Human Respiratory Syncytial Virus Memphis 37 Grown in HEp-2 Cells Causes more Severe Disease in Lambs than Virus Grown in Vero Cells
Abstract
:1. Introduction
2. Methods
2.1. Experimental Design
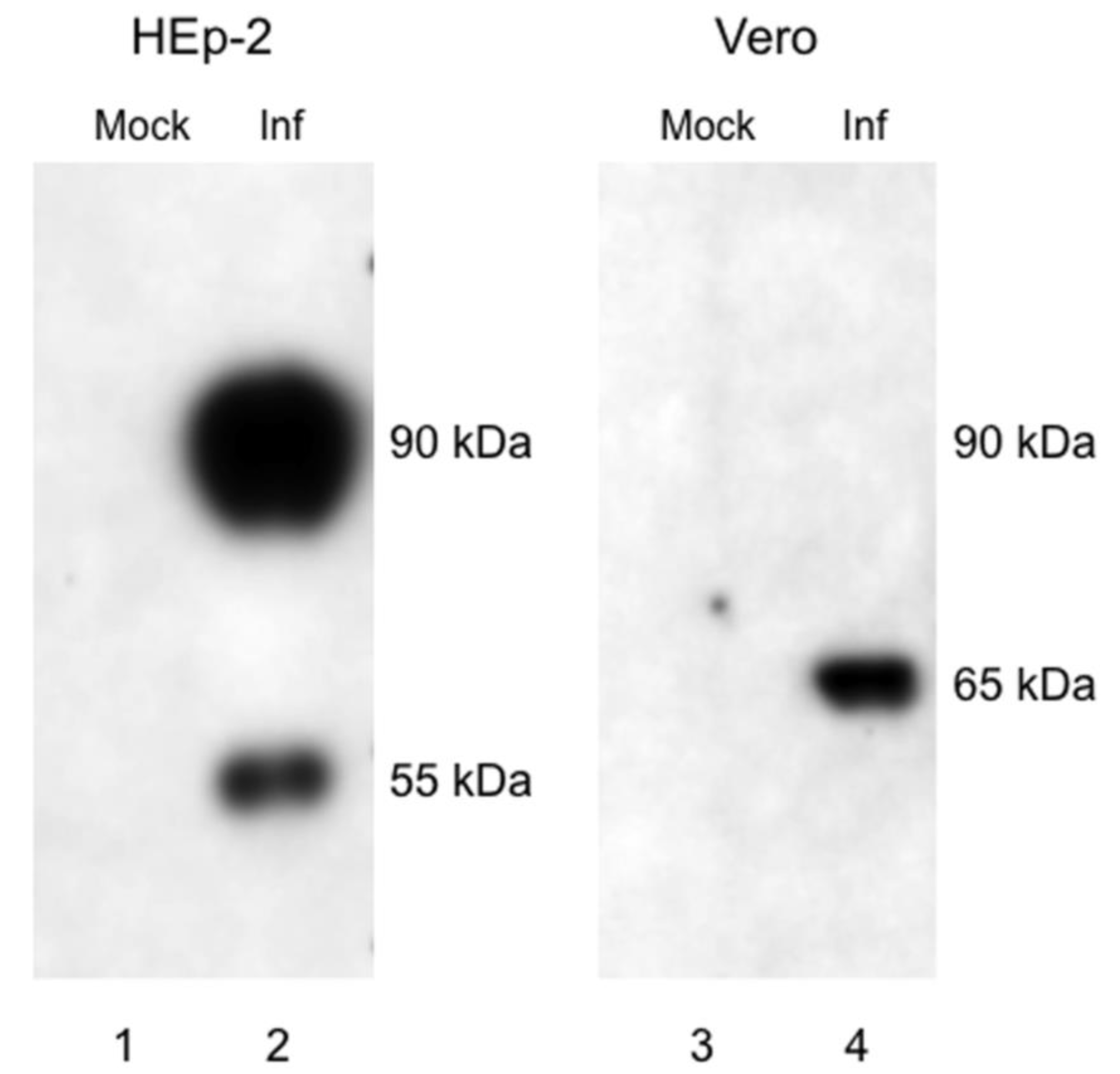
2.2. Western Blotting for G Protein
2.3. Post-Mortem
2.4. One-Step Reverse Transcription Quantitative PCR for RSV and Cytokine mRNA Levels
| M37 hRSV | Fwd: | GCTCTTAGCAAAGTCAAGTTGAACGA | IFN γ | Fwd: | TGGAGGACTTCAAAAGGCTGAT |
| Rev: | TGCTCCGTTGGATGGTGTATT | Rev: | GATGGCTTTGCGCTGGAT | ||
