Antiviral Effects of Black Raspberry (Rubus coreanus) Seed and Its Gallic Acid against Influenza Virus Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Viruses
2.2. Preparation of RCSF1
2.3. Cytotoxicity
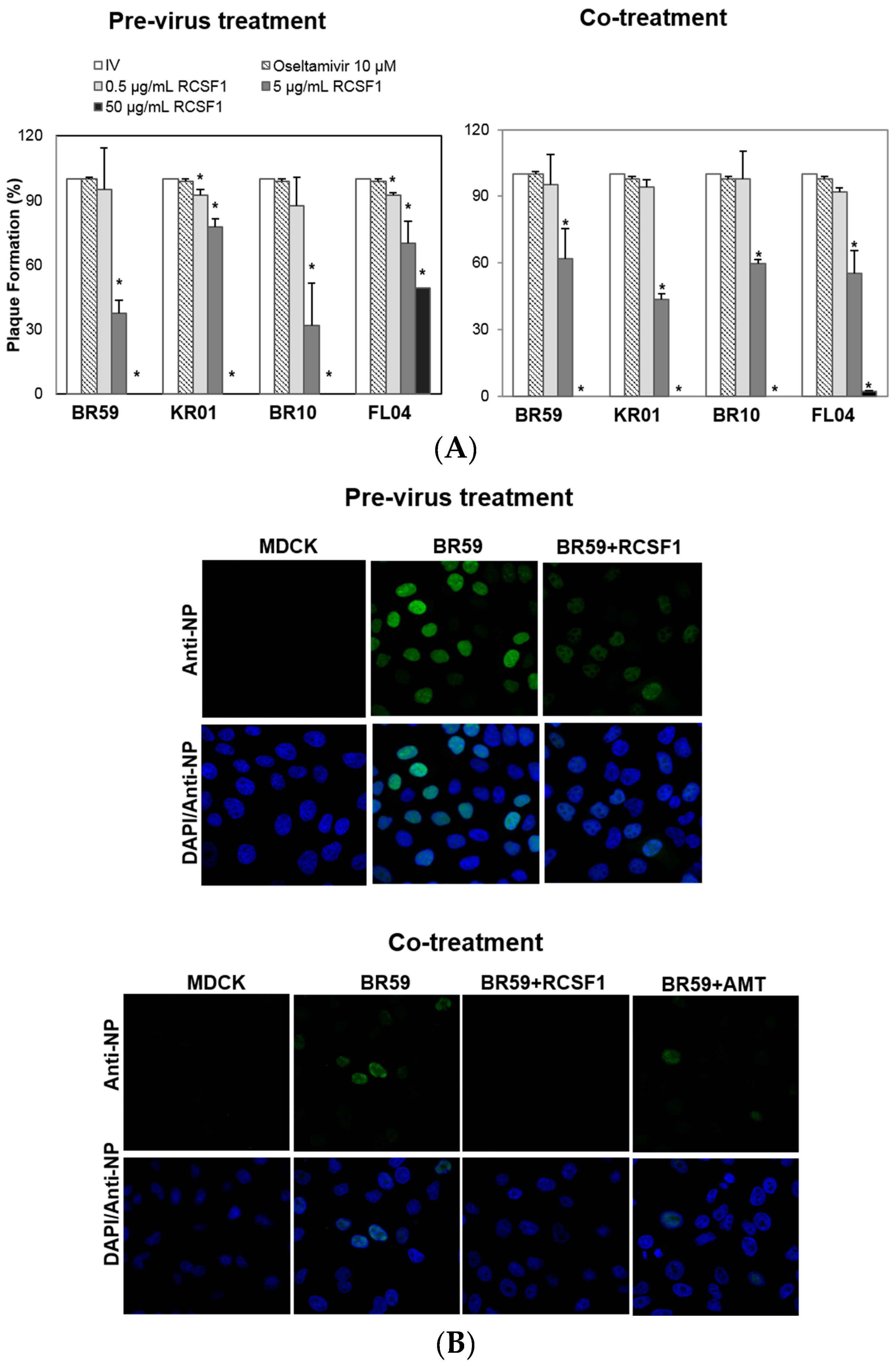
2.4. Time-of-Addition Plaque Reduction Assay
2.5. Confocal Microscopy
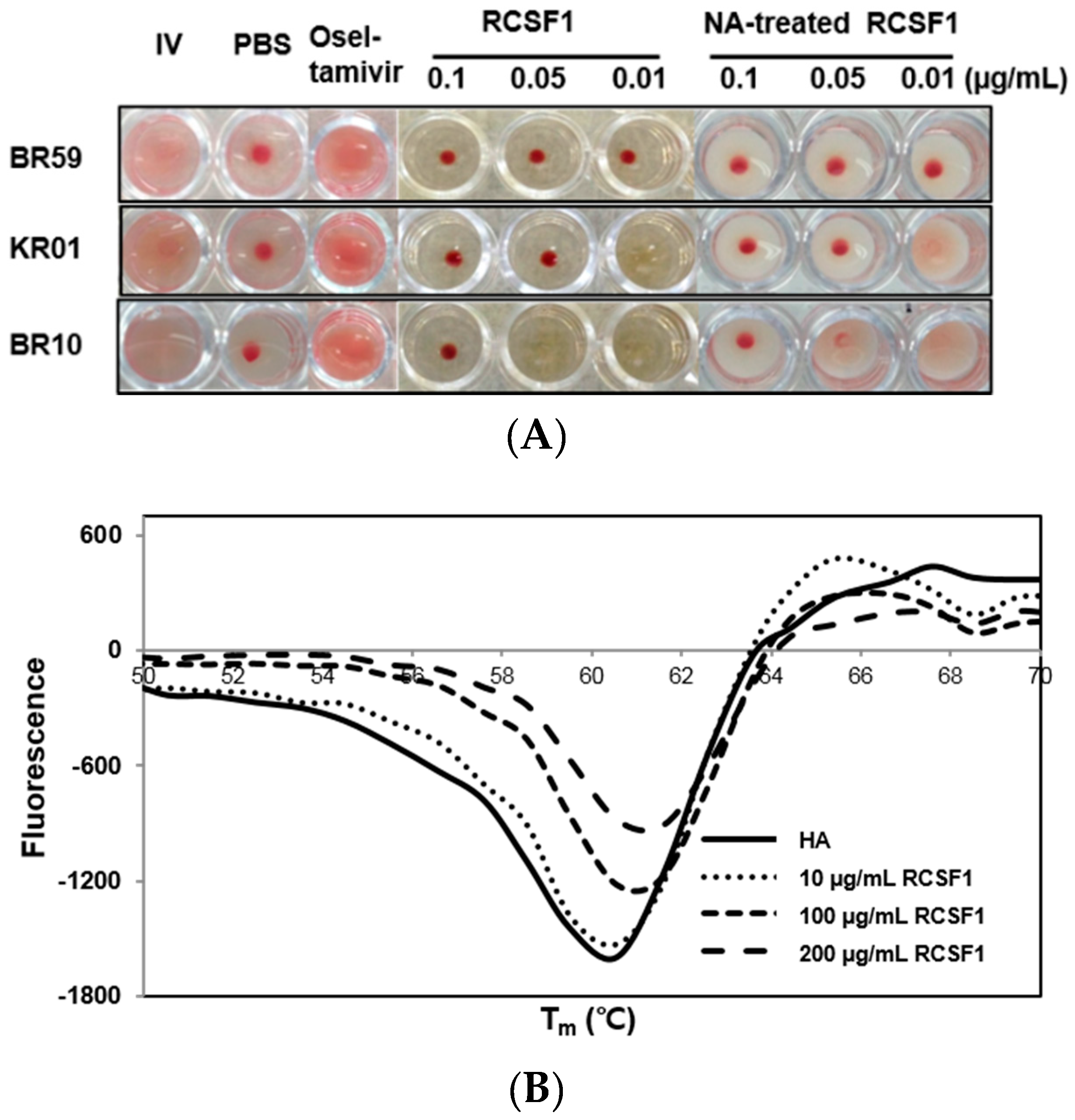
2.6. Hemagglutination Inhibition Assay
2.7. Differential Scanning Fluorometry and Fusion Inhibition Assay
2.8. Attachment and Penetration Assays
2.9. NA Treatment on RCSF1
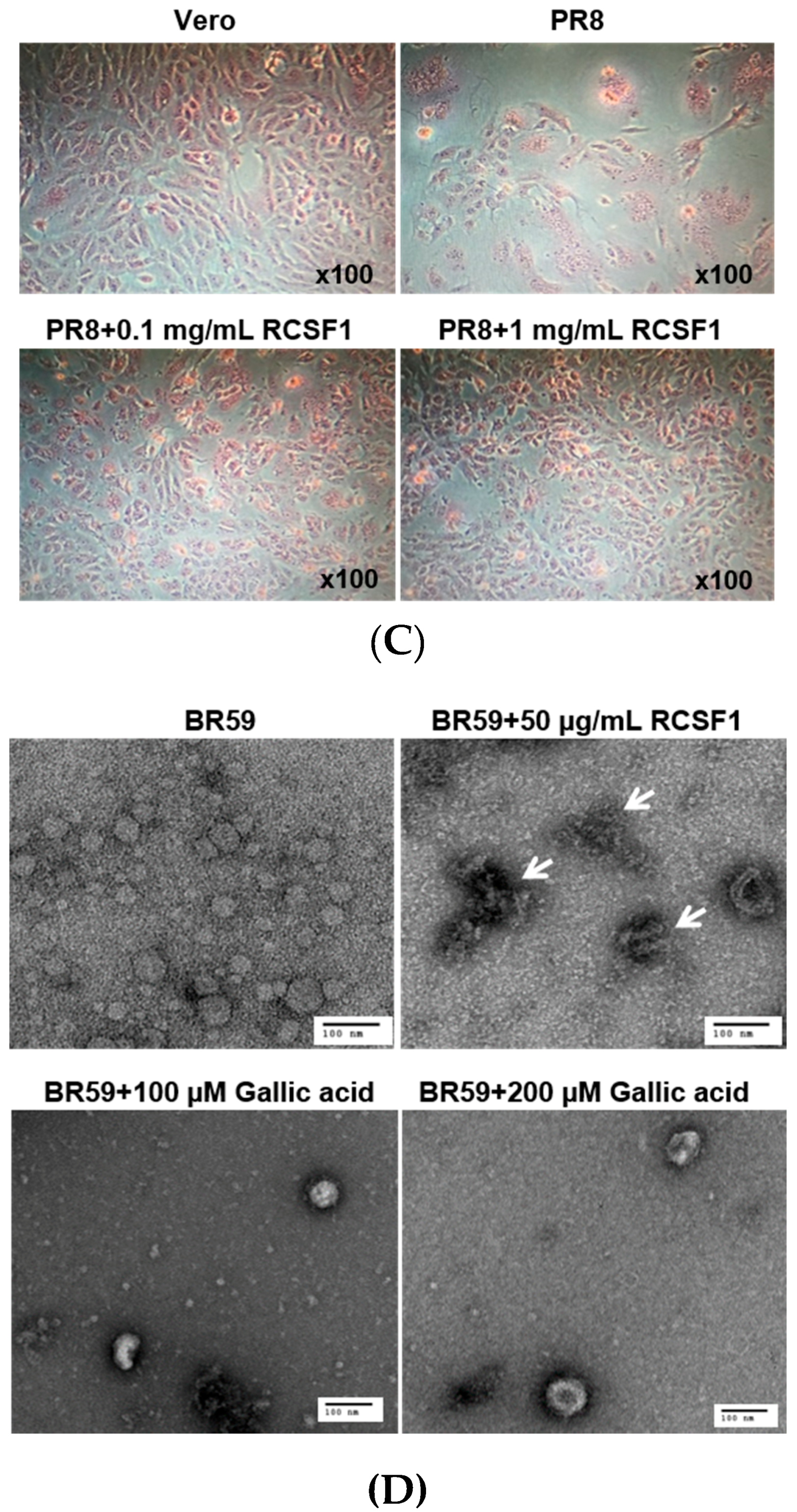
2.10. Transmission Electron Microscopy
2.11. Identification of Polyphenols by LC/MS/MS
2.12. In Vivo Mouse Experiments
2.13. Statistical Analysis
3. Results
3.1. RCSF1 Inhibits Early Stages of Influenza Viral Infection
