3.1. Generation of a Codon-Optimized Molecular Clone of a Reconstituted HERV-K(HML-2) Element
We previously described 25 non-synonymous post-insertional mutations in the HERV-K113 provirus originally characterised by Turner and co-workers [
11,
14,
15,
16]. Reversion of these 25 mutations and the reversion of three presumed additional post-insertional mutations in the 3'LTR of a molecular clone (to yield a clone termed oriHERV-K113) allowed the expression of replication-defective retroviral particles in transfected human cell lines [
11].
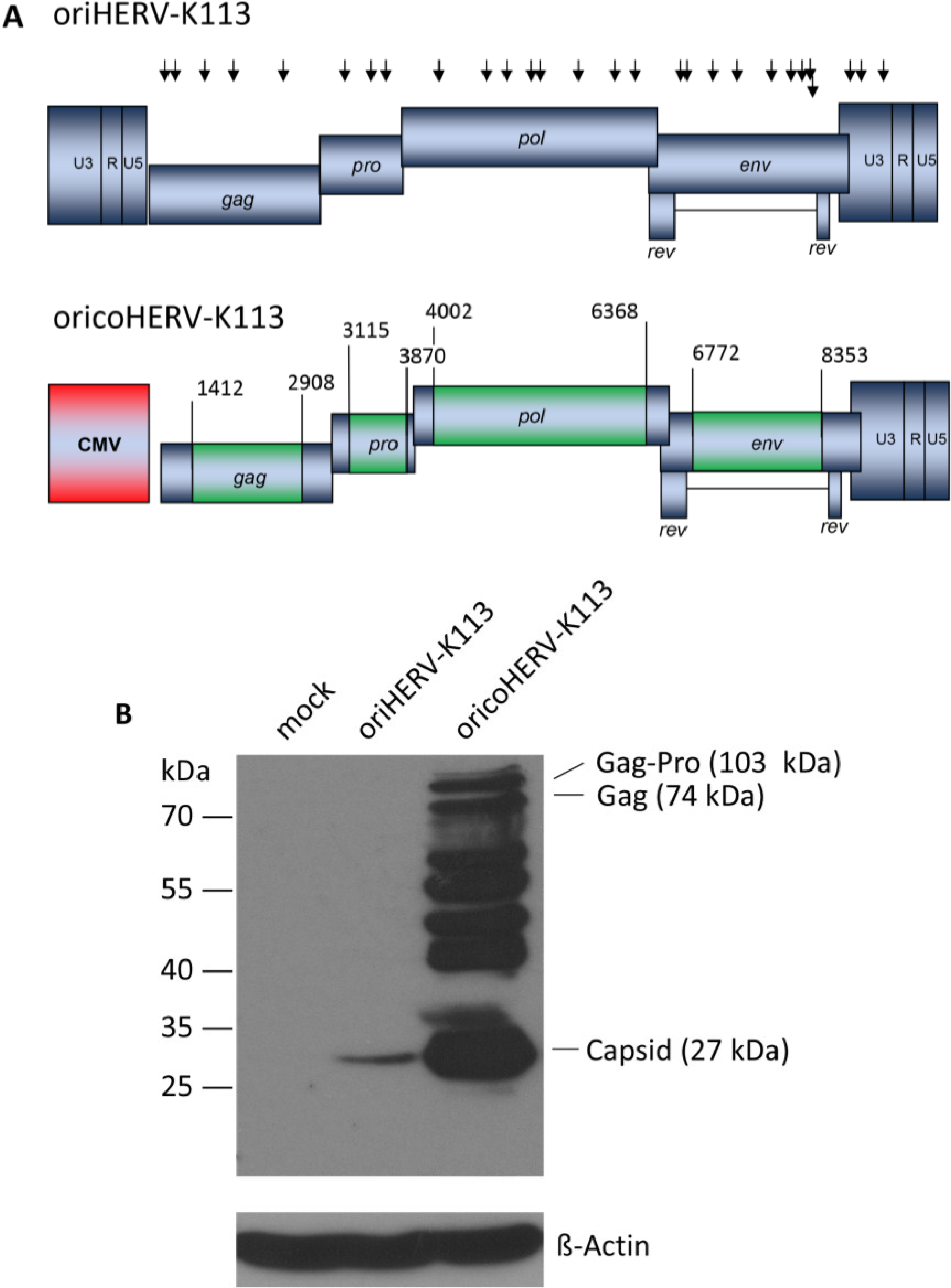
In order to boost the relatively low levels of particle expression, we also constructed a molecular clone in which parts of the oriHERV-K113 genome were codon-optimized, leaving potential regulatory sequences and regions of overlapping reading frames unchanged [
15]. To further enhance expression, we replaced the viral 5'LTR with a CMV promoter and named the resulting near-full-length molecular clone “ori
coHERV-K113” (
Figure 1A).
