Abstract
The global elimination of hepatitis C virus (HCV) has been prioritized by the World Health Organization (WHO) as a key public health target, with a deadline set for 2030. This initiative aims to significantly reduce both new infection rates and HCV-associated mortality. A major breakthrough in achieving this goal has been the development of direct-acting antiviral agents (DAAs), which offer cure rates exceeding 95%, along with excellent safety and tolerability. Nevertheless, transmission via parenteral routes continues to be the dominant pathway, particularly among high-risk groups, such as individuals who inject drugs, incarcerated populations, those exposed to unsafe medical practices, and healthcare professionals. Identifying, monitoring, and delivering tailored interventions to these groups is crucial to interrupt ongoing transmission and to reduce the burden of chronic liver disease. On a global scale, several nations have demonstrated measurable progress toward HCV elimination, with some nearing the targets set by WHO. These achievements have largely resulted from context-adapted policies that enhanced diagnostic and therapeutic access while emphasizing outreach to vulnerable communities. This review synthesizes current advancements in HCV prevention and control and proposes strategic frameworks to expedite global elimination efforts.
1. Introduction
HCV infection continues to represent a critical global health issue, despite significant advances in antiviral treatment and diagnostic methods over the past decade. As a blood-transmitted pathogen, HCV plays a critical role in the development of chronic liver diseases, notably cirrhosis and hepatocellular carcinoma (HCC), remaining a key contributor to liver-related morbidity and mortality on a global scale. According to recent estimates from the WHO, approximately one million new HCV infections occur annually, reflecting the ongoing challenges in controlling viral transmission at a global level [1,2]. In 2022, hepatitis C was associated with nearly 242,000 deaths, underscoring the severe consequences of delayed diagnosis and limited access to effective therapies [1].
These figures highlight an urgent need to reassess global strategies for prevention and control. The global distribution of HCV infection reveals considerable regional differences. Prevalence is highest in low- and middle-income countries (LMICs), where healthcare systems often struggle to provide comprehensive screening, timely diagnosis, and access to antiviral therapy. Importantly, nearly four out of five individuals living with chronic HCV remain unaware of their infection, limiting their opportunity to benefit from curative treatments. Even among those diagnosed, only about 5% initiate therapy, highlighting persistent gaps between diagnosis and treatment [2].
Historically, the standard of care involved PEGylated interferon alpha (PEG-IFNα) combined with ribavirin (RBV)—a regimen with moderate efficacy, long treatment duration, and frequent adverse effects, which negatively impacted adherence and outcomes [3]. The year 2014 marked a pivotal advancement in the therapeutic management of hepatitis C, with the introduction of DAAs revolutionizing treatment efficacy and clinical outcomes. These highly effective, well-tolerated oral regimens have transformed HCV management, allowing viral clearance in most patients within 8 to 12 weeks of therapy. Consequently, hepatitis C has evolved from a chronic, difficult-to-treat infection into one with curative potential for the majority of cases [4].
Today, two pangenotypic DAA combinations—sofosbuvir/velpatasvir and glecaprevir/pibrentasvir—are widely used as first-line treatments. Their proven efficacy across all HCV genotypes and favorable safety profiles make them essential tools in national and international elimination strategies. These regimens support large-scale treatment initiatives, even among patients with comorbidities or advanced liver disease [5,6,7].
In light of these advances, the global health community has intensified elimination efforts. During its sixty-ninth session in 2016, the World Health Assembly approved a resolution targeting the elimination of viral hepatitis as a pressing global public health issue by 2030. The WHO strategy set clear targets: a 65% reduction in hepatitis-related mortality, an 80% reduction in new infections, diagnosis of at least 90% of infected individuals, and treatment of at least 80% of those diagnosed [5]. To facilitate national monitoring, the WHO also established quantitative elimination benchmarks, including no more than five new HCV infections per 100,000 individuals annually and no more than two hepatitis C-related deaths per 100,000 people per year [8].
Although there has been important progress, several ongoing challenges still stand in the way of fully reaching the goals for eliminating hepatitis C. Among the most significant is the risk of HCV reinfection following successful antiviral therapy. Reinfection is suspected when viremia reappears after initial viral clearance, confirmed through sensitive molecular testing. Achieving an undetectable level of HCV RNA (hepatitis C virus ribonucleic acid) 12 weeks after completing therapy—commonly referred to as sustained virologic response (SVR12)—continues to serve as the primary indicator of successful hepatitis C treatment outcomes. However, recurrence of viremia may result from either relapse of the initial infection or a new exposure, particularly in high-risk populations [9].
Achieving sustained virologic response (SVR) is strongly associated with favorable clinical outcomes, including normalization of liver enzyme levels, fibrosis regression, and improved hepatic function. Nevertheless, viral eradication does not fully eliminate the risk of hepatocellular carcinoma or liver-related mortality, especially among patients with pre-existing cirrhosis or coexistent liver disease. Additional factors, such as metabolic syndrome, harmful alcohol use, and hepatitis B virus (HBV) co-infection, may further impact long-term liver health even after HCV clearance [10].
In recent decades, HCV epidemiology has evolved considerably, with marked shifts in its global distribution and transmission dynamics, shaped by demographic transitions, public health interventions, and changes in risk behaviors. Historically, unscreened blood transfusions and unsafe medical practices were the main transmission routes. Thanks to rigorous safety measures, these pathways have been largely controlled in high-income countries. In contrast, in many low-resource settings, these risks persist, reflecting broader healthcare disparities [11].
Currently, certain key populations remain central to the epidemic. People who inject drugs (PWIDs) and Human Immunodeficiency Virus (HIV)-positive men who have sex with men (MSMs) experience the highest rates of ongoing HCV transmission. These groups act as critical reservoirs for the virus, even where healthcare-associated transmission has been largely curtailed. Therefore, elimination strategies must include targeted interventions for these vulnerable groups, focusing on prevention, testing, and treatment programs. Additionally, vertical transmission remains the main source of HCV infection among children, highlighting the importance of maternal screening and appropriate management during pregnancy [12].
The incarcerated population represents a globally important high-risk group in the HCV epidemic. According to a recent meta-analysis, the global prevalence of HCV among people in prison is estimated at 17.7%, with regional variations reaching 28.4% in Oceania and 25.1% in Europe [13]. This elevated burden is primarily driven by overlapping risk factors such as injection drug use (IDU), unsafe tattooing, and limited access to healthcare services both prior to and during incarceration [14]. Despite the high risk, HCV testing and treatment coverage remain suboptimal in many correctional settings [15]. However, successful national models—such as those in Australia and Spain—demonstrate that prison-based programs incorporating universal screening and timely initiation of DAA therapy can achieve high cure rates and reduce post-release transmission [16,17]. The WHO emphasizes the inclusion of prison-based health services in national elimination plans, advocating for universal testing and linkage to care within carceral systems as a critical step toward achieving the 2030 elimination targets [15,18].
Although current strategies for eliminating HCV infection primarily focus on key populations, including PWIDs, MSMs, and prisoners, international guidelines and the recent literature emphasize the need to expand screening to the general population in settings where prevalence is significant. This broader approach can be implemented either through universal screening or by targeting specific birth cohorts, depending on the local epidemiological context. According to the European Centre for Disease Prevention and Control (ECDC) guidelines, population-based screening is recommended in countries with high prevalence, particularly among individuals born between 1945 and 1965 who are considered to have been exposed to iatrogenic risks in previous decades [19]. Furthermore, the WHO underscores that general population testing can play a decisive role in achieving the 2030 global elimination goals when adapted to the national context [20].
