Potential for Co-Infection of a Mosquito-Specific Flavivirus, Nhumirim Virus, to Block West Nile Virus Transmission in Mosquitoes
Abstract
:1. Introduction
2. Material and Methods
2.1. In Vitro Assessment of SIE
2.2. Experimental Infection of NHUV in Cx. pipiens and Cx. quinquefasciatus
2.3. RT-PCR and Immunofluorescence Detection of NHUV Infection
2.4. Vertical Transmission Assessment
2.5. Dual infection Vector Competence Assay
3. Results
3.1. In Vitro SIE Assessment
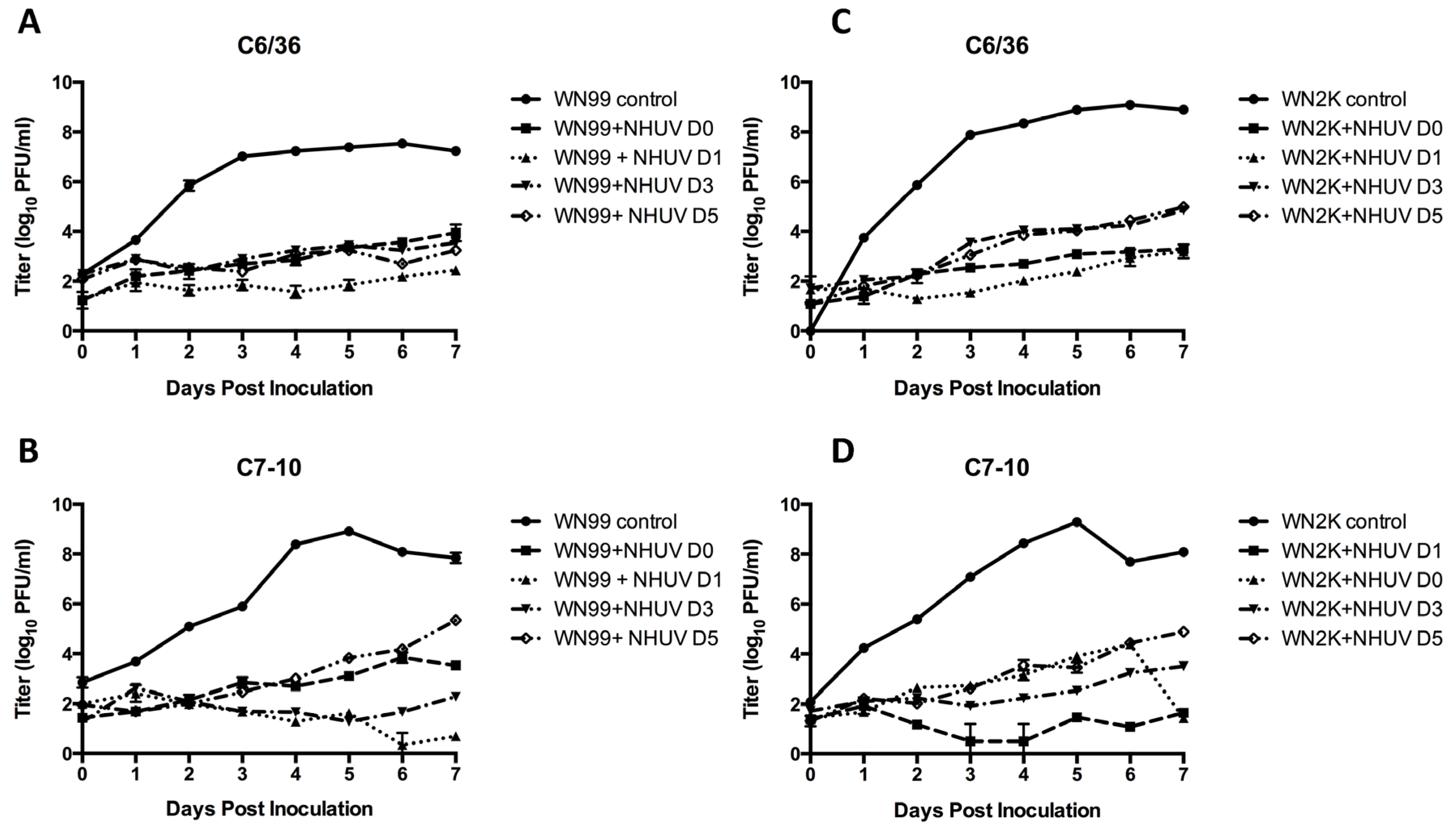
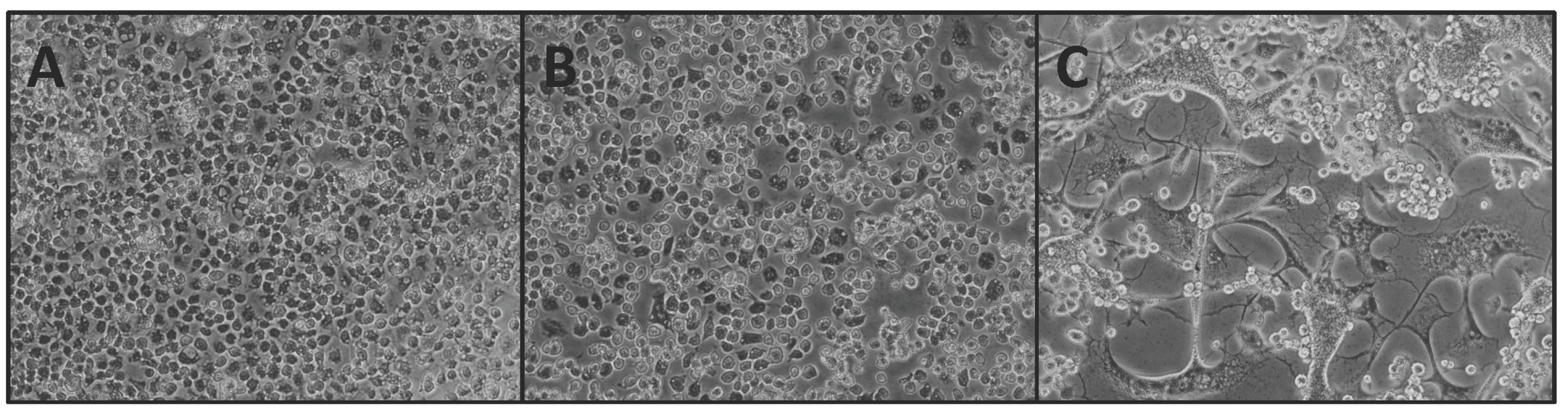
3.2. Experimental Infection of NHUV in Cx. pipiens and Cx. quinquefasciatus
3.3. Vertical Transmission Assay
3.4. Co-Infection of NHUV/WNV in Cx. pipiens mosquitoes
| Treatment Group | Infection (%) | Transmission (%) |
|---|---|---|
| NHUV + WNV | 33/33 (100) | 15/33 (45.5) |
| Sham + WNV | 11/11 (100) | 6/11 (54.6) |
| Treatment Group | Bodies (PFU/Mosquito) | Legs (PFU/Mosquito) | Saliva (PFU/Expectorant) |
|---|---|---|---|
| NHUV + WNV | 5.8 ± 1.1 * | 2.4 ± 0.7 | 0.6 ± 0.9 |
| Sham + WNV | 6.2 ± 0.2 | 2.5 ± 0.4 | 0.8 ± 1.0 |
3.5. Co-Infection of NHUV/WNV in Cx. quinquefasciatus mosquitoes
| Sampling | Treatment Group | Infection Rate (%) | Transmission Rate (%) |
|---|---|---|---|
| 3 dpi | NHUV + WNV | 19/21 (91) | 0/21 (0) |
| WNV | 32/32 (100) | 7/32 (21.8) | |
| 5 dpi | NHUV + WNV | 38/38 (100) | 19/38 (50) |
| WNV | 32/32 (100) | 11/32 (34) | |
| 7 dpi | NHUV + WNV | 30/30 (100) | 10/30 (33.3) |
| WNV | 25/25 (100) | 19/25 (76) * | |
| 9 dpi | NHUV + WNV | 21/21 (100) | 5/21 (23.8) |
| WNV | 27/27 (100) | 18/27 (66.7) * |
| Sampling | Treatment Group | Bodies (PFU/Mosquito) * | Saliva (PFU/Expectorant) * |
|---|---|---|---|
| 3 dpi | NHUV + WNV | 5.2 ± 1.9 | ND † |
| WNV | 6.5 ± 1.1 | 0.3 ± 0.6 | |
| 5 dpi | NHUV + WNV | 6.7 ± 0.5 | 1.1 ± 1.3 |
| WNV | 6.4 ± 0.5 | 0.7 ± 1.1 | |
| 7 dpi | NHUV + WNV | 6.2 ± 0.4 | 0.8 ± 0.2 |
| WNV | 6.2 ± 0.4 | 2.0 ± 1.4 | |
| 9 dpi | NHUV + WNV | 6.0 ± 0.3 | 0.4 ± 0.9 |
| WNV | 6.2 ± 0.3 | 1.8 ± 1.6 |
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chambers, T.J.; Hahn, C.S.; Galler, R.; Rice, C.M. Flavivirus genome organization, expression, and replication. Annu. Rev. Microbiol. 1990, 44, 649–688. [Google Scholar] [CrossRef] [PubMed]