Figure 1.
Genomic organization and expression of the reconstituted human endogenous retrovirus (HERV-K) human MMTV-like-2 HERV-K(HML-2) sequence “oriHERV-K113” and the partial codon-optimized variant with CMV promoter “oricoHERV-K113”. (A) For reconstitution of the potential infectious progenitor sequence of the HERV-K(HML-2) element, 25 non-synonymous putative post-insertional mutations (indicated by black arrows, lower arrow refers to rec reading frame) in the HERV-K113 genome sequence (AY037928) were reverted to generate oriHERV-K113. In oricoHERV-K113, parts of the oriHERV-K113 genome were codon-optimized (boxed in green) to achieve maximal expression in mammalian cells, leaving potential regulatory sequences or overlapping regions in the genome unaltered. The 5'LTR region was replaced by a CMV promoter (red). Numbers given for start and end of codon-optimized regions refer to genbank sequence AY037928. (B) Western blot of intracellular Gag proteins, processing fragments and degradation intermediates after transfection of the molecular clones in HEK 239T cells using a Gag (CA)-protein-specific antiserum for detection. Bands presumably representing the Gag-Pro, Gag and CA proteins are indicated. Stripping and re-probing of the membrane with ß-Actin specific monoclonal antibody demonstrated that the same amounts of cell lysate were loaded for mock, oriHERV-K113 and oricoHERV-K113 transfected cells, respectively.
Figure 1.
Genomic organization and expression of the reconstituted human endogenous retrovirus (HERV-K) human MMTV-like-2 HERV-K(HML-2) sequence “oriHERV-K113” and the partial codon-optimized variant with CMV promoter “oricoHERV-K113”. (A) For reconstitution of the potential infectious progenitor sequence of the HERV-K(HML-2) element, 25 non-synonymous putative post-insertional mutations (indicated by black arrows, lower arrow refers to rec reading frame) in the HERV-K113 genome sequence (AY037928) were reverted to generate oriHERV-K113. In oricoHERV-K113, parts of the oriHERV-K113 genome were codon-optimized (boxed in green) to achieve maximal expression in mammalian cells, leaving potential regulatory sequences or overlapping regions in the genome unaltered. The 5'LTR region was replaced by a CMV promoter (red). Numbers given for start and end of codon-optimized regions refer to genbank sequence AY037928. (B) Western blot of intracellular Gag proteins, processing fragments and degradation intermediates after transfection of the molecular clones in HEK 239T cells using a Gag (CA)-protein-specific antiserum for detection. Bands presumably representing the Gag-Pro, Gag and CA proteins are indicated. Stripping and re-probing of the membrane with ß-Actin specific monoclonal antibody demonstrated that the same amounts of cell lysate were loaded for mock, oriHERV-K113 and oricoHERV-K113 transfected cells, respectively.
![Viruses 06 04332 g001]()
Western blot analysis of transfected HEK 293T cells using a capsid-specific antiserum revealed that Gag expression from oricoHERV-K113 was at least 50-fold higher than that of oriHERV-K113 (
Figure 1B).
3.2. Differences in the Morphologies of oriHERV-K113 and oricoHERV-K113
We next set out to analyze the morphological impact of the modulated expression of retroviral proteins by thin-section EM analysis of human cell lines transfected with oricoHERV-K113.
