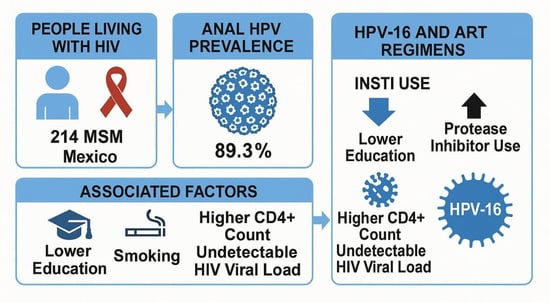
Prevalence and Associated Factors for HPV in People Living with HIV: Are INSTIs Protective Against HPV-16? The GAIA Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Measurements
- Sociodemographic characteristics: age, education level, occupation, and socioeconomic status.
- HIV-related parameters: baseline CD4+ cell count and HIV-RNA viral load (measured via PCR).
- Sexual practices: number of sexual partners, frequency of receptive anal sex, condom use, and history of sexually transmitted infections (STIs).
- HPV vaccination status: prior receipt of HPV vaccination and type of vaccine administered.
- High-risk genotypes: 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 69, 73, 82.
- Low-risk genotypes: 6, 11, 40, 42, 43, 44, 54, 61, 70.
2.4. Statistical Analysis
2.5. Ethical Considerations
3. Results
3.1. Study Population and Baseline Characteristics
3.2. Behavioral Factors Associated with HPV Infection
3.3. HPV Infection Prevalence
3.4. Factors Associated with HPV Infection
3.5. Immunovirological Factors Also Showed Associations
3.6. Educational Attainment Influenced HPV Infection Risk
3.7. Behavioral Factors Also Showed Associations
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3TC | Lamivudine |
| ABC | Abacavir |
| ART | Antiretroviral therapy |
| BIC | Bictegravir |
| CI | Confidence interval |
| DOR | Doravirine |
| DRV/c | Darunavir/cobicistat |
| DTG | Dolutegravir |
| FTC | Emtricitabine |
| HPV | Human papillomavirus |
| INSTI | Integrase strand transfer Inhibitor |
| NNRTI | Non-nucleoside retrotranscriptase inhibitor |
| OR | Odds ratio |
| PI | Protease inhibitor |
| TAF | Tenofovir alafenamide |
| TDF | Tenofovir disoproxil fumarate |
References
- De Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Parhizkar Roudsari, P.; Mousavi, S.; Saremian, J. A Brief Review of Anal Cancer Screening Methods for Prevention and Earlier Diagnosis. Cureus 2025, 17, e80686. [Google Scholar] [CrossRef] [PubMed]
- Kombe Kombe, A.J.; Li, B.; Zahid, A.; Mengist, H.M.; Bounda, G.A.; Zhou, Y.; Jin, T. Epidemiology and Burden of Human Papillomavirus and Related Diseases, Molecular Pathogenesis, and Vaccine Evaluation. Front. Public Health 2021, 8, 552028. [Google Scholar] [CrossRef] [PubMed]
- Uusküla, A.; Tisler, A.; DeHovitz, J.; Murenzi, G.; Castle, P.E.; Clifford, G. Prevention and control of HPV-related cancers in people living with HIV. Lancet HIV 2025, 12, e293–e302. [Google Scholar] [CrossRef]
- de Sanjosé, S.; Brotons, M.; Pavón, M.A. The natural history of human papillomavirus infection. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 47, 2–13. [Google Scholar] [CrossRef]
- Graham, S.V. The human papillomavirus replication cycle, and its links to cancer progression: A comprehensive review. Clin. Sci. 2017, 131, 2201–2221. [Google Scholar] [CrossRef]