Building on these global recommendations, this review provides a comparative analysis of national and regional strategies for HCV elimination, with a particular focus on high-performing programs in Egypt, Spain, and Australia. By evaluating the determinants of their success and the barriers encountered in different epidemiological and socioeconomic contexts, this study seeks to highlight existing knowledge gaps and outline future directions required to optimize screening, diagnosis, and treatment strategies. The ultimate goal is to inform evidence-based policies that can accelerate global progress toward achieving the WHO 2030 hepatitis C elimination targets.
2. Methodology
This structured literature review aimed to synthesize current evidence on global efforts to prevent, control, and eliminate HCV. Relevant, up-to-date research was identified through a comprehensive search of these major international databases—PubMed, Scopus, Web of Science—which are widely recognized for their extensive coverage of biomedical and public health research.
This research targeted English-language, open-access publications available in full-text format, published between 2010 and May 2025, including online-ahead-of-print papers. This timeframe ensured the inclusion of recent developments in HCV screening, elimination strategies, and public health interventions. Search terms included combinations such as “hepatitis C elimination”, “direct-acting antivirals”, “HCV screening”, “vulnerable populations”, and “global HCV control”, along with additional terms, including “HCV epidemiology”, “prevention”, and “risk factors”. The application of Boolean operators such as AND and OR was employed to refine the search strategy, ensuring an optimal balance between sensitivity and specificity.
An initial assessment of titles and abstracts was conducted to remove duplicate records, non-English language sources, and studies considered irrelevant to the objectives of the review. The inclusion criteria were limited to studies published within the past 15 years that provided unrestricted access to the full text. Eligible study types included original research, observational studies, intervention studies, systematic reviews, meta-analyses, clinical guidelines, and case studies addressing HCV elimination and related strategies.
Articles were excluded if they were older than 15 years, written in languages other than English, or not available in full text or focused on other types of viral hepatitis.
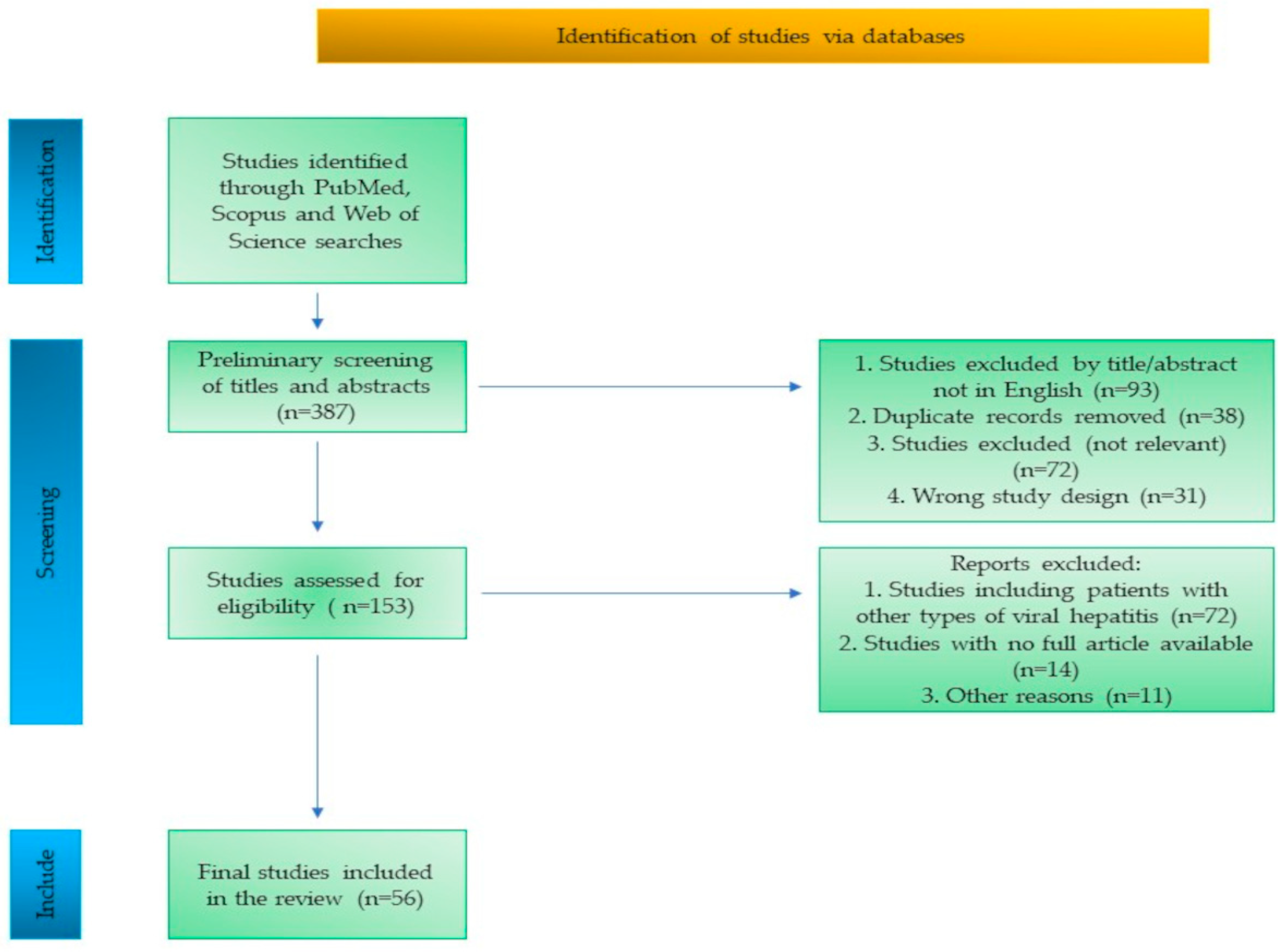
The study selection process is summarized in a flow diagram (Figure 1), outlining the identification, screening, eligibility assessment, and inclusion of studies.
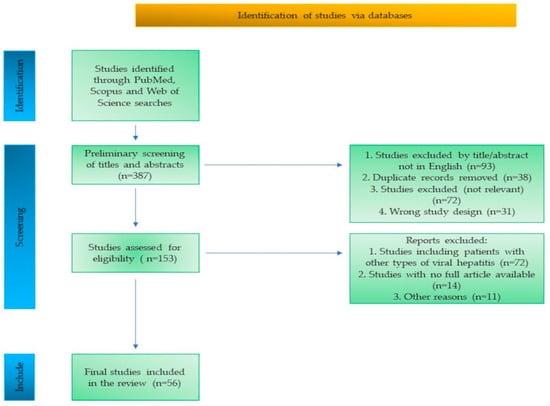
Figure 1.
Flow diagram of the study selection process.
Full-text versions of selected studies were retrieved and systematically analyzed. Data extraction focused on elimination strategies, intervention for high-risk populations, treatment approaches, implementation models, and progress toward WHO targets. The content of this manuscript is derived from a comprehensive synthesis of the reviewed literature.
