Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) Inhibits RNA-Mediated Gene Silencing by Targeting Ago-2
Abstract
:1. Introduction
2. Results
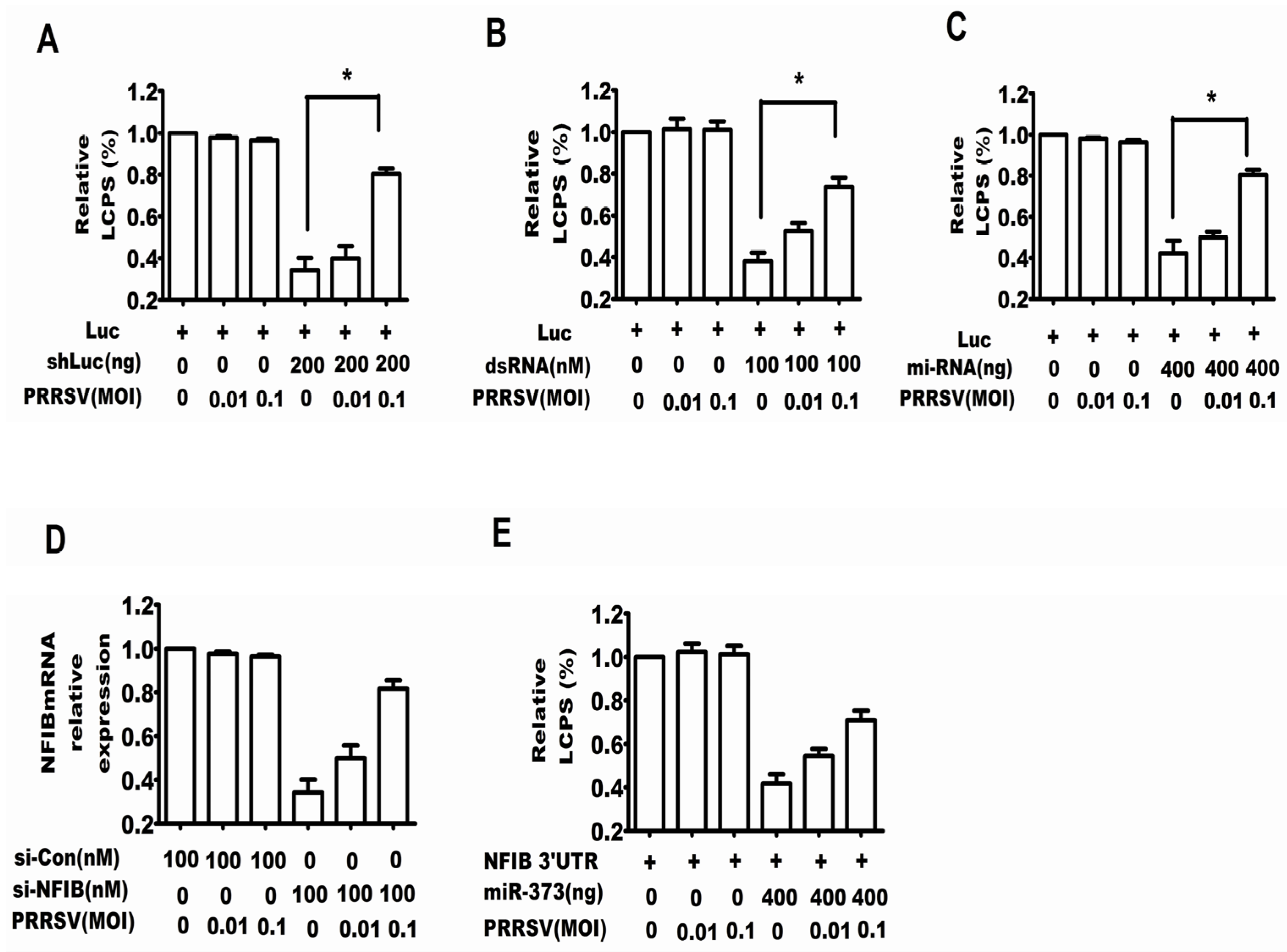
2.1. PRRSV Suppressed the RNA Silencing Induced by shRNA, dsRNA and miRNA
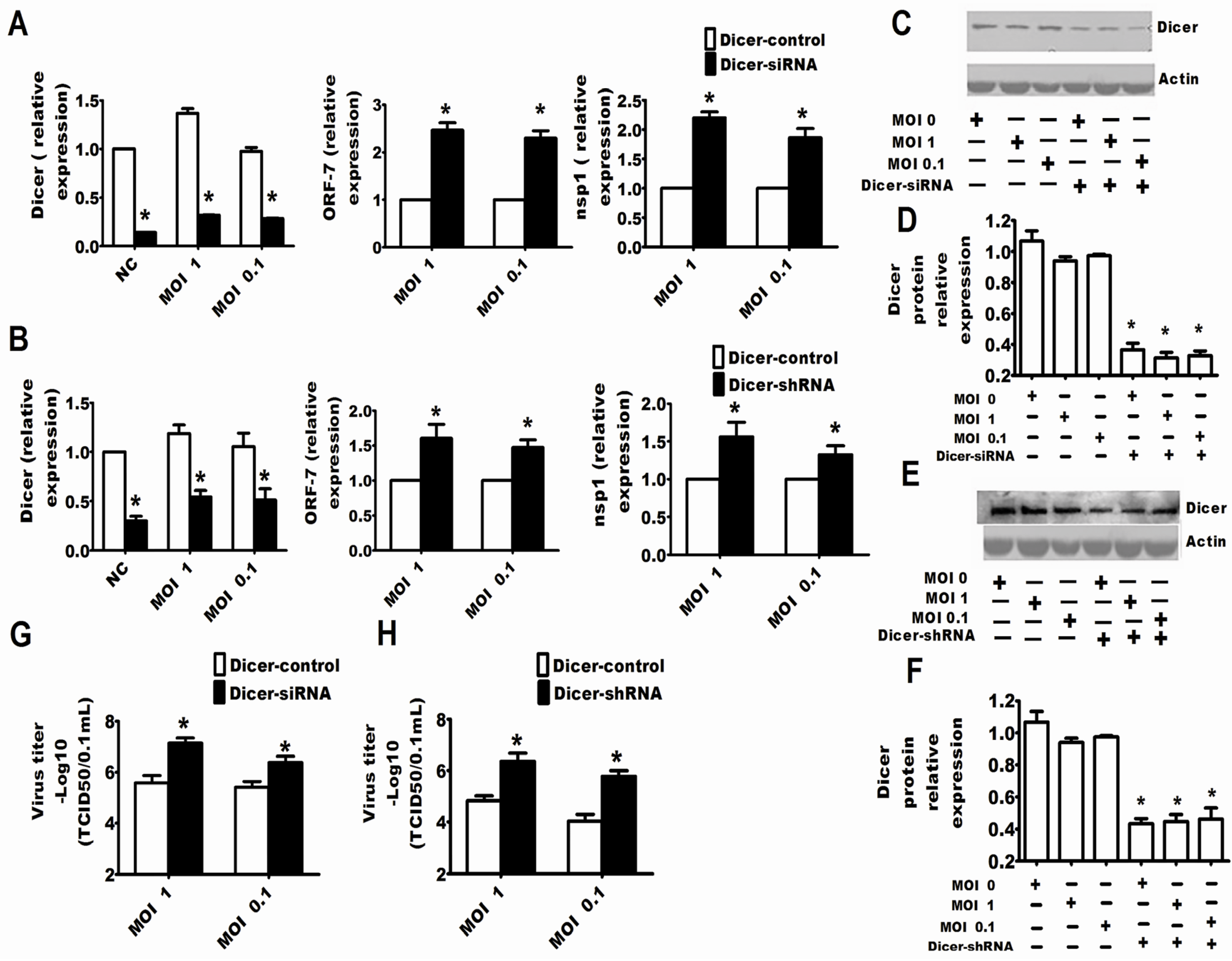
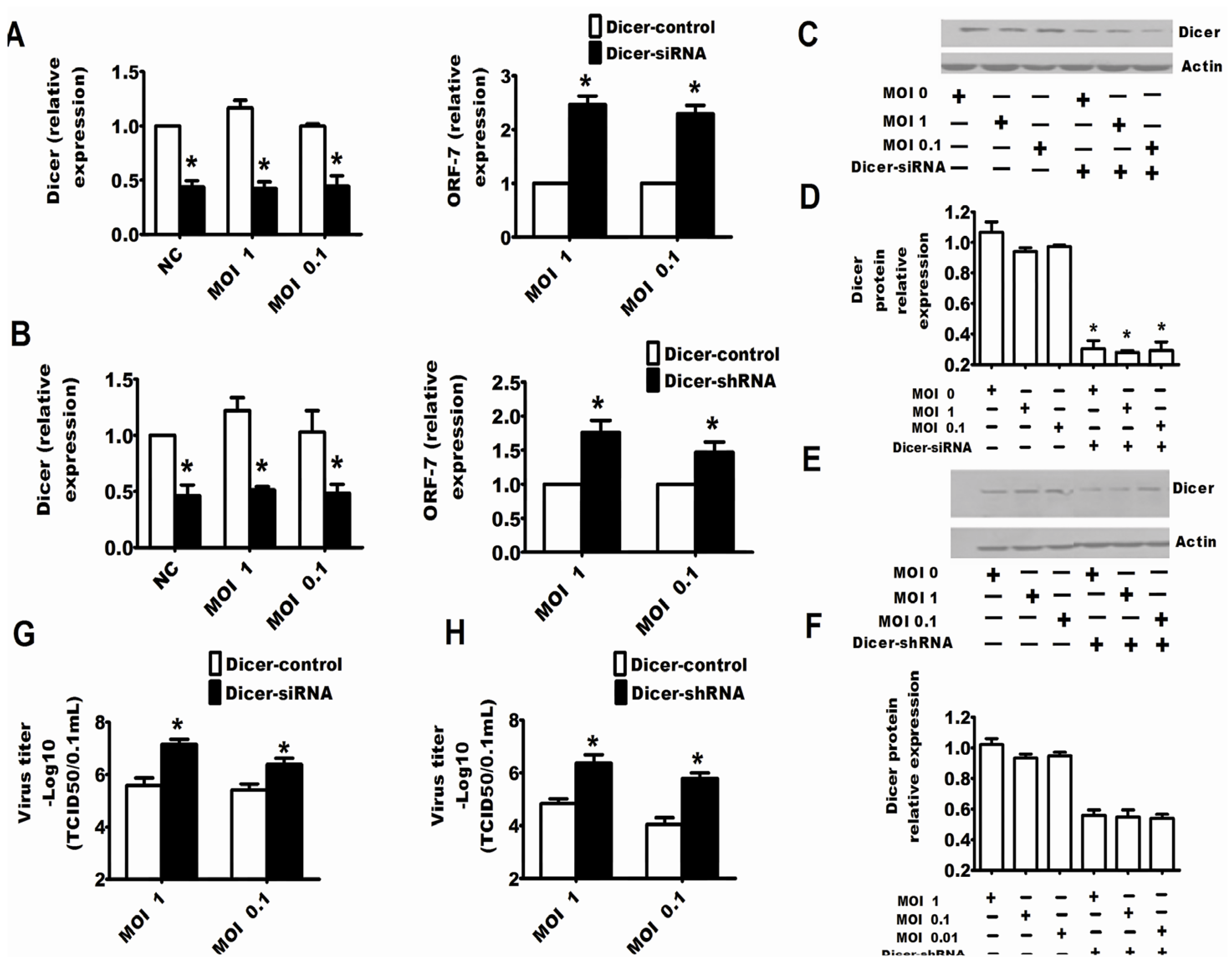
2.2. Dicer and Ago-2 Are Involved in Protection against PRRSV
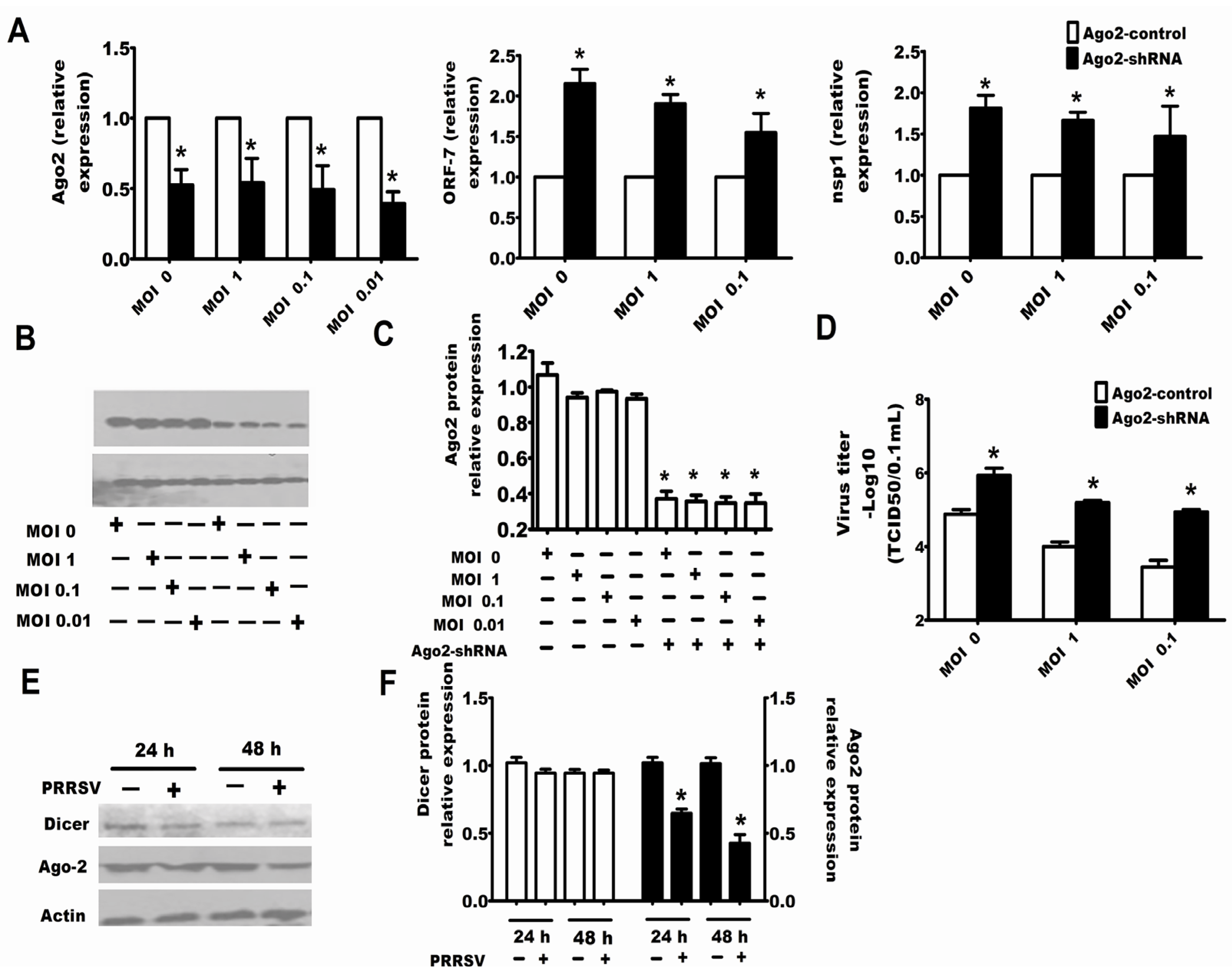
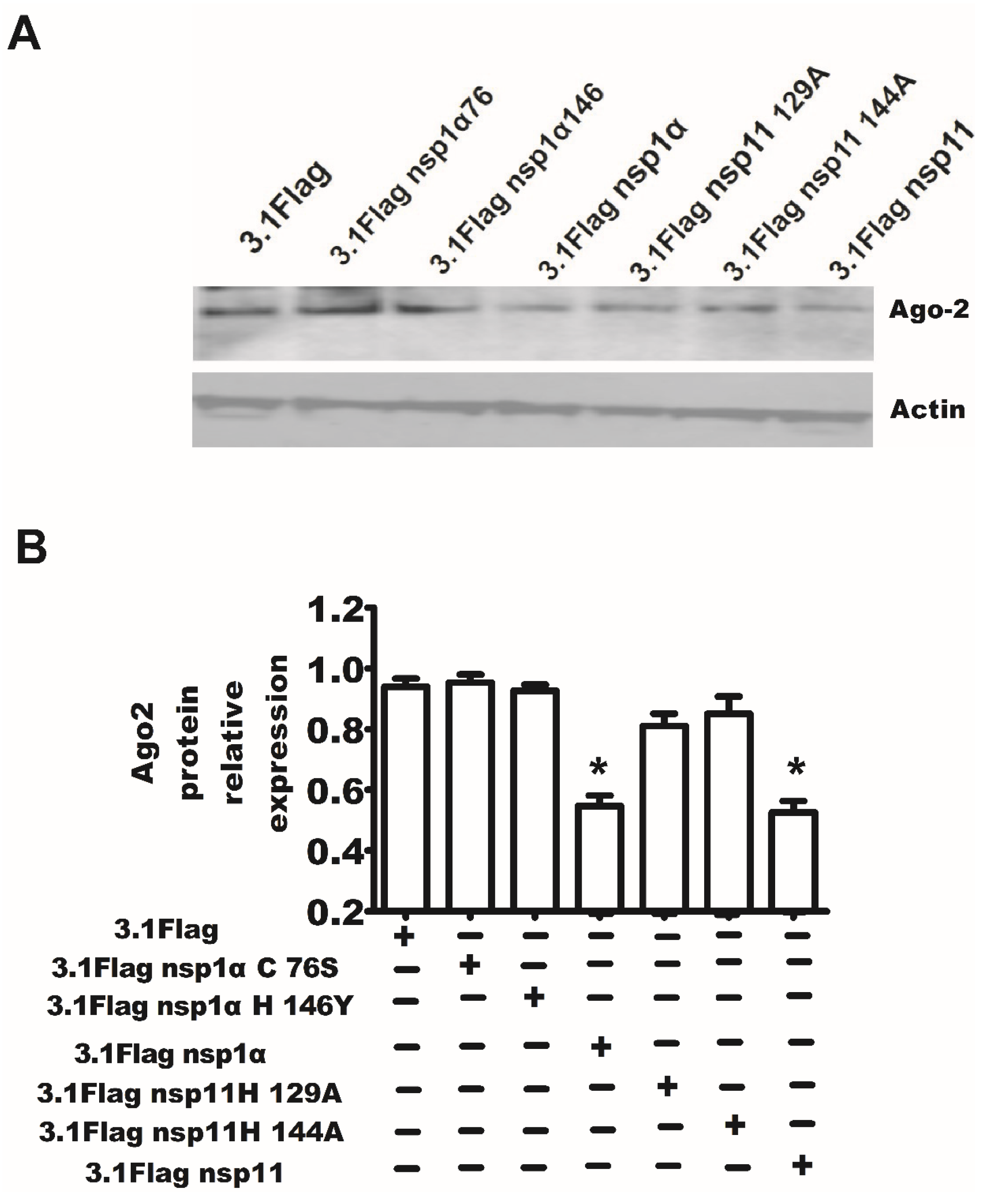
2.3. Downexpression of Ago-2 Protein Induced by PRRSV
2.4. PRRSV nsp1α and nsp11 as the Suppressors of RNA-Mediated Gene Silencing
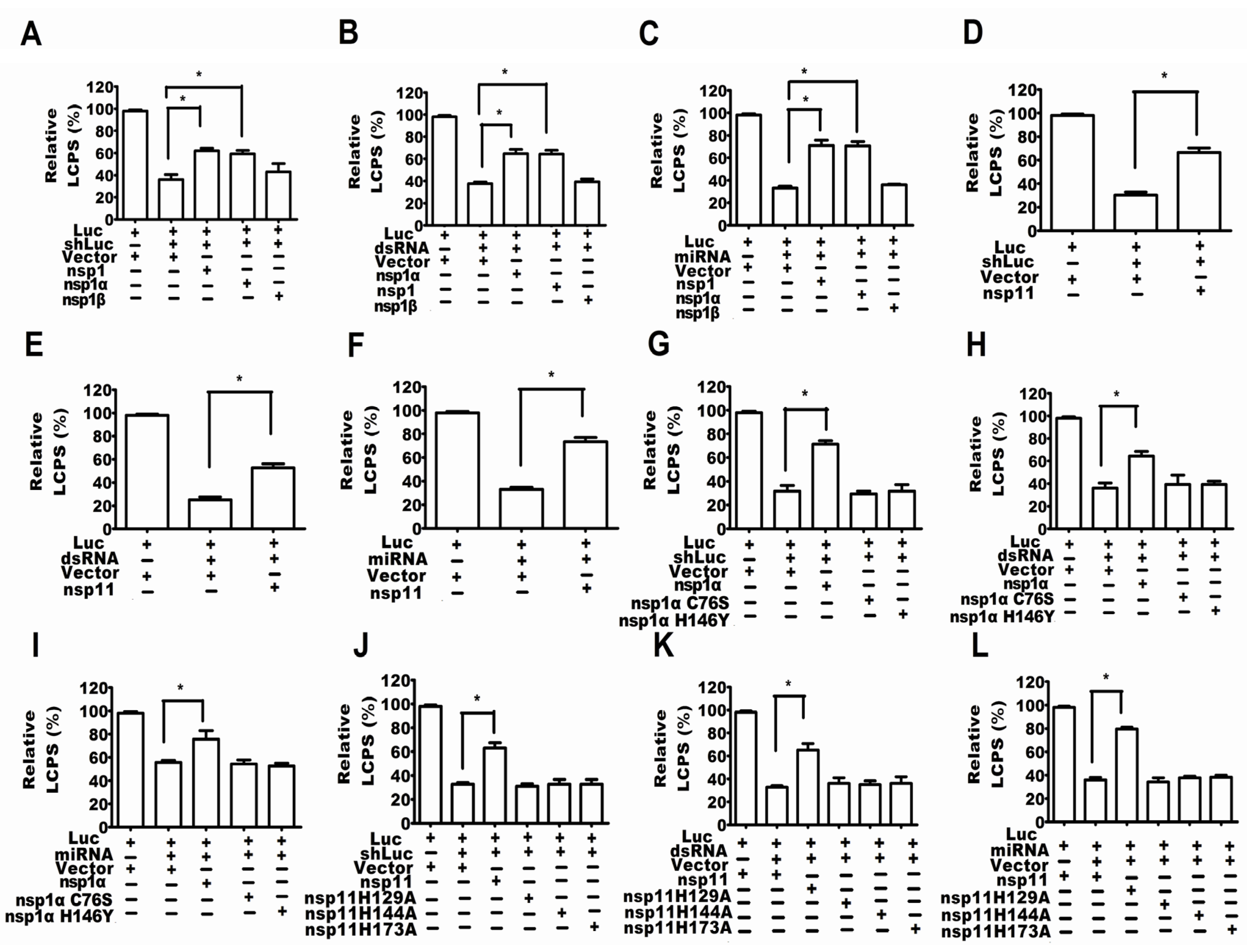
2.5. Activity of Papain-Like Cysteine Proteinase Was Essential for nsp1α as an RSS
2.6. The Endonuclease Activity Essential for nsp11 as a RSS
2.7. The Endonuclease Activity of nsp11 and the Activity of PCPα of nsp1α Were Essential for nsp11 and nsp1α to Downregulate Ago-2 Protein
3. Discussion
4. Materials and Methods
4.1. Animal Cell Culture and PRRSV Virus
4.2. Plasmids and siRNA
4.3. Antibody Used in This Study
4.4. Experiment for Transfection and the Luciferase Assay
4.5. Western Blot Experiments
4.6. RT-PCR
| Primers | Sequence (5′-3′) |
|---|---|
| Dicer-Forward | GCGGTCTGCCCTGGTCAACA |
| Dicer-Reverse | TCTCGCACAGGGGAACGGGG |
| ORF7-Forward | AAACCAGTCCAGAGGCAAGG |
| ORF7-Reverse | GCAAACTAAACTCCACAGTGTAA |
| nsp1-Forward | AGGGTGTTTATGGCGGAGGG |
| nsp1-Reverse | AACGTCCACCGGAGTGGCTC |
| GAPDH-Forward | TGACAACAGCCTCAAGATCG |
| GAPDH-Reverse | GTCTTCTGGGTGGCAGTGAT |
4.7. Viral Titers
