Anti-Glycoprotein G Antibodies of Herpes Simplex Virus 2 Contribute to Complete Protection after Vaccination in Mice and Induce Antibody-Dependent Cellular Cytotoxicity and Complement-Mediated Cytolysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Mice
2.3. Cells and Viruses
2.4. The mgG-2 Protein
2.5. Monoclonal Antibodies (MAbs) and Immune Sera
2.6. Adjuvant
2.7. Immunization and Challenge
2.8. Serum Samples and ELISA
2.9. CD4+ T Cell Proliferation Assay and Cytokine Detection
2.10. Passive Transfer of Immune Serum to Immunized µMT Mice
2.11. Passive Transfer of MAbs or Immune Serum to Naive Mice 48 h before Challenge
2.12. NT Assay
2.13. Antibody Dependent Cellular Cytotoxicity (ADCC) and Complement-Mediated Cytolysis (ACMC)
2.14. Statistics
3. Results
3.1. Detection of Antibodies in Vaccinated Mice
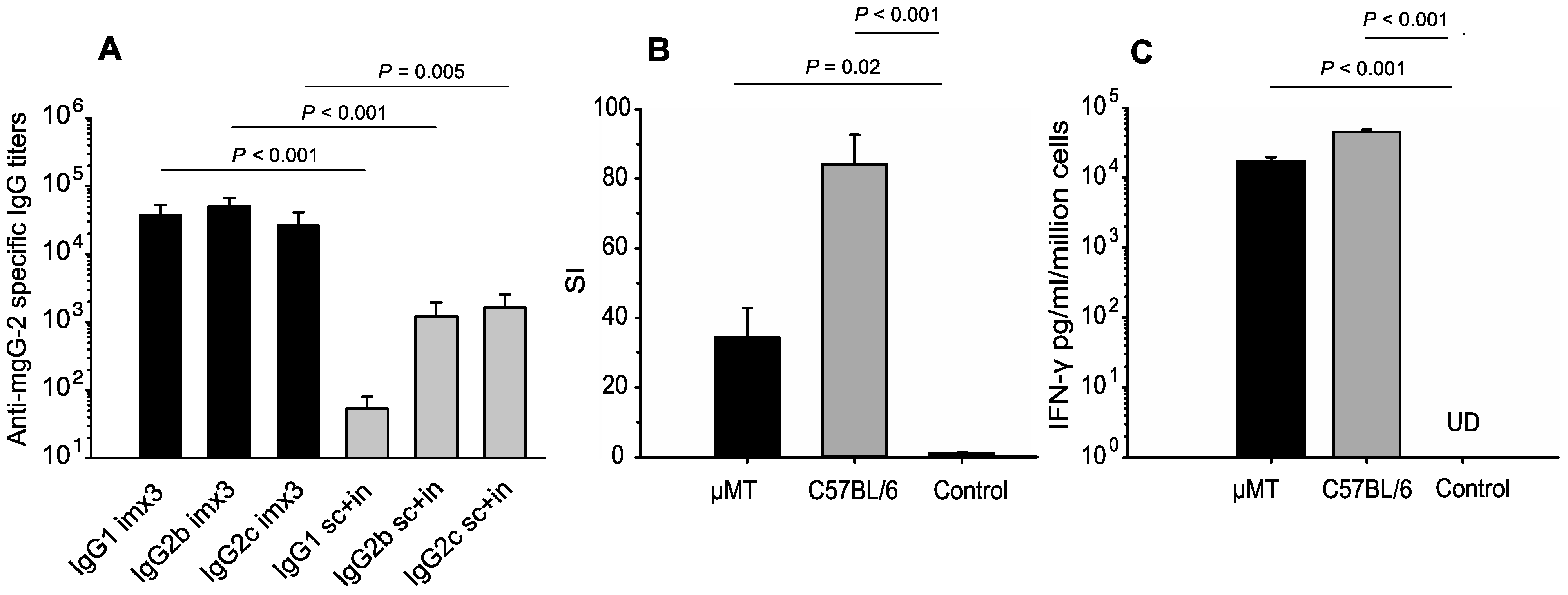
3.2. CD4+ T-Cell Responses Against mgG-2 and IFN-γ Production
3.3. Survival and Disease Score in Vaccinated C57BL/6 Mice
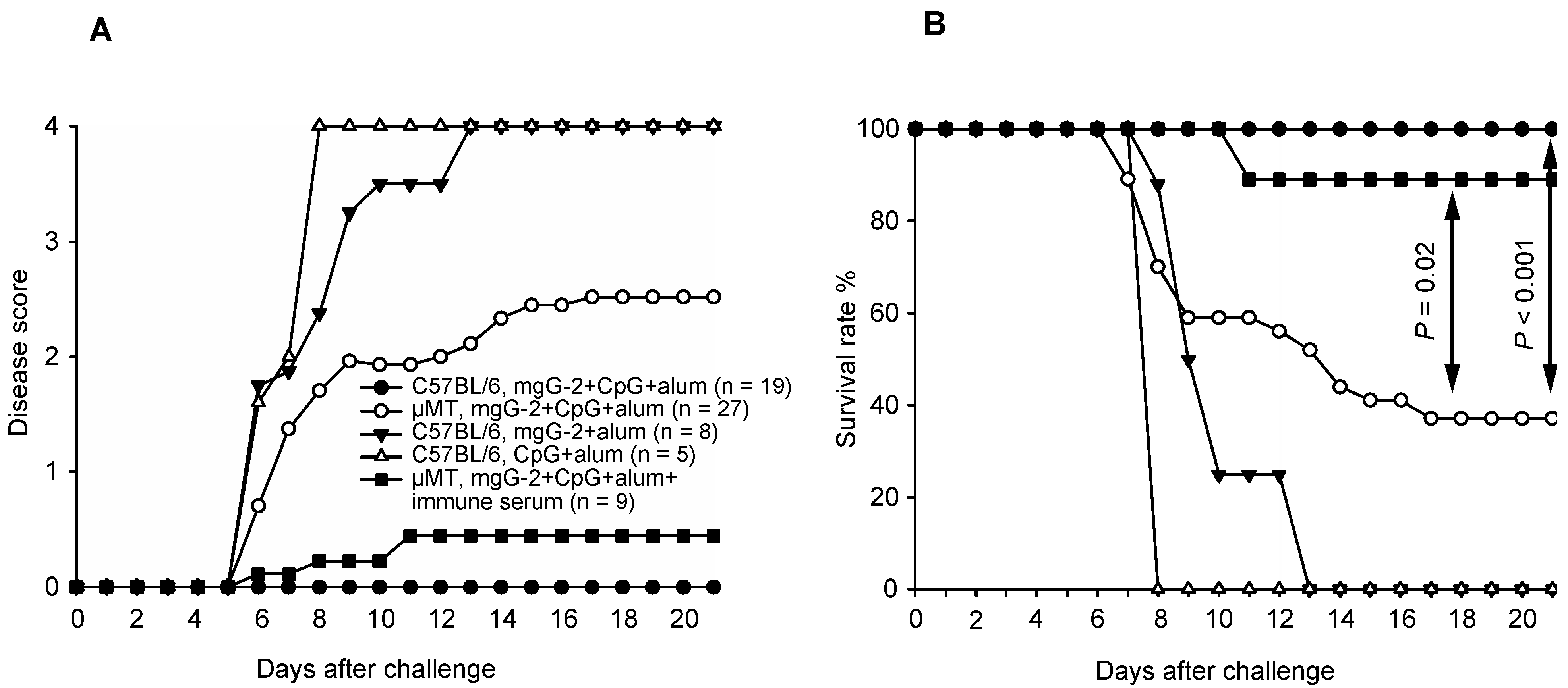
3.4. Survival and Disease Score in Vaccinated µMT Mice and Passive Transfer of Immune Serum
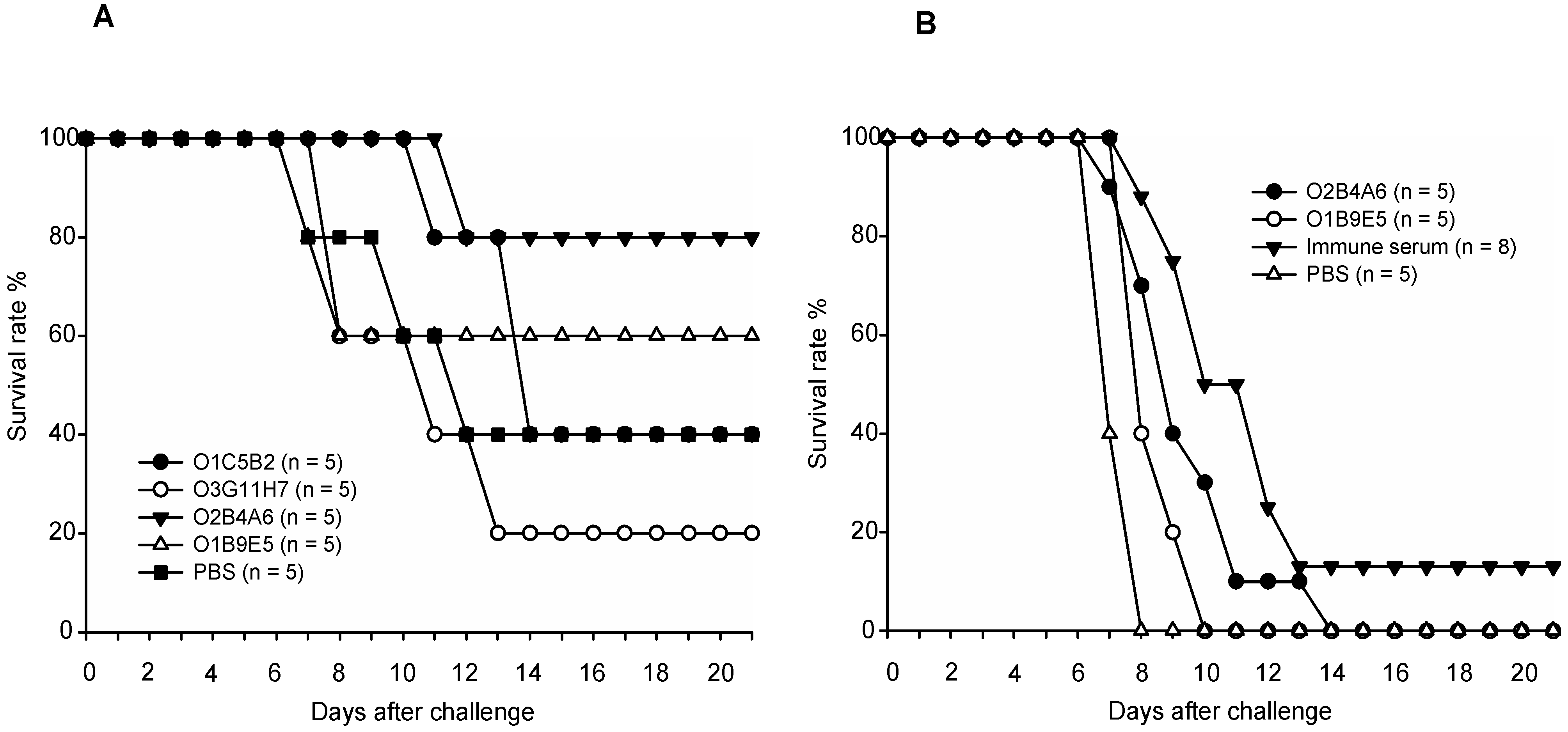
3.5. Passive Transfer of Anti-mgG-2 MAbs or Immune Serum to Naive C57BL/6 Mice
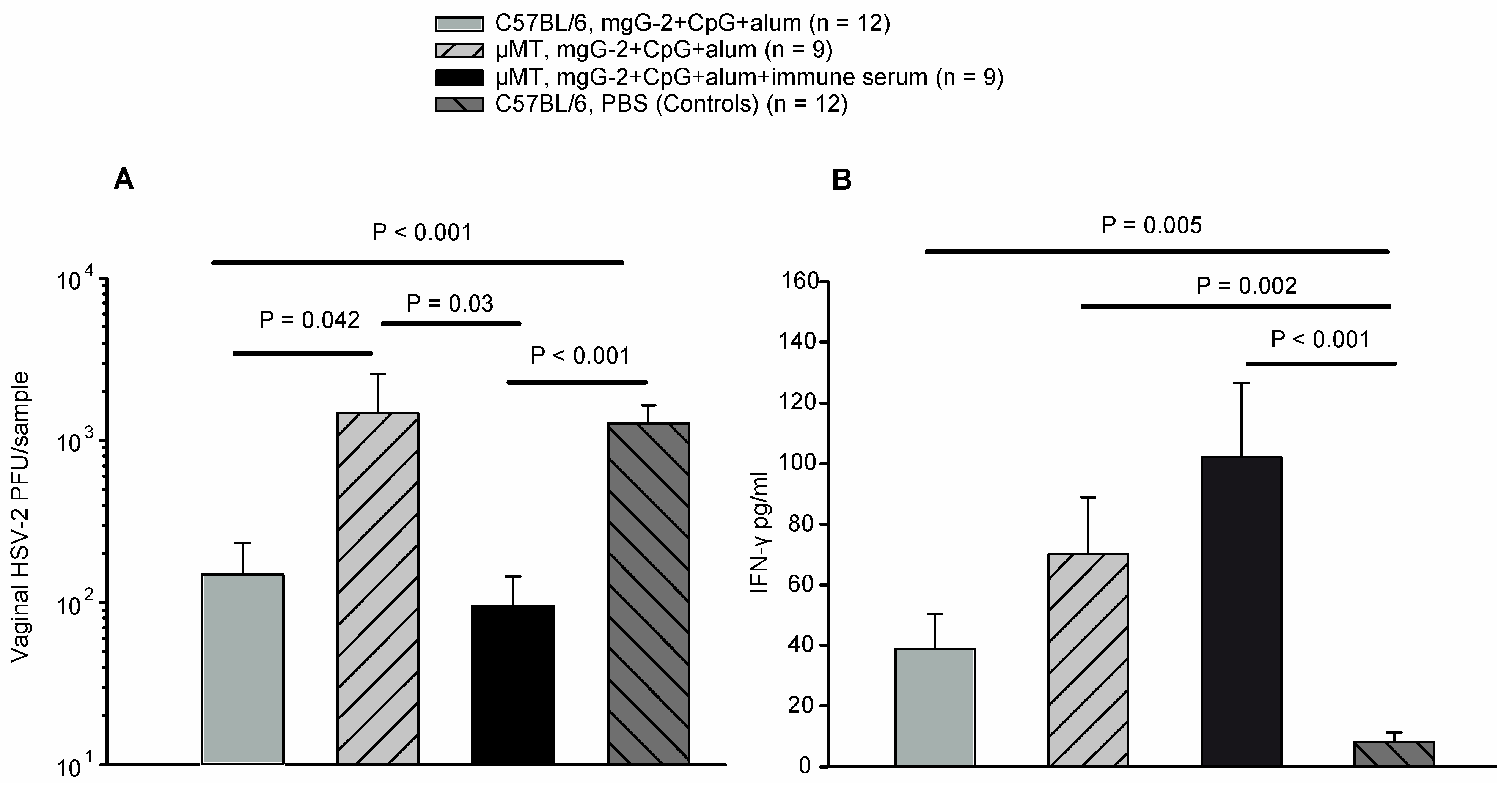
3.6. Viral Load and IFN-γ Response in Vaginal Washes