3. History
Efforts to uncover the viral causes of hepatitis began in the mid-20th century, representing a significant development in the field of hepatology. In 1965, Blumberg and his team identified a blood antigen later named the hepatitis B surface antigen (HBsAg), thereby confirming HBV as a major causative agent of serum hepatitis. This discovery led to the development of diagnostic assays for HBsAg detection and their widespread use in blood transfusion screening, reducing the incidence of post-transfusion hepatitis by nearly 50%. However, hepatitis cases still occurred despite HBsAg-negative blood donations, suggesting the presence of other unidentified agents [21].
In 1975, Alter and collaborators introduced the term “non-A, non-B hepatitis” to classify cases of viral liver inflammation that were not linked to hepatitis A or B viruses. Their research involved transfusing blood from chronically infected donors into chimpanzees, which subsequently developed liver inflammation and elevated alanine aminotransferase levels. These findings provided strong experimental evidence for a new infectious agent [22].
The identity of this agent remained unknown until 1989, when Houghton and his team successfully isolated and sequenced its genome, officially identifying it as HCV. The identification of HCV represented a critical milestone that facilitated the development of reliable diagnostic tools and the implementation of stringent blood donor screening practices, leading to a substantial decrease in transfusion-related infections [21].
In 2020, international recognition of the discovery’s impact came when Harvey J. Alter, Michael Houghton, and Charles M. Rice were honored with the Nobel Prize in Physiology or Medicine, highlighting their distinct but complementary roles in uncovering the virus responsible for previously unclassified cases of transfusion-related hepatitis. Through meticulous clinical studies, Harvey J. Alter demonstrated that certain post-transfusion hepatitis cases could not be explained by known hepatitis viruses, strongly suggesting the existence of an unidentified infectious cause. Houghton subsequently achieved its genetic isolation, while Rice validated the virus’s pathogenic potential using experimental model systems. Together, their contributions elucidated the cause of what had long been classified as non-A, non-B hepatitis, establishing a basis for advancements in its diagnosis, prevention, and therapy. The recognition of HCV as a distinct RNA virus reshaped the management of chronic viral hepatitis and continues to influence global public health strategies [2,23,24].
4. Characteristics of the HCV Genome
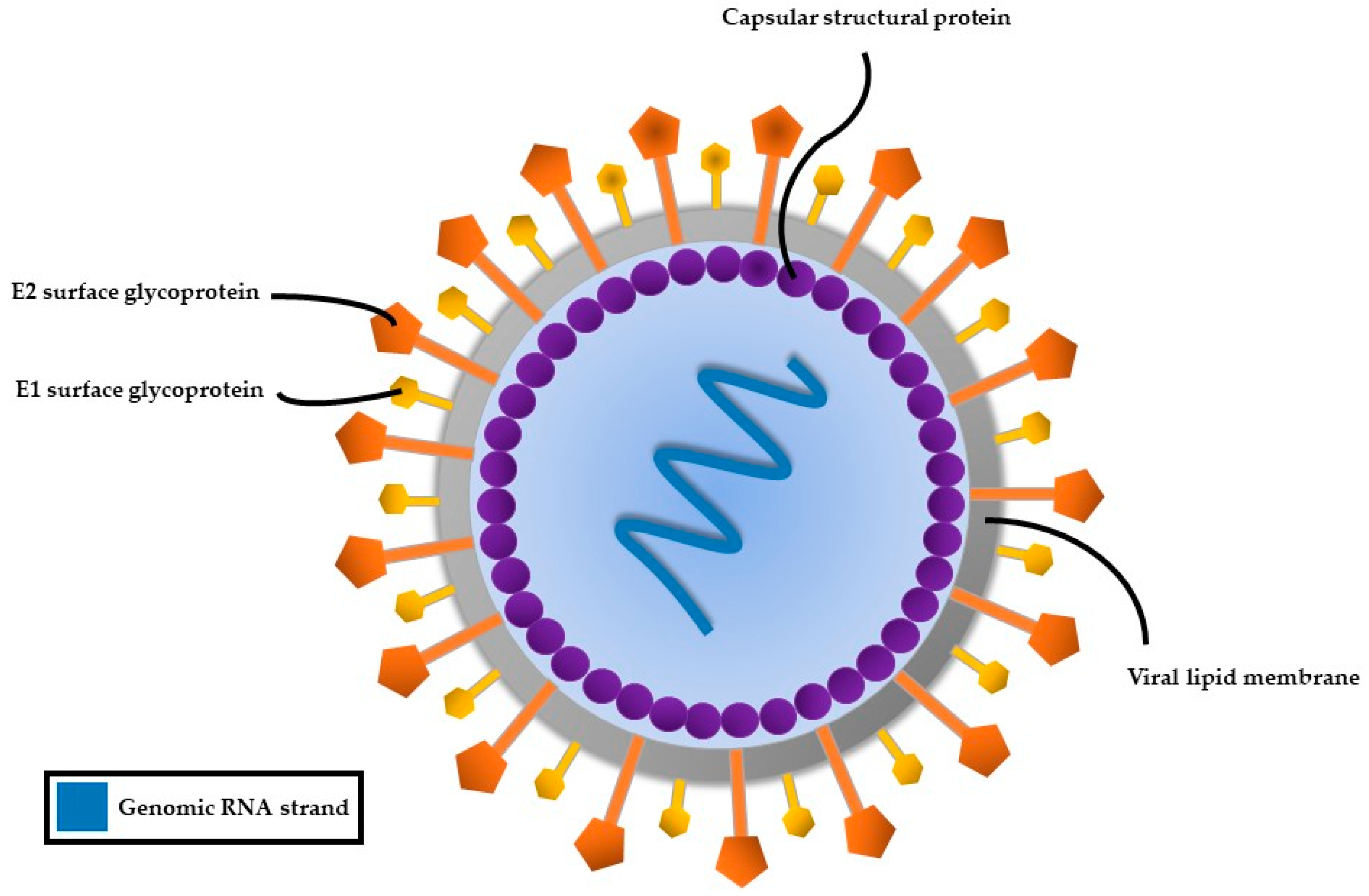
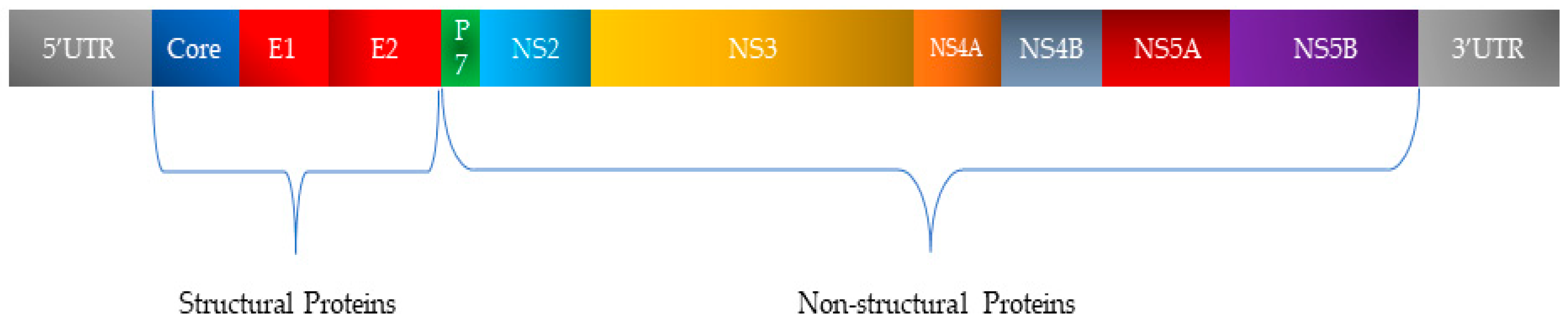
HCV, a member of the Flaviviridae family, possesses a single-stranded RNA genome enclosed in a small, enveloped virion approximately 50 nanometers in diameter (Figure 2). The RNA genome of HCV features one continuous coding region, which is translated into a precursor polyprotein of nearly 3100 amino acids. This polyprotein is cleaved by both host and viral proteases into individual structural and non-structural proteins required for viral replication and assembly (Figure 3). The principal structural components of the virus comprise the core protein, responsible for nucleocapsid formation, along with two envelope glycoproteins, E1 and E2, which are anchored in the surrounding lipid bilayer. These envelope proteins are essential for mediating the virus’s attachment to and penetration of host cells, with E2 protein playing an additional role in evading host immune responses [25].