| Probe: | 6FAM-ACACTCAACAAAGATCAACTTCTGTCATCCAGC | Probe: | 6FAM-CAAATTCCGGTGGATGATCTGC-TAMRA | ||
| CCSP | Fwd: | CAG CCC TGA CGA AGA CAT GA | TNF α | Fwd: | CAACCTGGGACACCCAGAAT |
| Rev: | GGG TGT CTA CCA GCG TCT TCA | Rev: | TCTCAAGGAACGTTGCGAAGT | ||
| Probe: | 6FAM-AGA GGC AAC AAG TCA G-MGBNFQ | Probe: | 6FAM-CAAGGGCCAGGGTTCTTACCGGAA-TAMRA | ||
| SP-A | Fwd: | TGA CCC TTA TGC TCC TCT GGA T | TGF β | Fwd: | TGTGTTCGTCAGCTCTACATTGAC |
| Rev: | GGG CTT CCA AGA CAA ACT TCC T | Rev: | TAGCCCTTGGGTTCGTGAAT | ||
| Probe: | 6FAM-TGG CTT CTG GCC TCG AGT GCG -TAMRA | Probe: | 6FAM-TCCAGCCCAGGTCCTTCCGGA-TAMRA | ||
| IL-6 | Fwd: | GCTGCTCCTGGTGATGACTTC | MCP1 α | Fwd: | GCTGTGATTTTCAAGACCATCCT |
| Rev: | GGTGGTGTCATTTTTGAAATCTTCT | Rev: | GGCGTCCTGGACCCATTT | ||
| Probe: | 6FAM-CTTTCCCTACCCCGGGTCCCCTG-MBGNFQ | Probe: | 6FAM-AAAGAGTTTTGTGCAGACCCCAACC-TAMRA | ||
| IL-8 | Fwd: | TTCCAAGCTGGCTGTTGCT | MIP1 α | Fwd: | CAGCAGCCAGTGCTCCAA |
| Rev: | TTGACAGAACTGCAGCTTCACA | Rev: | ACCTGCCGGCCTTTTTTG | ||