3.7. NT, ADCC and ACMC Activity in Sera from mgG-2 Vaccinated C57BL/6 Mice
| Method | Immunization schedule | |
|---|---|---|
| sc × 1 + in × 2 | im × 3 | |
| ADCC, titer ≥20 | 4/10 | 10/10a |
| ACMC, titer ≥25 | 5/10 | 19/19b |
| NTc, titer ≥20 | 0/10 | 0/19 |
4. Discussion
4.1. NT, ADCC and ACMC Activity of Anti-mgG-2 Antibodies
4.2. B-Cell KO Mice
4.3. Passive Transfer of Antibodies to Naive Mice
4.4. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Looker, K.J.; Garnett, G.P.; Schmid, G.P. An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. Bull. World Health Organ. 2008, 86, 805–812, A. [Google Scholar] [PubMed]
- Belshe, R.B.; Leone, P.A.; Bernstein, D.I.; Wald, A.; Levin, M.J.; Stapleton, J.T.; Gorfinkel, I.; Morrow, R.L.; Ewell, M.G.; Stokes-Riner, A.; et al. Efficacy results of a trial of a herpes simplex vaccine. N. Engl. J. Med. 2012, 366, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Immunology. Painful failure of promising genital herpes vaccine. Science 2010, 330, 304. [Google Scholar] [CrossRef] [PubMed]
- Corey, L.; Langenberg, A.G.; Ashley, R.; Sekulovich, R.E.; Izu, A.E.; Douglas, J.M., Jr.; Handsfield, H.H.; Warren, T.; Marr, L.; Tyring, S.; et al. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: Two randomized controlled trials. Chiron HSV vaccine study group. JAMA 1999, 282, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Stanberry, L.R.; Spruance, S.L.; Cunningham, A.L.; Bernstein, D.I.; Mindel, A.; Sacks, S.; Tyring, S.; Aoki, F.Y.; Slaoui, M.; Denis, M.; et al. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N. Engl. J. Med. 2002, 347, 1652–1661. [Google Scholar] [CrossRef] [PubMed]
- Gorander, S.; Svennerholm, B.; Liljeqvist, J.A. Secreted portion of glycoprotein G of herpes simplex virus type 2 is a novel antigen for type-discriminating serology. J. Clin. Microbiol. 2003, 41, 3681–3686. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.W.; Field, P.R.; Sjogren-Jansson, E.; Jeansson, S.; Cunningham, A.L. Indirect ELISA for the detection of HSV-2 specific IgG and IgM antibodies with glycoprotein G (gG-2). J. Virol. Methods 1992, 36, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Lee, F.K.; Coleman, R.M.; Pereira, L.; Bailey, P.D.; Tatsuno, M.; Nahmias, A.J. Detection of herpes simplex virus type 2-specific antibody with glycoprotein G. J. Clin. Microbiol. 1985, 22, 641–644. [Google Scholar] [PubMed]
- Svennerholm, B.; Olofsson, S.; Jeansson, S.; Vahlne, A.; Lycke, E. Herpes simplex virus type-selective enzyme-linked immunosorbent assay with helix pomatia lectin-purified antigens. J. Clin. Microbiol. 1984, 19, 235–239. [Google Scholar] [PubMed]