3.2. RCSF1 Inhibits Hemagglutination and Fusion and Disrupts Virus Particles
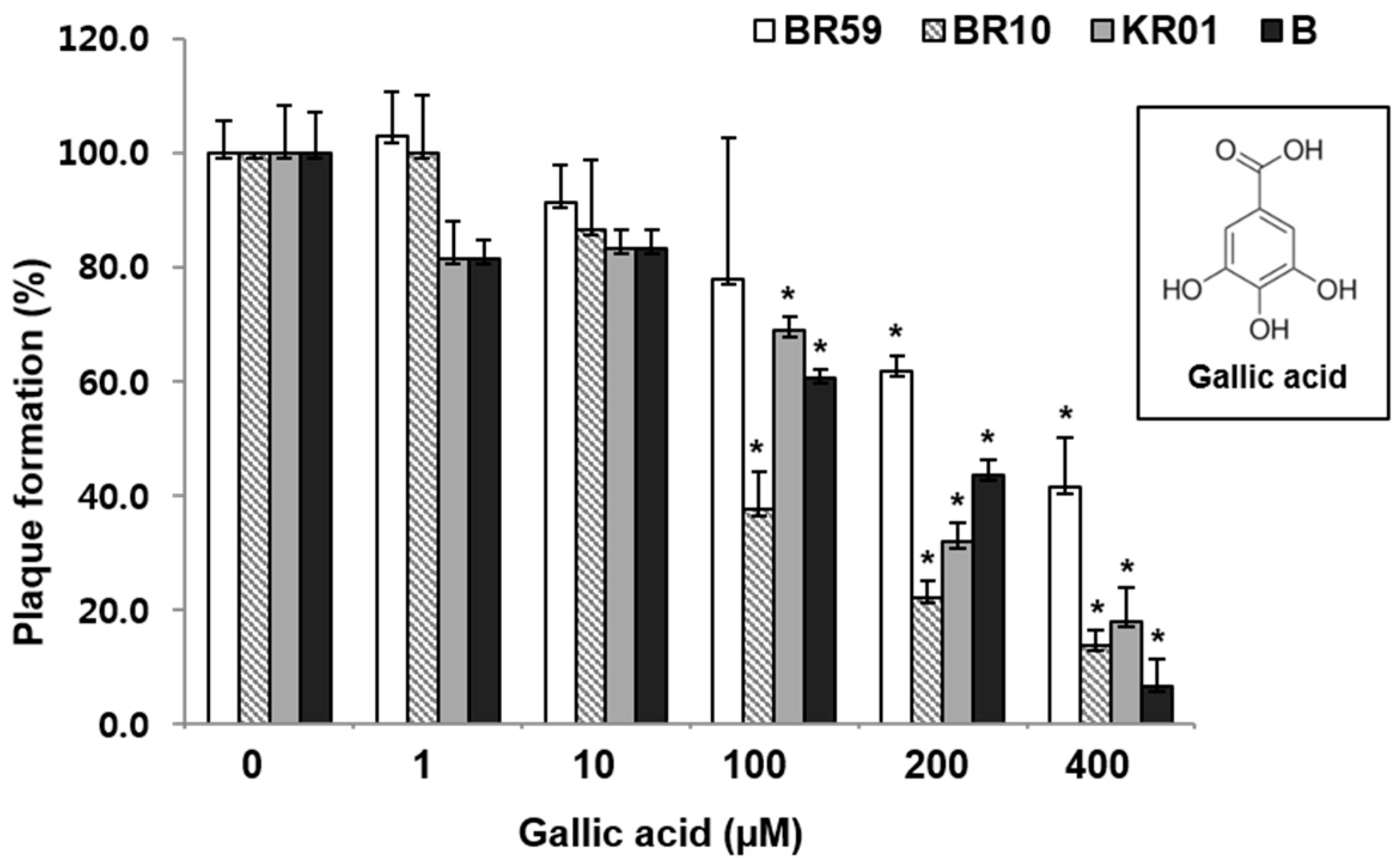
3.3. Polyphenols of RCSF1, GA, Have Anti-Influenza Activities against Various Strains
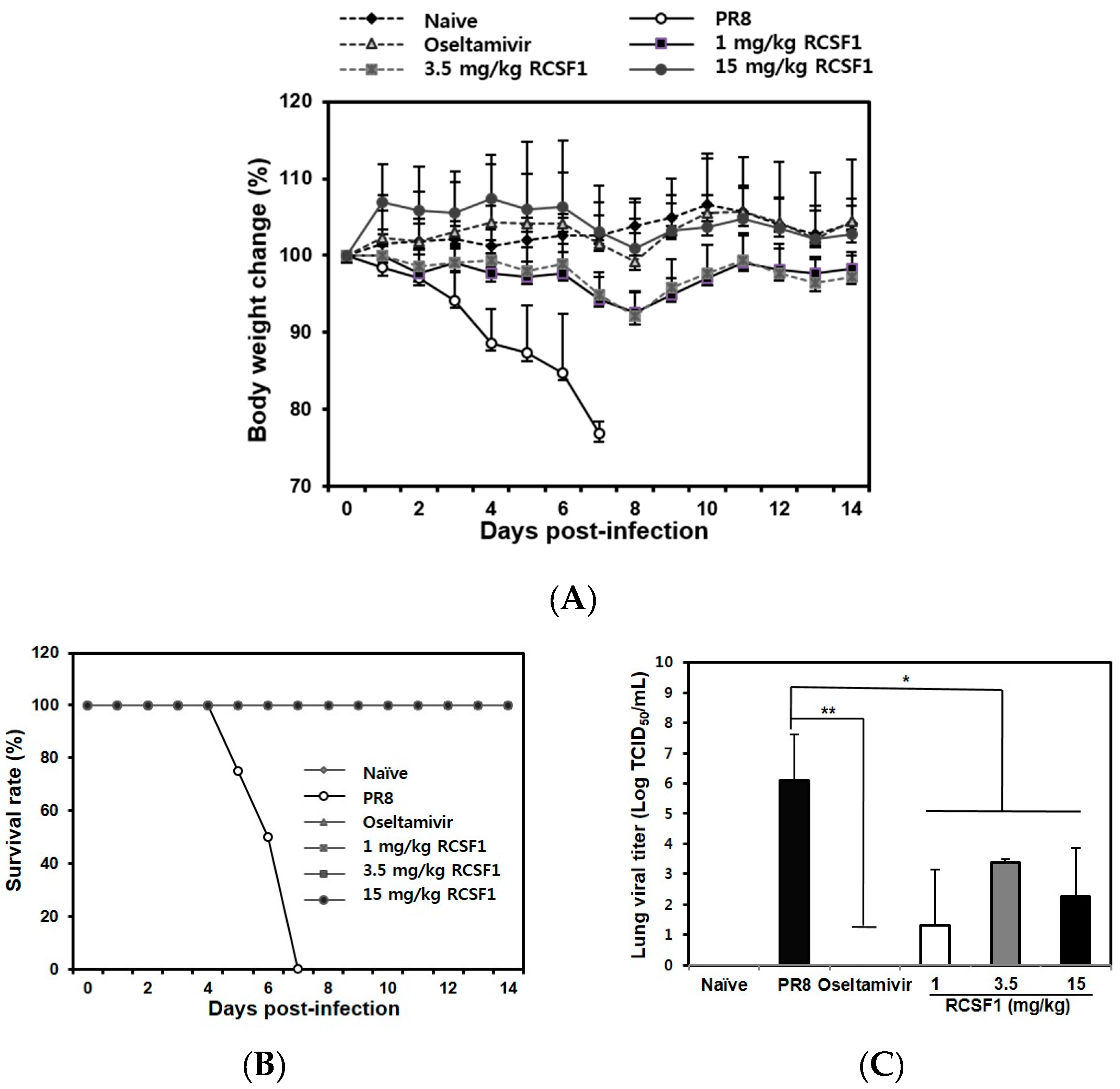
3.4. Oral Administration of RCSF1 Shows Anti-Influenza Activity in Mice
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jagger, B.W.; Wise, H.M.; Kash, J.C.; Walters, K.A.; Wills, N.M.; Xiao, Y.L.; Dunfee, R.L.; Schwartzman, L.M.; Ozinsky, A.; Bell, G.L.; et al. An overlapping protein-coding region in influenza A virus segment 3 modulates the host response. Science 2012, 337, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Ison, M.G. Optimizing antiviral therapy for influenza: Understanding the evidence. Expert