- Riddell, J., IV; Brouwer, A.F.; Walline, H.M.; Campredon, L.P.; Meza, R.; Eisenberg, M.C.; Andrus, E.C.; Delinger, R.L.; Yost, M.L.; McCloskey, J.K.; et al. Oral human papillomavirus prevalence, persistence, and risk-factors in HIV-positive and HIV-negative adults. Tumour Virus Res. 2022, 13, 200237. [Google Scholar] [CrossRef]
- McNeil, C.J.; Lee, J.S.; Cole, S.R.; Patel, S.A.; Martin, J.; Mathews, W.C.; Moore, R.D.; Mayer, K.H.; Eron, J.J.; Saag, M.S.; et al. Anal cancer incidence in men with HIV who have sex with men: Are black men at higher risk? AIDS 2022, 36, 657–664. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Rasizadeh, R.; Jafari, S.; Baghi, H.B. Prevalence of HPV in anal cancer: Exploring the role of infection and inflammation. Infect. Agents Cancer 2024, 19, 63. [Google Scholar] [CrossRef]
- Zhang, Z.; Ling, X.; Liu, L.; Xi, M.; Zhang, G.; Dai, J. Natural History of Anal Papillomavirus Infection in HIV-Negative Men Who Have SexWith Men Based on a Markov Model: A 5-Year Prospective Cohort Study. Front. Public Health 2022, 10, 891991. [Google Scholar] [CrossRef]
- Conde-Ferráez, L.; Chan-Mezeta, A.; Gómez-Carballo, J.G.; Ayora-Talavera, G.; González-Losa, M.D.R. Human Papillomavirus Genotypes Infecting the Anal Canal and Cervix in HIV+ Men andWomen, Anal Cytology, and Risk Factors for Anal Infection. Pathogens 2023, 12, 252. [Google Scholar] [CrossRef]
- Petit, B.; Epaulard, O. Men having sex with men and the HPV vaccine in France: A low vaccine coverage that may be due to its infrequent proposal by physicians. Vaccine 2020, 38, 2160–2165. [Google Scholar] [CrossRef]
- Pruski, D.; Millert-Kalińska, S.; Łagiedo, M.; Sikora, J.; Jach, R.; Przybylski, M. Effect of HPV Vaccination on Virus Disappearance in Cervical Samples of a Cohort of HPV-Positive Polish Patients. J. Clin. Med. 2023, 12, 7592. [Google Scholar] [CrossRef]
- Fracella, M.; Oliveto, G.; Roberto, P.; Cinti, L.; Gentile, M.; Coratti, E.; D’Ettorre, G.; Cavallari, E.N.; Romano, F.; Santinelli, L.; et al. The Epidemiology of Anal Human Papillomavirus (HPV) in HIV-Positive and HIV-NegativeWomen and Men: A Ten-Year Retrospective Observational Study in Rome (Italy). Pathogens 2024, 13, 163. [Google Scholar] [CrossRef]
- Khandwala, P.; Singhal, S.; Desai, D.; Parsi, M.; Potdar, R. HIV-associated anal cancer. Cureus 2021, 13, e14834. [Google Scholar] [CrossRef]
- Lin, C.; Franceschi, S.; Clifford, G.M. Human papillomavirus types from infection to cancer in the anus, according to sex and HIV status: A systematic review and meta-analysis. Lancet Infect. Dis. 2018, 18, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Sarıgül Yıldırım, F.; Candevir, A.; Akhan, S.; Kaya, S.; Çabalak, M.; Ersöz, G.; İnan, D.; Ceren, N.; Karaoğlan, İ.; Damar Çakırca, T.; et al. Comparison of Immunological and Virological Recovery with Rapid, Early, and Late Start of Antiretroviral Treatment in Naive Plwh: Real-World Data. Int. J. Gen. Med. 2023, 16, 1867–1877. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.H.; Kim, M.K.; Lee, I.H.; Kim, T.J.; Kwak, S.H.; Song, S.H.; Lee, J.K. Association between serum cytokine profiles and clearance or persistence of high-risk human papillomavirus infection: A prospective study. Int. J. Gynecol. Cancer 2010, 20, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Tenorio, C.; Gil-Anguita, C.; López Ruz, M.A.; Omar, M.; López-Hidalgo, J.; Pasquau, J. ART is key to clearing oncogenic HPV genotypes (HR-HPV) in anal mucosa of HIV-positive MSM. PLoS ONE 2019, 14, e0224183. [Google Scholar] [CrossRef]
- Hidalgo-Tenorio, C.; Calle-Gómez, I.; Moya-Megías, R.; Rodríguez-Granges, J.; Omar, M.; López Hidalgo, J.; García-Martínez, C. HPV Infection of the Oropharyngeal, Genital and Anal Mucosa and Associated Dysplasia in People Living with HIV. Viruses 2023, 15, 1170. [Google Scholar] [CrossRef]
- Israr, M.; Mitchell, D.; Alam, S.; Dinello, D.; Kishel, J.J.; Meyers, C. The HIV protease inhibitor lopinavir/ritonavir (Kaletra) alters the growth, differentiation and proliferation of primary gingival epithelium. HIV Med. 2011, 12, 145–156. [Google Scholar] [CrossRef]
- Mbang, P.A.; Kowalkowski, M.A.; Amirian, E.S.; Giordano, T.P.; Richardson, P.A.; Hartman, C.M.; Chiao, E.Y. Association between Time on Protease Inhibitors and the Incidence of Squamous Cell Carcinoma of the Anus among U.S. Male Veterans. PLoS ONE 2015, 10, e0142966. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H. HIV protease inhibitors induce endoplasmic reticulum stress and disrupt barrier integrity in intestinal epithelial cells. Methods Enzymol. 2011, 490, 107–119. [Google Scholar] [CrossRef]
- Park, S.; Auyeung, A.; Lee, D.L.; Lambert, P.F.; Carchman, E.H.; Sherer, N.M. HIV-1 Protease Inhibitors Slow HPV16-Driven Cell Proliferation through Targeted Depletion of Viral E6 and E7 Oncoproteins. Cancers 2021, 13, 949. [Google Scholar] [CrossRef]
- Pinto-Cardoso, S.; Klatt, N.R.; Reyes-Terán, G. Impact of antiretroviral drugs on the microbiome: Unknown answers to important questions. Curr. Opin. HIV AIDS 2018, 13, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Caputo, V.; Libera, M.; Sisti, S.; Giuliani, B.; Diotti, R.A.; Criscuolo, E. The initial interplay between HIV and mucosal innate immunity. Front. Immunol. 2023, 14, 1104423. [Google Scholar] [CrossRef]
- Patra, S.; Shand, H.; Ghosal, S.; Ghorai, S. HPV and Male Cancer: Pathogenesis, Prevention and Impact. J. Oman Med. Assoc. 2025, 2, 4. [Google Scholar] [CrossRef]
- Teixeira, M.F.; Sabidó, M.; Leturiondo, A.L.; de Oliveira Ferreira, C.; Torres, K.L.; Benzaken, A.S. High risk human papillomavirus prevalence and genotype distribution among women infected with HIV in Manaus, Amazonas. Virol. J. 2018, 15, 36. [Google Scholar] [CrossRef]
