The Correlation between the Virus- and Brain Antigen-Specific B Cell Response in the Blood of Patients with Multiple Sclerosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. ELISPOT
2.3. ELISA
2.4. Statistical Analysis
3. Results
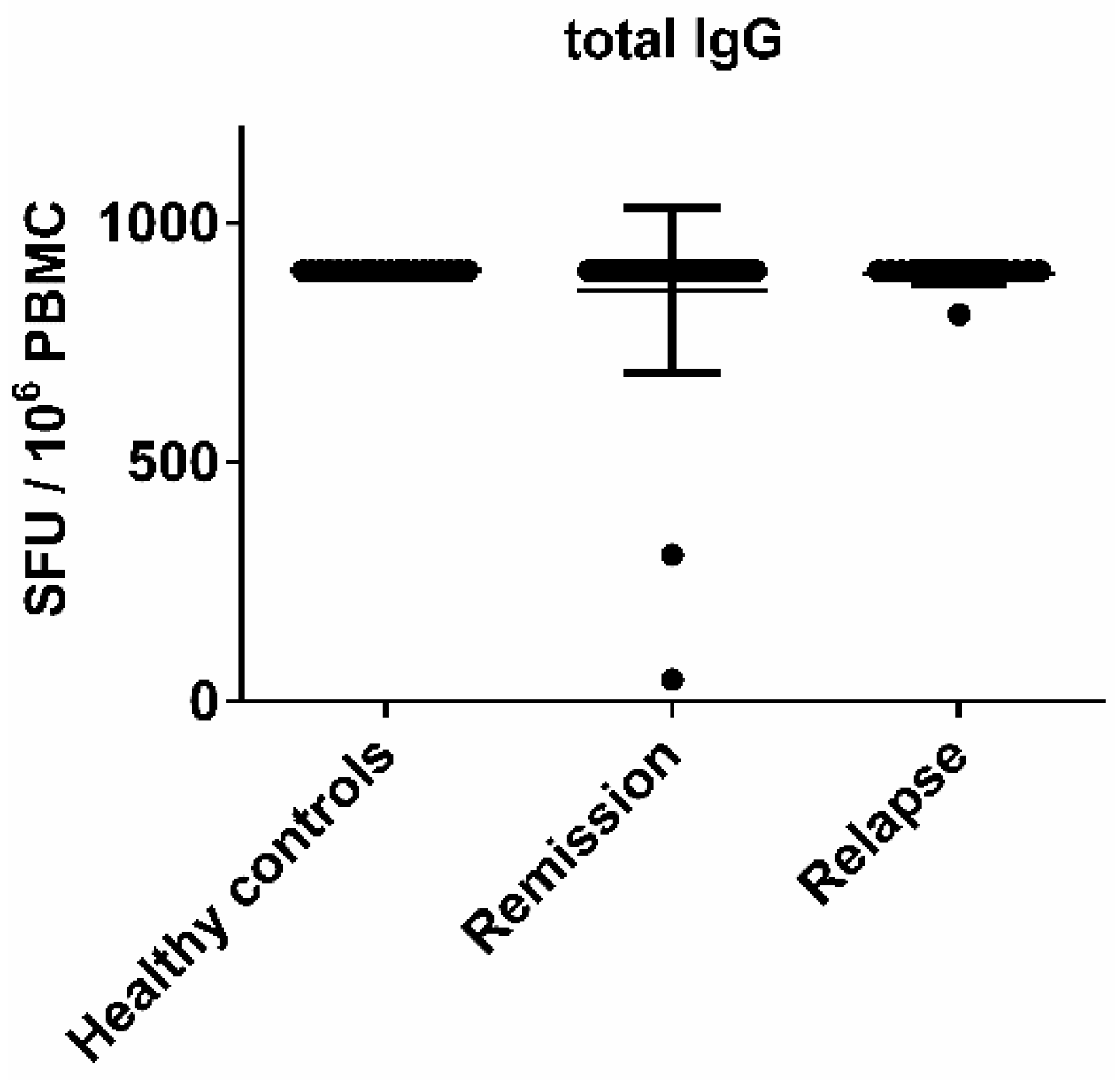
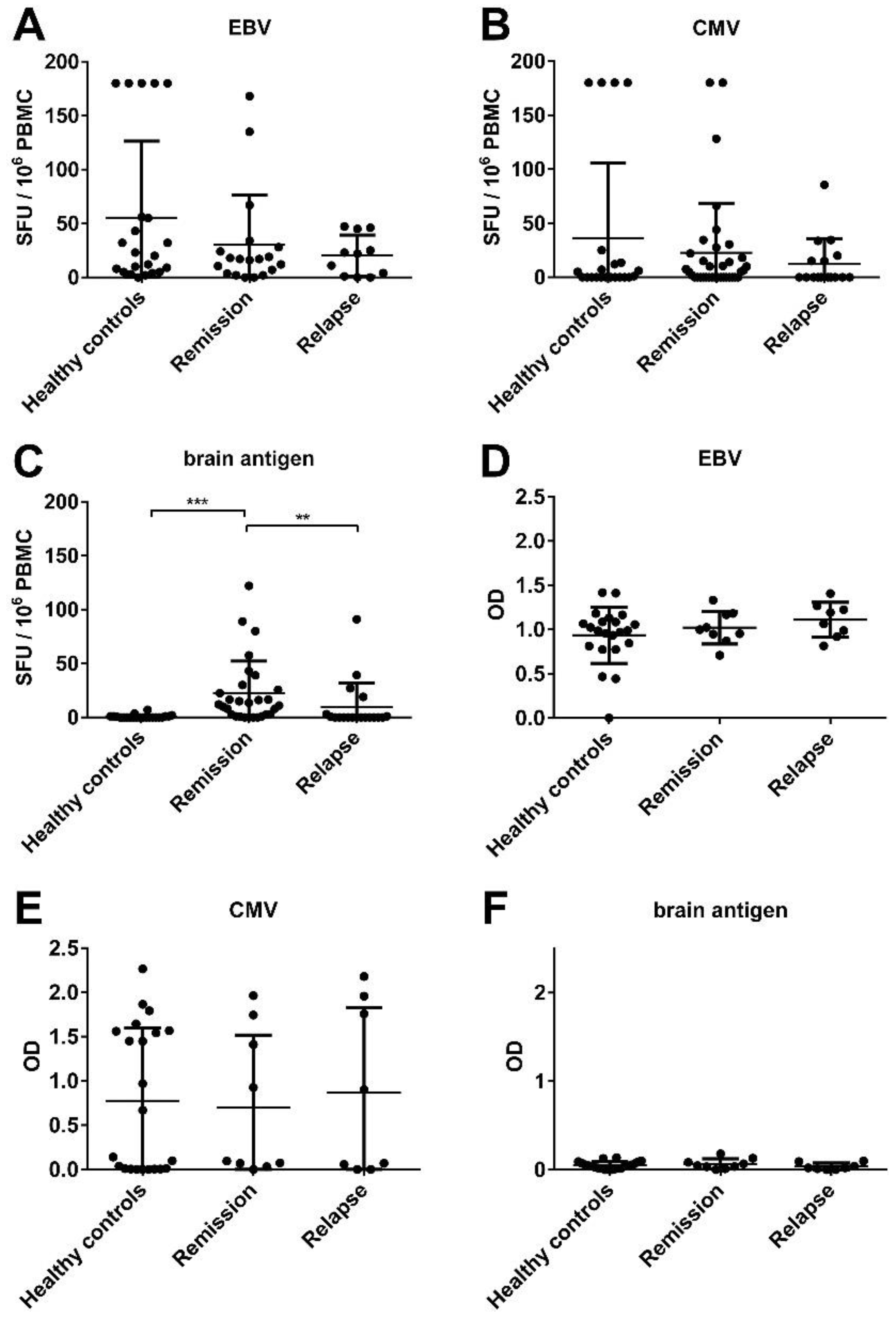
3.1. There Was No Significant Difference in the EBV-Specific B Cell Activity Comparing Healthy Controls and MS Patients
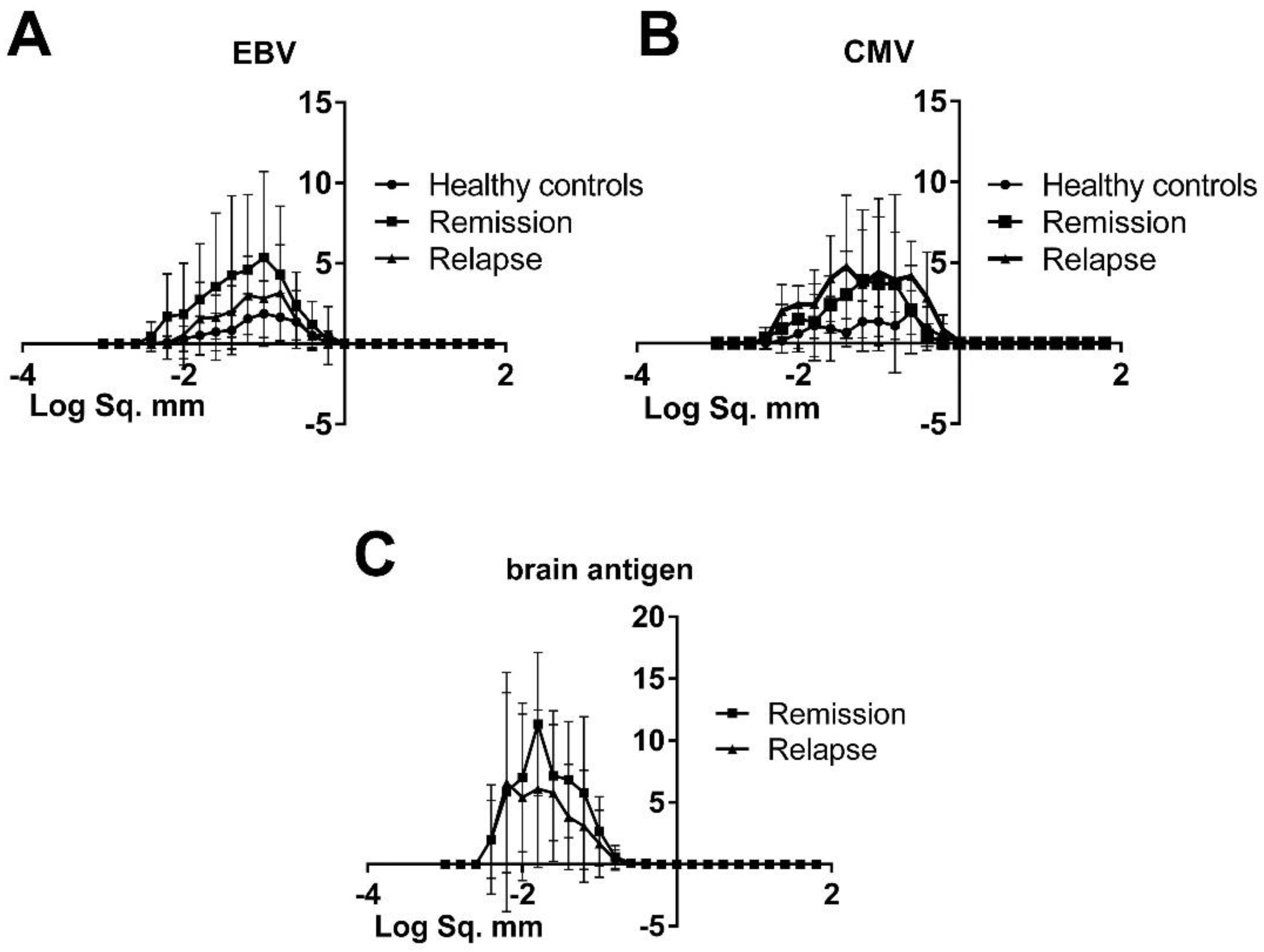
3.2. The Morphology of Spots Produced by Virus-Specific B Cells Was Similar in Healthy Controls and MS Patients, Hence There Was No Difference in Productivity and the Kinetics of Cell Secretory Activity
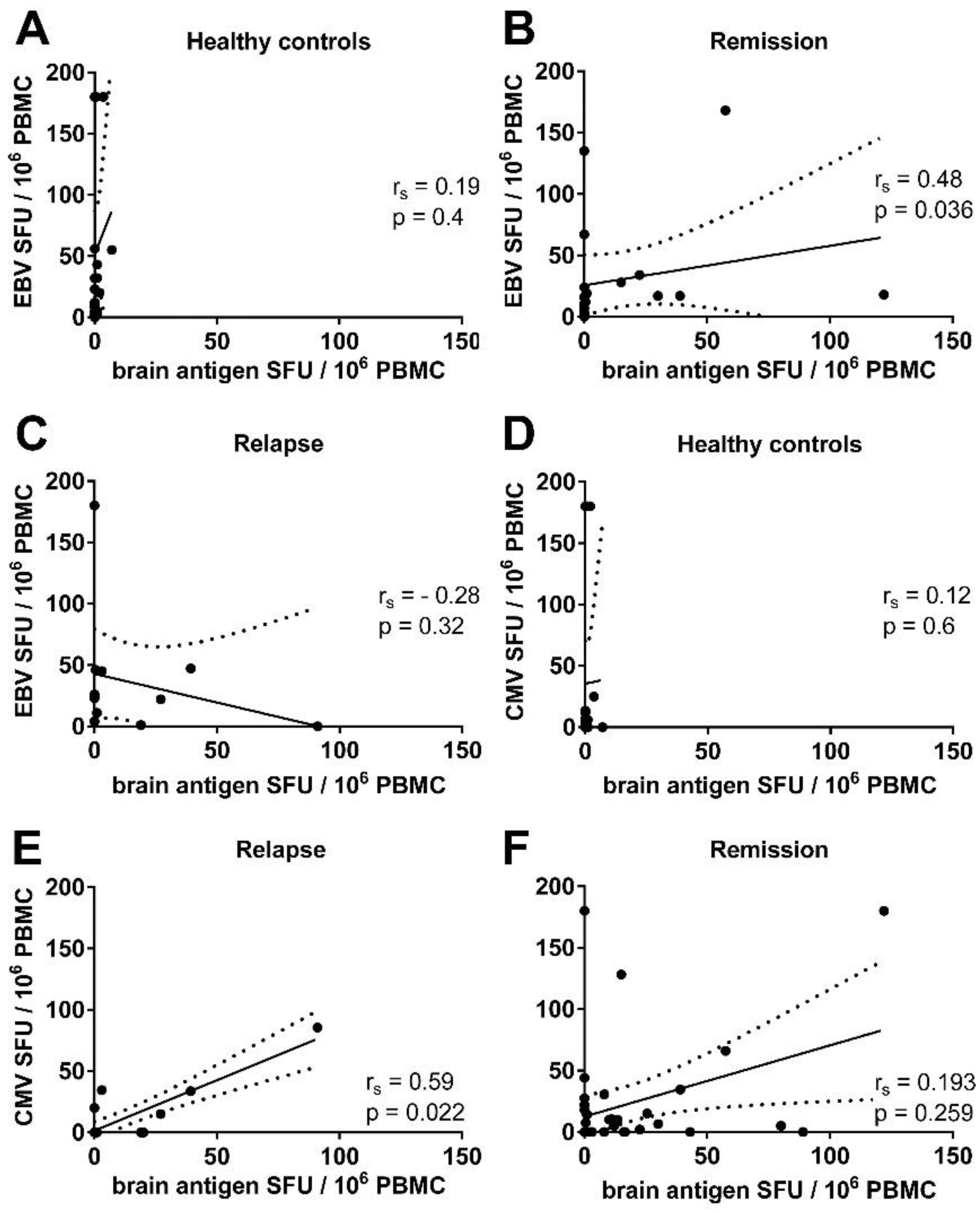
3.3. There Was a Correlation between the Frequencies of Virus- and Brain-Reactive B Cells in the Blood of MS Patients
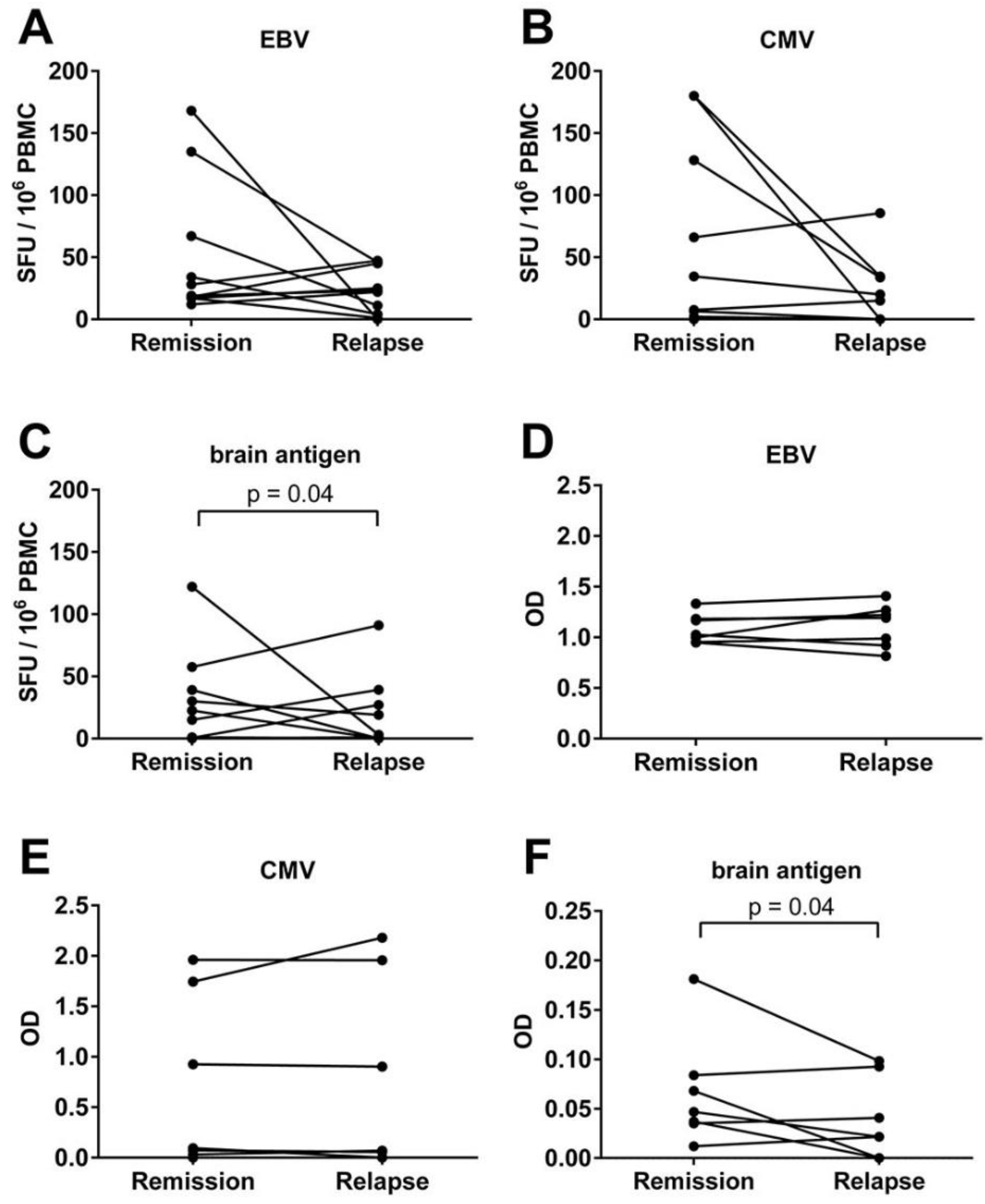
3.4. An Acute MS Relapse Was Not Associated with any Change in the Virus-Specific B Cell Activity, but with a Decrease in Brain-Reactive B Cell Responses Compared to the Remission
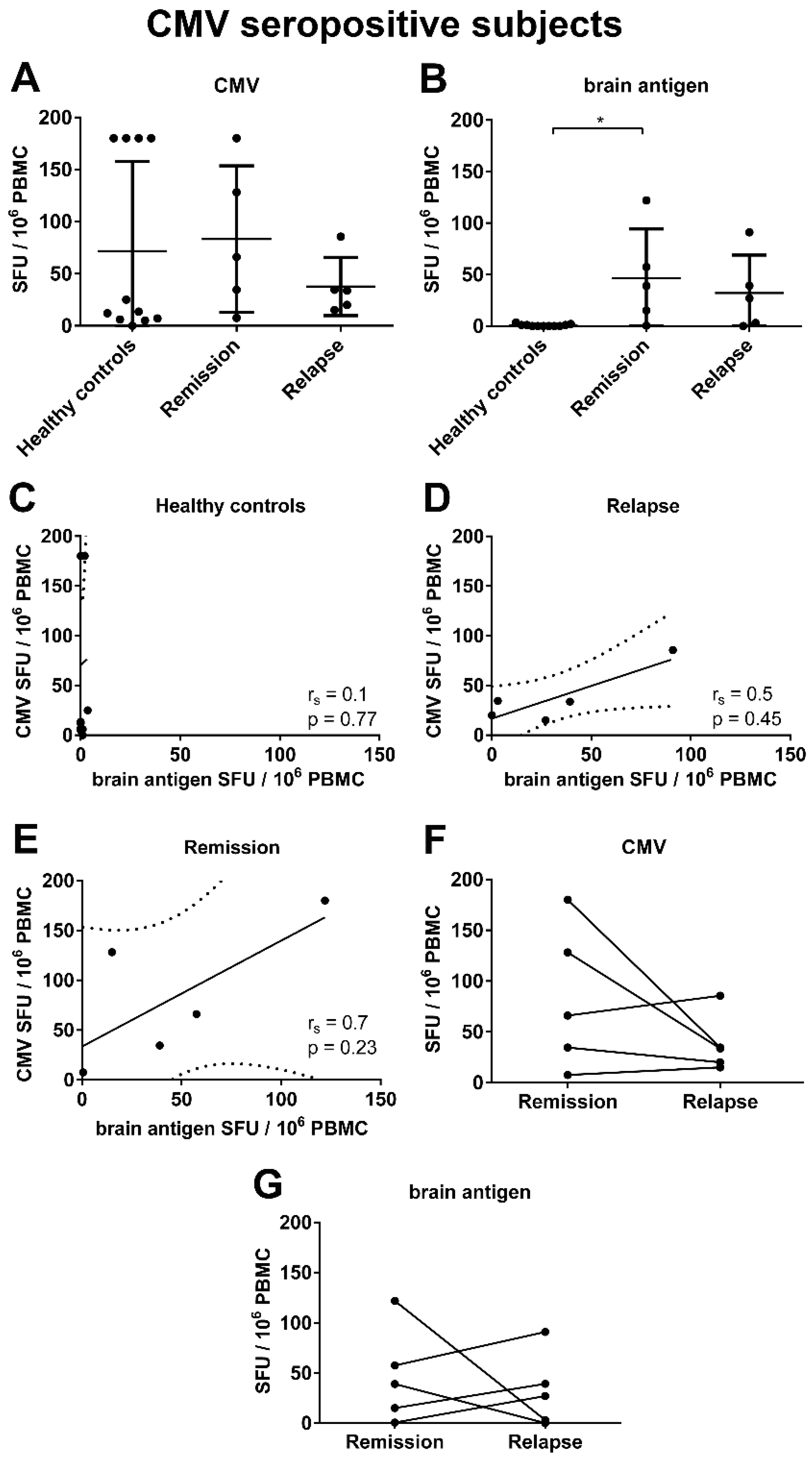
3.5. There Was a Trend for a Correlation between the Frequencies of CMV- and Brain-Reactive B Cells in the Blood of MS Patients Experiencing an Acute Relapse when Excluding CMV Seronegative Subjects
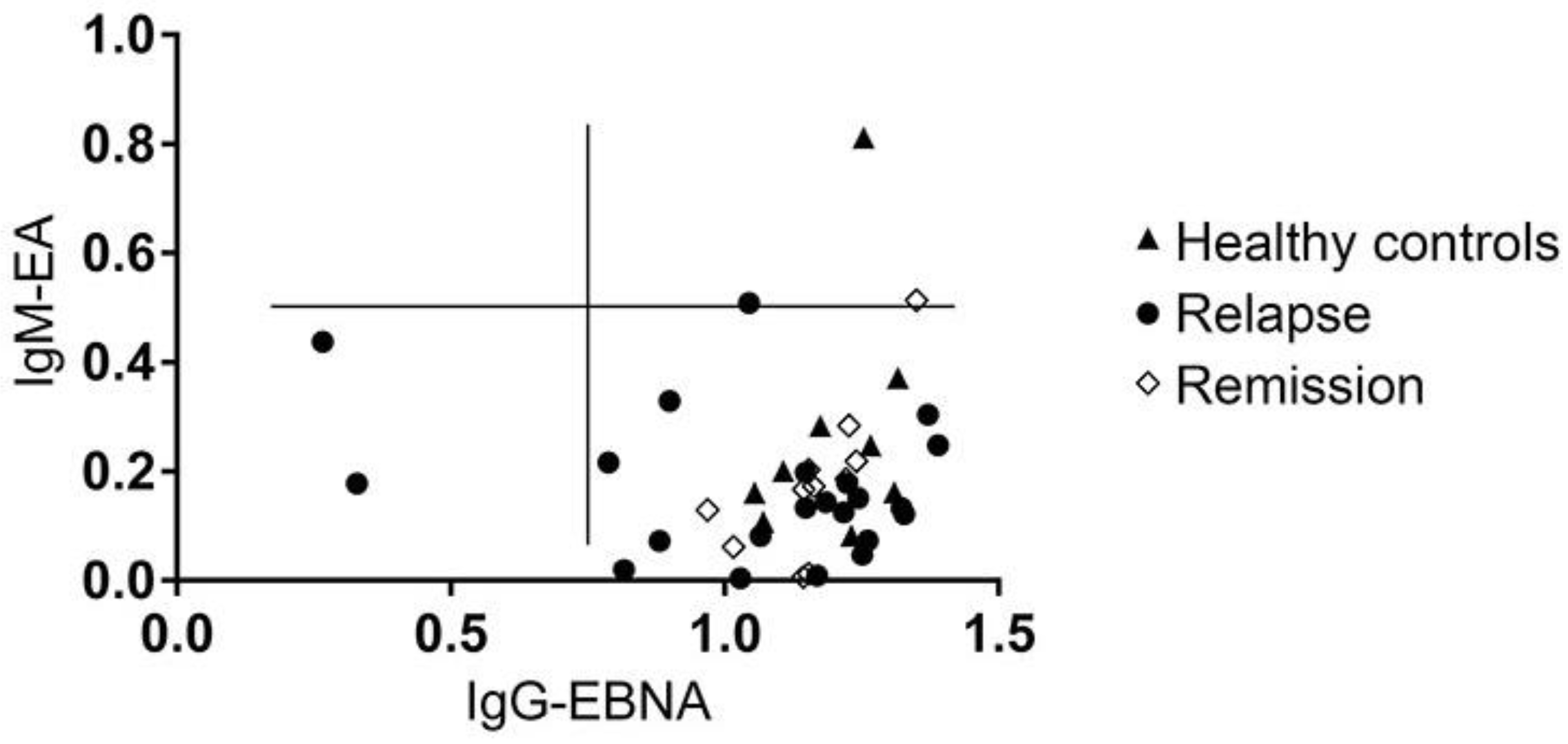
3.6. MS Is Not Associated with the Reactivation of a Latent EBV Infection
3.7. There Was a Correlation between the Virus-Specific B Cell Response and Clinical Disease Parameters
