.; Robert, P.; Nakorn, P.; Chawetsan, N.; Mark, D.S.; Elizabeth, A. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 2009, 361, 2209–2220. [Google Scholar]
- Brad, L.; Stephen, W.; Lauren, H.; Lindsey, G.; Maria Cecilia, H.; Cristillo, A.D. Nedd4-mediated increase in HIV-1 Gag and Env proteins and immunity following DNA-vaccination of BALB/c mice. PLoS ONE 2014, 9, e91267. [Google Scholar] [CrossRef]
- Alberto, C.; Simone, P.; Nicolas, R.; Stefano, S.; Rigmor, T.; Qiang, P.H.M.; Bo, H.; Anna, N.; Francesca, C. Relation of activation-induced deaminase (AID) expression with antibody response to A(H1N1)pdm09 vaccination in HIV-1 infected patients. Vaccine 2013, 31, 2231–2237. [Google Scholar]
- Haynes, B.F.; Gilbert, P.B.; Mcelrath, M.J.; Zollapazner, S.; Tomaras, G.D.; Alam, S.M.; Evans, D.T.; Montefiori, D.C.; Karnasuta, C.; Sutthent, R. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N. Engl. J. Med. 2012, 366, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Xiaohua, T.; Dan, S.; Wei, X. Prevention of HIV-1 infection with antiretroviral therapy. N. Engl. J. Med. 2011, 365, 486–487. [Google Scholar]
- Hazuda, D.J. HIV integrase as a target for antiretroviral therapy. Curr. Opin. HIV AIDS 2012, 7, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Ramcharan, J.; Colleluori, D.M.; Merkel, G.; Andrake, M.D.; Skalka, A.M. Mode of inhibition of HIV-1 Integrase by a C-terminal domain-specific monoclonal antibody. Retrovirology 2006, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Levy-Mintz, P.; Duan, L.; Zhang, H.; Hu, B.; Dornadula, G.; Zhu, M.; Kulkosky, J.; Bizub-Bender, D.; Skalka, A.M.; Pomerantz, R.J. Intracellular expression of single-chain variable fragments to inhibit early stages of the viral life cycle by targeting human immunodeficiency virus type 1 integrase. J. Virol. 1996, 70, 8821–8832. [Google Scholar] [PubMed]
- Quashie, P.K.; Sloan, R.D.; Wainberg, M.A. Novel therapeutic strategies targeting HIV integrase. BMC Med. 2012, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vasu, N.; Chi, G. HIV integrase inhibitors as therapeutic agents in AIDS. Rev. Med. Virol. 2007, 17, 277–295. [Google Scholar]
- Steinrigl, A.; Nosek, D.; Ertl, R.; Günzburg, W.H.; Salmons, B.; Klein, D. Mutations in the catalytic core or the C-terminus of murine leukemia virus (MLV) integrase disrupt virion infectivity and exert diverse effects on reverse transcription. Virology 2007, 362, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Mini, B.; Yant, S.R.; Luong, T.; Christopher, O.S.; Bam, R.A.; Angela, T.; Anita, N.M.; Stray, K.M.; Roman, S.; Tomas, C. Non-catalytic site HIV-1 integrase inhibitors disrupt core maturation and induce a reverse transcription block in target cells. PLoS ONE 2013, 8, e74163. [Google Scholar]