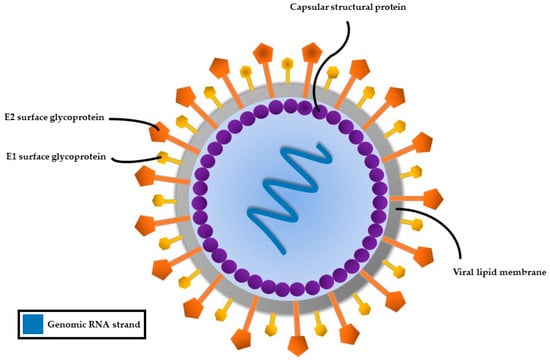
Figure 2.
Structure of the hepatitis C virus.
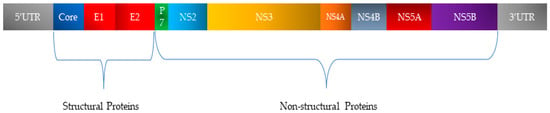
Figure 3.
Organization of HCV genetic material and classification of encoded proteins.
In addition, HCV encodes seven non-structural proteins (p7, NS2, NS3, NS4A, NS4B, NS5A, and NS5B) that coordinate RNA replication, virion assembly, and release from infected cells [25,26].
A key genomic feature is the presence of two hypervariable regions within the E2 protein. HCV exhibits a high rate of genetic variation, primarily due to its fast replication process and the lack of error-correcting ability in its RNA-dependent RNA polymerase. This results in significant genetic variability, enabling rapid adaptation and evasion of host immune responses [25,27].
After initial infection, HCV RNA can be identified in the bloodstream as early as one to four weeks post-exposure, with viral titers generally reaching their peak between the eighth and twelfth week. Although some individuals spontaneously clear the infection, 50–85% develop chronic hepatitis C, often due to insufficient CD4+ T-lymphocyte and CD8+ cytotoxic T-lymphocyte responses. HCV does not directly induce hepatocyte death. Instead, liver injury arises from persistent immune-mediated inflammation. Chronic immune activation promotes hepatic fibrosis, which can progress to cirrhosis over time. Disease progression is influenced by factors such as excessive alcohol intake, co-infection with HIV or HBV, HCV genotype 3 infection, obesity, insulin resistance, and non-alcoholic fatty liver disease (NAFLD). These molecular and biological features contribute to HCV’s persistence and help explain why chronic infection frequently leads to severe liver complications [28].
5. The Worldwide Prevalence of Hepatitis C Virus Genotypes
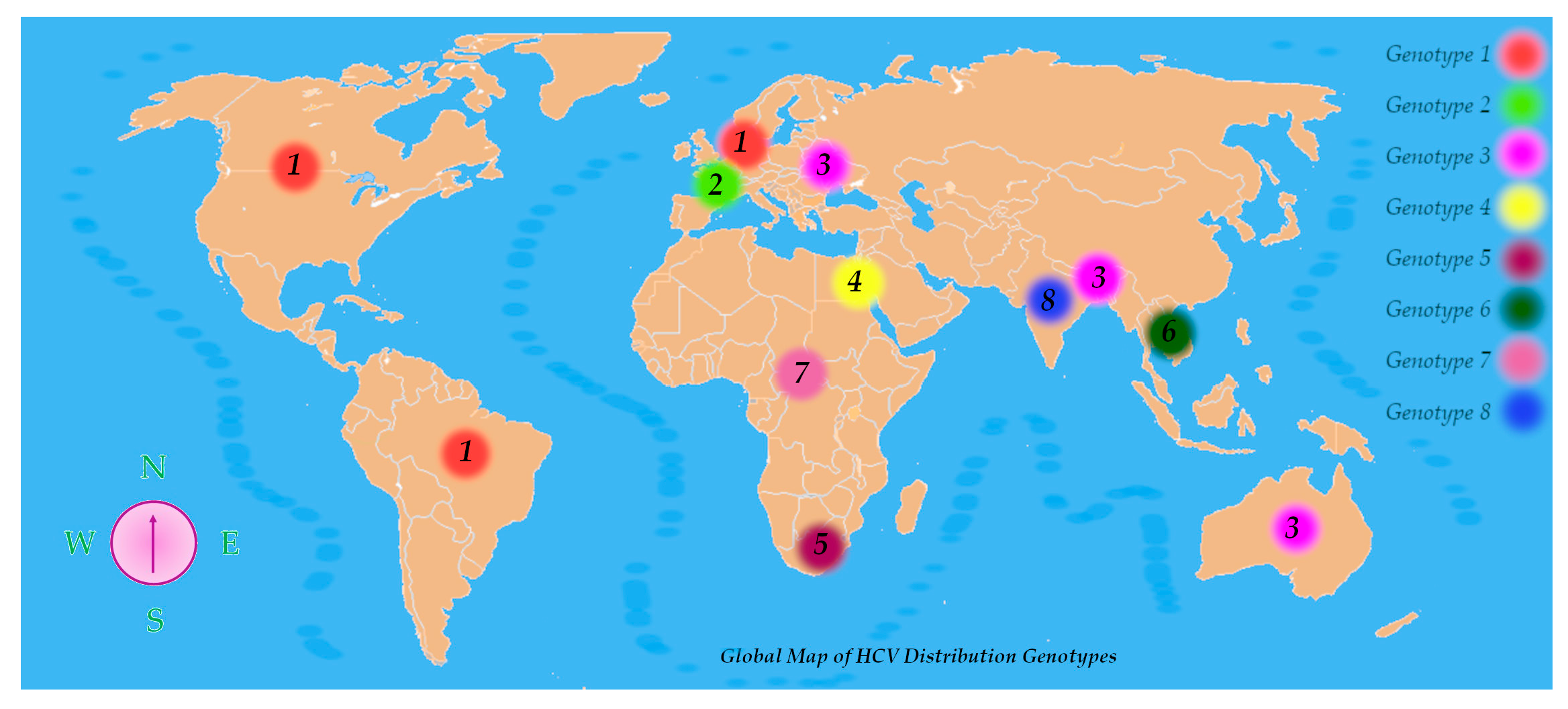
HCV exhibits remarkable genetic diversity, which has led to its classification into eight genotypes and at least 93 subtypes, with a minimum nucleotide sequence divergence of 30% between genotypes. This genetic heterogeneity not only defines viral classification but also influences epidemiological patterns, clinical progression, and therapeutic responses [29].