4.8. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Filipowicz, W.; Bhattacharyya, S.N.; Sonenberg, N. Mechanisms of post-transcriptional regulation by microRNAs: Are the answers in sight? Nat. Rev. Genet. 2008, 9, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, P. Renaissance of mammalian endogenous RNAi. FEBS Lett. 2014, 588, 2550–2556. [Google Scholar] [CrossRef] [PubMed]
- Elkayam, E.; Kuhn, C.D.; Tocilj, A.; Haase, A.D.; Greene, E.M.; Hannon, G.J.; Joshua-Tor, L. The structure of human argonaute-2 in complex with miR-20a. Cell 2012, 150, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Schirle, N.T.; MacRae, I.J. The crystal structure of human Argonaute2. Science 2012, 336, 1037–1040. [Google Scholar] [CrossRef] [PubMed]
- Bivalkar-Mehla, S.; Vakharia, J.; Mehla, R.; Abreha, M.; Kanwar, J.R.; Tikoo, A.; Chauhan, A. Viral RNA silencing suppressors (RSS): Novel strategy of viruses to ablate the host RNA interference (RNAi) defense system. Virus Res. 2011, 155, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.W.; Voinnet, O. Antiviral immunity directed by small RNAs. Cell 2007, 130, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Chen, Z.J. Intrinsic antiviral immunity. Nat. Immunol. 2012, 13, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wei, C.; Li, Y. Viral suppression of RNA silencing. Sci. China Life Sci. 2012, 55, 109–118. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chinnappan, M.; Singh, A.K.; Kakumani, P.K.; Kumar, G.; Rooge, S.B.; Kumari, A.; Varshney, A.; Rastogi, A.; Singh, A.K.; Sarin, S.K.; et al. Key elements of the RNAi pathway are regulated by hepatitis B virus replication and HBx acts as a viral suppressor of RNA silencing. Biochem. J. 2014, 462, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Parameswaran, P.; Sklan, E.; Wilkins, C.; Burgon, T.; Samuel, M.A.; Lu, R.; Ansel, K.M.; Heissmeyer, V.; Einav, S.; Jackson, W.; et al. Six RNA viruses and forty-one hosts: Viral small RNAs and modulation of small RNA repertoires in vertebrate and invertebrate systems. PLoS Pathog. 2010, 6, e1000764. [Google Scholar] [CrossRef] [PubMed]