- Boso, G.; Tasaki, T.; Yong, T.K.; Somia, N.V. The N-end rule and retroviral infection: No effect on integrase. Rev. Sci. Instrum. 2012, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ao, Z.; Fowke, K.R.; Cohen, É.A.; Yao, X. Contribution of the C-terminal tri-lysine regions of human immunodeficiency virus type 1 integrase for efficient reverse transcription and viral DNA nuclear import. Retrovirology 2005, 2, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ruikang, L.; Juan, T.; Yongquan, L.; Rui, J.; Wei, Y.; Chen, L.; Yunqi, G.; Wentao, Q. HIV-1 Vpr activates both canonical and noncanonical NF-κB pathway by enhancing the phosphorylation of IKKα/β. Virology 2013, 439, 47–56. [Google Scholar]
- Serio, D.; Rizvi, T.A.; Cartas, M.; Kalyanaraman, V.S.; Weber, I.T.; Koprowaki, H.; Srinivasan, A. Development of a novel anti-HIV-1 agent from within: Effect of chimeric Vpr-containing protease cleavage site residues on virus replication. Proc. Natl. Acad. Sci. USA 1997, 94, 3346–3351. [Google Scholar] [CrossRef] [PubMed]
- Jain, B.; Chaturvedi, U.C.; Jain, A. Role of intracellular events in the pathogenesis of dengue: An overview. Microb. Pathog. 2014, 69, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Green, A.M.; Beatty, P.R.; Alexandros, H.; Eva, H. Innate immunity to dengue virus infection and subversion of antiviral responses. J. Mol. Biol. 2014, 426, 1148–1160. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.T.; Lixin, M.; Burgner, J.W.; Groesch, T.D.; Post, C.B.; Kuhn, R.J. Flavivirus capsid is a dimeric alpha-helical protein. J. Virol. 2003, 77, 7143–7149. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, R.J.; Wei, Z.; Rossmann, M.G.; Pletnev, S.V.; Jeroen, C.; Edith, L.; Jones, C.T.; Suchetana, M.; Chipman, P.R.; Strauss, E.G. Structure of dengue virus: Implications for flavivirus organization, maturation, and fusion. Cell 2007, 108, 717–725. [Google Scholar] [CrossRef]
- Qin, C.F. Capsid-targeted Viral Inactivation for dengue virus infection. Acta Microbiol. Sin. 2005, 45, 111–115. [Google Scholar]
- Lopez, C.; Gil, L.L.; Menendez, I.; Marcos, E.; Sanchez, J.; Valdes, I.; Falcon, V.; de la Rosa, M.; Marquez, G.; Guillen, G. In vitro assembly of nucleocapsid-like particles from purified recombinant capsid protein of dengue-2 virus. Arch. Virol. 2009, 154, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, M.; Wang, H.; Liang, G. Japanese encephalitis and Japanese encephalitis virus in mainland China. Rev. Med. Virol. 2012, 22, 301–322. [Google Scholar] [CrossRef] [PubMed]