| Probe: | 6FAM-CCGCTTTCCTGCTCTCTGCAGCTC-TAMRA | Probe: | 6FAM-CCTGGTGTCATCTTCCAGA-MGBNFQ | ||
| IL-10 | Fwd: | GTCGGAAATGATCCAGTTTTACCT | RANTES | Fwd: | TGCTTCTGCCTCCCCATATG |
| Rev: | GTCAGGCCCATGGTTCTCA | Rev: | GGGCGGGAGATATAGGCAAA | ||
| Probe: | 6FAM-AGGAGGTGATGCCACAGG-MGBNFQ | Probe: | 6FAM-CACCACGCCCTGCT-MGBNFQ | ||
| IFN β | Fwd: | TGGTTCTCCTGCTGTGTTTCTC | |||
| Rev: | CGTTGTTGGAATCGAAGCAA | ||||
| Probe: | 6FAM-ACCACAGCTCTTTCCAGGAGCTACA-TAMRA |
2.5. Gross Lesion Evaluation and Scoring
2.6. Histologic Evaluation and Scoring
2.7. Immunohistochemical Detection of RSV Antigen
2.8. Statistical Analysis
3. Results
3.1. G Protein Expression in RSV Memphis 37-infected Vero and HEp-2 Cells
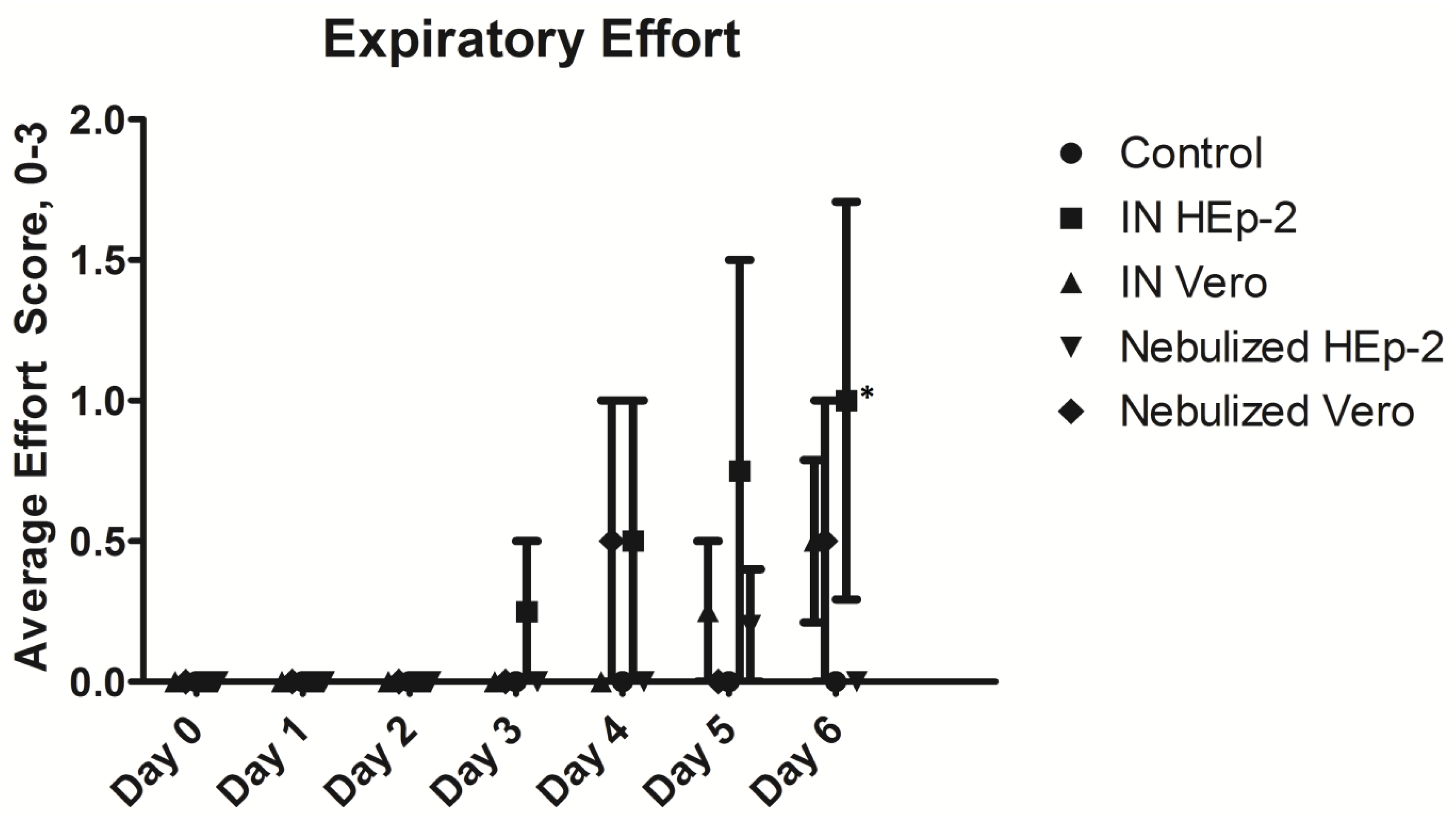
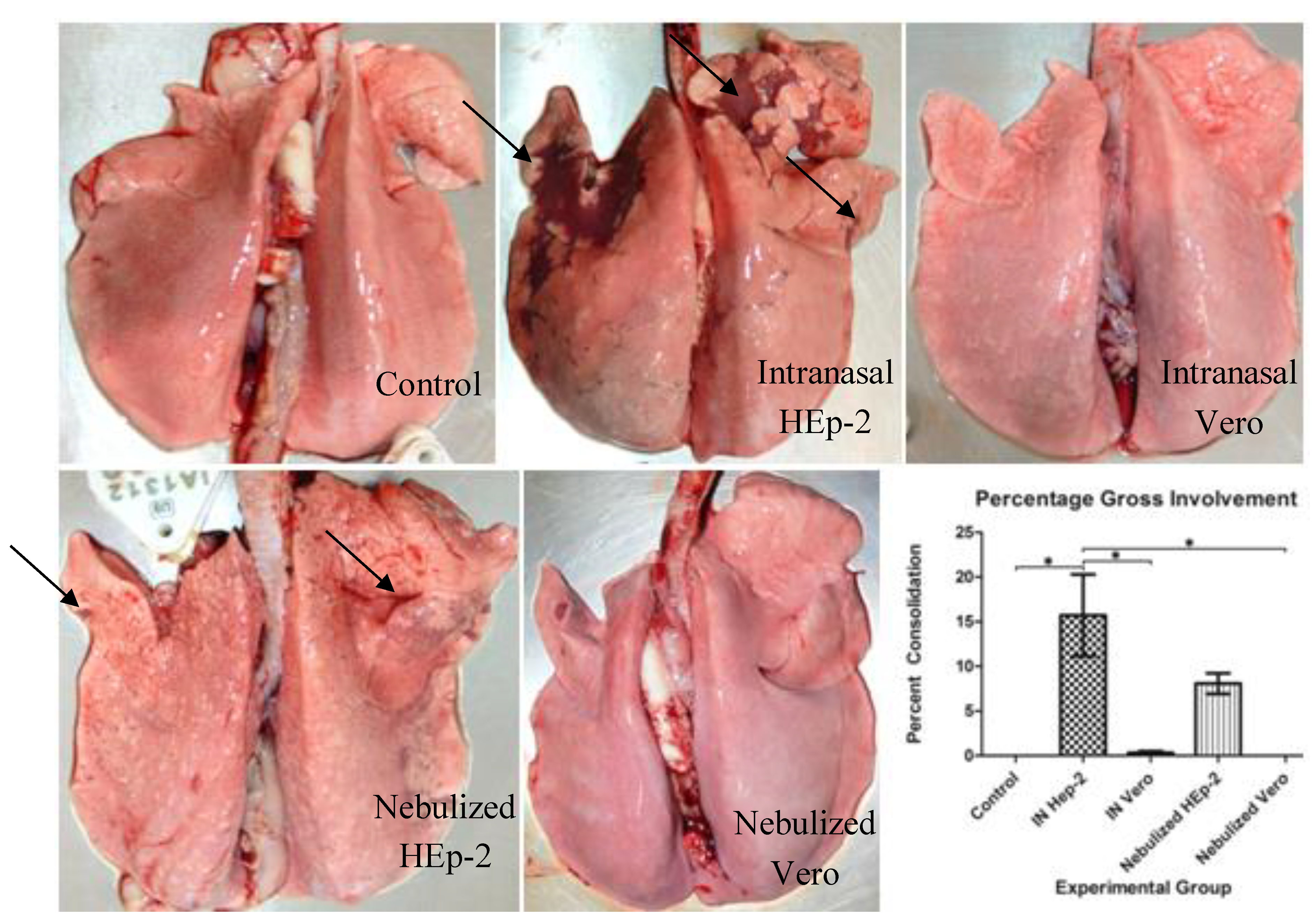
3.2. Clinical and Post-Mortem Findings
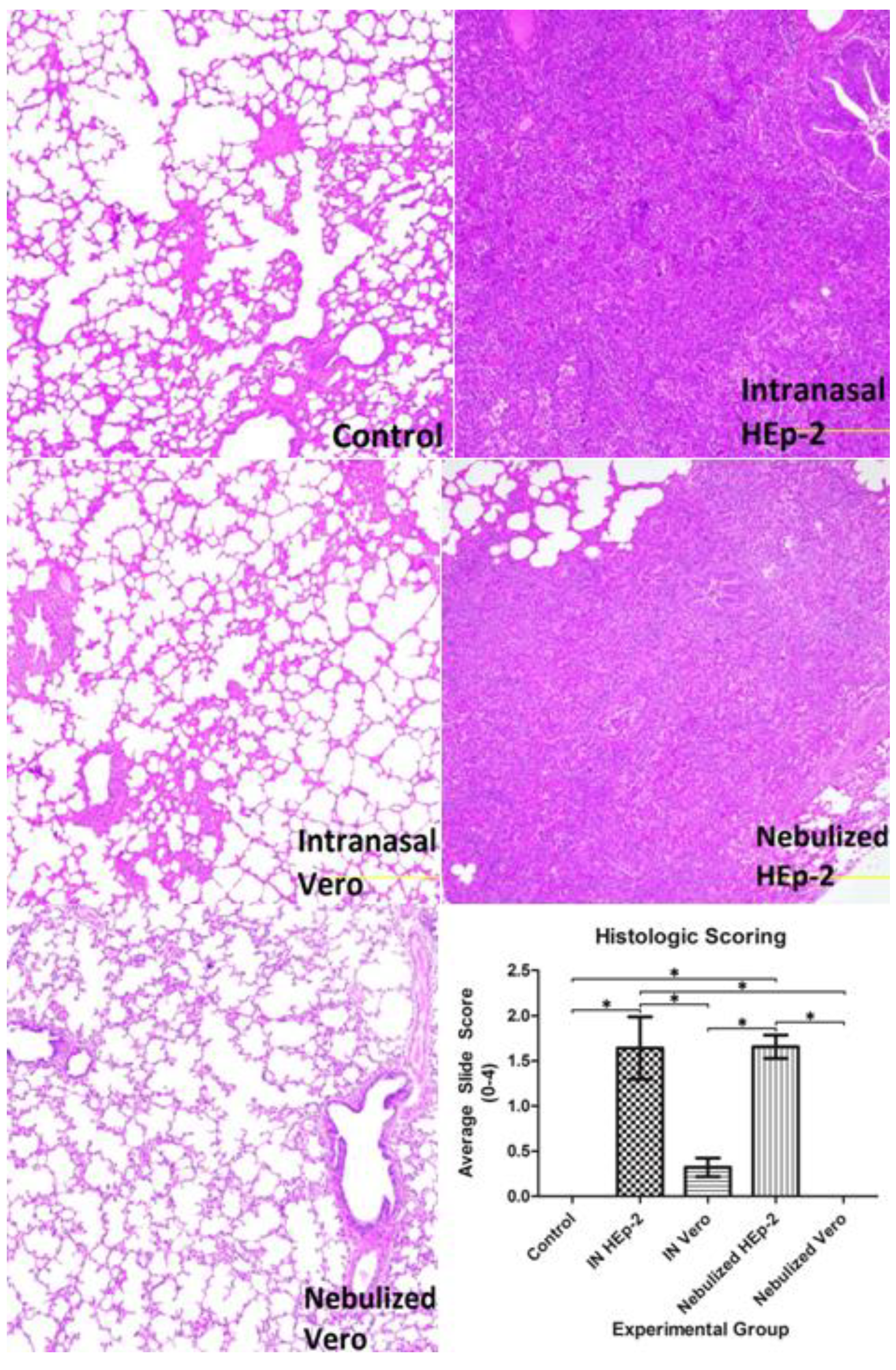
3.3. Histopathology Findings and Scores
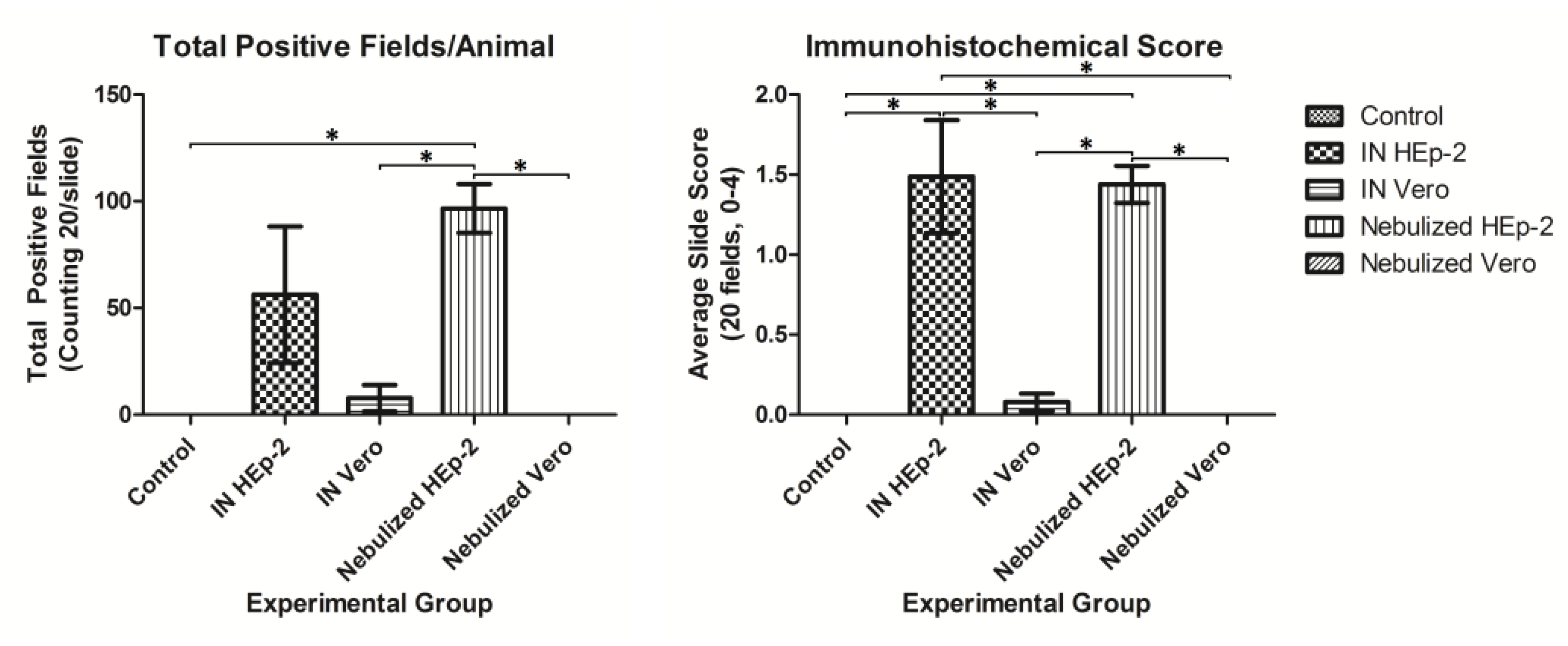
3.4. Immunohistochemical Detection of RSV Antigen
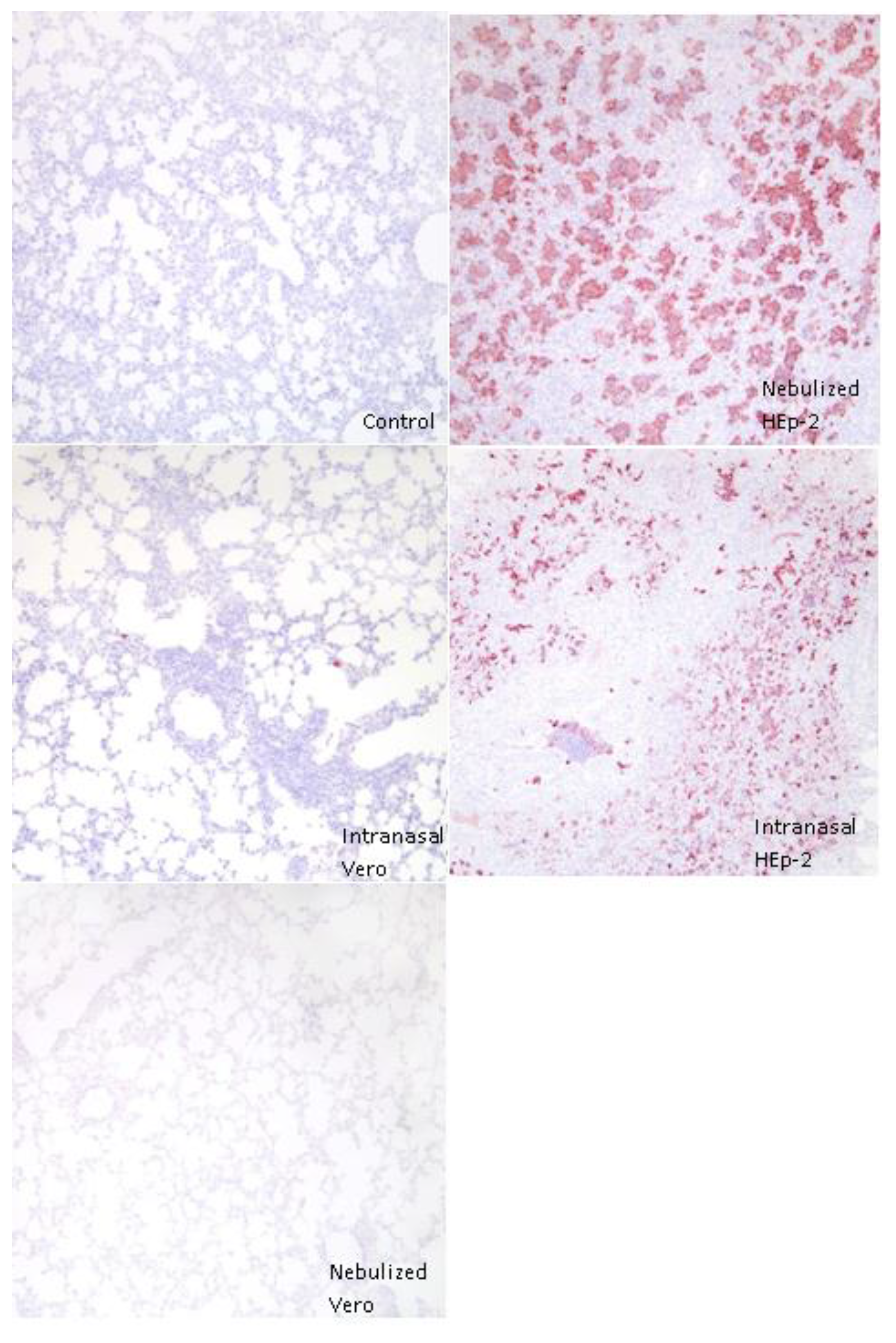
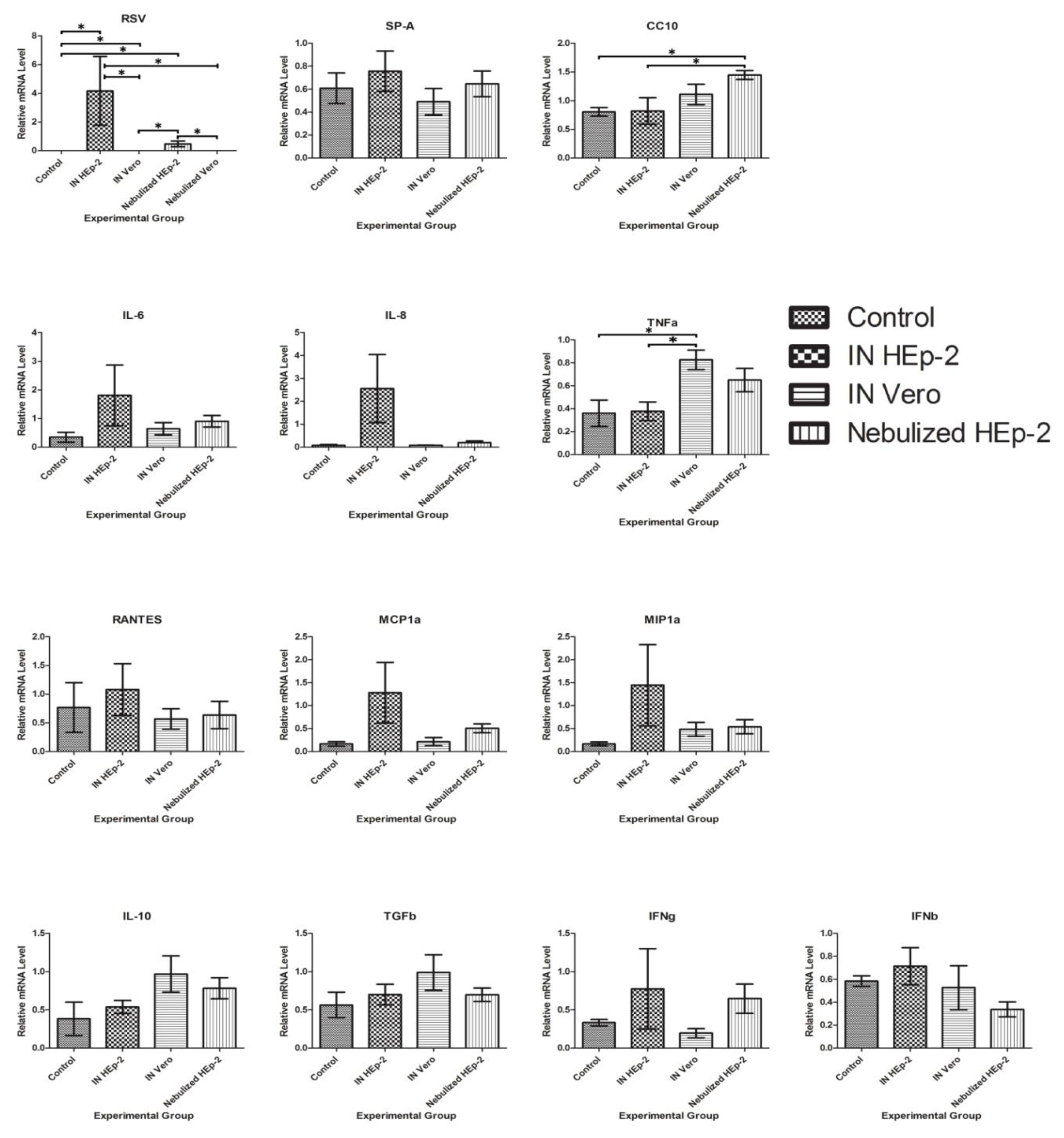
3.5. RSV and Cytokine mRNA Levels
4. Discussion
Acknowledgments
Conflicts of Interest
References
- Nair, H.; Nokes, D.J.; Gessner, B.D.; Dherani, M.; Madhi, S.A.; Singleton, R.J.; O’Brien, K.L.; Roca, A.; Wright, P.F.; Bruce, N.; et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet 2010, 375, 1545–1555. [Google Scholar] [CrossRef]
- WHO. Acute respiratory infections (update September 2009), 2009 09/09 [cited 2013]. Available online: http://www.who.int/vaccine_research/diseases/ari/en/index2.html (accesssed on 12 March 2012).