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Mouhieddine, T.H.; Darwish, H.; Fawaz, L.; Yamout, B.; Tamim, H.; Khoury, S.J. Risk factors for Multiple Sclerosis and associations with anti-EBV antibody titers. Clin. Immunol. 2015, 158, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Castellazzi, M.; Contini, C.; Tamborino, C.; Fasolo, F.; Roversi, G.; Seraceni, S.; Rizzo, R.; Baldi, E.; Tola, M.; Bellini, T.; et al. Epstein-Barr virus-specific intrathecal oligoclonal IgG production in relapsing-remitting multiple sclerosis is limited to a subset of patients and is composed of low-affinity antibodies. J. Neuroinflammation 2014, 11, 188. [Google Scholar] [CrossRef] [PubMed]
- Serafini, B.; Rosicarelli, B.; Franciotta, D.; Magliozzi, R.; Reynolds, R.; Cinque, P.; Andreoni, L.; Trivedi, P.; Salvetti, M.; Faggioni, A.; et al. Dysregulated Epstein-Barr virus infection in the multiple sclerosis brain. J. Exp. Med. 2007, 204, 2899–2912. [Google Scholar] [CrossRef] [PubMed]
- Cocuzza, C.E.; Piazza, F.; Musumeci, R.; Oggioni, D.; Andreoni, S.; Gardinetti, M.; Fusco, L.; Frigo, M.; Banfi, P.; Rottoli, M.R.; et al. EBV-MS Italian Study Group. Quantitative detection of epstein-barr virus DNA in cerebrospinal fluid and blood samples of patients with relapsing-remitting multiple sclerosis. PLoS ONE 2014, 9, e94497. [Google Scholar] [CrossRef] [PubMed]
- Ascherio, A.; Munger, K.L. Epstein-barr virus infection and multiple sclerosis: A review. J. Neuroimmune Pharmacol. 2010, 5, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Balfour, H.H., Jr.; Odumade, O.A.; Schmeling, D.O.; Mullan, B.D.; Ed, J.A.; Knight, J.A.; Vezina, H.E.; Thomas, W.; Hogquist, K.A. Behavioral, virologic, and immunologic factors associated with acquisition and severity of primary Epstein-Barr virus infection in university students. J. Infect. Dis. 2013, 207, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Murata, T. Regulation of Epstein-Barr virus reactivation from latency. Microbiol. Immunol. 