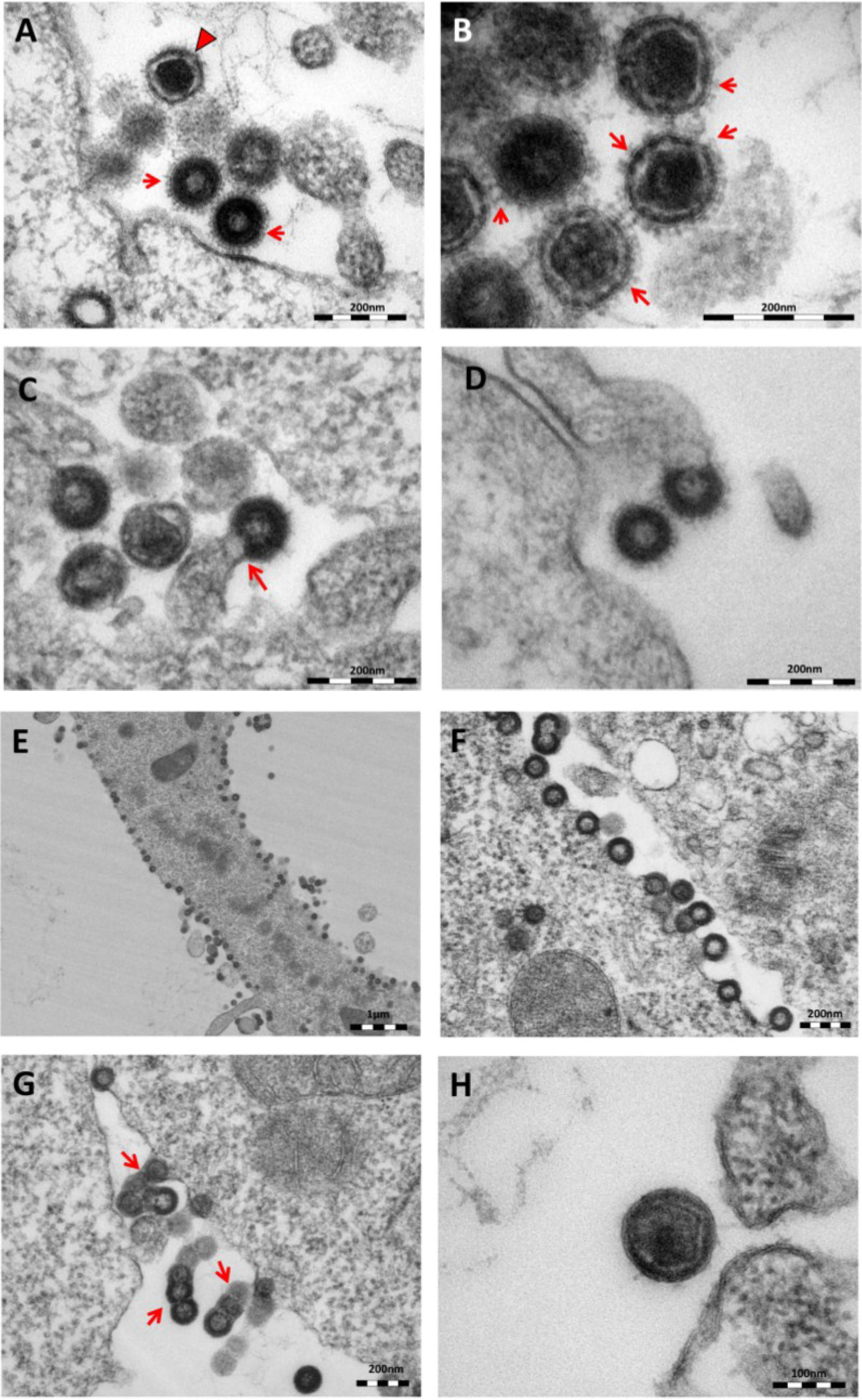
As previously reported [
11], oriHERV-K113 produces particles with immature and condensed mature cores and many envelope spikes clearly visible at the viral membrane (
Figure 2A,B).
Figure 2.