- Maillard, P.V.; Ciaudo, C.; Marchais, A.; Li, Y.; Jay, F.; Ding, S.W.; Voinnet, O. Antiviral RNA interference in mammalian cells. Science 2013, 342, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, J.; Han, Y.; Fan, X.; Ding, S.W. RNA interference functions as an antiviral immunity mechanism in mammals. Science 2013, 342, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Bennasser, Y.; Le, S.Y.; Benkirane, M.; Jeang, K.T. Evidence that HIV-1 encodes an siRNA and a suppressor of RNA silencing. Immunity 2005, 22, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Triboulet, R.; Mari, B.; Lin, Y.L.; Chable-Bessia, C.; Bennasser, Y.; Lebrigand, K.; Cardinaud, B.; Maurin, T.; Barbry, P.; Baillat, V.; et al. Suppression of microRNA-silencing pathway by HIV-1 during virus replication. Science 2007, 315, 1579–1582. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, M.; Jing, Q.; Georgel, P.; New, L.; Chen, J.; Mols, J.; Kang, Y.J.; Jiang, Z.; Du, X.; Cook, R.; et al. Hypersusceptibility to vesicular stomatitis virus infection in Dicer1-deficient mice is due to impaired miR24 and miR93 expression. Immunity 2007, 27, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Matskevich, A.A.; Moelling, K. Dicer is involved in protection against influenza A virus infection. J. Gen. Virol. 2007, 88, 2627–2635. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, D. Nidovirales: A new order comprising Coronaviridae and Arteriviridae. Arch. Virol. 1997, 142, 629–633. [Google Scholar] [PubMed]