2014, 58, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Thacker, E.L.; Mirzaei, F.; Ascherio, A. Infectious mononucleosis and risk for multiple sclerosis: A meta-analysis. Ann. Neurol. 2006, 59, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Munger, K.L.; Levin, L.I.; O’Reilly, E.J.; Falk, K.I.; Ascherio, A. Anti-Epstein-Barr virus antibodies as serological markers of multiple sclerosis: A prospective study among United States military personnel. Mult. Scler. 2011, 17, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Buljevac, D.; van Doornum, G.J.; Flach, H.Z.; Groen, J.; Osterhaus, A.D.; Hop, W.; van Doorn, P.A.; van der Meché, F.G.; Hintzen, R.Q. Epstein-Barr virus and disease activity in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1377–1381. [Google Scholar] [CrossRef] [PubMed]
- Kvistad, S.; Myhr, K.M.; Holmøy, T.; Bakke, S.; Beiske, A.G.; Bjerve, K.S.; Hovdal, H.; Løken-Amsrud, K.I.; Lilleås, F.; Midgard, R.; et al. Antibodies to Epstein-Barr virus and MRI disease activity in multiple sclerosis. Mult Scler. 2014, 20, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Kuerten, S.; Pommerschein, G.; Barth, S.K.; Hohmann, C.; Milles, B.; Sammer, F.W.; Duffy, C.E.; Wunsch, M.; Rovituso, D.M.; Schroeter, M.; et al. Identification of a B cell-dependent subpopulation of multiple sclerosis by measurements of brain-reactive B cells in the blood. Clin. Immunol. 2014, 152, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, C.; Milles, B.; Schinke, M.; Schroeter, M.; Ulzheimer, J.; Kraft, P.; Kleinschnitz, C.; Lehmann, P.V.; Kuerten, S. Categorization of multiple sclerosis relapse subtypes by B cell profiling in the blood. Acta Neuropathol. Commun. 