- Ashley, R.L.; Militoni, J.; Lee, F.; Nahmias, A.; Corey, L. Comparison of western blot (immunoblot) and glycoprotein G-specific immunodot enzyme assay for detecting antibodies to herpes simplex virus type 1 and 2 in human sera. J. Clin. Microbiol. 1988, 26, 662–667. [Google Scholar] [PubMed]
- Gorander, S.; Harandi, A.M.; Lindqvist, M.; Bergstrom, T.; Liljeqvist, J.A. Glycoprotein G of herpes simplex virus 2 as a novel vaccine antigen for immunity to genital and neurological disease. J. Virol. 2012, 86, 7544–7553. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, N.; Bacchetti, S.; Rawls, W.E. Protection against lethal challenge of BALB/c mice by passive transfer of monoclonal antibodies to five glycoproteins of herpes simplex virus type 2. Infect. Immun. 1982, 37, 1132–1137. [Google Scholar] [PubMed]
- Bystricka, M.; Petrikova, M.; Zatovicova, M.; Solarikova, L.; Kostolansky, F.; Mucha, V.; Russ, G. Monoclonal antibodies to the distinct antigenic sites on glycoproteins C and B and their protective abilities in herpes simplex virus infection. Acta Virol. 1997, 41, 5–12. [Google Scholar] [PubMed]