- Firth, A.E.; Atkins, J.F. A conserved predicted pseudoknot in the NS2A-encoding sequence of West Nile and Japanese encephalitis flaviviruses suggests NS1′ may derive from ribosomal frameshifting. Virol. J. 2009, 6. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, R.L.; Mackenzie, J.M. A conserved peptide in West Nile virus NS4A protein contributes to proteolytic processing and is essential for replication. J. Virol. 2011, 85, 11274–11282. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.; Moureau, G.; Kitchen, A.; Gould, E.A.; de Lamballerie, X.; Holmes, E.C.; Harbach, R.E. Molecular evolution of the insect-specific flaviviruses. J. Gen. Virol. 2012, 93, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Gaunt, M.W.; Sall, A.A.; de Lamballerie, X.; Falconar, A.K.; Dzhivanian, T.I.; Gould, E.A. Phylogenetic relationships of flaviviruses correlate with their epidemiology, disease association and biogeography. J. Gen. Virol. 2001, 82, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Kenney, J.L.; Solberg, O.D.; Langevin, S.A.; Brault, A.C. Characterization of a novel insect-specific flavivirus from Brazil: Potential for inhibition of infection of arthropod cells with medically important flaviviruses. J. Gen. Virol. 2014, 95, 2796–2808. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Grubaugh, N.D.; Kondig, J.P.; Turell, M.J.; Kim, H.C.; Klein, T.A.; O’Guinn, M.L. Isolation and genomic characterization of Chaoyang virus strain ROK144 from Aedes vexans nipponii from the Republic of Korea. Virology 2013, 435, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.S.; An, S.Y.; Wang, Y.; Han, Y.; Guo, J.Q. A new virus of Flavivirus: Chaoyang virus isolated in Liaoning province. Chin. Public Health 2009, 26, 769–772. [Google Scholar]
- Blitvich, B.; Firth, A. Insect-Specific Flaviviruses: A Systematic Review of Their Discovery, Host Range, Mode of Transmission, Superinfection Exclusion Potential and Genomic Organization. Viruses 2015, 7, 1927–1959. [Google Scholar] [CrossRef] [PubMed]
- Kolodziejek, J.; Pachler, K.; Bin, H.; Mendelson, E.; Shulman, L.; Orshan, L.; Nowotny, N. Barkedji virus, a novel mosquito-borne flavivirus identified in Culex perexiguus mosquitoes, Israel, 2011. J. Gen. Virol. 2013, 94 Pt 11, 2449–2457. [Google Scholar] [CrossRef] [PubMed]
- Junglen, S.; Kopp, A.; Kurth, A.; Pauli, G.; Ellerbrok, H.; Leendertz, F.H. A new flavivirus and a new vector: Characterization of a novel flavivirus isolated from uranotaenia mosquitoes from a tropical rain forest. J. Virol. 2009, 83, 4462–4468. [Google Scholar] [CrossRef] [PubMed]
