Direct oHSV Infection Induces DC Maturation and a Tumor Therapeutic Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Flow Cytometry
2.3. Viral Recovery and IncuCyte® Viral Spread Assay
2.4. Co-Culture Experiment and Tumor Killing Assay
2.5. Syngeneic Murine Model
2.6. Statistical Analysis
3. Results
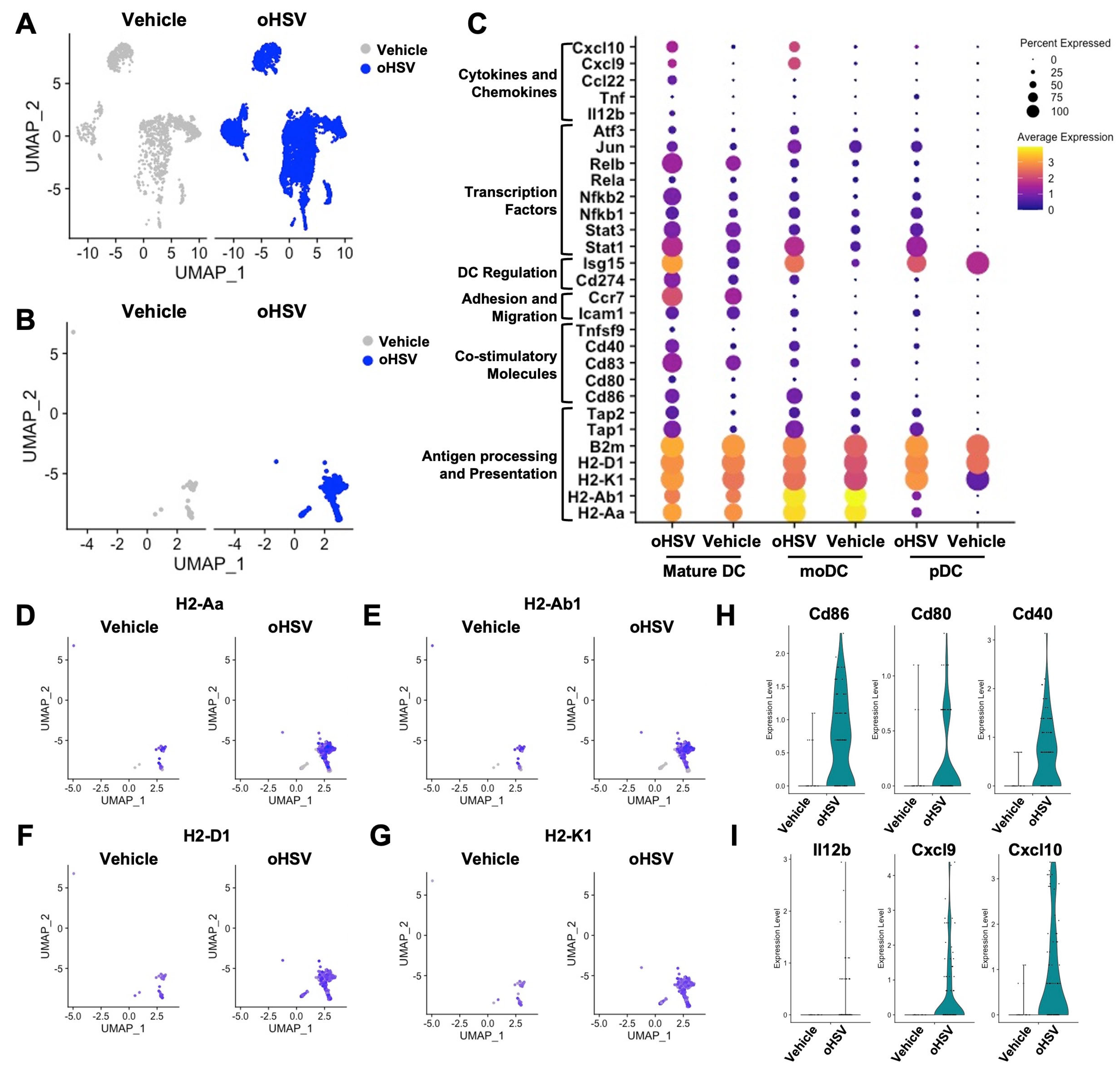
3.1. Secondary Analysis of Pre-Clinical Results Suggests That oHSV Infection Promotes DC Maturation
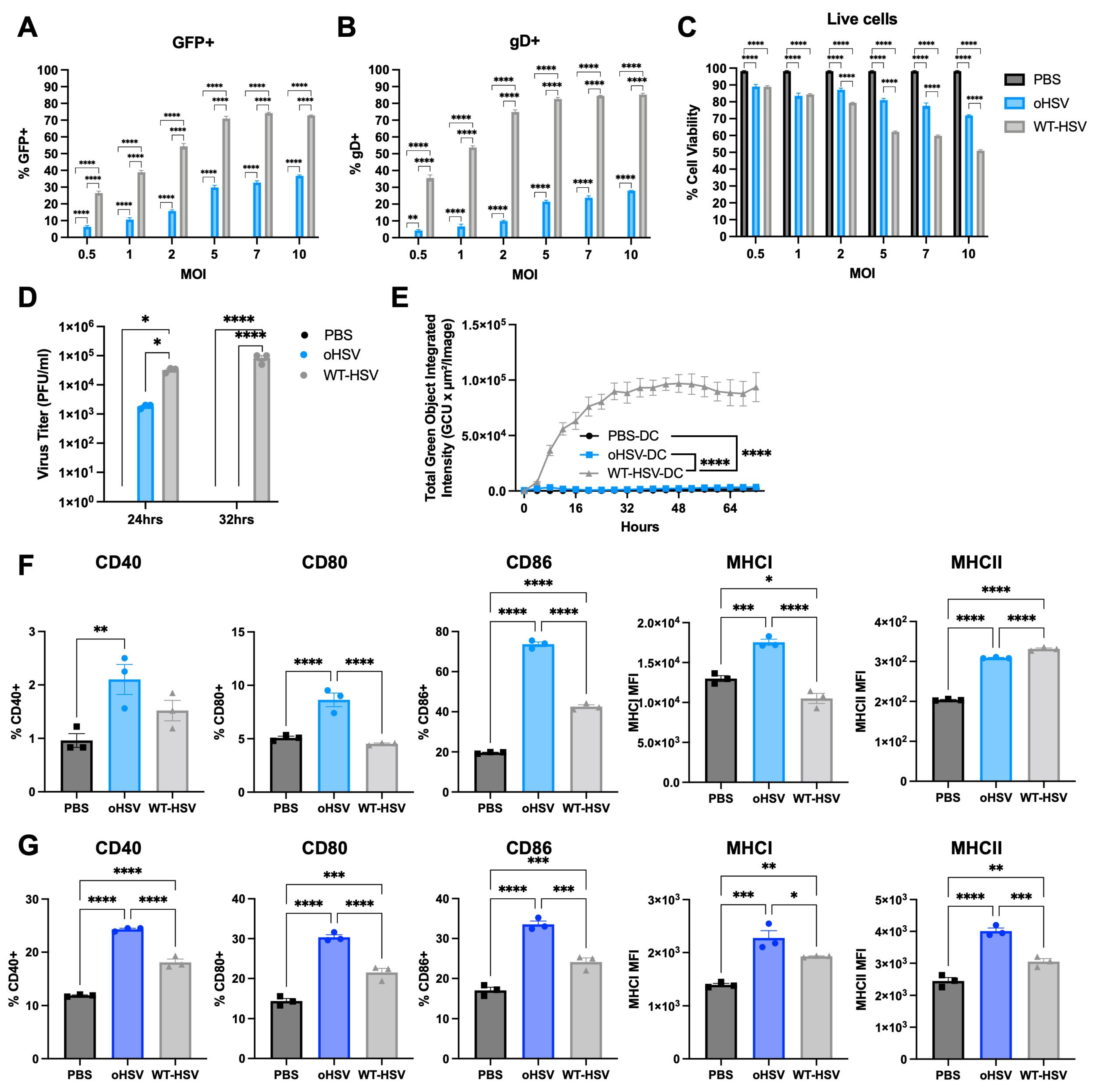
3.2. oHSV Can Directly Infect DCs but oHSV Replication Is Restricted
3.3. oHSV Infection Alters DC Maturation
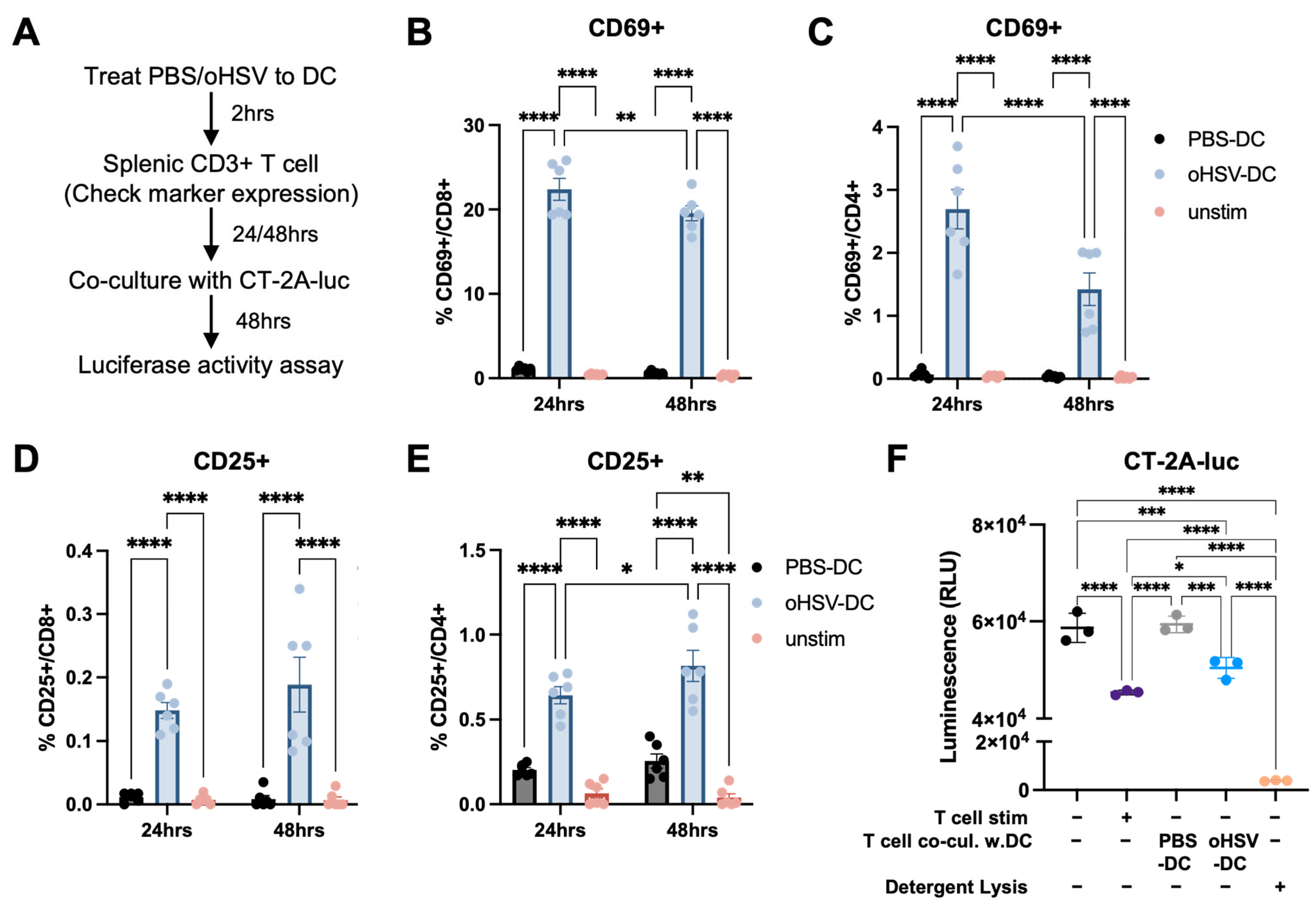
3.4. oHSV-Infected DCs Can Prime T Cells That Can Mediate a Functional Anti-Tumor Response
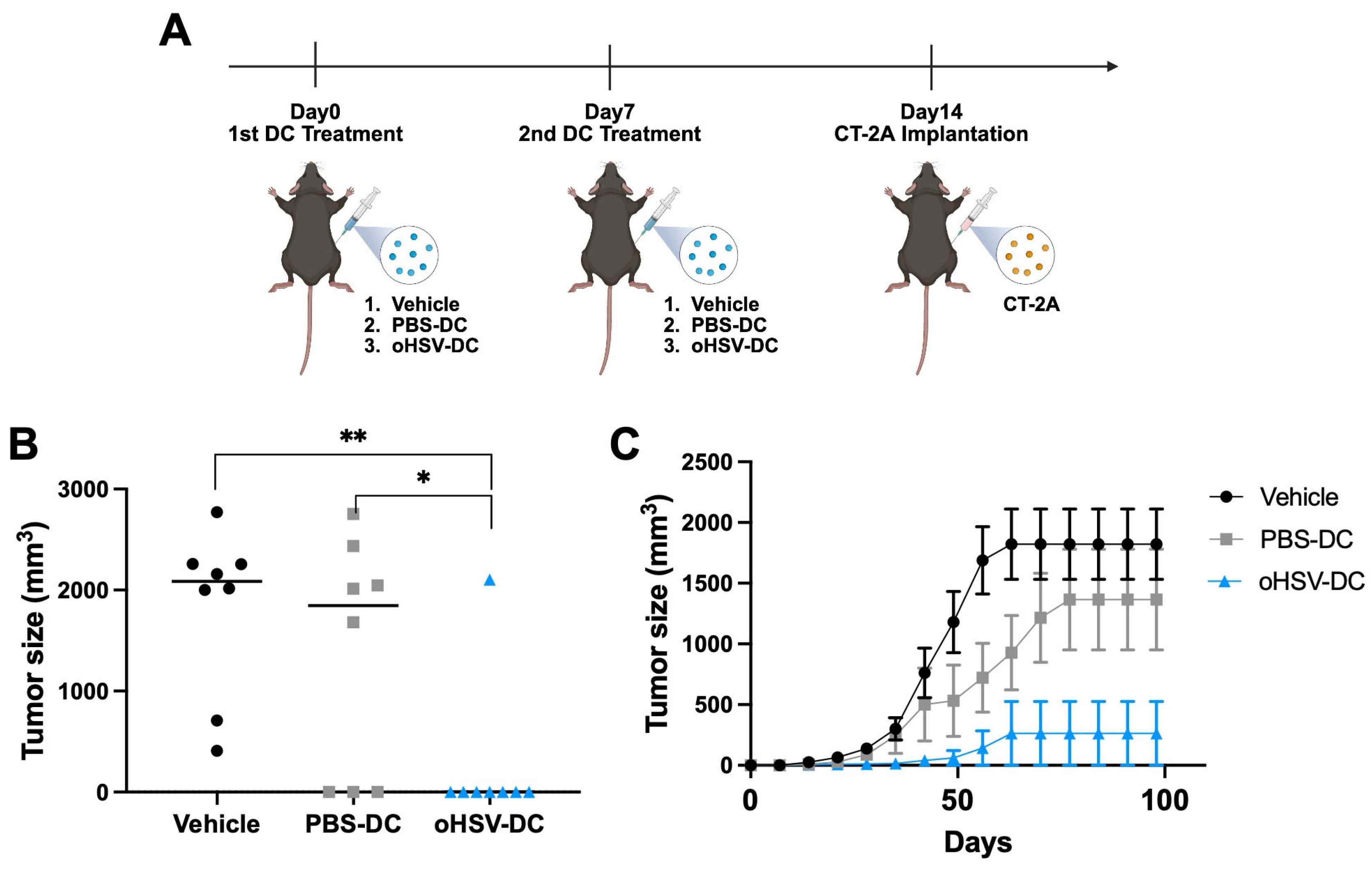
3.5. oHSV-Infected DC Mediates Therapeutic Effect Against Malignant Brain Tumor
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Frosina, G. Recapitulating the Key Advances in the Diagnosis and Prognosis of High-Grade Gliomas: Second Half of 2021 Update. Int. J. Mol. Sci. 2023, 24, 6375. [Google Scholar] [CrossRef] [PubMed]
- Lenting, K.; Verhaak, R.; Ter Laan, M.; Wesseling, P.; Leenders, W. Glioma: Experimental models and reality. Acta Neuropathol. 2017, 133, 263–282. [Google Scholar] [CrossRef]
- Rong, L.; Li, N.; Zhang, Z. Emerging therapies for glioblastoma: Current state and future directions. J. Exp. Clin. Cancer Res. 2022, 41, 142. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Garancher, A.; Ramaswamy, V.; Wechsler-Reya, R.J. Medulloblastoma: From Molecular Subgroups to Molecular Targeted Therapies. Annu. Rev. Neurosci. 2018, 41, 207–232. [Google Scholar] [CrossRef]