HCV genotypes display distinct global distributions. Genotypes 1, 2, and 3 are the most prevalent HCV variations found worldwide among those that have been characterized. Genotype 1 is most frequently encountered in regions such as North America, Northern and Western Europe, and areas of South America. Genotype 2 is also common in Western nations. Genotype 3 prevails in South Asia, Australia, and certain parts of Europe. Genotype 4 shows a high prevalence across the Middle East and North Africa, with notably elevated rates in Egypt and Saudi Arabia. Genotype 5 has been reported mainly in Southern Africa, genotype 6 in Southeast Asia, and genotype 7 has been detected primarily in Central African populations. The recent identification of genotype 8 in India further underscores HCV’s genetic complexity (Figure 4) [29].
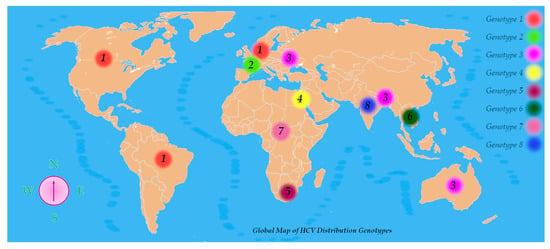
Figure 4.
Global map of HCV distribution genotypes.
This variability has significant clinical implications. Among the various HCV genotypes, type 3 is consistently correlated with a more aggressive clinical course of liver disease. Patients infected with genotype 3 have a higher rate of fibrosis progression and earlier onset of cirrhosis compared to those with other genotypes [26,30].
Prior to the development of DAAs, interferon- and ribavirin-based therapies showed variable efficacy by genotype: genotypes 1a and 1b had lower response rates, whereas 3a and 3b responded more favorably. Although DAA regimens have significantly improved overall cure rates and safety profiles, genotype 3 still presents challenges, particularly in treatment response and resistance patterns [29,30].
Consequently, accurate genotyping remains a critical component in clinical management of HCV, guiding the selection of antiviral regimens and treatment duration. A comprehensive understanding of the global distribution and biological behavior of HCV genotypes is essential for optimizing patient care and informing hepatitis C elimination strategies worldwide.
6. Modes of Transmission of HCV
The principal route of HCV transmission involves direct contact with blood contaminated by the virus. Historically, the main route of transmission was through blood transfusions and the use of unscreened blood products. With the implementation of advanced serological and molecular screening methods, the risk associated with transfusions has been significantly reduced. IDU remains the primary pathway for HCV transmission today, with the sharing of contaminated needles and injection equipment posing the highest risk among PWIDs [2,31].
To provide a more comprehensive understanding of the epidemiological dynamics of HCV transmission, it is essential to examine data derived from original research conducted in various global contexts. These studies offer granular insights into how specific transmission routes operate within distinct population groups and settings, revealing the interplay between medical practices, behavioral risks, and social determinants. Table 1 presents a selection of original research articles, each focused on a particular mode of HCV transmission, along with details regarding the study population, geographic region, year of publication, and main methodological limitations (sample size, design constraints, and potential sources of bias). This synthesis supports the formulation of evidence-based, context-sensitive public health strategies aimed at interrupting transmission chains and advancing toward HCV elimination.

Table 1.
Identified risk factors for HCV infection.
Among the additional and context-specific transmission routes highlighted in the literature are unsafe medical or dental procedures involving unsterilized instruments, as well as cosmetic practices such as tattooing, body piercing, and acupuncture when performed without proper hygiene standards. Healthcare workers are also at risk of accidental exposure via needlestick injuries or contact with sharp instruments used on infected patients.
Each year, an estimated 112 million blood donations are collected worldwide, highlighting the importance of robust screening procedures to ensure transfusion safety. WHO recommends that all blood intended for transfusion be systematically screened for infections such as HIV, HBV, HCV, and syphilis to ensure safety [39].
IDU constitutes a leading factor in the global spread of HCV, with PWIDs being disproportionately affected. Genotypes 1a and 3a are the most frequently detected in this population. Subtype 3a, originally endemic in Southeast Asia, has spread among drug users in Europe and North America. Data from several European countries reveal an increasing circulation of HCV genotypes 1a and 3a, particularly in Germany, France, Italy, and Portugal. Instances of dual HCV genotype infections have been reported across Europe, including combinations like 1b and 3a in Italy; 2a and 3b in Germany; and 1a, alongside 1b, in Sweden. The rapid expansion of HCV in Eastern Europe and Central Asia is linked to heroin injection and the emergence of synthetic drugs. Genotype 3a has shown a rising prevalence in countries such as Romania, Bulgaria, and Poland, and across the Balkan region [40,41].
PWIDs play a significant role in shaping the transmission patterns of HCV, influencing not only high-risk groups but also broader population networks [42]. This dynamic is exacerbated by factors such as poverty, incarceration, and co-infection with HIV. In rural parts of the United States, PWIDs are at increased risk due to limited healthcare infrastructure, lack of harm reduction programs, and reduced access to opioid use disorder treatments. Rural populations experience increased HCV prevalence as a consequence of these inequities, when compared to urban settings [43].
MSMs represent another high-risk group, with multiple HCV outbreaks reported across Europe, North America, Australia, and Asia. In regions where homosexuality is stigmatized, such as parts of the Arabian Peninsula, barriers to care and social discrimination further increase vulnerability. HIV and HCV co-infections are frequently reported in PWIDs and MSMs, reflecting shared transmission risks in these key populations. In some regions, up to 25% of individuals living with HIV are also infected with HCV. Among MSMs living with HIV, the rate of HCV co-infection is notably elevated, reflecting the overlapping modes of transmission [40].
Although sexual transmission of HCV is best documented among MSMs, recent evidence indicates that this route is not exclusive to this population. Heterosexual transmission, while less efficient than for other viruses, such as HIV, is not negligible under certain circumstances, including unprotected sexual contact, the presence of genital lesions, drug use during sexual activity, or co-infection with other sexually transmitted infections [38,44,45]. Furthermore, the 2020 ECDC report documented sexual transmission in heterosexual populations engaging in high-risk behaviors, confirming the need for a broader preventive approach [46].
HCV prevalence is highly concentrated geographically. Around 30 countries account for 80% of global cases, with the highest burdens observed in China, India, Pakistan, Ukraine, Russia, and the United States of America (USA). Over 70% of people with active HCV infection reside in LMICs, where access to testing and treatment remains limited. For example, infection rates in Ukraine and Romania reach 3.1% and 2.3%, respectively, compared to less than 1% in most Western European nations [11].
The highest numbers of PWIDs are reported in countries like China, Russia, the USA, and Brazil, with significant populations also found in Mexico, Pakistan, and Thailand. In nearly all European countries, HCV seroprevalence among PWIDs is high, with particularly elevated rates in Latvia, Portugal, Turkey, and Cyprus. In contrast, lower prevalence (<30%) is reported in the Czech Republic, Hungary, and Slovenia. A favorable decline in HCV transmission has been observed in Germany, France, the United Kingdom (UK), and Italy in recent years [40].
In LMICs, unsafe medical practices—including reuse of unsterilized syringes and inadequately screened blood transfusions—remain significant contributors to HCV transmission. Among hemodialysis patients, infection rates can exceed 40% in some areas of the Arabian Peninsula and in China. Co-infection with HBV is also reported, with HCV co-infection rates ranging from 3% in Thailand to 30% in Spain [40].