2014, 2, 138. [Google Scholar] [CrossRef] [PubMed]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Polman, C.H.; Reingold, S.C.; Edan, G.; Filippi, M.; Hartung, H.P.; Kappos, L.; Lublin, F.D.; Metz, L.M.; McFarland, H.F.; O’Connor, P.W.; et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann. Neurol. 2005, 58, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An Expanded Disability Status Scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Pinna, D.; Corti, D.; Jarrossay, D.; Sallusto, F.; Lanzavecchia, A. Clonal dissection of the human memory B-cell repertoire following infection and vaccination. Eur. J. Immunol. 2009, 39, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- Karulin, A.Y.; Lehmann, P.V. How ELISPOT morphology reflects on the productivity and kinetics of cells’ secretory activity. Methods Mol. Biol. 2012, 792, 125–143. [Google Scholar] [PubMed]
- Obel, N.; Høier-Madsen, M.; Kangro, H. Serological and clinical findings in patients with serological evidence of reactivated Epstein-Barr virus infection. APMIS 1996, 104, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Meffre, E.; Wardemann, H. B-cell tolerance checkpoints in health and autoimmunity. Curr. Opin. Immunol. 2008, 20, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.M.; Zhang, X.H. Cross-reactivity between human cytomegalovirus peptide 981–1003 and myelin oligodendroglia glycoprotein peptide 35–55 in experimental autoimmune encephalomyelitis in Lewis rats. Biochem. Biophys. Res. Commun. 2014, 443, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Sanadgol, N.; Ramroodi, N.; Ahmadi, G.A.; Komijani, M.; Moghtaderi, A.; Bouzari, M. Prevalence of cytomegalovirus infection and its role in total immunoglobulin pattern in Iranian patients with different subtypes of multiple sclerosis. New Microbiol. 2011, 34, 263–274. [Google Scholar] [PubMed]