- Abel, S.; Najioullah, F.; Voluménie, J.L.; Accrombessi, L.; Carles, G.; Catherine, D.; Chiappetta, D.; Clavel, C.; Codjo-Sodokine, A.; El Guedj, M.; et al. High prevalence of human papillomavirus infection in HIV-infected women living in French Antilles and French Guiana. PLoS ONE 2019, 14, e0221334. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, X.; Ye, Y.; Shen, W.; Ye, X.; Lin, Y.; Lin, Z.; Tan, S.; Gao, M.; Ding, Y.; et al. Increased CD4+ T cell count is associated with lower anal human papillomavirus prevalence among HIV-positive male cohort in Taizhou, China: A cross-sectional study. BMC Infect. Dis. 2022, 22, 250. [Google Scholar] [CrossRef] [PubMed]
- Vaccarella, S.; Lazcano-Ponce, E.; Castro-Garduño, J.A.; Cruz-Valdez, A.; Díaz, V.; Schiavon, R.; Hernández, P.; Kornegay, J.R.; Hernández-Avila, M.; Franceschi, S. Prevalence and determinants of human papillomavirus infection in men attending vasectomy clinics in Mexico. Int. J. Cancer 2006, 119, 1934–1939. [Google Scholar] [CrossRef]
- Symer, M.M.; Yeo, H.L. Recent advances in the management of anal cancer. F1000Research 2018, 7, 1572. [Google Scholar] [CrossRef]
- Xi, L.F.; Koutsky, L.A.; Castle, P.E.; Edelstein, Z.R.; Meyers, C.; Ho, J.; Schiffman, M. Relationship between cigarette smoking and human papilloma virus types 16 and 18 DNA load. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 3490–3496. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.Y.; Kim, M.K.; Seo, S.; Lee, D.O.; Chung, Y.K.; Lim, M.C.; Kim, J.; Lee, C.W.; Park, S. Alcohol consumption and persistent infection of high-risk human papillomavirus. Epidemiol. Infect. 2015, 143, 1442–1450. [Google Scholar] [CrossRef] [PubMed]
- Stephens, E.S.; Dema, E.; McGee-Avila, J.K.; Shiels, M.S.; Kreimer, A.R.; Shing, J.Z. Human Papillomavirus Awareness by Educational Level and by Race and Ethnicity. JAMA Netw. Open 2023, 6, e2343325. [Google Scholar] [CrossRef] [PubMed]
- Anguzu, G.; Flynn, A.; Musaazi, J.; Kasirye, R.; Atuhaire, L.K.; Kiragga, A.N.; Kabagenyi, A.; Mujugira, A. Relationship between socioeconomic status and risk of sexually transmitted infections in Uganda: Multilevel analysis of a nationally representative survey. Int. J. STD AIDS 2019, 30, 284–291. [Google Scholar] [CrossRef]
- Gholamzad, A.; Khakpour, N.; Hashemi, M.; Gholamzad, M. Prevalence of high and low risk HPV genotypes among vaccinated and non-vaccinated people in Tehran. Virol. J. 2024, 21, 9. [Google Scholar] [CrossRef]

| Characteristics | n | % | Median (IQR) |
|---|---|---|---|
| Demographic Characteristics | |||
| Age (years) | - | - | 30 (25–36) |
| Race/Ethnicity | |||
| Hispanic | 185 | 86.4 | - |
| White | 23 | 10.7 | - |
| Other | 6 | 2.8 | - |
| Education | |||
| Elementary school | 3 | 1.4 | - |
| High school | 100 | 46.7 | - |
| College | 84 | 39.3 | - |
| Postgraduate | 27 | 12.6 | - |
| Clinical Characteristics | |||