Vertical transmission of HCV is more likely when the mother has detectable levels of viral RNA, and this risk increases significantly if she is also infected with HIV. Antiretroviral therapy for HIV during pregnancy reduces the risk, aligning transmission rates with those seen in mothers with HCV monoinfection [47].
A nuanced understanding of transmission dynamics is essential for effective prevention strategies and global elimination efforts.
7. Prognosis
The clinical course of HCV infection is marked by significant variability, influenced by viral persistence and host-specific factors. Although spontaneous clearance can occur, it is observed in only 10–15% of cases. In the remaining majority, the infection persists and may progress to chronic liver disease [28]. About one in five people with chronic HCV develops cirrhosis within two decades [48]. In this group, HCC risk over three decades is 1–5%. Progression may accelerate in older adults or those with metabolic comorbidities. Liver damage risk increases with high alcohol intake. Pre-existing cirrhosis or HBV co-infection further amplifies this risk and leads to poorer outcomes [28,49].
Viral eradication, marked by undetectable HCV RNA post-treatment, is key to long-term success. Achieving SVR significantly reduces cirrhosis, HCC, and liver-related mortality. Patients with SVR have better survival and slower liver function decline, regardless of disease stage [28,50].
8. Screening, Prevention, and Control Strategies
Timely diagnosis represents a fundamental component in the effective management of HCV, serving as the initial step within the comprehensive care pathway. When followed by prompt linkage to treatment, it facilitates access to curative therapy and helps reduce viral transmission. In recognition of its critical role, the WHO recommends universal HCV testing within correctional facilities, where prevalence rates are significantly elevated [51].
Diagnosis typically begins with antibody screening for HCV, followed by RNA-based testing to confirm ongoing viral activity. This dual-step strategy ensures both diagnostic precision and informed clinical decision-making [5].
One of the main obstacles in HCV control is the high proportion of asymptomatic individuals who remain undiagnosed [52]. Broadening access to testing is essential—not only for case identification but also to reduce community-level spread. Reflecting this need, professional associations such as the American Association for the Study of Liver Diseases advocate routine HCV screening for all adults aged 18 and older [53].
Advancements in diagnostic approaches continue to enhance detection accuracy. For example, recent findings have shown that pairing two complementary screening methods significantly improves sensitivity and specificity, surpassing the reliability of single-test protocols. This supports adopting dual-testing strategies, especially in high-burden or resource-limited settings [39,54,55].
Approaches to HCV screening vary significantly across countries, influenced by healthcare system capacity, policy frameworks and population risk profiles. Table 2 presents a comparative overview of national HCV screening strategies and the diagnostic methods currently implemented.

Table 2.
HCV screening approaches and challenges in various populations.
Rapid tests used at the point of care offer a viable option, especially where conventional lab diagnostics are not accessible. Particularly in remote or underserved regions, these devices enable faster diagnosis and improved outreach to hard-to-reach populations.
An increasingly recognized component of strategies aimed to eliminate blood-borne viral (BBV) infections in the implementation of screening programs in emergency departments. Evidence shows that integrating HCV testing into emergency care can significantly increase early diagnosis rates in densely populated urban settings [57]. Systematic BBV screening in emergency departments in Spain led to a substantial increase in diagnosis and referral rates for treatment [67], while studies conducted in South Korea indicate that this approach is sustainable, cost effective, and well accepted by patients, particularly when supported by educational interventions and integrated health information systems [68].
Preventive interventions are equally vital in curbing new HCV infections. Key measures include rigorous infection control practices in clinical settings—such as safe injection techniques, appropriate disposal of medical waste, and thorough sterilization of equipment. For PWIDs, essential harm-reduction services—like needle exchange programs, OAT, and counseling—are proven to reduce transmission. Continued mandatory screening of donated blood, alongside public education on safe sexual behaviors, reinforces a comprehensive prevention framework [1].
Some countries, such as Italy, have adopted integrated public health policies that provide free HCV and HIV screening and treatment. These programs are often jointly coordinated by health and justice ministries, though their implementation may vary regionally depending on local healthcare infrastructure [69].
In high-income nations, routine screening at blood donation centers typically involves antibody testing using enzyme-linked immunosorbent assay (ELISA) or chemiluminescence immunoassay (CLIA), followed by nucleic acid amplification testing (NAT) for RNA confirmation. Since their broad implementation in the 1990s, these testing protocols have almost entirely removed the threat of HCV transmission through blood transfusions [70].
Together, these diagnostic and preventive strategies serve as the foundation for global HCV control efforts. Continued refinement and equitable expansion of these programs remain central to achieving long-term elimination goals.
9. Economic and Health Outcomes of Hepatitis C Screening and Treatment Programs
The elimination of HCV as a public health threat by 2030, a target set by the WHO, requires not only epidemiologically validated strategies but also economically sustainable interventions. Health economics and outcomes research (HEOR) approach is essential for assessing the cost-effectiveness, budget impact, and long-term social value of screening, diagnostic, and treatment strategies for HCV. As more countries expand access to testing and DAA therapy, integrating economic analyses will become crucial for informing evidence-based public health policy decisions. Multiple studies have demonstrated that HCV elimination is not only clinically feasible but also cost-effective in diverse settings. A global modeling study indicates that expanding prevention and treatment interventions could prevent 15.1 million new infections and 1.5 million deaths by 2030, resulting in substantial healthcare savings [71].
Recent national-level evaluations further support these findings. In South Korea, Choi and his colleagues demonstrated that universal screening of adults aged 40–65 years, followed by DAA treatment, is highly cost-effective, well below the national willingness-to-pay threshold [68]. In Europe, a multicenter analysis conducted in Spain showed that large-scale treatment is cost-effective even in countries with low prevalence, particularly when combined with decentralized screening models and simplified care pathways [67].
From a healthcare-system perspective, integrating HCV testing into emergency departments, prisons, and primary care represents a strategic opportunity for early case identification by leveraging existing infrastructure. An evaluation conducted in the UK demonstrated that “opt-out” BBV testing in emergency services not only increased diagnostic rates but also proved to be cost-saving in the medium term due to early linkage to treatment [72].
Moreover, economic evaluations also highlight the benefits regarding health equity. “Test-and-treat” strategies targeting disadvantaged populations not only improve clinical outcomes but also reduce health disparities, supporting the inclusion of social justice principles in cost-effectiveness analyses [73]. These conclusions are perfectly aligned with WHO priorities on universal health coverage and equitable access to care [74].
10. Discussion
For many years, Egypt has been considered one of the countries most heavily affected by HCV infection, historically reporting the highest global prevalence rates. The widespread introduction of DAA therapies has significantly transformed the national response to this public health crisis. These effective treatment regimens have enabled notable progress in Egypt’s HCV control strategy, substantially reducing the disease burden. This success underscores how sustained political support, strategic planning, and broad treatment access can drive national-level epidemic control [75].
In line with the WHO targets for HCV elimination—diagnosing at least 80% of infected individuals and treating 70% of those diagnosed—Egypt has surpassed these benchmarks. The national screening campaign identified approximately 87% of those with HCV, with 93% receiving curative antiviral therapy. Consequently, more than four million individuals with chronic hepatitis C have been successfully treated, making Egypt the first country to receive WHO validation for being on the path toward HCV elimination. This achievement underscores the strength of Egypt’s healthcare system and provides a model for other nations aiming to integrate screening, treatment, and policy interventions [75].