- Tran, C.; Du Pasquier, R.A.; Cavassini, M.; Guex-Crosier, Y.; Meuli, R.; Ciuffreda, D. Neuromyelitis optica following CMV primo-infection. J. Intern. Med. 2007, 261, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Barzilai, O.; Sherer, Y.; Ram, M.; Izhaky, D.; Anaya, J.M.; Shoenfeld, Y. Epstein-Barr virus and cytomegalovirus in autoimmune diseases: Are they truly notorious? A preliminary report. Ann. N. Y. Acad. Sci. 2007, 1108, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Owens, G.P.; Bennett, J.L. Trigger, pathogen, or bystander: The complex nexus linking Epstein- Barr virus and multiple sclerosis. Mult. Scler. 2012, 18, 1204–1208. [Google Scholar] [CrossRef] [PubMed]
- Libbey, J.E.; McCoy, L.L.; Fujinami, R.S. Molecular mimicry in multiple sclerosis. Int. Rev. Neurobiol. 2007, 79, 127–147. [Google Scholar] [PubMed]
- Wucherpfennig, K.W.; Strominger, J.L. Molecular mimicry in T cell-mediated autoimmunity: Viral peptides activate human T cell clones specific for myelin basic protein. Cell 1995, 80, 695–705. [Google Scholar] [CrossRef]
- Fujinami, R.S.; von Herrath, M.G.; Christen, U.; Whitton, J.L. Molecular mimicry, bystander activation, or viral persistence: Infections and autoimmune disease. Clin. Microbiol. Rev. 2006, 19, 80–94. [Google Scholar] [CrossRef] [PubMed]
- Loebel, M.; Strohschein, K.; Giannini, C.; Koelsch, U.; Bauer, S.; Doebis, C.; Thomas, S.; Unterwalder, N.; von Baehr, V.; Reinke, P.; et al. Deficient EBV-specific B- and T-cell response in patients with chronic fatigue syndrome. PLoS ONE 2014, 9, e85387. [Google Scholar] [CrossRef] [PubMed]