| Time since HIV diagnosis (months) | - | - | 18 (12–29) |
| CD4+ T-cell count (cells/mm3) | - | - | 625 (431–879) |
| Nadir CD4+ T-Cell Count (cells/mm3) | |||
| <200 | 89 | 41.6 | - |
| 200–500 | 97 | 45.3 | - |
| >500 | 28 | 13.1 | - |
| HIV-1 RNA < 40 copies/mL | 187 | 87.4 | - |
| ART Duration (months) | - | - | 12 (6–24) |
| Treatment History | |||
| First treatment regimen | 199 | 93 | - |
| ART-naïve | 14 | 6.5 | - |
| Optimized regimen due to previous treatment failure | 1 | 0.5 | - |
| ART Regimen | |||
| DTG/3TC/ABC | 52 | 24.3 | - |
| BIC/TAF/FTC | 59 | 27.6 | - |
| DRV/c + TDF/FTC | 47 | 22.0 | - |
| DTG + TDF/FTC | 11 | 5.2 | - |
| DRV/c + 3TC | 22 | 10.3 | - |
| DOR/TDF/3TC | 5 | 2.3 | - |
| DTG/3TC | 3 | 1.4 | - |
| DRV/c + DTG + TDF/FTC | 1 | 0.5 | - |
| None | 14 | 6.5 | - |
| ART Regimen by Pharmacological Group | |||
| INSTI-based | 126 | 58.9 | - |
| PI-based | 69 | 32.2 | - |
| NNRTI-based | 5 | 2.3 | - |
| None | 14 | 6.5 | - |
| Behavioral Characteristics | |||
| Age at first sexual intercourse (years) | - | - | 17 (15–19) |
| Age at first receptive anal intercourse (years) | - | - | 18 (16–21) |
| Sexual Partners per Year | |||
| 0 | 22 | 10.3 | - |
| 1–4 | 120 | 56.1 | - |
| 5–9 | 31 | 14.5 | - |
| 10–19 | 21 | 9.8 | - |
| ≥20 | 15 | 7.0 | - |
| Unknown | 5 | 2.3 | - |
| Consistent Condom Use | 182 | 85.0 | - |
| Smoking | 57 | 26.6 | - |
| Alcohol Consumption (past 12 months) | 184 | 86.0 | - |
| HPV Vaccination | |||
| Not vaccinated | 155 | 72.4 | - |
| 1 Dose | 28 | 13.1 | - |
| 2 Doses | 16 | 7.5 | - |
| 3 Doses | 14 | 6.5 | - |
| Variable | HPV Genotype | OR | 95% CI | p-Value |
|---|---|---|---|---|
| Antiretroviral Therapy (ART) | ||||
| No INSTI use | - | 1.00 | Referent | - |
| INSTI use | 16 | 0.42 | 0.21–0.83 | 0.011 |
| 33 | 0.39 | 0.13–1.11 | 0.071 | |
| 43 | 0.47 | 0.21–1.05 | 0.065 | |
| No PI use | - | 1.00 | Referent | - |
| PI use | 16 | 2.16 | 1.09–4.29 | 0.025 |
| 6 | 2.38 | 1.06–5.32 | 0.031 | |
| No ART | - | 1.00 | Referent | - |
| ART use | 39 | 0.18 | 0.05–0.62 | 0.012 |
| 53 | 0.24 | 0.07–0.88 | 0.044 | |
| 66 | 0.20 | 0.06–0.68 | 0.016 | |
| 40 | 0.17 | 0.04–0.73 | 0.035 | |
| INSTI Use < 12 months | - | 1.00 | Referent | - |
| INSTI Use > 12 months | 53 | 0.39 | 0.15–0.99 | 0.043 |
| Clinical Characteristics | ||||
| CD4+ < 500 cells/mm3 | - | 1.00 | Referent | - |
| CD4+ ≥ 500 cells/mm3 | High-risk genotypes | 0.48 | 0.27–0.86 | 0.013 |
| Low-risk genotypes | 0.45 | 0.24–0.83 | 0.010 | |
| CD4+ < 200 cells/mm3 | - | 1.00 | Referent | - |
| CD4+ ≥ 200 cells/mm3 | 82 | 0.10 | 0.01–0.61 | 0.039 |
| HIV-1 RNA > 40 copies/mL | - | 1.00 | Referent | - |
| HIV-1 RNA < 40 copies/mL | High-risk genotypes | 0.40 | 0.15–0.95 | 0.049 |
| 45 | 0.22 | 0.06–0.82 | 0.036 | |
| 53 | 0.22 | 0.08–0.58 | 0.001 | |
| 44 | 0.32 | 0.12–0.86 | 0.019 | |
| Behavioral and Demographic Characteristics | ||||