Across Europe, various elimination strategies have emerged at both local and national levels. The French Ministry of Health piloted a test-and-treat program in Perpignan which subsequently guided nationwide adoption. Similarly, the Hep Care project, led by University College Dublin with European Union (EU) funding, showed that targeted micro-elimination programs can reduce HCV prevalence among high-risk groups within multiple European contexts. These initiatives underscore the importance of adapting local approaches within comprehensive elimination frameworks [76].
Spain has also made substantial progress toward WHO’s HCV elimination targets, which include an annual incidence of five or fewer cases per 100,000 people, two or fewer per 100 among PWIDs, and HCV-related mortality rate limited to a maximum of two deaths per 100,000 individuals. Achieving these goals depends on consistent implementation of prevention, high diagnostic coverage, and widespread treatment. Spain’s coordinated efforts align with global targets and offer valuable insights for other countries [77,78].
Correctional facilities are a focal point in WHO’s HCV elimination plan, as people in prison are at significantly higher risk—often tenfold—compared to the general population. This increased vulnerability is primarily linked to behaviors such as IDU. HCV continues to pose a major health challenge in institutional settings, with recent figures indicating a prevalence of 14.8% in facilities under the Ministry of the Interior and nearly 12% in prisons across Catalonia. In recent years, these prevalence rates have declined, coinciding with reduced HIV incidence and lower levels of substance use among this population, likely influenced by prevention programs and expanded antiviral-treatment access [79].
The high turnover and movement of detained individuals between prisons and communities contribute to the risk of HCV spread. Effective prison-based screening and treatment represent essential components of comprehensive public health strategies. Treating this population reduces overall transmission and supports national and global elimination efforts [77,80].
Following the introduction of DAA therapy in Catalonian prisons starting in 2015, more than 1000 incarcerated people have successfully reached SVR. However, reinfection remains a challenge among individuals who continue engaging in high-risk behaviors, especially IDU, as viral clearance does not confer immunity. Ongoing risk reduction strategies and reinfection monitoring are thus vital for maintaining treatment success [77].
A study by Cambianica et al. investigated the hepatitis C care pathway within two penitentiary institutions located in Brescia, Northern Italy. The findings confirmed higher HCV seroprevalence among people in detention than in the general population and revealed poor screening adherence. The authors recommended enhanced education and test-and-treat strategies to improve engagement and outcomes [69].
Another study, led by Saludes and colleagues, conducted across eight Catalan prisons, showed low reinfection rates following DAA treatment. Nevertheless, reinfection risk persists, particularly among newly incarcerated individuals or those from vulnerable groups, including people living with HIV, homeless individuals, and PWIDs. Prisons offer a valuable opportunity to deliver care to populations often excluded from mainstream services, thus also contributing to community-level transmission reduction [77].
In the Catalan study, over 80% of participants reported having injected drugs at some point, and 28% of viremic individuals had done so during prior incarcerations. Among those monitored for reinfection, one-third continued intravenous drug use during treatment. These findings reaffirm that IDU remains the main driver of ongoing HCV transmission in correctional settings globally. Integration of harm-reduction services with medical treatment is critical in mitigating these risks [77].
Global differences in prison reinfection rates stem not only from local HCV prevalence but also from the public health models used. Effective harm-reduction strategies play a critical role in this context. Needle exchange programs and opioid substitution therapy within prisons are recognized as key measures for reducing reinfection and supporting sustained treatment success [77,81].
In its national strategy for eliminating HCV, Canada has designated individuals with a history of incarceration as a priority group for intervention. They account for roughly 10% of the national HCV burden. According to the recent data from the Public Health Agency of Canada, an estimated 10% of incarcerated individuals—equivalent to approximately 38,000 people—have had prior exposure to HCV. This highlights the urgent need to improve access to testing and medical care within correctional institutions. The frequent movement between prison and community settings further highlights the importance of comprehensive prison-based care in achieving national elimination goals [51,82,83,84,85,86,87].
An estimated 55,354 individuals in San Diego County, California, have been infected with HCV, underscoring the region’s significant epidemiological burden. In 2018, the Owen Clinic at the University of California San Diego launched a targeted micro-elimination program focused on managing HIV-HCV co-infection among vulnerable groups. This initiative later developed into the “Eliminate Hepatitis C Initiative”, a collaborative effort between public institutions and the American Liver Foundation. Formalized in 2021, the plan aligns with WHO goals by promoting awareness, screening, linkage to care, and treatment throughout the care continuum [88,89].
To achieve an 80% reduction in HCV incidence, modeling suggests that without expanded harm-reduction measures, treatment coverage must reach 60% annually among HCV-positive individuals without HIV. These findings stress the need for combining scaled-up therapy with interventions that reduce transmission risk [88].
Research led by Wedemeyer et al. found that treating 10% of infected individuals annually could lead to a 90% reduction in HCV infections by 2030. Large-scale implementation of high-SVR antiviral regimens holds great potential to reduce deaths and disease burden. Even modest improvements in diagnosis and treatment can significantly reduce the disease burden [90].
In Mexico, a study by Jose Abrego et al. reported an HCV prevalence rate of 36.4% among individuals diagnosed with HIV. The primary transmission route was IDU, linked to intense drug trafficking and high incarceration rates in the western region [91].
Taiwan introduced an HCV screening and treatment initiative targeting the incarcerated population at Yulin Prison. Individuals testing positive for antibodies were promptly treated with DAAs, achieving nearly 100% SVR. The program’s success underscores the importance of collaboration between correctional and medical staff [92]. For individuals with a history of IDU, OAT remains a cornerstone of care. An on-site “test-and-treat” approach was developed, integrating rapid HCV/HIV screening with prompt initiation of DAA therapy. Over three years, the program reduced HCV prevalence from 38% to 7%, highlighting the impact of targeted prison-based interventions [5,93].
Recent findings from both qualitative and interventional research highlight key challenges and opportunities in addressing hepatitis C among PWIDs. In rural areas of Northern New England, limited access to sterile syringes and significant gaps in the understanding of HCV transmission, diagnosis, and treatment have been linked to high-risk behaviors such as syringe sharing, underscoring the need for expanded education and harm-reduction services [94]. Meanwhile, a hospital-based trial in Oslo, Norway, demonstrated that initiating HCV treatment as part of inpatient care can reduce time to viral clearance, offering a promising strategy for engaging marginalized populations who may otherwise drop out of care [95]. However, persistent reinfection—particularly among PWIDs—reinforces the importance of integrating treatment with robust, sustained prevention efforts [94,95].
Integrating clinical interventions with an understanding of community-level risk factors is essential. Recent studies emphasize this complementary: one demonstrates the success of inpatient treatment strategies [94], while the other reveals vulnerabilities on the ground, particularly in rural areas.
In Australia, a study by Scott et al. highlighted the need to significantly increase HCV testing to meet WHO elimination goals. The study estimated that testing coverage must grow by at least 50% to capture undiagnosed cases and halt transmission [96].
A similar study by Gountas and colleagues in Greece concluded that large-scale screening programs are essential for elimination. Passive detection and low awareness lead to undiagnosed cases and advanced disease, making them economically inefficient. Comprehensive elimination strategies are more cost-effective by preventing long-term outcomes [97].