- Collins, P.L.; Melero, J.A. Progress in understanding and controlling respiratory syncytial virus: still crazy after all these years. Virus Res. 2011, 162, 80–99. [Google Scholar] [CrossRef]
- Utsumi, M.; Makimoto, K.; Quroshi, N.; Ashida, N. Types of infectious outbreaks and their impact in elderly care facilities: a review of the literature. Age Ageing 2010, 39, 299–305. [Google Scholar] [CrossRef]
- Derscheid, R.J.; Ackermann, M.R. Perinatal lamb model of respiratory syncytial virus (RSV) infection. Viruses 2012, 4, 2359–2378. [Google Scholar] [CrossRef]
- Alcorn, D.G.; Adamson, T.M.; Maloney, J.E.; Robinson, P.M. A morphologic and morphometric analysis of fetal lung development in the sheep. Anat. Rec. 1981, 201, 655–667. [Google Scholar] [CrossRef]
- Smith, L.J.; McKay, K.O.; van Asperen, P.P.; Selvadurai, H.; Fitzgerald, D.A. Normal development of the lung and premature birth. Paediatr. Respir. Rev. 2010, 11, 135–142. [Google Scholar] [CrossRef]
- Parker, D.; Prince, A. Innate immunity in the respiratory epithelium. Am. J. Respir. Cell Mol. Biol. 2011, 45, 189–201. [Google Scholar] [CrossRef]
- Meyerholz, D.K.; Grubor, B.; Fach, S.J.; Sacco, R.E.; Lehmkuhl, H.D.; Gallup, J.M.; Ackermann, M.R. Reduced clearance of respiratory syncytial virus in a preterm lamb model. Microbes Infect. 2004, 6, 1312–1319. [Google Scholar] [CrossRef]
- Olivier, A.; Gallup, J.M.; de Macedo, M.M.; Varga, S.M.; Ackermann, M.R. Human respiratory syncytial virus A2 strain replicates and induces innate immune responses by respiratory epithelia of neonatal lambs. Int. J. Exp. Pathol. 2009, 90, 431–438. [Google Scholar] [CrossRef]
- Bukreyev, A; Yang, L.; Fricke, J.; Cheng, L.; Ward, J.M.; Murphy, B.R; Collins, P.L. The secreted form of respiratory syncytial virus G glycoprotein helps the virus evade antibody-mediated restriction of replication by acting as an antigen decoy and through effects on Fc receptor-bearing leukocytes. J. Virol. 2008, 82, 12191–12204. [Google Scholar]
- Gruber, C.; Levine, S. Respiratory syncytial virus polypeptides. III. The envelope-associated proteins. J. Gen. Virol. 1983, 64, 825–832. [Google Scholar] [CrossRef]
- Levine, S. Polypeptides of respiratory syncytial virus. J. Virol. 1977, 21, 427–431. [Google Scholar]
- Levine, S.; Klaiber-Franco, R.; Paradiso, P.R. Demonstration that glycoprotein G is the attachment protein of respiratory syncytial virus. J. Gen. Virol. 1987, 68, 2521–2524. [Google Scholar] [CrossRef]
- Collins, P.L.; Mottet, G. Oligomerization and post-translational processing of glycoprotein G of human respiratory syncytial virus: Altered O-glycosylation in the presence of brefeldin. A. J. Gen. Virol. 1992, 73, 849–863. [Google Scholar] [CrossRef]
- Ding, M.X.; Wen, D.Z.; Schlesinger, M.J.; Wertz, G.W.; Ball, L.A. Expression and glycosylation of the respiratory syncytial virus G protein in Saccharomyces cerevisiae. Virology 1987, 159, 450–453. [Google Scholar] [CrossRef]
- Lamb, R.A.; Jardetzky, T.S. Structural basis of viral invasion: Lessons from paramyxovirus F. Curr. Opin. Struct. Biol. 2007, 17, 427–436. [Google Scholar] [CrossRef]
- Johnson, T.R.; McLellan, J.S.; Graham, B.S. Respiratory syncytial virus glycoprotein G interacts with DC-SIGN and L-SIGN to activate ERK1 and ERK2. J. Virol. 2012, 86, 1339–1347. [Google Scholar] [CrossRef]
- Batonick, M.; Oomens, A.G.; Wertz, G.W. Human respiratory syncytial virus glycoproteins are not required for apical targeting and release from polarized epithelial cells. J. Virol. 2008, 82, 8664–8672. [Google Scholar] [CrossRef]
- Karron, R.A; Buonagurio, D.A.; Georgiu, A.F; Whitehead, S.S.; Adamus, J.E.; Clements-Mann, M.L.; Harris, D.O.; Randolph, V.B.; Udem, S.A.; Murphy, B.R.; et al. Respiratory syncytial virus (RSV) SH and G proteins are not essential for viral replication in vitro: clinical evaluation and molecular characterization of a cold-passaged, attenuated RSV subgroup B mutant. Proc. Natl. Acad. Sci. 1997, 94, 13961–13966. [Google Scholar] [CrossRef]