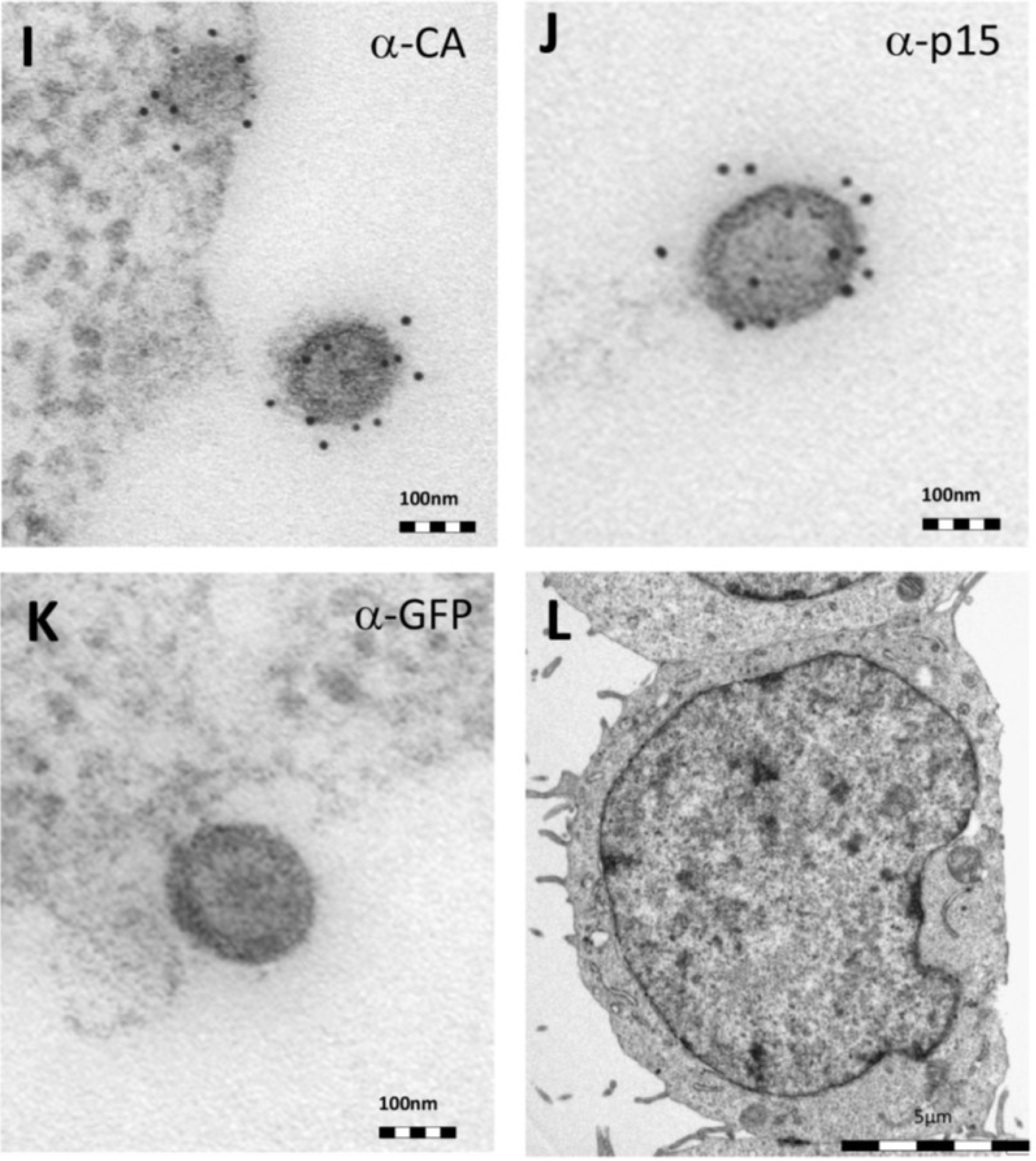
Thin-section electron micrographs of oriHERV-K113 and oricoHERV-K113 particle production after transfection with full-length molecular clones. (A) In HEK 293T cells, both immature oriHERV-K113 derived particles with ring-like structures of Gag proteins (marked by arrows) and mature forms with the condensed cores (marked by an arrowhead) are seen. (B) Env is incorporated strongly into the viral membrane (arrows). (C) Budding of oriHERV-K113 particles is of typical C-type morphology with the assembly of the Gag shells at the cellular membrane during budding (an arrow indicates a budding stage). (D) oriHERV-K113 particles produced in HeLa cells. (E,F) oricoHERV-K113 transfected HEK 293T cells produce large numbers of particles that line up with the cellular membrane and display an A-type morphology, predominantly with preassembled rings of Gag protein attached to the membranes. (G) oricoHERV-K113 occasionally forms budding chains with structures resembling C-type morphology (arrows). (H) Incorporation of Env into the viral membrane of oricoHERV-K113 appears to be very low. Shown is a released mature particle produced by HeLa cells (I) Immunostaining EM with a CA specific antiserum. (J) Immunostaining EM with a p15-specific antiserum and a GFP control antiserum (K). (L) No transfection control.