- Millard, N.E.; De Braganca, K.C. Medulloblastoma. J. Child Neurol. 2016, 31, 1341–1353. [Google Scholar] [CrossRef]
- Griesinger, A.M.; Birks, D.K.; Donson, A.M.; Amani, V.; Hoffman, L.M.; Waziri, A.; Wang, M.; Handler, M.H.; Foreman, N.K. Characterization of distinct immunophenotypes across pediatric brain tumor types. J. Immunol. 2013, 191, 4880–4888. [Google Scholar] [CrossRef]
- Bockmayr, M.; Mohme, M.; Klauschen, F.; Winkler, B.; Budczies, J.; Rutkowski, S.; Schuller, U. Subgroup-specific immune and stromal microenvironment in medulloblastoma. Oncoimmunology 2018, 7, e1462430. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; Wick, W.; Aldape, K.; Brada, M.; Berger, M.; Pfister, S.M.; Nishikawa, R.; Rosenthal, M.; Wen, P.Y.; Stupp, R.; et al. Glioma. Nat. Rev. Dis. Primers 2015, 1, 15017. [Google Scholar] [CrossRef]
- Totsch, S.K.; Schlappi, C.; Kang, K.D.; Ishizuka, A.S.; Lynn, G.M.; Fox, B.; Beierle, E.A.; Whitley, R.J.; Markert, J.M.; Gillespie, G.Y.; et al. Oncolytic herpes simplex virus immunotherapy for brain tumors: Current pitfalls and emerging strategies to overcome therapeutic resistance. Oncogene 2019, 38, 6159–6171. [Google Scholar] [CrossRef]
- Jackson, J.W.; Hall, B.L.; Marzulli, M.; Shah, V.K.; Bailey, L.; Chiocca, E.A.; Goins, W.F.; Kohanbash, G.; Cohen, J.B.; Glorioso, J.C. Treatment of glioblastoma with current oHSV variants reveals differences in efficacy and immune cell recruitment. Mol. Ther. Oncolytics 2021, 22, 444–453. [Google Scholar] [CrossRef]
- Thompson, E.M.; Kang, K.D.; Stevenson, K.; Zhang, H.; Gromeier, M.; Ashley, D.; Brown, M.; Friedman, G.K. Elucidating cellular response to treatment with viral immunotherapies in pediatric high-grade glioma and medulloblastoma. Transl. Oncol. 2024, 40, 101875. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.J.; Peng, K.W.; Bell, J.C. Oncolytic virotherapy. Nat. Biotechnol. 2012, 30, 658–670. [Google Scholar] [CrossRef]