Rev. Anti-Infect. Ther. 2015, 13, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.K.; Leung, T.W.; Ho, E.C.; Leung, P.C.; Ng, A.Y.; Lai, M.Y.; Lim, W.W. Oseltamivir- and amantadine-resistant influenza viruses A (H1N1). Emerg. Infect. Dis. 2009, 15, 966–968. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, J.I.; Lee, I.; Lee, S.; Hwang, M.W.; Bae, J.Y.; Heo, J.; Kim, D.; Han, S.Z.; Park, M.S. Aronia melanocarpa and its components demonstrate antiviral activity against influenza viruses. Biochem. Biophys. Res. Commun. 2013, 440, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Bahramsoltani, R.; Sodagari, H.R.; Farzaei, M.H.; Abdolghaffari, A.H.; Gooshe, M.; Rezaei, N. The preventive and therapeutic potential of natural polyphenols on influenza. Expert Rev. Anti-Infect. Ther. 2016, 14, 57–80. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, Y.N.; Youn, H.N.; Lee, D.H.; Kwak, J.H.; Seong, B.L.; Lee, J.B.; Park, S.Y.; Choi, I.S.; Song, C.S. Anti-influenza virus activity of green tea by-products in vitro and efficacy against influenza virus infection in chickens. Poult. Sci. 2012, 91, 66–73. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Han, H.M.; Wang, W.; Gao, B. Anti-influenza virus effect of aqueous extracts from dandelion. Virol. J. 2011, 8. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Morimoto, K.; Hirayama, M.; Hori, K. High mannose-specific lectin (KAA-2) from the red alga Kappaphycus alvarezii potently inhibits influenza virus infection in a strain-independent manner. Biochem. Biophys. Res. Commun. 