- Le, F.G.; Porphyre, V.; Barbazan, P.; Gonzalez, J.P. Review of climate, landscape, and viral genetics as drivers of the Japanese encephalitis virus ecology. PLoS Negl. Trop. Dis. 2013, 7, e2208–e2208. [Google Scholar]
- Shen, T.; Ke, L.; Miao, D.; Cao, R.; Chen, P. Effective inhibition of Japanese encephalitis virus replication by shRNAs targeting various viral genes in vitro and in vivo. Virology 2014, 454–455, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Gromowski, G.D.; Cai-Yen, F.; Hanson, C.T.; Whitehead, S.S. Japanese encephalitis virus vaccine candidates generated by chimerization with dengue virus type 4. Vaccine 2014, 32, 3010–3018. [Google Scholar] [CrossRef] [PubMed]
- Ting, S.; Ke, L.; Denian, M.; Ruibing, C.; Bin, Z.; Puyan, C. Lentivirus-mediated RNA interference against Japanese encephalitis virus infection in vitro and in vivo. Antivir. Res. 2014, 108, 56–64. [Google Scholar]
- Yang, D.; Li, X.F.; Ye, Q.; Wang, H.J.; Deng, Y.Q.; Zhu, S.Y.; Zhang, Y.; Li, S.H.; Qin, C.F. Characterization of live-attenuated Japanese encephalitis vaccine virus SA14-14-2. Vaccine 2014, 32, 2675–2681. [Google Scholar] [CrossRef] [PubMed]
- Orito, E.; Ichida, T.; Sakugawa, H.; Sata, M.; Horiike, N.; Hino, K.; Okita, K.; Okanoue, T.; Iino, S.; Tanaka, E. Geographic distribution of hepatitis B virus (HBV) genotype in patients with chronic HBV infection in Japan. Hepatology 2001, 34, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Carolina, B.; Paola, F.; Caterina, V.; Barbara, A.; Paola, D.V.; Tiziana, G.; Diletta, L.; Alessandro, Z.; Albertina, C.; Gabriele, M. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J. Virol. 2007, 81, 21–30. [Google Scholar]
- Fattovich, G.; Farci, P.; Rugge, M.; Brollo, L.; Mandas, A.; Pontisso, P.; Giustina, G.; Lai, M.E.; Belussi, F.; Busatto, G. A randomized controlled trial of lymphoblastoid interferon-alpha in patients with chronic hepatitis B lacking HBeAg. Hepatology 1992, 15, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Guo, S.M.; Liu, B. A randomized controlled trial of kurorinone versus interferon-alpha2a treatment in patients with chronic hepatitis B. J. Viral Hepat. 2000, 7, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Kristie, B.; Abdullah, E.; Claudio, M.; Toni, C.; Patrick, A. Inactivation of hepatitis B virus replication in cultured cells and in vivo with engineered transcription activator-like effector nucleases. Mol. Ther. J. Am. Soc. Gene Ther. 2013, 21, 1889–1897. [Google Scholar]