- Huhtamo, E.; Putkuri, N.; Kurkela, S.; Manni, T.; Vaheri, A.; Vapalahti, O.; Uzcategui, N.Y. Characterization of a novel flavivirus from mosquitoes in northern europe that is related to mosquito-borne flaviviruses of the tropics. J. Virol. 2009, 83, 9532–9540. [Google Scholar] [CrossRef] [PubMed]
- Huhtamo, E.; Cook, S.; Moureau, G.; Uzcategui, N.Y.; Sironen, T.; Kuivanen, S.; Putkuri, N.; Kurkela, S.; Harbach, R.E.; Firth, A.E.; et al. Novel flaviviruses from mosquitoes: Mosquito-specific evolutionary lineages within the phylogenetic group of mosquito-borne flaviviruses. Virology 2014, 464–465, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, J.; Cruz, C.; Guevara, C.; Astete, H.; Carey, C.; Kochel, T.J.; Morrison, A.C.; Williams, M.; Halsey, E.S.; Forshey, B.M. Characterization of a novel flavivirus isolated from Culex (Melanoconion) ocossa mosquitoes from Iquitos, Peru. J. Gen. Virol. 2013, 94, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, A.; Sanchez-Seco, M.P.; Palacios, G.; Molero, F.; Reyes, N.; Ruiz, S.; Aranda, C.; Marques, E.; Escosa, R.; Moreno, J.; et al. Novel flaviviruses detected in different species of mosquitoes in Spain. Vector-Borne Zoonotic Dis. 2012, 12, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, F.; Cerutti, F.; Ballardini, M.; Mosca, A.; Vitale, N.; Radaelli, M.C.; Desiato, R.; Prearo, M.; Pautasso, A.; Casalone, C.; et al. Molecular characterization of flaviviruses from field-collected mosquitoes in northwestern Italy, 2011–2012. Parasites Vectors 2014, 7. [Google Scholar] [CrossRef] [PubMed]
- Pauvolid-Correa, A.; Solberg, O.; Couto-Lima, D.; Kenney, J.; Serra-Freire, N.; Brault, A.; Nogueira, R.; Langevin, S.; Komar, N. Nhumirim virus, a novel flavivirus isolated from mosquitoes from the Pantanal, Brazil. Arch. Virol. 2015, 160, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Whitehorn, J.; Simmons, C.P. The pathogenesis of dengue. Vaccine 2011, 29, 7221–7228. [Google Scholar] [CrossRef] [PubMed]
- Solomon, T.; Vaughn, D.W. Pathogenesis and clinical features of Japanese encephalitis and West Nile virus infections. Curr. Top. Microbiol. Immunol. 2002, 267, 171–194. [Google Scholar] [PubMed]