- Mateu, E.; Diaz, I. The challenge of PRRS immunology. Vet. J. 2008, 177, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Muylkens, B.; Coupeau, D.; Dambrine, G.; Trapp, S.; Rasschaert, D. Marek’s disease virus microRNA designated Mdv1-pre-miR-M4 targets both cellular and viral genes. Arch. Virol. 2010, 155, 1823–1837. [Google Scholar] [CrossRef] [PubMed]
- Meister, G.; Landthaler, M.; Patkaniowska, A.; Dorsett, Y.; Teng, G.; Tuschl, T. Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol. Cell 2004, 15, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Meister, G.; Tuschl, T. Mechanisms of gene silencing by double-stranded RNA. Nature 2004, 431, 343–349. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Hannon, G.J. MicroRNAs: Small RNAs with a big role in gene regulation. Nat. Rev. Genet. 2004, 5, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, L.; Li, X.; Zhang, G.; Guo, J.; Zhao, D.; Chai, S.; Deng, R. Endoribonuclease activities of porcine reproductive and respiratory syndrome virus nsp11 was essential for nsp11 to inhibit IFN-β induction. Mol. Immunol. 2011, 48, 1568–1572. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, L.; Zhi, Y.; Xing, G.; Zhao, D.; Deng, R.; Zhang, G. Porcine reproductive and respiratory syndrome virus (PRRSV) could be sensed by professional β interferon-producing system and had mechanisms to inhibit this action in MARC-145 cells. Virus Res. 2010, 153, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Beura, L.K.; Sarkar, S.N.; Kwon, B.; Subramaniam, S.; Jones, C.; Pattnaik, A.K.; Osorio, F.A. Porcine reproductive and respiratory syndrome virus nonstructural protein 1β modulates host innate immune response by antagonizing IRF3 activation. J. Virol. 