- Latham, L.B.; Lee, M.J.; Lincoln, J.A.; Ji, N.; Forsthuber, T.G.; Lindsey, J.W. Antivirus immune activity in multiple sclerosis correlates with MRI activity. Acta Neurol. Scand. 2015. [Google Scholar] [CrossRef] [PubMed]
- Levin, L.I.; Munger, K.L.; Rubertone, M.V.; Peck, C.A.; Lennette, E.T.; Spiegelmann, D.; Ascherio, A. Temporal relationship between elevation of Epstein–Barr virus antibody titers and initial onset of neurological symptoms in multiple sclerosis. JAMA 2005, 293, 2496–2500. [Google Scholar] [CrossRef] [PubMed]
- Serafini, B.; Severa, M.; Columba-Cabezas, S.; Rosicarelli, B.; Veroni, C.; Chiappetta, G.; Magliozzi, R.; Reynolds, R.; Coccia, E.M.; Aloisi, F. Epstein-Barr virus latent infection and BAFF expression in B cells in the multiple sclerosis brain: Implications for viral persistence and intrathecal B-cell activation. J. Neuropathol. Exp. Neurol. 2010, 69, 677–693. [Google Scholar] [CrossRef] [PubMed]
- Willis, S.N.; Stadelmann, C.; Rodig, S.J.; Caron, T.; Gattenloehner, S.; Mallozzi, S.S.; Roughan, J.E.; Almendinger, S.E.; Blewett, M.M.; Brück, W.; et al. Epstein-Barr virus infection is not a characteristic feature of multiple sclerosis brain. Brain 2009, 132, 3318–3328. [Google Scholar] [CrossRef] [PubMed]
- Sargsyan, S.A.; Shearer, A.J.; Ritchie, A.M.; Burgoon, M.P.; Anderson, S.; Hemmer, B.; Stadelmann, C.; Gattenlöhner, S.; Owens, G.P.; Gilden, D.; et al. Absence of Epstein–Barr virus in the brain and CSF of patients with multiple sclerosis. Neurology 2010, 74, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Scotet, E.; Peyrat, M.A.; Saulquin, X.; Retiere, C.; Coudedel, C.; Davodeau, F.; Dulphy, N.; Toubert, A.; Bignon, J.D.; Lim, A.; et al. Frequent enrichment for CD8 T cells reactive against common herpes viruses in chronic inflammatory lesions: Towards a reassessment of the physiopathological significance of T cell clonal expansions found in autoimmune inflammatory processes. Eur. J. Immunol. 1999, 29, 973–985. [Google Scholar] [CrossRef]
- Horakova, D.; Zivadinov, R.; Weinstock-Guttmann, B.; Havrdova, E.; Qu, J.; Tamano-Blanco, M.; Badgett, D.; Tyblova, M.; Bergsland, N.; Hussein, S.; et al. Environmental factors associated with disease progression after the first demyelinating event: Results from the multi-center SET study. PLoS ONE 2013, 8, e53996. [Google Scholar] [CrossRef] [PubMed]
- Sundqvist, E.; Bergström, T.; Daialhosein, H.; Nyström, M.; Sundström, P.; Hillert, J.; Alfredsson, L.; Kockum, I.; Olsson, T. Cytomegalovirus seropositivity is negatively associated with multiple sclerosis. Mult. Scler. 2014, 20, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Zivadinov, R.; Nasuelli, D.; Tommasi, M.A.; Serafin, M.; Bratina, A.; Ukmar, M.; Pirko, I.; Johnson, A.J.; Furlan, C.; Pozzi-Mucelli, R.S.; et al. Positivity of cytomegalovirus antibodies predicts a better clinical and radiological outcome in multiple sclerosis patients. Neurol. Res. 2006, 28, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Terlutter, F.; Nowacki, T.M.; Caspell, R.; Li, R.; Horvath, K.; von Gall, C.; Kuerten, S.; Sundararaman, S.; Lehmann, P.V. Direct detection of T and B memory lymphocytes reveals HCMV exposure that serum antibodies fail to identify. Cancer Immunol. Immunother. (under review).