- Schirrmacher, V.; Fournier, P. Harnessing oncolytic virus-mediated anti-tumor immunity. Front. Oncol. 2014, 4, 337. [Google Scholar] [CrossRef] [PubMed]
- Foreman, P.M.; Friedman, G.K.; Cassady, K.A.; Markert, J.M. Oncolytic Virotherapy for the Treatment of Malignant Glioma. Neurotherapeutics 2017, 14, 333–344. [Google Scholar] [CrossRef]
- Cassady, K.A. Human cytomegalovirus TRS1 and IRS1 gene products block the double-stranded-RNA-activated host protein shutoff response induced by herpes simplex virus type 1 infection. J. Virol. 2005, 79, 8707–8715. [Google Scholar] [CrossRef] [PubMed]
- Ghonime, M.G.; Jackson, J.; Shah, A.; Roth, J.; Li, M.; Saunders, U.; Coleman, J.; Gillespie, G.Y.; Markert, J.M.; Cassady, K.A. Chimeric HCMV/HSV-1 and Deltagamma(1)34.5 oncolytic herpes simplex virus elicit immune mediated antigliomal effect and antitumor memory. Transl. Oncol. 2018, 11, 86–93. [Google Scholar] [CrossRef]
- Miller, K.E.; Cassady, K.A.; Roth, J.C.; Clements, J.; Schieffer, K.M.; Leraas, K.; Miller, A.R.; Prasad, N.; Leavenworth, J.W.; Aban, I.B.; et al. Immune Activity and Response Differences of Oncolytic Viral Therapy in Recurrent Glioblastoma: Gene Expression Analyses of a Phase IB Study. Clin. Cancer Res. 2022, 28, 498–506. [Google Scholar] [CrossRef]
- Cassady, K.A.; Haworth, K.B.; Jackson, J.; Markert, J.M.; Cripe, T.P. To Infection and Beyond: The Multi-Pronged Anti-Cancer Mechanisms of Oncolytic Viruses. Viruses 2016, 8, 43. [Google Scholar] [CrossRef]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2020, 20, 7–24. [Google Scholar] [CrossRef]