- Kwilas, S.; Liesman, R.M.; Zhang, L.; Walsh, E.; Pickles, R.J.; Peeples, M.E. Respiratory syncytial virus grown in Vero cells contains a truncated attachment protein that alters its infectivity and dependence on glycosaminoglycans. J. Virol. 2009, 83, 10710–10718. [Google Scholar] [CrossRef]
- Wunner, W.H.; Pringle, C.R. Respiratory syncytial virus proteins. Virology 1976, 73, 228–243. [Google Scholar] [CrossRef]
- Moussa, A. Assembly of enveloped respiratory syncytial virus particles within the cytoplasm of infected Vero cells. Arch. Virol. 1994, 134, 205–211. [Google Scholar] [CrossRef]
- Norrby, E.; Marusyk, H.; Orvell, C. Morphogenesis of respiratory syncytial virus in a green monkey kidney cell line (Vero). J. Virol. 1970, 6, 237–242. [Google Scholar]
- DeVincenzo, J.P.; Wilkinson, T.; Vaishnaw, A.; Cehelsky, J.; Meyers, R.; Nochur, S.; Harrison, L.; Meeking, P.; Mann, A.; Moane, E.; et al. Viral load drives disease in humans experimentally infected with respiratory syncytial virus. Am. J. Respir. Crit. Care Med. 2010, 182, 1305–1314. [Google Scholar]
- Gallup, J.M.; Ackermann, M.R. The ‘PREXCEL-Q Method’ for qPCR. Int. J. Biomed. Sci. 2008, 4, 273–293. [Google Scholar]
- Gruber, C.; Levine, S. Respiratory syncytial virus polypeptides IV. The oligosaccharides of the glycoproteins. J. Gen. Virol. 1985, 66, 417–432. [Google Scholar] [CrossRef]
- Gruber, C.; Levine, S. Respiratory syncytial virus polypeptides V. The kinetics of glycoprotein synthesis. J. Gen. Virol. 1985, 66, 1241–1247. [Google Scholar] [CrossRef]
- Wertz, G.W.; Kreiger, M.; Bell, L.A. Structure and cell surface maturation of the attachment glycoprotein of human respiratory syncytial virus in cell line deficient in O glycosylation. J. Virol. 1989, 63, 4767–4776. [Google Scholar]
- Vieira, R.A.; Diniz, E.M.; Ceccon, M.E. Correlation between inflammatory mediators in the nasopharyngeal secretion and in the serum of children with lower respiratory tract infection caused by respiratory syncytial virus and disease severity. J. Bras. Pneumol. 2010, 36, 59–66. [Google Scholar] [CrossRef]
- Sow, F.B.; Gallup, J.M.; Krishnan, S.; Patera, A.C.; Suzich, J.; Ackermann, M.R. Respiratory syncytial virus infection is associated with an altered innate immunity and a heightened pro-inflammatory response in the lungs of preterm lambs. Respir. Res. 2011, 12, 106. [Google Scholar] [CrossRef]
- Derscheid, R.J.; van Geelen, A; Berkebile, A.R. ; Gallup, J.M.; Hostetter, S.J.; Banfi, B.; McCray, P.B., Jr.; Ackermann, M.R. Increased concentration of iodide in airway secretions is associated with reduced RSV disease severity. Am. J. Respir. Cell Mol. Biol. 2013. (Epub). [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Derscheid, R.J.; Van Geelen, A.; McGill, J.L.; Gallup, J.M.; Cihlar, T.; Sacco, R.E.; Ackermann, M.R. Human Respiratory Syncytial Virus Memphis 37 Grown in HEp-2 Cells Causes more Severe Disease in Lambs than Virus Grown in Vero Cells. Viruses 2013, 5, 2881-2897. https://doi.org/10.3390/v5112881
Derscheid RJ, Van Geelen A, McGill JL, Gallup JM, Cihlar T, Sacco RE, Ackermann MR. Human Respiratory Syncytial Virus Memphis 37 Grown in HEp-2 Cells Causes more Severe Disease in Lambs than Virus Grown in Vero Cells. Viruses. 2013; 5(11):2881-2897. https://doi.org/10.3390/v5112881
Chicago/Turabian StyleDerscheid, Rachel J., Albert Van Geelen, Jodi L. McGill, Jack M. Gallup, Tomas Cihlar, Randy E. Sacco, and Mark R. Ackermann. 2013. "Human Respiratory Syncytial Virus Memphis 37 Grown in HEp-2 Cells Causes more Severe Disease in Lambs than Virus Grown in Vero Cells" Viruses 5, no. 11: 2881-2897. https://doi.org/10.3390/v5112881
APA StyleDerscheid, R. J., Van Geelen, A., McGill, J. L., Gallup, J. M., Cihlar, T., Sacco, R. E., & Ackermann, M. R. (2013). Human Respiratory Syncytial Virus Memphis 37 Grown in HEp-2 Cells Causes more Severe Disease in Lambs than Virus Grown in Vero Cells. Viruses, 5(11), 2881-2897. https://doi.org/10.3390/v5112881