- Reisen, W.K. St. Louis Encephalitis. In Encyclopedia of Virology, 3rd ed.; Van Regenmortel, M.H.V., Ed.; Elsevier: Oxford, UK, 2008; pp. 652–659. [Google Scholar]
- Mansfield, K.L.; Johnson, N.; Phipps, L.P.; Stephenson, J.R.; Fooks, A.R.; Solomon, T. Tick-borne Encephalitis Virus—A Review of an Emerging Zoonosis. J. Gen. Virol. 2009, 90 Pt 8, 1781–1794. [Google Scholar] [CrossRef] [PubMed]
- Zou, G.; Zhang, B.; Lim, P.Y.; Yuan, Z.; Bernard, K.A.; Shi, P.Y. Exclusion of West Nile virus superinfection through RNA replication. J. Virol. 2009, 83, 11765–11776. [Google Scholar] [CrossRef] [PubMed]
- Bolling, B.G.; Olea-Popelka, F.J.; Eisen, L.; Moore, C.G.; Blair, C.D. Transmission dynamics of an insect-specific flavivirus in a naturally infected Culex pipiens laboratory colony and effects of co-infection on vector competence for West Nile virus. Virology 2012, 427, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Hobson-Peters, J.; Yam, A.W.; Lu, J.W.; Setoh, Y.X.; May, F.J.; Kurucz, N.; Walsh, S.; Prow, N.A.; Davis, S.S.; Weir, R.; et al. A new insect-specific flavivirus from northern Australia suppresses replication of West Nile virus and Murray Valley encephalitis virus in co-infected mosquito cells. PLoS ONE 2013, 8, e56534. [Google Scholar] [CrossRef] [PubMed]
- Kent, R.J.; Crabtree, M.B.; Miller, B.R. Transmission of West Nile virus by Culex quinquefasciatus say infected with Culex Flavivirus Izabal. PLoS Negl. Trop. Dis. 2010, 4, e671. [Google Scholar] [CrossRef] [PubMed]
- Kuwata, R.; Isawa, H.; Hoshino, K.; Sasaki, T.; Kobayashi, M.; Maeda, K.; Sawabe, K. Analysis of Mosquito-Borne Flavivirus Superinfection in Culex tritaeniorhynchus (Diptera: Culicidae) Cells Persistently Infected with Culex Flavivirus (Flaviviridae). J. Med. Entomol. 2015, 52, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.L.; Smith, D.R.; Sanchez-Vargas, I.; Zhang, B.; Shi, P.Y.; Ebel, G.D. A positively selected mutation in the WNV 2K peptide confers resistance to superinfection exclusion in vivo. Virology 2014, 464C–465C, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Maharaj, P.D.; Bolling, B.G.; Anishchenko, M.; Reisen, W.K.; Brault, A.C. Genetic Determinants of Differential Oral Infection Phenotypes of West Nile and St. Louis Encephalitis Viruses in Culex spp. Mosquitoes. Am. J. Trop. Med. Hyg. 2014, 91, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Kuno, G. Universal diagnostic RT-PCR protocol for arboviruses. J. Virol. Methods 1998, 72, 27–41. [Google Scholar] [CrossRef]