| No. of healthy controls | 22 |
|---|---|
| Female sex—No. (%) | 13 (59.1) |
| Mean (SE) age—years | 34.1 (12.2) |
| No. of patients | 41 |
|---|---|
| Female sex—No. (%) | 25 (61) |
| Mean (SE) age—years | 38 (10.8) |
| Treatment | na | CMV Positive | EBV Positive | Brain Antigen Positive |
|---|---|---|---|---|
| untreated | 16 | 9/16 (56.3%) | 8/11 (72.7%) | 5/16 (31.3%) |
| IFN-β | 14 | 4/14 (28.6%) | 5/5 (100%) | 8/14 (57.1%) |
| glatiramer acetate | 5 | 4/5 (80%) | 1/1 (100%) | 3/5 (60%) |
| fumaric acid | 1 | 0/1 (0%) | n. a. | 0/1 (0%) |
| natalizumab | 8 | 4/8 (50%) | 7/7 (100%) | 1/8 (12.5%) |
| fingolimod | 1 | 1/1 (100%) | 1/1 (100%) | 0/1 (0%) |
| mitoxantrone | 2 | 0/2 (0%) | 2/2 (100%) | 1/2 (50%) |
| rituximab | 2 | 0/2 (0%) | 0 (0%) | 0/2 (0%) |
| Correlation | Relapse | Remission |
|---|---|---|
| EBV—Disease activity a | rs = 0.39; p = 0.24 (n = 11) | rs = 0.58; p = 0.009 (n = 19) |
| EBV—Age at time of diagnosis | rs = −0.31; p = 0.32 (n = 11) | rs = 0.023; p = 0.93 (n = 19) |
| EBV—Remission EDSS | rs = 0.053; p = 0.88 (n = 11) | rs = −0.05; p = 0.84 (n = 19) |
| CMV—Disease activity a | rs = 0.68; p = 0.004 (n = 16) | rs = 0.39; p = 0.02 (n = 35) |
| CMV—Age at time of diagnosis | rs = −0.2; p = 0.12 (n = 16) | rs = 0.01; p = 0.94 (n = 35) |
| CMV—Remission EDSS | rs = −0.18; p = 0.12 (n = 16) | rs = 0.15; p = 0.38 (n = 35) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wunsch, M.; Hohmann, C.; Milles, B.; Rostermund, C.; Lehmann, P.V.; Schroeter, M.; Bayas, A.; Ulzheimer, J.; Mäurer, M.; Ergün, S.; et al. The Correlation between the Virus- and Brain Antigen-Specific B Cell Response in the Blood of Patients with Multiple Sclerosis. Viruses 2016, 8, 105. https://doi.org/10.3390/v8040105
Wunsch M, Hohmann C, Milles B, Rostermund C, Lehmann PV, Schroeter M, Bayas A, Ulzheimer J, Mäurer M, Ergün S, et al. The Correlation between the Virus- and Brain Antigen-Specific B Cell Response in the Blood of Patients with Multiple Sclerosis. Viruses. 2016; 8(4):105. https://doi.org/10.3390/v8040105
Chicago/Turabian StyleWunsch, Marie, Christopher Hohmann, Bianca Milles, Christina Rostermund, Paul V. Lehmann, Michael Schroeter, Antonios Bayas, Jochen Ulzheimer, Mathias Mäurer, Süleyman Ergün, and et al. 2016. "The Correlation between the Virus- and Brain Antigen-Specific B Cell Response in the Blood of Patients with Multiple Sclerosis" Viruses 8, no. 4: 105. https://doi.org/10.3390/v8040105
APA StyleWunsch, M., Hohmann, C., Milles, B., Rostermund, C., Lehmann, P. V., Schroeter, M., Bayas, A., Ulzheimer, J., Mäurer, M., Ergün, S., & Kuerten, S. (2016). The Correlation between the Virus- and Brain Antigen-Specific B Cell Response in the Blood of Patients with Multiple Sclerosis. Viruses, 8(4), 105. https://doi.org/10.3390/v8040105







