IPNV Antigen Uptake and Distribution in Atlantic Salmon Following Oral Administration
Abstract
:1. Introduction
2. Results
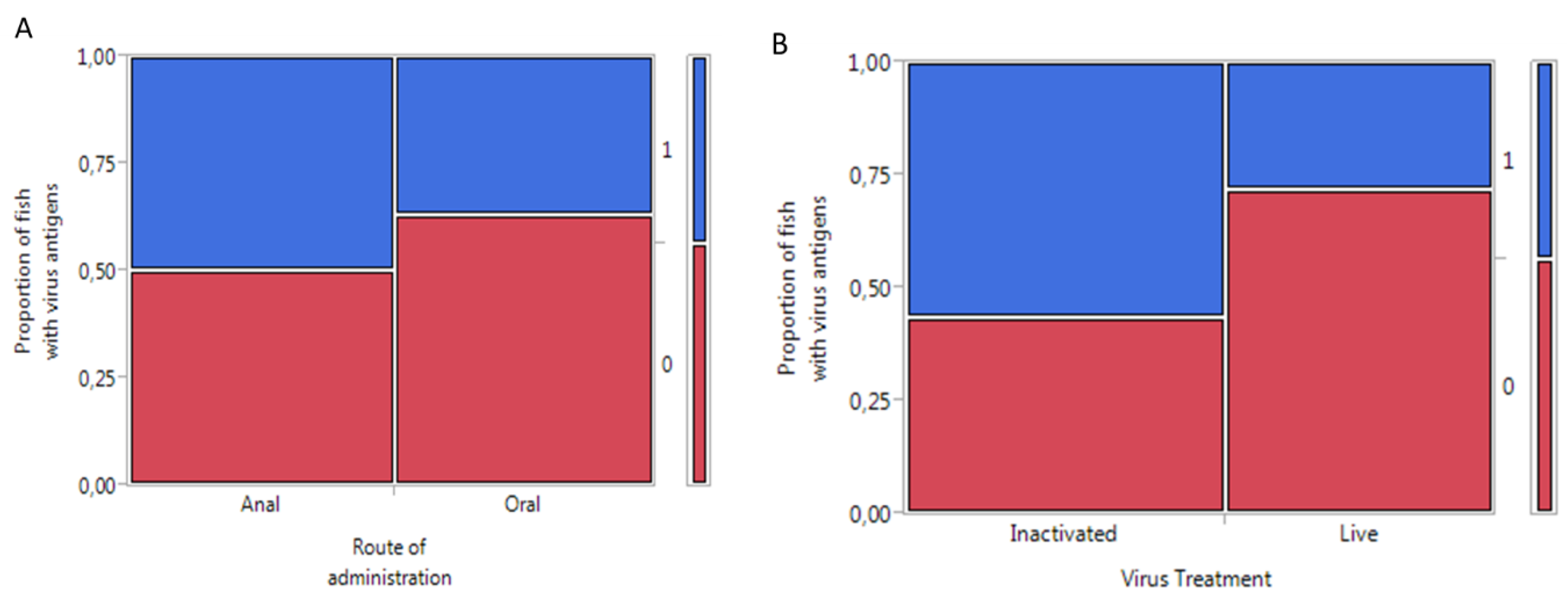
2.1. Higher IPNV Antigens Were Detected in Orally-Compared to Anally-Intubated Fish by ELISA
2.2. Detection of IPNV Antigens in Different Tissues by Immunohistochemistry
| Tissue | Time | Live Virus | Inactivated Virus | ||
|---|---|---|---|---|---|
| (Hours p.i.) | Anal Intubation | Oral Intubation | Anal Intubation | Oral Intubation | |
| Posterior intestine | 1 | 0 * | 0 ** | 1 | 1 |
| 24 | 0 | 0 | 0 | 0 | |
| 72 | 0 | 0 | 1 | 1 | |
| Head kidney | 1 | 3 * | 0 ** | 2 | 3 |
| 24 | 0 | 1 | 2 | 0 | |
| 72 | 0 | 3 | 3 | 0 | |
| Spleen | 1 | 2 * | 0 ** | 1 | 2 |
| 24 | 0 | 0 | 3 | 1 | |
| 72 | 0 | 0 | 2 | 0 | |
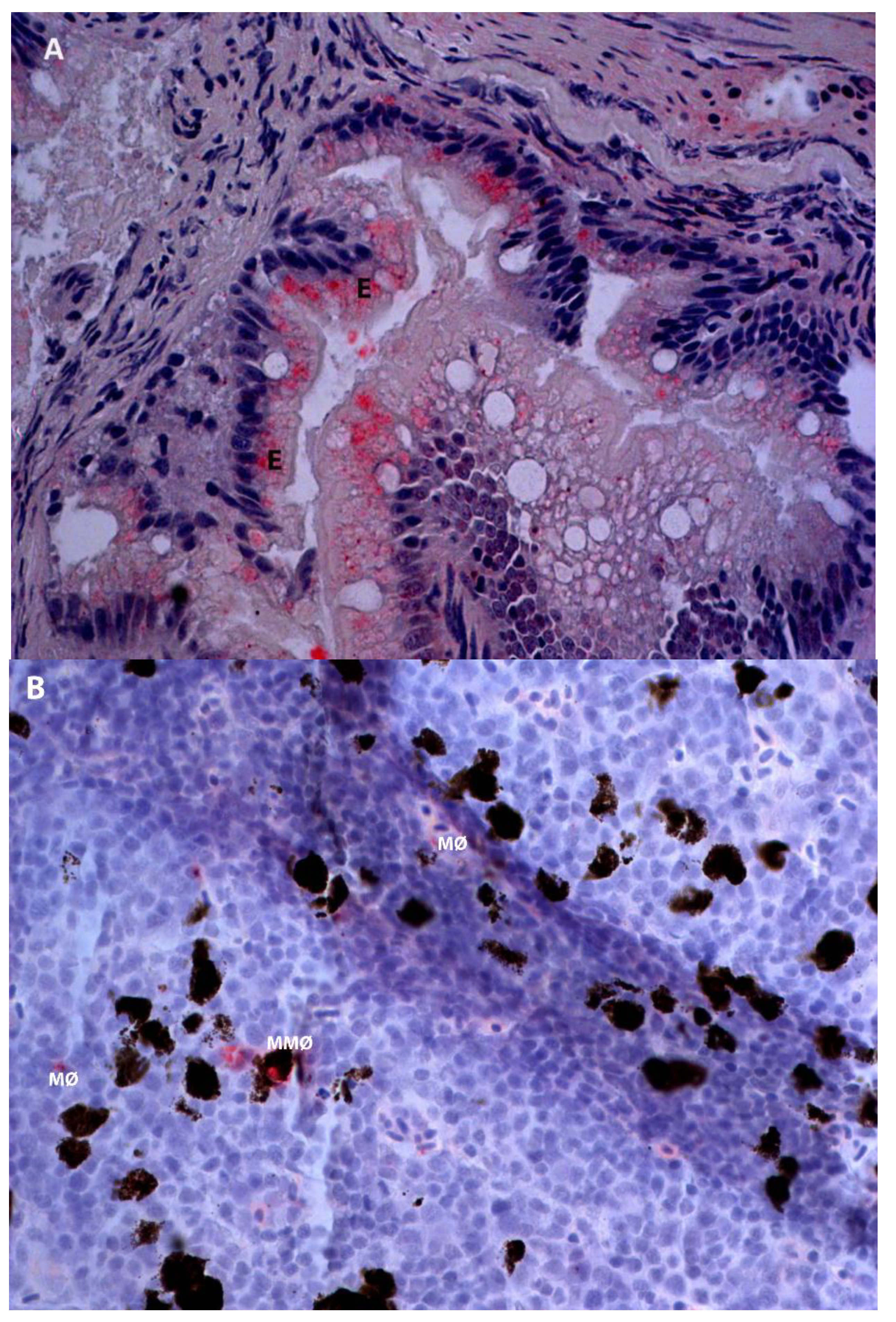
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Viruses
4.2. Fish and Rearing Conditions
4.3. Antigen Administration/Infection of the Fish
4.4. Sampling
4.5. Enzyme-Linked Immunosorbent Assay (ELISA)
4.6. Immunohistochemistry
4.7. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rombout, J.H.; Abelli, L.; Picchietti, S.; Scapigliati, G.; Kiron, V. Teleost intestinal immunology. Fish Shellfish Immunol. 2011, 31, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, G.W. Oral vaccines for finfish: Academic theory or commercial reality? Anim. Health Res. Rev. 2004, 5, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Adelmann, M.; Kollner, B.; Bergmann, S.M.; Fischer, U.; Lange, B.; Weitschies, W.; Enzmann, P.J.; Fichtner, D. Development of an oral vaccine for immunisation of rainbow trout (Oncorhynchus mykiss) against viral haemorrhagic septicaemia. Vaccine 2008, 26, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Dhar, A.K.; Allnutt, F.C.T. Challenges and opportunities in developming oral vaccines against viral diseases of fish. J. Mar. Sci. Res. Dev. 2011. [Google Scholar] [CrossRef]
- Centrovet Inactivated Vaccine for Salmon Rickettsial Syndrome and Subunit Vaccine Against Infectious Salmon Anaemia Freeze-Dried Oral Powder. 2014. Available online: http://www.centrovet.com/index.php/products-en/fish. (accessed on 8 December 2014).