- Belshe, R.B.; Heineman, T.C.; Bernstein, D.I.; Bellamy, A.R.; Ewell, M.; van der Most, R.; Deal, C.D. Correlate of immune protection against HSV-1 genital disease in vaccinated women. J. Infect. Dis. 2014, 209, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, S.; Belshe, R.B.; Friedman, H.M. Better neutralization of herpes simplex virus type 1 (HSV-1) than HSV-2 by antibody from recipients of glaxosmithkline HSV-2 glycoprotein D2 subunit vaccine. J. Infect. Dis. 2014, 210, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, D.; Roes, J.; Kuhn, R.; Rajewsky, K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature 1991, 350, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, S.; Jeansson, S.; Lycke, E. Unusual lectin-binding properties of a herpes simplex virus type 1-specific glycoprotein. J. Virol. 1981, 38, 564–570. [Google Scholar] [PubMed]
- Liljeqvist, J.A.; Trybala, E.; Svennerholm, B.; Jeansson, S.; Sjogren-Jansson, E.; Bergstrom, T. Localization of type-specific epitopes of herpes simplex virus type 2 glycoprotein G recognized by human and mouse antibodies. J. Gen. Virol 1998, 79, 1215–1224. [Google Scholar] [PubMed]
- Kohl, S.; Cahall, D.L.; Walters, D.L.; Schaffner, V.E. Murine antibody-dependent cellular cytotoxicity to herpes simplex virus-infected target cells. J. Immunol. 1979, 123, 25–30. [Google Scholar] [PubMed]
- Sigma plot 12; 12.0 Build 12.0.0.182; Systat Software Inc: San Jose, CA, USA, 2010.