- Yin, X.; Chen, S.; Eisenbarth, S.C. Dendritic Cell Regulation of T Helper Cells. Annu. Rev. Immunol. 2021, 39, 759–790. [Google Scholar] [CrossRef]
- Jhunjhunwala, S.; Hammer, C.; Delamarre, L. Antigen presentation in cancer: Insights into tumour immunogenicity and immune evasion. Nat. Rev. Cancer 2021, 21, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Ness, S.; Lin, S.; Gordon, J.R. Regulatory Dendritic Cells, T Cell Tolerance, and Dendritic Cell Therapy for Immunologic Disease. Front. Immunol. 2021, 12, 633436. [Google Scholar] [CrossRef]
- Kim, Y.; Clements, D.R.; Sterea, A.M.; Jang, H.W.; Gujar, S.A.; Lee, P.W. Dendritic Cells in Oncolytic Virus-Based Anti-Cancer Therapy. Viruses 2015, 7, 6506–6525. [Google Scholar] [CrossRef]
- Benencia, F.; Courreges, M.C.; Fraser, N.W.; Coukos, G. Herpes virus oncolytic therapy reverses tumor immune dysfunction and facilitates tumor antigen presentation. Cancer Biol. Ther. 2008, 7, 1194–1205. [Google Scholar] [CrossRef]
- O’Donoghue, C.; Doepker, M.P.; Zager, J.S. Talimogene laherparepvec: Overview, combination therapy and current practices. Melanoma Manag. 2016, 3, 267–272. [Google Scholar] [CrossRef]
- Toda, M.; Martuza, R.L.; Rabkin, S.D. Tumor growth inhibition by intratumoral inoculation of defective herpes simplex virus vectors expressing granulocyte-macrophage colony-stimulating factor. Mol. Ther. 2000, 2, 324–329. [Google Scholar] [CrossRef]
- Shah, A.C.; Parker, J.N.; Gillespie, G.Y.; Lakeman, F.D.; Meleth, S.; Markert, J.M.; Cassady, K.A. Enhanced antiglioma activity of chimeric HCMV/HSV-1 oncolytic viruses. Gene Ther. 2007, 14, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Hedberg, J.; Studebaker, A.; Smith, L.; Chen, C.Y.; Westfall, J.J.; Cam, M.; Gross, A.; Hernandez-Aguirre, I.; Martin, A.; Kim, D.; et