2010, 84, 1574–1584. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Krell, P.; Yoo, D. Nonstructural protein 1α subunit-based inhibition of NF-κB activation and suppression of interferon-β production by porcine reproductive and respiratory syndrome virus. Virology 2010. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhang, G.; Wang, L.; Li, X.; Zhi, Y.; Wang, F.; Fan, J.; Deng, R. The nonstructural protein 1 papain-like cysteine protease was necessary for porcine reproductive and respiratory syndrome virus nonstructural protein 1 to inhibit interferon-beta induction. DNA Cell. Biol. 2011, 30, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Kroese, M.V.; Zevenhoven-Dobbe, J.C.; Bos-de Ruijter, J.N.; Peeters, B.P.; Meulenberg, J.J.; Cornelissen, L.A.; Snijder, E.J. The nsp1α and nsp1 papain-like autoproteinases are essential for porcine reproductive and respiratory syndrome virus RNA synthesis. J. Gen. Virol. 2008, 89, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xue, F.; Guo, Y.; Ma, M.; Hao, N.; Zhang, X.C.; Lou, Z.; Li, X.; Rao, Z. Crystal structure of porcine reproductive and respiratory syndrome virus leader protease Nsp1alpha. J. Virol. 2009, 83, 10931–10940. [Google Scholar] [CrossRef] [PubMed]
- den Boon, J.A.; Faaberg, K.S.; Meulenberg, J.J.; Wassenaar, A.L.; Plagemann, P.G.; Gorbalenya, A.E.; Snijder, E.J. Processing and evolution of the N-terminal region of the arterivirus replicase ORF1a protein: identification of two papainlike cysteine proteases. J. Virol. 1995, 69, 4500–4505. [Google Scholar] [PubMed]