- Choi, J.; Ryoo, J.; Oh, C.; Hwang, S.; Ahn, K. SAMHD1 specifically restricts retroviruses through its RNase activity. Retrovirology 2015, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Cheng, L.; Chen, J.; Jiang, X.; Zou, S. Construction of transgenic P_0 grass carp by capsid-targeted viral inactivation of reovirus. J. Fish. China 2014, 38, 1956–1963. [Google Scholar]
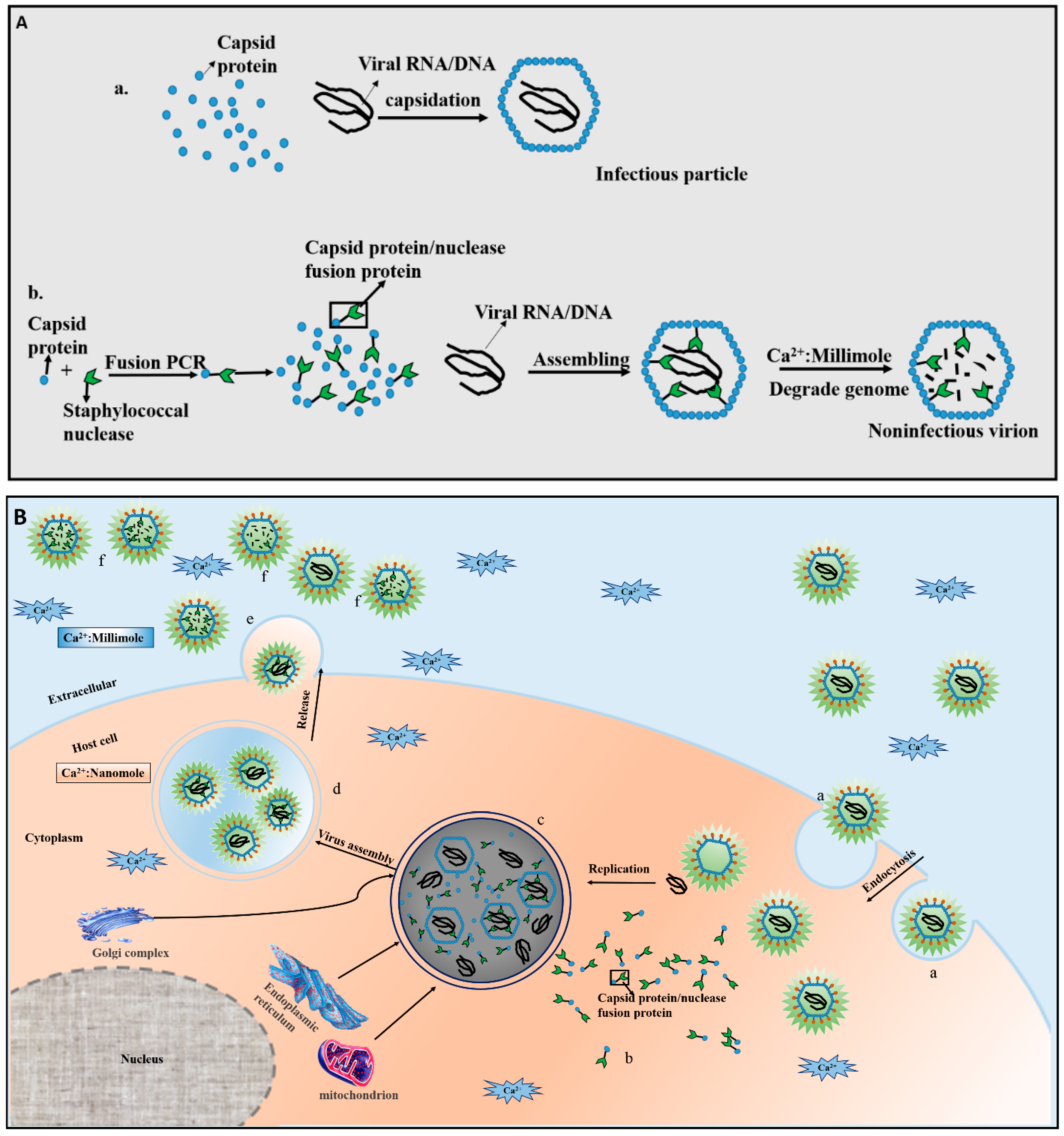
| Viral Type | Virus | Genome | Viral Protein | Foreign Molecule | Fusion Protein | Location of Enzyme | Plasmid Vector | Cell | Antibiotic | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Retroviruses | Mo-MLV | RNA | Gag/Gag-Pol | SN/RNase HI | Gag-SN/Gag-RNase HI | C-terminus | pGN1600 | RCASBP | [18] | |
| CSFV | RNA | Capsid | SN | Capsid-SN | C-terminus | pcDNA | PK-15 | G418 | [19] | |
| HIV-1 | RNA | Vpr | scAb | Vpr-scAb | C-terminus | pCXN2 | 293T | G418 | [20,21] | |
| Vpr | SN | Vpr-SN | C-terminus | pLR2P | HeLa | [22] | ||||
| Flavivirus | JEV | RNA | Capsid | SN | Capsid-SN | C-terminus | pcDNA3.1 | BHK-21 | [12,23] | |
| DENV2 | RNA | Capsid | SN | Capsid-SN | C-terminus | pcDNA6/V5-His | BHK-21 | blasticidin | [16,17,24] | |
| Circovirus | PCV2 | DNA | Capsid | SN | Capsid-SN | C/N-terminus | pIRESneo | PK15 | G418 | |
| Hepadnaviruses | HBV | DNA | Capsid | Ribonuclease | p/TN | C-terminus | pcDNA3.1 (−) | HepG2.2.15 | [13] | |
| Capsid | SN | Capsid-SN | C-terminus | pcDNA6/Myc-His | Huh7 | [14,25] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Jia, R.; Zhou, J.; Wang, M.; Yin, Z.; Cheng, A. Capsid-Targeted Viral Inactivation: A Novel Tactic for Inhibiting Replication in Viral Infections. Viruses 2016, 8, 258. https://doi.org/10.3390/v8090258
Zhang X, Jia R, Zhou J, Wang M, Yin Z, Cheng A. Capsid-Targeted Viral Inactivation: A Novel Tactic for Inhibiting Replication in Viral Infections. Viruses. 2016; 8(9):258. https://doi.org/10.3390/v8090258
Chicago/Turabian StyleZhang, Xingcui, Renyong Jia, Jiakun Zhou, Mingshu Wang, Zhongqiong Yin, and Anchun Cheng. 2016. "Capsid-Targeted Viral Inactivation: A Novel Tactic for Inhibiting Replication in Viral Infections" Viruses 8, no. 9: 258. https://doi.org/10.3390/v8090258
APA StyleZhang, X., Jia, R., Zhou, J., Wang, M., Yin, Z., & Cheng, A. (2016). Capsid-Targeted Viral Inactivation: A Novel Tactic for Inhibiting Replication in Viral Infections. Viruses, 8(9), 258. https://doi.org/10.3390/v8090258