- Awasthi, S.; Lubinski, J.M.; Shaw, C.E.; Barrett, S.M.; Cai, M.; Wang, F.; Betts, M.; Kingsley, S.; Distefano, D.J.; Balliet, J.W.; et al. Immunization with a vaccine combining herpes simplex virus 2 (HSV-2) glycoprotein C (gC) and gD subunits improves the protection of dorsal root ganglia in mice and reduces the frequency of recurrent vaginal shedding of HSV-2 DNA in guinea pigs compared to immunization with gD alone. J. Virol. 2011, 85, 10472–10486. [Google Scholar] [CrossRef] [PubMed]
- Khodai, T.; Chappell, D.; Christy, C.; Cockle, P.; Eyles, J.; Hammond, D.; Gore, K.; McCluskie, M.J.; Evans, D.M.; Lang, S.; et al. Single and combination herpes simplex virus type 2 glycoprotein vaccines adjuvanted with CpG oligodeoxynucleotides or monophosphoryl lipid A exhibit differential immunity that is not correlated to protection in animal models. Clin. Vaccine Immunol. 2011, 18, 1702–1709. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, N.; Oba, D.E.; Hutt-Fletcher, L.M. Antigenic cross-reactions among herpes simplex virus types 1 and 2, epstein-barr virus, and cytomegalovirus. J. Virol. 1987, 61, 1125–1135. [Google Scholar] [PubMed]
- Para, M.F.; Parish, M.L.; Noble, A.G.; Spear, P.G. Potent neutralizing activity associated with anti-glycoprotein D specificity among monoclonal antibodies selected for binding to herpes simplex virions. J. Virol. 1985, 55, 483–488. [Google Scholar] [PubMed]
- Chu, C.F.; Meador, M.G.; Young, C.G.; Strasser, J.E.; Bourne, N.; Milligan, G.N. Antibody-mediated protection against genital herpes simplex virus type 2 disease in mice by Fc gamma receptor-dependent and -independent mechanisms. J. Reprod. Immunol. 2008, 78, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Kohl, S.; Charlebois, E.D.; Sigouroudinia, M.; Goldbeck, C.; Hartog, K.; Sekulovich, R.E.; Langenberg, A.G.; Burke, R.L. Limited antibody-dependent cellular cytotoxicity antibody response induced by a herpes simplex virus type 2 subunit vaccine. J. Infect. Dis. 2000, 181, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Ravetch, J.V.; Kinet, J.P. Fc receptors. Annu. Rev. Immunol. 1991, 9, 457–492. [Google Scholar] [CrossRef] [PubMed]
- Dudley, K.L.; Bourne, N.; Milligan, G.N. Immune protection against HSV-2 in B-cell-deficient mice. Virology 2000, 270, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Parr, M.B.; Parr, E.L. Immunity to vaginal herpes simplex virus-2 infection in B-cell knockout mice. Immunology 2000, 101, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Morrison, L.A.; Zhu, L.; Thebeau, L.G. Vaccine-induced serum immunoglobin contributes to protection from herpes simplex virus type 2 genital infection in the presence of immune T cells. J. Virol. 2001, 75, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Homann, D.; Tishon, A.; Berger, D.P.; Weigle, W.O.; von Herrath, M.G.; Oldstone, M.B. Evidence for an underlying CD4 helper and CD8 T-cell defect in B-cell-deficient mice: Failure to clear persistent virus infection after adoptive immunotherapy with virus-specific memory cells from muMT/muMT mice. J. Virol. 1998, 72, 9208–9216. [Google Scholar] [PubMed]
- Bourne, N.; Pyles, R.B.; Bernstein, D.I.; Stanberry, L.R. Modification of primary and recurrent genital herpes in guinea pigs by passive immunization. J. Gen. Virol. 2002, 83, 2797–2801. [Google Scholar] [PubMed]