Figure 2.
Thin-section electron micrographs of oriHERV-K113 and oricoHERV-K113 particle production after transfection with full-length molecular clones. (A) In HEK 293T cells, both immature oriHERV-K113 derived particles with ring-like structures of Gag proteins (marked by arrows) and mature forms with the condensed cores (marked by an arrowhead) are seen. (B) Env is incorporated strongly into the viral membrane (arrows). (C) Budding of oriHERV-K113 particles is of typical C-type morphology with the assembly of the Gag shells at the cellular membrane during budding (an arrow indicates a budding stage). (D) oriHERV-K113 particles produced in HeLa cells. (E,F) oricoHERV-K113 transfected HEK 293T cells produce large numbers of particles that line up with the cellular membrane and display an A-type morphology, predominantly with preassembled rings of Gag protein attached to the membranes. (G) oricoHERV-K113 occasionally forms budding chains with structures resembling C-type morphology (arrows). (H) Incorporation of Env into the viral membrane of oricoHERV-K113 appears to be very low. Shown is a released mature particle produced by HeLa cells (I) Immunostaining EM with a CA specific antiserum. (J) Immunostaining EM with a p15-specific antiserum and a GFP control antiserum (K). (L) No transfection control.
![Viruses 06 04332 g002a]()
![Viruses 06 04332 g002b]()
The overall production of viral particles is low, with only few budding stages or clusters of released immature and mature particles per cell. Cell sections with oriHERV-K113 virions were relatively rare. Only about one out of 20–30 cell sections contained particles. The budding morphology of oriHERV-K113 is typically C-type, despite it being a betaretrovirus according to its
pol region sequence [
18]. The Gag molecules commence assembly at the cell membrane in a convex formation that resembles in form the letter “C” (
Figure 2C). We never observed a complete closure of the Gag ring during the late budding stages, indicating a virtually concomitant completion of the Gag shell and pinching off of the virion. There were also no immature virus-like particles present in the cytoplasm of cells transfected with oriHERV-K113. The results following transfection of the oriHERV-K113 full-length molecular clone into HeLa cells were similar to those with HEK 293T cells (
Figure 2D).
The picture is different in cells transfected with oricoHERV-K113. In these cells, particles with completely closed Gag shells line up at the inner leaflet of the plasma membrane (
Figure 2E,F) and in some cells also at the cytoplasmic side of vesicular membranes. The cytoplasmic assembly of particles having completely closed immature cores is reminiscent of the regular retroviral A- and B-type morphology and is comparable to the mode of assembly of typical betaretroviruses including mouse MMTV and Mason Pfizer Monkey Virus (MPMV) [
19]. Many of the transfected cells display budding chains in plasma membrane protrusions (
Figure 2G). Although intracellular particles predominate, immature and mature virions are also present outside of the cells (
Figure 2G,H), indicating some degree of particle release from the cell. The incorporation of Env in the viral membrane appears to be significantly lower compared to that in particles produced by oriHERV-K113, with very few spikes being present on budding structures or released particles (
Figure 2G,H). Expressing oricoHERV-K113 in HeLa cells results in morphologies similar to those observed in HEK 293T cells (
Figure 2H). To confirm the identity of the oricoHERV-K(HML-2) particles we have performed immunostaining EM on thin-sections of transfected HEK 293T cells embedded in Lowicryl. Intra- and extracellular viral particles were specifically labelled with a CA and p15 specific antiserum, but not with an anti-GFP control (
Figure 2I–K) No virus particles were observed in untransfected cells (
Figure 2L).