- Nedialkova, D.D.; Ulferts, R.; van den Born, E.; Lauber, C.; Gorbalenya, A.E.; Ziebuhr, J.; Snijder, E.J. Biochemical characterization of arterivirus nonstructural protein 11 reveals the nidovirus-wide conservation of a replicative endoribonuclease. J. Virol. 2009, 83, 5671–5682. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, K.A.; Hertzig, T.; Rozanov, M.; Bayer, S.; Thiel, V.; Gorbalenya, A.E.; Ziebuhr, J. Major genetic marker of nidoviruses encodes a replicative endoribonuclease. Proc. Natl. Acad. Sci. USA 2004, 101, 12694–12699. [Google Scholar] [CrossRef] [PubMed]
- Laneve, P.; Altieri, F.; Fiori, M.E.; Scaloni, A.; Bozzoni, I.; Caffarelli, E. Purification, cloning, and characterization of XendoU, a novel endoribonuclease involved in processing of intron-encoded small nucleolar RNAs in Xenopus laevis. J. Biol. Chem. 2003, 278, 13026–13032. [Google Scholar] [CrossRef] [PubMed]
- Ricagno, S.; Egloff, M.P.; Ulferts, R.; Coutard, B.; Nurizzo, D.; Campanacci, V.; Cambillau, C.; Ziebuhr, J.; Canard, B. Crystal structure and mechanistic determinants of SARS coronavirus nonstructural protein 15 define an endoribonuclease family. Proc. Natl. Acad. Sci. USA 2006, 103, 11892–11897. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kwang, J.; Yoon, I.J.; Joo, H.S.; Frey, M.L. Enhanced replication of porcine reproductive and respiratory syndrome (PRRS) virus in a homogeneous subpopulation of MA-104 cell line. Arch. Virol. 1993, 133, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Bi, Y.; Li, J.; Xie, Q.; Yang, H.; Liu, W. Cellular microRNA miR-26a suppresses replication of porcine reproductive and respiratory syndrome virus by activating innate antiviral immunity. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- He, Y.X.; Hua, R.H.; Zhou, Y.J.; Qiu, H.J.; Tong, G.Z. Interference of porcine reproductive and respiratory syndrome virus replication on MARC-145 cells using DNA-based short interfering RNAs. Antiviral Res. 2007, 74, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Chang, Y.; Zhang, X.; Wang, L.; Li, C.; Jiang, K.; Chen, J.; Wang, C.; Deng, R.; Fan, J.; et al. Small interfering RNA targeting nonstructural protein1 alpha (nsp1alpha) of porcine reproductive and respiratory syndrome virus (PRRSV) can reduce the replication of PRRSV in MARC-145 cells. Res. Vet. Sci. 2015, 99, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Jiang, P.; Li, Y.; Xu, J.; Jiang, W.; Wang, X. Inhibition of porcine reproductive and respiratory syndrome virus replication by short hairpin RNA in MARC-145 cells. Vet. Microbiol. 2006, 115, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Guo, X.K.; Wang, L.; Zhang, Q.; Li, N.; Chen, X.X.; Wang, Y.; Feng, W.H. MicroRNA 181 suppresses porcine reproductive and respiratory syndrome virus (PRRSV) infection by targeting PRRSV receptor CD163. J. Virol. 2013, 87, 8808–8812. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Guo, X.K.; Gao, L.; Huang, C.; Li, N.; Jia, X.; Liu, W.; Feng, W.H. MicroRNA-23 inhibits PRRSV replication by directly targeting PRRSV RNA and possibly by upregulating type I interferons. Virology 2014, 450, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Wan, B.; Qiao, S.; Li, P.; Jin, Q.; Liu, Y.; Bao, D.; Liu, M.; Wang, Y.; Zhang, G. Impairment of the antibody-dependent phagocytic function of PMNs through regulation of the FcgammaRs expression after porcine reproductive and respiratory syndrome virus infection. PLoS ONE 2013, 8, e66965. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Liu, H.; Mitchelson, K.; Rao, H.; Luo, M.; Xie, L.; Sun, Y.; Zhang, L.; Lu, Y.; Liu, R.; et al. MicroRNAs-372/373 promote the expression of hepatitis B virus through the targeting of nuclear factor I/B. Hepatology 2011, 54, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Qin, L.; Liu, G.; Zhao, S.; Peng, N.; Chen, X. Dynamic balance of pSTAT1 and pSTAT3 in C57BL/6 mice infected with lethal or nonlethal Plasmodium yoelii. Cell. Mol. Immunol. 2008, 5, 341–348. [Google Scholar] [CrossRef] [PubMed][Green Version]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Shi, X.; Zhang, X.; Wang, L.; Luo, J.; Xing, G.; Deng, R.; Yang, H.; Li, J.; Wang, A.; et al. Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) Inhibits RNA-Mediated Gene Silencing by Targeting Ago-2. Viruses 2015, 7, 5539-5552. https://doi.org/10.3390/v7102893
Chen J, Shi X, Zhang X, Wang L, Luo J, Xing G, Deng R, Yang H, Li J, Wang A, et al. Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) Inhibits RNA-Mediated Gene Silencing by Targeting Ago-2. Viruses. 2015; 7(10):5539-5552. https://doi.org/10.3390/v7102893
Chicago/Turabian StyleChen, Jing, Xibao Shi, Xiaozhuan Zhang, Li Wang, Jun Luo, Guangxu Xing, Ruiguang Deng, Hong Yang, Jinting Li, Aiping Wang, and et al. 2015. "Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) Inhibits RNA-Mediated Gene Silencing by Targeting Ago-2" Viruses 7, no. 10: 5539-5552. https://doi.org/10.3390/v7102893
APA StyleChen, J., Shi, X., Zhang, X., Wang, L., Luo, J., Xing, G., Deng, R., Yang, H., Li, J., Wang, A., & Zhang, G. (2015). Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) Inhibits RNA-Mediated Gene Silencing by Targeting Ago-2. Viruses, 7(10), 5539-5552. https://doi.org/10.3390/v7102893