| Non-smoking | - | 1.00 | Referent | - |
| Smoking | 53 | 2.64 | 1.11–6.31 | 0.024 |
| 44 | 2.72 | 1.17–6.31 | 0.016 | |
| Inconsistent use | - | 1.00 | Referent | - |
| Consistent condom use | 39 | 0.84 | 0.79–0.89 | 0.030 |
| Higher educational level | - | 1.00 | Referent | - |
| Having a lower educational level (high school or less) | 16 | 2.15 | 1.09–4.23 | 0.025 |
| 53 | 2.75 | 1.16–6.54 | 0.018 | |
| 59 | 4.12 | 1.41–12.01 | 0.006 | |
| Lower educational level | - | 1.00 | Referent | - |
| Having a higher educational level (college school or higher) | 53 | 0.19 | 0.05–0.66 | 0.004 |
| HPV-16 Model (n = 43/214) | aOR | 95% (CI) | p-Value |
|---|---|---|---|
| INSTI | 0.43 | 0.21–0.86 | 0.018 |
| IP | 1.06 | 0.33–3.45 | 0.913 |
| Lower educational level | 2.05 | 1.03–4.08 | <0.001 |
| Higher educational level | 2.89 | 0.78–10.75 | 0.040 |
| HPV-53 Model (n = 24/214) | aOR | 95% (CI) | p-Value |
| HIV-1 RNA < 40 copies/mL | 0.172 | 0.05–0.50 | <0.001 |
| Smoking | 3.025 | 1.16–7.88 | 0.024 |
| Having a lower educational level | 16.810 | 1.33–212.47 | 0.029 |
| Having a higher educational level | 0.176 | 0.48–0.65 | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-López, O.; González-Contreras, B.C.; Cano-Díaz, A.L.; Mata-Marín, J.A.; Pompa-Mera, E.N.; Noyola-Gómez, J.V.; Triana-González, S.; Padilla-Noguera, P.E.; Chaparro-Sánchez, A.; García-Gutiérrez, S.A.; et al. Prevalence and Associated Factors for HPV in People Living with HIV: Are INSTIs Protective Against HPV-16? The GAIA Study. Viruses 2025, 17, 1147. https://doi.org/10.3390/v17081147
Hernández-López O, González-Contreras BC, Cano-Díaz AL, Mata-Marín JA, Pompa-Mera EN, Noyola-Gómez JV, Triana-González S, Padilla-Noguera PE, Chaparro-Sánchez A, García-Gutiérrez SA, et al. Prevalence and Associated Factors for HPV in People Living with HIV: Are INSTIs Protective Against HPV-16? The GAIA Study. Viruses. 2025; 17(8):1147. https://doi.org/10.3390/v17081147
Chicago/Turabian StyleHernández-López, Omar, Brenda Clara González-Contreras, Ana Luz Cano-Díaz, José Antonio Mata-Marín, Ericka Nelly Pompa-Mera, Javier Vicente Noyola-Gómez, Salma Triana-González, Paola Edith Padilla-Noguera, Alberto Chaparro-Sánchez, Sócrates Alberto García-Gutiérrez, and et al. 2025. "Prevalence and Associated Factors for HPV in People Living with HIV: Are INSTIs Protective Against HPV-16? The GAIA Study" Viruses 17, no. 8: 1147. https://doi.org/10.3390/v17081147
APA StyleHernández-López, O., González-Contreras, B. C., Cano-Díaz, A. L., Mata-Marín, J. A., Pompa-Mera, E. N., Noyola-Gómez, J. V., Triana-González, S., Padilla-Noguera, P. E., Chaparro-Sánchez, A., García-Gutiérrez, S. A., Barriga-Angulo, G., & Gaytan-Martinez, J. E. (2025). Prevalence and Associated Factors for HPV in People Living with HIV: Are INSTIs Protective Against HPV-16? The GAIA Study. Viruses, 17(8), 1147. https://doi.org/10.3390/v17081147