To further demonstrate virion release from cells transfected with oricoHERV-K113, the supernatant of transfected HEK 293T cells was pelleted by ultracentrifugation and embedded for thin-section EM. As shown in
Figure 3, the pellets contain high proportions of exclusively mature virions with relatively large, typically concentric spherical or polygonal electron dense core structures against a background of contaminating microsomes (
Figure 3). In agreement with the observations made in the previous experiment, spikes are barely visible on the surface of oricoHERV-K113 particles. Our data therefore demonstrate the efficient release and maturation of retroviral particles from cells transfected with oricoHERV-K113.
Figure 3.
Virus particles in the supernatant of transfected HEK 293T cells. Cells were transfected with the full-length molecular clone oricoHERV-K113 and virions in the supernatant were pelleted by ultracentrifugation and embedded for thin-section EM. Nearly all particles show the condensed core of mature virions.
Figure 3.
Virus particles in the supernatant of transfected HEK 293T cells. Cells were transfected with the full-length molecular clone oricoHERV-K113 and virions in the supernatant were pelleted by ultracentrifugation and embedded for thin-section EM. Nearly all particles show the condensed core of mature virions.
3.3. Enhancement of oriHERV-K113 Expression by Staufen-1 Overexpression Does Not Result in a Morphology Switch from C- to A-Type
We recently described the interaction of the HERV-K(HML-2) encoded proteins Rec and Gag with the human Staufen-1 protein and noticed a 20-fold increase in oriHERV-K113 production in cells overexpressing Staufen-1 [
17].
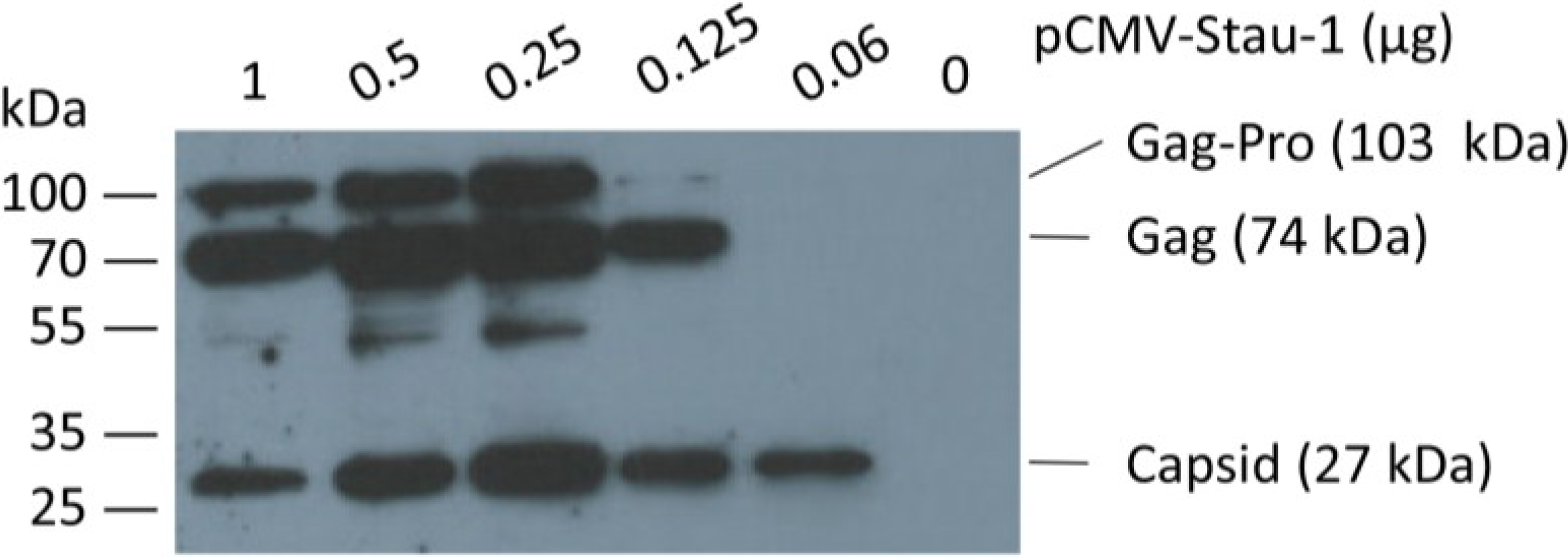
In order to boost oriHERV-K113 expression by Staufen-1 as strongly as possible and to evaluate a potential switch in morphology, we first optimized the transfection ratio of oriHERV-K113 and Staufen-1 expression plasmids. As shown in
Figure 4, the peak of Gag expression is associated with an intermediate amount of Staufen-1 DNA;
i.e., with a 4:1 ratio of oriHERV-K113 to Staufen-1 DNA.