- de las Heras, A.I.; Rodriguez Saint-Jean, S.; Perez-Prieto, S.I. Immunogenic and protective effects of an oral DNA vaccine against infectious pancreatic necrosis virus in fish. Fish Shellfish Immunol. 2010, 28, 562–570. [Google Scholar]
- Villumsen, K.R.; Neumann, L.; Ohtani, M.; Strom, H.K.; Raida, M.K. Oral and anal vaccination confers full protection against enteric redmouth disease (ERM) in rainbow trout. PLoS ONE 2014, 9, e93845. [Google Scholar] [CrossRef] [PubMed]
- Rombout, J.H.W.M.; Kiron, V. Mucosal vaccination of fish. In Fish Vaccination, 1st ed.; Gudding, R.L.A., Evensen, O., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2014; pp. 56–67. [Google Scholar]
- Rombout, J.H.; Lamers, C.H.; Helfrich, M.H.; Dekker, A.; Taverne-Thiele, J.J. Uptake and transport of intact macromolecules in the intestinal epithelium of carp (Cyprinus carpio L.) and the possible immunological implications. Cell Tissue Res. 1985, 239, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Rombout, J.H.W.M.; Bot, H.E.; Taverne-Thiele, J.J. Immunological importance of the second gut segment of carp. II. Characterization of mucosal leucocytes. J. Fish Bio. 1989, 35, 167–178. [Google Scholar] [CrossRef]
- Maurice, S.; Nussinovitch, A.; Jaffe, N.; Shoseyov, O.; Gertler, A. Oral immunization of Carassius auratus with modified recombinant A-layer proteins entrapped in alginate beads. Vaccine 2004, 23, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, N.A.; Rodriguez Saint-Jean, S.; Perez-Prieto, S.I. Food pellets as an effective delivery method for a DNA vaccine against infectious pancreatic necrosis virus in rainbow trout (Oncorhynchus mykiss, Walbaum). Fish Shellfish Immunol. 2014, 37, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, N.A.; Castro, R.; Abos, B.; Rodriguez Saint-Jean, S.S.; Perez-Prieto, S.I.; Tafalla, C. The pyloric caeca area is a major site for IgM(+) and IgT(+) B cell recruitment in response to oral vaccination in rainbow trout. PLoS ONE 2013, 8, e66118. [Google Scholar] [CrossRef] [PubMed]
- Joosten, P.H.; Engelsma, M.Y.; van der Zee, M.D.; Rombout, J.H. Induction of oral tolerance in carp (Cyprinus carpio L.) after feeding protein antigens. Vet. Immunol. Immunopathol. 1997, 60, 187–196. [Google Scholar]
- Novoa, B.; Barja, J.L.; Figueras, A. Entry and sequential distribution of an aquatic birnavirus in turbot (Scophthdmus maximus). Aquaculture 1995, 131, 1–9. [Google Scholar] [CrossRef]
- Rombout, J.W.; Blok, L.J.; Lamers, C.H.; Egberts, E. Immunization of carp (Cyprinus carpio) with a Vibrio anguillarum bacterin: Indications for a common mucosal immune system. Dev. Comp. Immunol. 1986, 10, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Rombout, J.H.W.M.; van den Berg, A.A.; van den Berg, C.T.G.A.; Witte, P.; Egberts, E. Immunological importance of the second gut segment of carp. III. Systemic and/or mucosal immune responses after immunization with soluble or particulate antigen. J. Fish Bio. 1989, 35, 179–186. [Google Scholar] [CrossRef]
- Sundh, H.; Olsen, R.E.; Fridell, F.; Gadan, K.; Evensen, O.; Glette, J.; Taranger, G.L.; Myklebust, R.; Sundell, K. The effect of hyperoxygenation and reduced flow in fresh water and subsequent infectious pancreatic necrosis virus challenge in sea water, on the intestinal barrier integrity in Atlantic salmon, Salmo salar L. J. Fish Dis. 2009, 32, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Christie, K.E.; Hjeltnes, B. Infeksiøs pankreas nekrose-IPN. In Fiskehelse-Sykdommer, Forebygging Behandling; Poppe, T., Ed.; John Grieg Forlag AS: Fyllingsdalen, Norway, 1990; pp. 190–192. [Google Scholar]
- Myrmel, M.; Modahl, I.; Nygaard, H.; Lie, K.M. Infectious pancreatic necrosis virus in fish by-products is inactivated with inorganic acid (pH 1) and base (pH 12). J. Fish Dis. 2014, 37, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, H.; Modahl, I.; Myrmel, M. Thermal inactivation of infectious pancreatic necrosis virus in a peptone-salt medium mimicking the water-soluble phase of hydrolyzed fish by-products. Appl. Environ. Microbiol. 2012, 78, 2446–2448. [Google Scholar] [CrossRef] [PubMed]