al. Oncolytic virus-driven immune remodeling revealed in mouse medulloblastomas at single cell resolution. Mol. Ther. Oncolytics 2023, 30, 39–55. [Google Scholar] [CrossRef]
- Jackson, J.D.; Markert, J.M.; Li, L.; Carroll, S.L.; Cassady, K.A. STAT1 and NF-kappaB Inhibitors Diminish Basal Interferon-Stimulated Gene Expression and Improve the Productive Infection of Oncolytic HSV in MPNST Cells. Mol. Cancer Res. 2016, 14, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.S.; Shin, E.C. The activation of bystander CD8+ T cells and their roles in viral infection. Exp. Mol. Med. 2019, 51, 1–9. [Google Scholar] [CrossRef]
- Vitiello, G.A.F.; Ferreira, W.A.S.; Cordeiro de Lima, V.C.; Medina, T.D.S. Antiviral Responses in Cancer: Boosting Antitumor Immunity Through Activation of Interferon Pathway in the Tumor Microenvironment. Front. Immunol. 2021, 12, 782852. [Google Scholar] [CrossRef]
- Tsuboi, N.; Rivera-Caraballo, K.A.; Sahu, U.; Pacholczyk, R.; Douglass, E.; Johnson, T.S.; Wang, Q.; Kolhe, R.; Hedrick, C.C.; Munn, D.H.; et al. Blocking Feedback Immunosuppression of Antigen Presentation in Brain Tumor During Oncolytic Virotherapy with oHSV-mshPKR. Mol. Cancer Ther. 2025, 24, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Markert, J.M.; Leavenworth, J.W. Modulation of the Intratumoral Immune Landscape by Oncolytic Herpes Simplex Virus Virotherapy. Front. Oncol. 2017, 7, 136. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.; Sahu, U.; Mullarkey, M.P.; Kaur, B. Replication and Spread of Oncolytic Herpes Simplex Virus in Solid Tumors. Viruses 2022, 14, 118. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Li, Z.; Chiocca, E.A.; Caligiuri, M.A.; Yu, J. The emerging field of oncolytic virus-based cancer immunotherapy. Trends Cancer 2023, 9, 122–139. [Google Scholar] [CrossRef]