- Kinney, R.M.; Huang, C.Y.; Whiteman, M.C.; Bowen, R.A.; Langevin, S.A.; Miller, B.R.; Brault, A.C. Avian virulence and thermostable replication of the North American strain of West Nile virus. J. Gen. Virol. 2006, 87 Pt 12, 3611–3622. [Google Scholar] [CrossRef] [PubMed]
- Saiyasombat, R.; Bolling, B.G.; Brault, A.C.; Bartholomay, L.C.; Blitvich, B.J. Evidence of efficient transovarial transmission of Culex flavivirus by Culex pipiens (Diptera: Culicidae). J. Med. Entomol. 2011, 48, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Newman, C.M.; Cerutti, F.; Anderson, T.K.; Hamer, G.L.; Walker, E.D.; Kitron, U.D.; Ruiz, M.O.; Brawn, J.D.; Goldberg, T.L. Culex Flavivirus and West Nile Virus Mosquito Coinfection and Positive Ecological Association in Chicago, United States. Vector-Borne Zoonotic Dis. 2011, 11, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Bolling, B.G.; Eisen, L.; Moore, C.G.; Blair, C.D. Insect-Specific Flaviviruses from Culex Mosquitoes in Colorado, with Evidence of Vertical Transmission. Am. J. Trop. Med. Hyg. 2011, 85, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Pepin, K.M.; Hanley, K.A. Density-dependent competitive suppression of sylvatic dengue virus by endemic dengue virus in cultured mosquito cells. Vector-Borne Zoonotic Dis. 2008, 8, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Beaty, B.J.; Sundin, D.R.; Chandler, L.J.; Bishop, D.H. Evolution of bunyaviruses by genome reassortment in dually infected mosquitoes (Aedes triseriatus). Science 1985, 230, 548–550. [Google Scholar] [CrossRef] [PubMed]
- Davies, C.R.; Jones, L.D.; Nuttall, P.A. Viral interference in the tick, Rhipicephalus appendiculatus. I. Interference to oral superinfection by Thogoto virus. J. Gen. Virol. 1989, 70 Pt 9, 2461–2468. [Google Scholar] [CrossRef] [PubMed]
- Eaton, B.T. Heterologous interference in Aedes albopictus cells infected with alphaviruses. J. Virol. 1979, 30, 45–55. [Google Scholar] [PubMed]
- El Hussein, A.; Ramig, R.F.; Holbrook, F.R.; Beaty, B.J. Asynchronous mixed infection of Culicoides variipennis with bluetongue virus serotypes 10 and 17. J. Gen. Virol. 1989, 70 Pt 12, 3355–3362. [Google Scholar] [CrossRef] [PubMed]