- Khimmakthong, U.; Deshmukh, S.; Chettri, J.K.; Bojesen, A.M.; Kania, P.W.; Dalsgaard, I.; Buchmann, K. Tissue specific uptake of inactivated and live Yersinia ruckeri in rainbow trout (Oncorhynchus mykiss): Visualization by immunohistochemistry and in situ hybridization. Microb. Pathog. 2013, 59–60, 33–41. [Google Scholar]
- Ohtani, M.; Villumsen, K.R.; Koppang, E.O.; Raida, M.K. Global 3D Imaging of Yersinia ruckeri Bacterin Uptake in Rainbow Trout Fry. PLoS ONE 2015, 10, e0117263. [Google Scholar] [CrossRef] [PubMed]
- Mutoloki, S.; Alexandersen, S.; Evensen, O. Sequential study of antigen persistence and concomitant inflammatory reactions relative to side-effects and growth of Atlantic salmon (Salmo salar L.) following intraperitoneal injection with oil-adjuvanted vaccines. Fish Shellfish Immunol. 2004, 16, 633–644. [Google Scholar]
- Agius, C.; Roberts, R.J. Melano-macrophage centres and their role in fish pathology. J. Fish Dis. 2003, 26, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Espenes, A.; Press, C.M.; Dannevig, B.H.; Landsverk, T. Immune-complex trapping in the splenic ellipsoids of rainbow trout (Oncorhynchus mykiss). Cell Tissue Res. 1995, 282, 41–48. [Google Scholar] [PubMed]
- Ellis, A.E.; Sousa, M.D. Phylogeny of Lymphoid System .1. Study of Fate of Circulating Lymphocytes in Plaice. Eur. J. Immunol. 1974, 4, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Munang'andu, H.M.; Fredriksen, B.N.; Mutoloki, S.; Dalmo, R.A.; Evensen, O. Antigen dose and humoral immune response correspond with protection for inactivated infectious pancreatic necrosis virus vaccines in Atlantic salmon (Salmo salar L). Vet. Res. 2013, 44, e7. [Google Scholar] [CrossRef]
- Kryvi, H.; Totland, G.K. Fiskeanatomi; Høyskoleforlaget AS-Norwegian Academic Press: Kristiansand, Norway, 1997. [Google Scholar]
- Petersen, L.H.; Dzialowski, E.; Huggett, D.B. The interactive effects of a gradual temperature decrease and long-term food deprivation on cardiac and hepatic blood flows in rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2011, 160, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Thorarensen, H.; McLean, E.; Donaldson, E.M.; Farrell, A.P. The blood vasculature of the gastrointestinal tract in chinook, Oncorhynchus tshawytscha (Walbaum), and coho, O. kisutch (Walbaum), salmon. J. Fish Biol. 1991, 38, 525–531. [Google Scholar] [CrossRef]
- Munang'andu, H.M.; Fredriksen, B.N.; Mutoloki, S.; Brudeseth, B.; Kuo, T.Y.; Marjara, I.S.; Dalmo, R.A.; Evensen, O. Comparison of vaccine efficacy for different antigen delivery systems for infectious pancreatic necrosis virus vaccines in Atlantic salmon (Salmo salar L.) in a cohabitation challenge model. Vaccine 2012, 30, 4007–4016. [Google Scholar] [CrossRef] [PubMed]
- Evensen, O.; Rimstad, E. Immunohistochemical identification of infectious pancreatic necrosis virus in paraffin-embedded tissues of Atlantic salmon (Salmo salar). J. Vet. Diagn. Invest. 1990, 2, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Evensen, O.; Lorenzen, E. An immunohistochemical study of Flexibacter psychrophilus infection in experimentally and naturally infected rainbow trout (Oncorhynchus mykiss) fry. Dis. Aquat. Organ. 1996, 25, 53–61. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Evensen, Ø.; Mutoloki, S. IPNV Antigen Uptake and Distribution in Atlantic Salmon Following Oral Administration. Viruses 2015, 7, 2507-2517. https://doi.org/10.3390/v7052507
Chen L, Evensen Ø, Mutoloki S. IPNV Antigen Uptake and Distribution in Atlantic Salmon Following Oral Administration. Viruses. 2015; 7(5):2507-2517. https://doi.org/10.3390/v7052507
Chicago/Turabian StyleChen, Lihan, Øystein Evensen, and Stephen Mutoloki. 2015. "IPNV Antigen Uptake and Distribution in Atlantic Salmon Following Oral Administration" Viruses 7, no. 5: 2507-2517. https://doi.org/10.3390/v7052507
APA StyleChen, L., Evensen, Ø., & Mutoloki, S. (2015). IPNV Antigen Uptake and Distribution in Atlantic Salmon Following Oral Administration. Viruses, 7(5), 2507-2517. https://doi.org/10.3390/v7052507