- Heilingloh, C.S.; Muhl-Zurbes, P.; Steinkasserer, A.; Kummer, M. Herpes simplex virus type 1 ICP0 induces CD83 degradation in mature dendritic cells independent of its E3 ubiquitin ligase function. J. Gen. Virol. 2014, 95, 1366–1375. [Google Scholar] [CrossRef]
- Jin, H.; Yan, Z.; Ma, Y.; Cao, Y.; He, B. A herpesvirus virulence factor inhibits dendritic cell maturation through protein phosphatase 1 and Ikappa B kinase. J. Virol. 2011, 85, 3397–3407. [Google Scholar] [CrossRef]
- Ayele, K.; Wakimoto, H.; Nauwynck, H.J.; Kaufman, H.L.; Rabkin, S.D.; Saha, D. Understanding the interplay between oHSV and the host immune system: Implications for therapeutic oncolytic virus development. Mol. Ther. 2025, 33, 1327–1343. [Google Scholar] [CrossRef]
- Gao, Y.; Cheng, J.; Xu, X.; Li, X.; Zhang, J.; Ma, D.; Jiang, G.; Liao, Y.; Fan, S.; Niu, Z.; et al. HSV-1 Infection of Epithelial Dendritic Cells is a Critical Strategy for Interfering with Antiviral Immunity. Viruses 2022, 14, 1046. [Google Scholar] [CrossRef]
- Sato, A.; Linehan, M.M.; Iwasaki, A. Dual recognition of herpes simplex viruses by TLR2 and TLR9 in dendritic cells. Proc. Natl. Acad. Sci. USA 2006, 103, 17343–17348. [Google Scholar] [CrossRef]
- Gujar, S.; Pol, J.G.; Kim, Y.; Lee, P.W.; Kroemer, G. Antitumor Benefits of Antiviral Immunity: An Underappreciated Aspect of Oncolytic Virotherapies. Trends Immunol. 2018, 39, 209–221. [Google Scholar] [CrossRef]