Figure 4.
Co-expression of the human Staufen-1 protein enhances oriHERV-K113 protein production. Western blot detection by using a Gag (CA)-specific antiserum of intracellular HERV Gag protein 48 h after co-transfection of HEK 293T cells with a fixed amount of oriHERV-K113 full-length molecular clone and decreasing amounts of the Staufen-1 expression vector. The presumed Gag-Pro, Gag and processed capsid proteins are indicated. The exposure time was optimized to show expression differences. It was too short to visualize Gag proteins on lane 6 (no Staufen-1 overexpression).
Figure 4.
Co-expression of the human Staufen-1 protein enhances oriHERV-K113 protein production. Western blot detection by using a Gag (CA)-specific antiserum of intracellular HERV Gag protein 48 h after co-transfection of HEK 293T cells with a fixed amount of oriHERV-K113 full-length molecular clone and decreasing amounts of the Staufen-1 expression vector. The presumed Gag-Pro, Gag and processed capsid proteins are indicated. The exposure time was optimized to show expression differences. It was too short to visualize Gag proteins on lane 6 (no Staufen-1 overexpression).
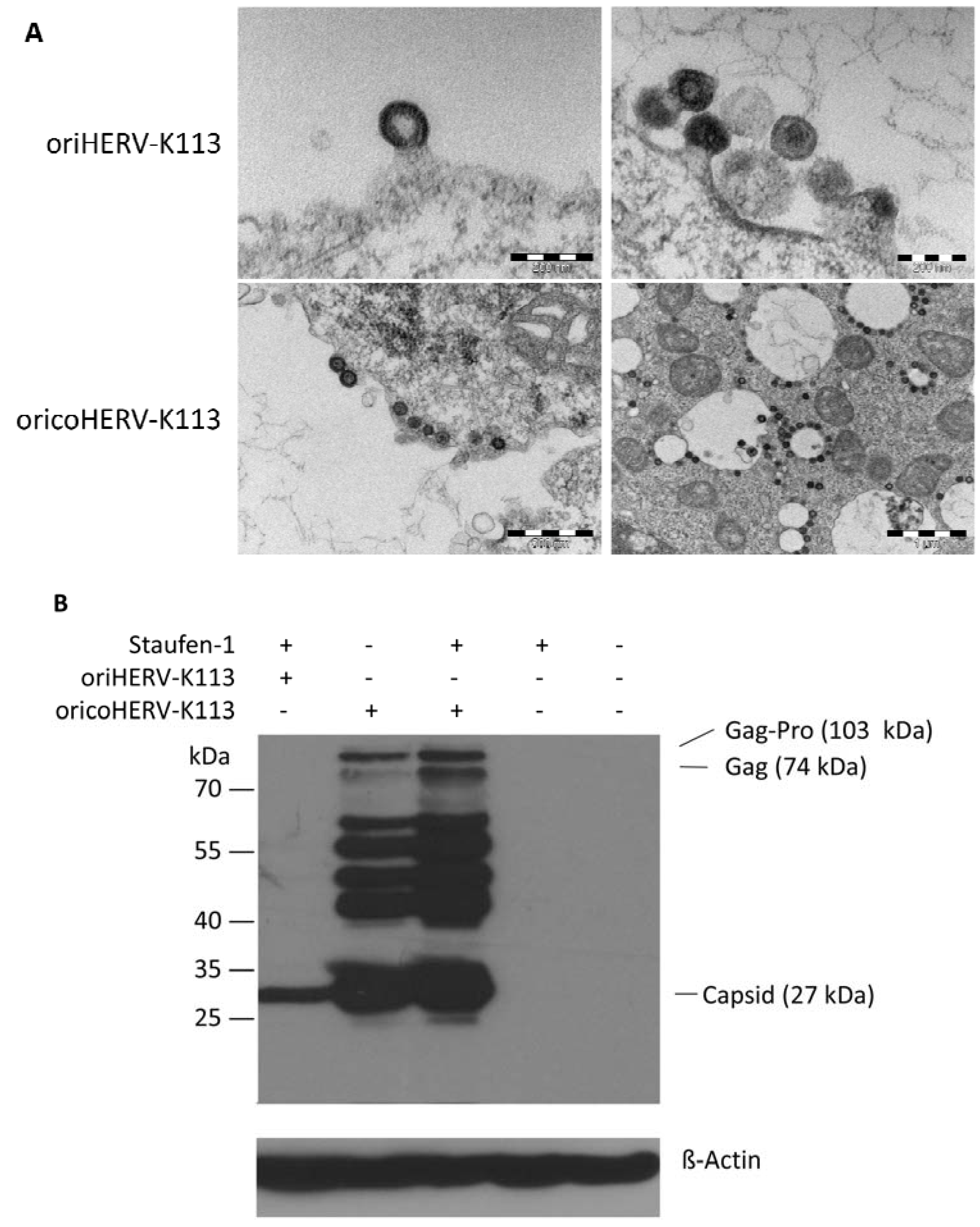
The morphology of viral particles produced by transfected HEK 293T cells under optimized Staufen-1 mediated enhancement conditions were also analyzed by thin-section EM. Despite Staufen-1 mediated enhancement resulting in significantly more viral particles, there was no evidence for a C- to A-type switch (
Figure 5A, upper panel). However, even when enhanced by Staufen-1, the levels of Gag expression were still lower compared to that in cells transfected with oricoHERV-K113 (
Figure 5B). As expected, co-transfection of Staufen-1 did not alter the morphology of oricoHERV-K113 particles (
Figure 5A, lower panel) and had only a minimal enhancing effect on the expression of the oricoHERV-K113 Gag protein (
Figure 5B), which is probably due to the high baseline level of protein expression from this construct.
Figure 5.