2011, 405, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Zu, M.; Yang, F.; Zhou, W.L.; Liu, A.L.; Du, G.H.; Zheng, L.S. In vitro anti-influenza virus and anti-inflammatory activities of theaflavin derivatives. Antivir. Res. 2012, 94, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Song, J.M.; Lee, K.H.; Seong, B.L. Antiviral effect of catechins in green tea on influenza virus. Antivir. Res. 2005, 68, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.W.; Hwang, H.J.; Shin, C.S. Polyphenol compounds and anti-inflammatory activities of Korean black raspberry (Rubus coreanus Miquel) wines produced from juice supplemented with pulp and seed. J. Agric. Food Chem. 2012, 60, 5121–5127. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alonso, M.; de Pascual-Teresa, S.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Evaluation of the antioxidant properties of fruits. Food Chem. 2004, 84, 13–18. [Google Scholar] [CrossRef]
- Ku, C.S.; Mun, S.P. Antioxidant activities of ethanol extracts from seeds in fresh Bokbunja (Rubus coreanus Miq.) and wine processing waste. Bioresour. Technol. 2008, 99, 4503–4509. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Jang, H.G. Lactic acid fermentation and biological activities of Rubus coreanus. J. Korean Soc. Agric. Chem. Biotechnol. 2003, 46, 367–375. (In Korean) [Google Scholar]
- World Health Organization. Manual for the Laboratory Diagnosis and Virological Surveillance of Influenza. Available online: http://apps.who.int/iris/bitstream/10665/44518/1/9789241548090_eng.pdf (accessed on 14 April 2014).