- Karpf, A.R.; Lenches, E.; Strauss, E.G.; Strauss, J.H.; Brown, D.T. Superinfection exclusion of alphaviruses in three mosquito cell lines persistently infected with Sindbis virus. J. Virol. 1997, 71, 7119–7123. [Google Scholar] [PubMed]
- Singh, I.R.; Suomalainen, M.; Varadarajan, S.; Garoff, H.; Helenius, A. Multiple mechanisms for the inhibition of entry and uncoating of superinfecting Semliki Forest virus. Virology 1997, 231, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Sundin, D.R.; Beaty, B.J. Interference to oral superinfection of Aedes triseriatus infected with La Crosse virus. Am. J. Trop. Med. Hyg. 1988, 38, 428–432. [Google Scholar] [PubMed]
- Brackney, D.E.; Beane, J.E.; Ebel, G.D. RNAi targeting of West Nile virus in mosquito midguts promotes virus diversification. PLoS Pathog. 2009, 5, e1000502. [Google Scholar] [CrossRef]
- Brackney, D.E.; Scott, J.C.; Sagawa, F.; Woodward, J.E.; Miller, N.A.; Schilkey, F.D.; Mudge, J.; Wilusz, J.; Olson, K.E.; Blair, C.D.; et al. C6/36 Aedes albopictus cells have a dysfunctional antiviral RNA interference response. PLoS Negl. Trop. Dis. 2010, 4, e856. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goenaga, S.; Kenney, J.L.; Duggal, N.K.; Delorey, M.; Ebel, G.D.; Zhang, B.; Levis, S.C.; Enria, D.A.; Brault, A.C. Potential for Co-Infection of a Mosquito-Specific Flavivirus, Nhumirim Virus, to Block West Nile Virus Transmission in Mosquitoes. Viruses 2015, 7, 5801-5812. https://doi.org/10.3390/v7112911
Goenaga S, Kenney JL, Duggal NK, Delorey M, Ebel GD, Zhang B, Levis SC, Enria DA, Brault AC. Potential for Co-Infection of a Mosquito-Specific Flavivirus, Nhumirim Virus, to Block West Nile Virus Transmission in Mosquitoes. Viruses. 2015; 7(11):5801-5812. https://doi.org/10.3390/v7112911
Chicago/Turabian StyleGoenaga, Silvina, Joan L. Kenney, Nisha K. Duggal, Mark Delorey, Gregory D. Ebel, Bo Zhang, Silvana C. Levis, Delia A. Enria, and Aaron C. Brault. 2015. "Potential for Co-Infection of a Mosquito-Specific Flavivirus, Nhumirim Virus, to Block West Nile Virus Transmission in Mosquitoes" Viruses 7, no. 11: 5801-5812. https://doi.org/10.3390/v7112911
APA StyleGoenaga, S., Kenney, J. L., Duggal, N. K., Delorey, M., Ebel, G. D., Zhang, B., Levis, S. C., Enria, D. A., & Brault, A. C. (2015). Potential for Co-Infection of a Mosquito-Specific Flavivirus, Nhumirim Virus, to Block West Nile Virus Transmission in Mosquitoes. Viruses, 7(11), 5801-5812. https://doi.org/10.3390/v7112911