Thin-section EM of oriHERV-K113 and oricoHERV-K113 particle production after co-transfection of full-length molecular clones with Staufen-1 in a 4:1 ratio to enhance protein production. (A) Upper panel: EM of particles produced by oriHERV-K113 after co-transfection with Staufen-1. No changes in C-type morphology can be seen despite enhancement of protein production by Staufen-1. Lower panel: Same experiment with oricoHERV-K113, no influence on the A-type morphology can be seen as a result of moderate Staufen-1 enhancement. (B) Western blot of Staufen-1-mediated enhanced Gag protein expression in HEK 293T cell lysate. Cells were transfected with oriHERV-K113 and Staufen-1 (4:1 ratio), oricoHERV-K113, oricoHERV-K113 and Staufen-1 (4:1 ratio), Staufen-1 alone and with empty vector as mock control (left to right). Intracellular Gag was detected by Gag (CA)-protein specific antiserum. As loading control, the membrane was stripped and re-probed with a ß-Actin specific monoclonal antibody.
Figure 5.
Thin-section EM of oriHERV-K113 and oricoHERV-K113 particle production after co-transfection of full-length molecular clones with Staufen-1 in a 4:1 ratio to enhance protein production. (A) Upper panel: EM of particles produced by oriHERV-K113 after co-transfection with Staufen-1. No changes in C-type morphology can be seen despite enhancement of protein production by Staufen-1. Lower panel: Same experiment with oricoHERV-K113, no influence on the A-type morphology can be seen as a result of moderate Staufen-1 enhancement. (B) Western blot of Staufen-1-mediated enhanced Gag protein expression in HEK 293T cell lysate. Cells were transfected with oriHERV-K113 and Staufen-1 (4:1 ratio), oricoHERV-K113, oricoHERV-K113 and Staufen-1 (4:1 ratio), Staufen-1 alone and with empty vector as mock control (left to right). Intracellular Gag was detected by Gag (CA)-protein specific antiserum. As loading control, the membrane was stripped and re-probed with a ß-Actin specific monoclonal antibody.
![Viruses 06 04332 g005]()