- Bruggisser, R.; von Daeniken, K.; Jundt, G.; Schaffner, W.; Tullberg-Reinert, H. Interference of plant extracts, phytoestrogens and antioxidants with the MTT tetrazolium assay. Planta Med. 2002, 68, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Furuta, Y.; Takhashi, K.; Kuno-Maekawa, M.; Sangawa, H.; Uehara, S.; Kozaki, K.; Nomura, N.; Egawa, H.; Shiraki, K. Mechanism of action of T-705 against influenza virus. Antimicrob. Agents Chemother. 2005, 49, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.J.; Lee, J.H.; Hong, K.W.; Kim, S.H.; Park, Y.; Lee, J.Y.; Kang, S.; Kim, S.; Yang, J.H.; Kim, E.K.; et al. Insight into structural diversity of influenza virus haemagglutinin. J. Gen. Virol. 2013, 94, 1712–1722. [Google Scholar] [CrossRef] [PubMed]
- Reed, M.L.; Yen, H.L.; DuBois, R.M.; Bridges, O.A.; Salomon, R.; Webster, R.G.; Russell, C.J. Amino acid residues in the fusion peptide pocket regulate the pH of activation of the H5N1 influenza virus hemagglutinin protein. J. Virol. 2009, 83, 3568–3580. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rajasekaran, D.; Palombo, E.A.; Yeo, T.C.; Ley, D.L.S.; Tu, C.L.; Malherbe, F.; Grollo, L. Identification of traditional medicinal plant extracts with novel anti-influenza activity. PLoS ONE 2013, 8, e79293. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar]
- Varki, A. Diversity in the sialic acids. Glycobiology 1992, 2, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Thorlund, K.; Awad, T.; Boivin, G.; Yhabane, L. Systematic review of influenza resistance to the neuraminidase inhibitors. BMC Infect. Dis. 2011, 11, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Song, M.S.; Kumar, G.; Shadrick, W.R.; Zhou, W.; Jeevan, T.; Li, Z.; Slavish, P.J.; Fabrizio, T.P.; Yoon, S.W.; Webb, T.R.; et al. Identification and characterization of influenza variants resistant to a viral endonuclease inhibitor. Proc. Natl. Acad. Sci. USA 2016, 113, 3669–3674. [Google Scholar] [CrossRef] [PubMed]
- Rowse, M.; Qiu, S.; Tsao, J.; Xian, T.; Khawaja, S.; Yamauchi, Y.; Yang, Z.; Wang, G.; Luo, M. Characterization of potent fusion inhibitors of influenza virus. PLoS ONE 2015, 10, e0122536. [Google Scholar] [CrossRef] [PubMed]
- Vigant, F.; Lee, J.; Hollmann, A.; Tanner, L.B.; Akyol Ataman, Z.; Yun, T.; Shui, G.; Aguilar, H.C.; Zhang, D.; Meriwether, D.; et al. A mechanistic paradigm for broad-spectrum antivirals that target virus-cell fusion. PLoS Pathog. 2013, 9, e1003297. [Google Scholar] [CrossRef] [PubMed]