- Liau, L.M.; Prins, R.M.; Kiertscher, S.M.; Odesa, S.K.; Kremen, T.J.; Giovannone, A.J.; Lin, J.W.; Chute, D.J.; Mischel, P.S.; Cloughesy, T.F.; et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin. Cancer Res. 2005, 11, 5515–5525. [Google Scholar] [CrossRef] [PubMed]
- Belmans, J.; Van Woensel, M.; Creyns, B.; Dejaegher, J.; Bullens, D.M.; Van Gool, S.W. Immunotherapy with subcutaneous immunogenic autologous tumor lysate increases murine glioblastoma survival. Sci. Rep. 2017, 7, 13902. [Google Scholar] [CrossRef]
- Liau, L.M.; Ashkan, K.; Tran, D.D.; Campian, J.L.; Trusheim, J.E.; Cobbs, C.S.; Heth, J.A.; Salacz, M.; Taylor, S.; D’Andre, S.D.; et al. First results on survival from a large Phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J. Transl. Med. 2018, 16, 142. [Google Scholar] [CrossRef]
- Rolig, A.S.; Rose, D.C.; McGee, G.H.; Rubas, W.; Kivimae, S.; Redmond, W.L. Combining bempegaldesleukin (CD122-preferential IL-2 pathway agonist) and NKTR-262 (TLR7/8 agonist) improves systemic antitumor CD8+ T cell cytotoxicity over BEMPEG+RT. J. Immunother. Cancer 2022, 10, e004218. [Google Scholar] [CrossRef]
- Hong, Y.; Robbins, Y.; Yang, X.; Mydlarz, W.K.; Sowers, A.; Mitchell, J.B.; Gulley, J.L.; Schlom, J.; Gameiro, S.R.; Sievers, C.; et al. Cure of syngeneic carcinomas with targeted IL-12 through obligate reprogramming of lymphoid and myeloid immunity. JCI Insight 2022, 7, e157448. [Google Scholar] [CrossRef]
- Akter, F.; Simon, B.; de Boer, N.L.; Redjal, N.; Wakimoto, H.; Shah, K. Pre-clinical tumor models of primary brain tumors: Challenges and opportunities. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188458. [Google Scholar] [CrossRef] [PubMed]
- Sahu, U.; Barth, R.F.; Otani, Y.; McCormack, R.; Kaur, B. Rat and Mouse Brain Tumor Models for Experimental Neuro-Oncology Research. J. Neuropathol. Exp. Neurol. 2022, 81, 312–329. [Google Scholar] [CrossRef] [PubMed]