- Parr, E.L.; Parr, M.B. Immunoglobulin G is the main protective antibody in mouse vaginal secretions after vaginal immunization with attenuated herpes simplex virus type 2. J. Virol. 1997, 71, 8109–8115. [Google Scholar] [PubMed]
- Eis-Hubinger, A.M.; Schmidt, D.S.; Schneweis, K.E. Anti-glycoprotein B monoclonal antibody protects T cell-depleted mice against herpes simplex virus infection by inhibition of virus replication at the inoculated mucous membranes. J. Gen. Virol. 1993, 74, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, A.; Krauss, J.; Eis-Hubinger, A.M.; Daumer, M.P.; Schwarzenbacher, R.; Dittmer, U.; Schneweis, K.E.; Jager, D.; Roggendorf, M.; Arndt, M.A. Impact of valency of a glycoprotein B-specific monoclonal antibody on neutralization of herpes simplex virus. J. Virol. 2011, 85, 1793–1803. [Google Scholar] [CrossRef] [PubMed]
- Halford, W.P.; Geltz, J.; Gershburg, E. Pan-HSV-2 IgG antibody in vaccinated mice and guinea pigs correlates with protection against herpes simplex virus 2. PLoS One 2013, 8, e65523. [Google Scholar] [CrossRef] [PubMed]
- McDermott, M.R.; Brais, L.J.; Evelegh, M.J. Mucosal and systemic antiviral antibodies in mice inoculated intravaginally with herpes simplex virus type 2. J. Gen. Virol. 1990, 71, 1497–1504. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, S.; Friedman, H.M. A paradigm shift: Vaccine-induced antibodies as an immune correlate of protection against herpes simplex virus type 1 genital herpes. J. Infect. Dis. 2014, 209, 813–815. [Google Scholar] [CrossRef] [PubMed]
- Excler, J.L.; Ake, J.; Robb, M.L.; Kim, J.H.; Plotkin, S.A. Nonneutralizing functional antibodies: A new “old” paradigm for HIV vaccines. Clin. Vaccine Immunol. 2014, 21, 1023–1036. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Görander, S.; Ekblad, M.; Bergström, T.; Liljeqvist, J.-Å. Anti-Glycoprotein G Antibodies of Herpes Simplex Virus 2 Contribute to Complete Protection after Vaccination in Mice and Induce Antibody-Dependent Cellular Cytotoxicity and Complement-Mediated Cytolysis. Viruses 2014, 6, 4358-4372. https://doi.org/10.3390/v6114358
Görander S, Ekblad M, Bergström T, Liljeqvist J-Å. Anti-Glycoprotein G Antibodies of Herpes Simplex Virus 2 Contribute to Complete Protection after Vaccination in Mice and Induce Antibody-Dependent Cellular Cytotoxicity and Complement-Mediated Cytolysis. Viruses. 2014; 6(11):4358-4372. https://doi.org/10.3390/v6114358
Chicago/Turabian StyleGörander, Staffan, Maria Ekblad, Tomas Bergström, and Jan-Åke Liljeqvist. 2014. "Anti-Glycoprotein G Antibodies of Herpes Simplex Virus 2 Contribute to Complete Protection after Vaccination in Mice and Induce Antibody-Dependent Cellular Cytotoxicity and Complement-Mediated Cytolysis" Viruses 6, no. 11: 4358-4372. https://doi.org/10.3390/v6114358
APA StyleGörander, S., Ekblad, M., Bergström, T., & Liljeqvist, J.-Å. (2014). Anti-Glycoprotein G Antibodies of Herpes Simplex Virus 2 Contribute to Complete Protection after Vaccination in Mice and Induce Antibody-Dependent Cellular Cytotoxicity and Complement-Mediated Cytolysis. Viruses, 6(11), 4358-4372. https://doi.org/10.3390/v6114358