- Haidari, M.; Ali, M.; Casscells, S.W.; Madjid, M. Pomegranate (Punica granatum) purified polyphenol extract inhibits influenza virus and has a synergistic effect with oseltamivir. Phytomedicine 2009, 16, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, R.; Li, X.; He, J.; Jiang, S.; Liu, S.; Yang, J. Quercetin as an antiviral agent inhibits influenza A virus (IAV) entry. Viruses 2016, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Theisen, L.L.; Erdelmeier, C.A.; Spoden, G.A.; Boukhallouk, F.; Sausy, A.; Florin, L.; Muller, C.P. Tannins from Hamamelis virginiana bark extract: Characterization and improvement of the antiviral efficacy against influenza A virus and human papillomavirus. PLoS ONE 2014, 31, e88062. [Google Scholar] [CrossRef] [PubMed]
- Atigadda, V.R.; Brouillette, W.J.; Duarte, F.; Ali, S.M.; Babu, Y.S.; Bantia, S.; Chand, P.; Chu, N.; Montgomery, J.A.; Walsh, D.A.; et al. Potent inhibition of influenza sialidase by a benzoic acid containing a 2-pyrrolidinone substituent. J. Med. Chem. 1999, 13, 2332–2343. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, D.; Jin, J.; Xu, J.; Li, M.; Wang, H.; Dou, J.; Zhou, C. Antiviral activity of SA-2 against influenza A virus in vitro/vivo and its inhibition of RNA polymerase. Antivir. Res. 2016, 127, 68–78. [Google Scholar] [CrossRef] [PubMed]
| Class | Polyphenols | Contents |
|---|---|---|
| Flavonoids | Cyanidin-3-glucoside | 0.15 ± 0.00 |
| Cyanidin-3-rutinoside | 8.50 ± 0.20 | |
| Quercetin | 0.27 ± 0.01 | |
| Myricetin | ND 1 | |
| Rutin | 0.53 ± 0.00 | |
| Catechin | 34.55 ± 0.82 | |
| Epigallocatechin gallate | ND | |
| Ellagic acid | 11.43 ± 0.99 | |
| Phenolic acid | Gallic acid | 1.30 ± 0.04 |
| 3,4-Dihydroxybenzoic acid | 1.99 ± 0.05 | |
| Caffeic acid | 0.22 ± 0.03 | |
| trans-Ferulic acid | 0.13 ± 0.01 | |
| p-Coumaric acid | 0.44 ± 0.00 | |
| Chlorogenic acid | 0.02 ± 0.00 | |
| Stilbenoids | trans-Resveratrol | 0.02 ± 0.00 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-H.; Oh, M.; Seok, J.H.; Kim, S.; Lee, D.B.; Bae, G.; Bae, H.-I.; Bae, S.Y.; Hong, Y.-M.; Kwon, S.-O.; et al. Antiviral Effects of Black Raspberry (Rubus coreanus) Seed and Its Gallic Acid against Influenza Virus Infection. Viruses 2016, 8, 157. https://doi.org/10.3390/v8060157
Lee J-H, Oh M, Seok JH, Kim S, Lee DB, Bae G, Bae H-I, Bae SY, Hong Y-M, Kwon S-O, et al. Antiviral Effects of Black Raspberry (Rubus coreanus) Seed and Its Gallic Acid against Influenza Virus Infection. Viruses. 2016; 8(6):157. https://doi.org/10.3390/v8060157
Chicago/Turabian StyleLee, Ji-Hye, Mi Oh, Jong Hyeon Seok, Sella Kim, Dan Bi Lee, Garam Bae, Hae-In Bae, Seon Young Bae, Young-Min Hong, Sang-Oh Kwon, and et al. 2016. "Antiviral Effects of Black Raspberry (Rubus coreanus) Seed and Its Gallic Acid against Influenza Virus Infection" Viruses 8, no. 6: 157. https://doi.org/10.3390/v8060157
APA StyleLee, J.-H., Oh, M., Seok, J. H., Kim, S., Lee, D. B., Bae, G., Bae, H.-I., Bae, S. Y., Hong, Y.-M., Kwon, S.-O., Lee, D.-H., Song, C.-S., Mun, J. Y., Chung, M. S., & Kim, K. H. (2016). Antiviral Effects of Black Raspberry (Rubus coreanus) Seed and Its Gallic Acid against Influenza Virus Infection. Viruses, 8(6), 157. https://doi.org/10.3390/v8060157