- Brandner, S. Rodent models of tumours of the central nervous system. Mol. Oncol. 2024, 18, 2842–2870. [Google Scholar] [CrossRef]
- Hicks, W.H.; Bird, C.E.; Traylor, J.I.; Shi, D.D.; El Ahmadieh, T.Y.; Richardson, T.E.; McBrayer, S.K.; Abdullah, K.G. Contemporary Mouse Models in Glioma Research. Cells 2021, 10, 712. [Google Scholar] [CrossRef] [PubMed]
- Frederico, S.C.; Zhang, X.; Hu, B.; Kohanbash, G. Pre-clinical models for evaluating glioma targeted immunotherapies. Front. Immunol. 2022, 13, 1092399. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Kelly, M.; Hedberg, J.; Martin, A.K.; Hernandez-Aguirre, I.; Kim, Y.; Cain, L.R.; Dhital, R.; Cassady, K.A. Direct oHSV Infection Induces DC Maturation and a Tumor Therapeutic Response. Viruses 2025, 17, 1134. https://doi.org/10.3390/v17081134
Kim D, Kelly M, Hedberg J, Martin AK, Hernandez-Aguirre I, Kim Y, Cain LR, Dhital R, Cassady KA. Direct oHSV Infection Induces DC Maturation and a Tumor Therapeutic Response. Viruses. 2025; 17(8):1134. https://doi.org/10.3390/v17081134
Chicago/Turabian StyleKim, Doyeon, Michael Kelly, Jack Hedberg, Alexia K. Martin, Ilse Hernandez-Aguirre, Yeaseul Kim, Lily R. Cain, Ravi Dhital, and Kevin A. Cassady. 2025. "Direct oHSV Infection Induces DC Maturation and a Tumor Therapeutic Response" Viruses 17, no. 8: 1134. https://doi.org/10.3390/v17081134
APA StyleKim, D., Kelly, M., Hedberg, J., Martin, A. K., Hernandez-Aguirre, I., Kim, Y., Cain, L. R., Dhital, R., & Cassady, K. A. (2025). Direct oHSV Infection Induces DC Maturation and a Tumor Therapeutic Response. Viruses, 17(8), 1134. https://doi.org/10.3390/v17081134