Several studies from various global regions, including correctional facilities, have evaluated progress toward HCV elimination. While some countries are on track to meet WHO benchmarks, others face structural or epidemiological challenges.
Table 3A,B summarize studies evaluating HCV elimination efforts at national and local levels. For clarity, studies were organized according to the level of intervention: macro-level national strategies (Table 3A) and targeted micro-elimination initiatives in high-risk populations (Table 3B). This structure underscores the heterogeneity of outcomes influenced by policy frameworks, healthcare infrastructure, and population risk profiles. Such comparative evidence is relevant for guiding future implementation strategies adapted to different healthcare settings.

Table 3.
(A) Macro-level national strategies for hepatitis C elimination. (B) Micro-elimination initiatives targeting high-risk populations.
While Table 3A focuses on national-level strategies and policy-oriented interventions, Table 3B highlights micro-elimination approaches implemented in high-risk or underserved populations. These targeted strategies are essential complements to national programs, as they address persistent transmission pockets and improve equity in access to HCV diagnosis and treatment.
The exclusive focus of current strategies on vulnerable populations should be complemented by screening measures targeting the general population, as this is crucial for a comprehensive and sustainable approach to HCV elimination. Several countries have already adopted such integrated models. According to the Centers for Disease Control and Prevention (CDC) guidelines in the US, all adults should receive a one-time HCV test, and people born between 1945 and 1965 should be screened more regularly [108]. Evidence consistently demonstrates that these approaches significantly increase diagnostic and treatment initiation rates while remaining cost effective in the era of highly effective direct-acting antivirals [71]. Notably, cohort-based testing has been shown to perform comparably to risk-based testing in identifying infected individuals, while offering greater feasibility for large-scale, system-wide implementation [109]. These findings emphasize the practical feasibility and public health value of integrating population-level screening into routine healthcare services.
To eliminate hepatitis C at national and global levels, strategic frameworks must prioritize universal access to DAAs and the integration of robust prevention efforts. These include systematic screening, safe medical practices, harm-reduction services, and public health education to combat stigma. A coordinated approach combining early diagnosis, immediate treatment, and prevention is the most effective path to ending HCV as a public health threat [110].
11. Remaining Barriers and Knowledge Gaps in HCV Elimination
Despite important progress in screening, diagnosis, and treatment, several knowledge gaps continue to limit the optimization of HCV elimination strategies. Data on hard-to-reach groups, such as PWIDs, prisoners, and migrants, are often incomplete or inconsistent, making it difficult to design effective programs. Long-term studies on reinfection rates, treatment adherence, and the population-level impact of DAA therapy are still limited. Research on cost-effectiveness is also limited in LMICs, where decisions on how to use resources are crucial. The integration of HCV screening and treatment into primary healthcare is poorly documented, and digital tools for patient follow-up and linkage to care are rarely used. Closing these gaps will require coordinated international studies, improved patient registries, and outreach strategies adapted to local epidemiological and socioeconomic conditions [46,111,112]. The analysis of national strategies illustrates how these gaps translate into context-specific barriers (Table 4). In Egypt, although mass testing campaigns achieved very wide coverage, important challenges were reported, including the initial stigmatization of the population and the high logistical costs required for large-scale implementation [113]. In Spain, the inclusion of screening within primary healthcare services was limited by a lack of human resources and marked regional differences, which affected the consistency and effectiveness of the program [98,114]. In Australia, while the universal treatment program was an important step in improving access to care, it continued to struggle to reach marginalized groups, particularly PWIDs [115]. In all three settings, financial sustainability and the continued implementation of prevention campaigns remain shared and significant challenges, highlighting the need for coordinated and durable public health strategies.

Table 4.
Comparative analysis of the barriers and limitations of national strategies.
12. Conclusions
The elimination of HCV infection remains an important global public health goal, supported by major progress in diagnosis, treatment, and prevention. The availability of DAA therapies—effective and well-tolerated—has changed how the disease is managed, turning hepatitis C into a curable and potentially eliminable infection.
A key part of this effort is the wide use of screening programs that help detect infections early, especially in high-risk groups. These include PWIDs, those who had blood transfusions before routine screening was introduced, people in prison, and underserved communities with limited access to healthcare. Finding and treating cases in these groups is essential to reducing the spread of the virus and to increasing access to therapy.
To reach elimination targets, screening must be matched by equitable and sustained access to treatment. Everyone affected—regardless of income, stage of illness, or personal risk factors—should be able to receive antiviral therapy. This helps reduce health inequalities and supports progress at both national and global levels.
It is also important to keep monitoring risk factors and to develop long-term, practical public health strategies. Utilizing digital tools such as patient tracking systems to improve follow-up and program planning can help increase the effectiveness of these efforts, and significant barriers and knowledge gaps still limit progress.
Despite these advances, HCV elimination is still limited by significant barriers and knowledge gaps. Disparities in healthcare infrastructure, funding sustainability, and social acceptance continue to restrict program expansion, while hard-to-reach populations remain insufficiently engaged. Robust data on reinfection rates, long-term adherence to antiviral therapy, and cost-effectiveness in LMICs are still lacking. Successful national programs in Egypt, Spain, and Australia show that political will and financial investment alone are not enough. Tailored interventions adapted to local epidemiological and socioeconomic contexts, targeted research, and stronger global cooperation are essential to overcoming these barriers and improving program effectiveness.
This review aims to support the foundation of prevention and control initiatives by offering a comprehensive analysis of the most relevant aspects related to the epidemiology, diagnosis, treatment, and prevention of HCV infection. By synthesizing current evidence and outlining the necessary strategic directions, this work contributes to the global coordination efforts required to transform the elimination of hepatitis C from an aspirational goal into a tangible achievement with meaningful impact on worldwide public health. Sustained global commitment and coordinated implementation of these strategies are essential to achieving this public health milestone.
13. Future Directions
13.1. Telemedicine—Expanding Access to Diagnosis and Treatment
Limited access to specialized healthcare services remains a major obstacle to the elimination of HCV, particularly for individuals living in remote areas or belonging to vulnerable groups. In this context, telemedicine represents a promising strategy to facilitate access to care and ensure continuity throughout the diagnostic and treatment process.
Remote consultations can support the initial evaluation of individuals at risk for HCV infection by enabling the interpretation of laboratory results and directing further investigations. Moreover, they allow real-time monitoring during DAA therapy, enabling timely treatment adjustments in response to therapeutic efficacy and adverse effects.
Beyond clinical management, digital platforms can provide psychological support and educational content through online sessions that improve adherence and support successful treatment outcomes. A practical example is the implementation of structured hepatology teleconsultation programs, in collaboration with general practitioners, which may shorten the time between diagnosis and treatment initiation by removing geographic and logistical barriers. This approach enhances interdisciplinary communication, supports shared decision-making, and ensures secure transfer of medical data throughout the care pathway [116].
13.2. Medical Informatics—Optimizing Data Management and Patient Flow
The digitalization of healthcare systems offers a strong foundation for streamlining clinical workflows and enabling long-term follow-up of patients with HCV infection. Electronic registries of diagnosed cases, accessible to primary care providers, specialists, and public health authorities, can support coordinated monitoring and allow for timely interventions in cases of treatment discontinuation or non-adherence [117].
Automated alert systems embedded in electronic health records (EHRs) can flag abnormal laboratory values indicative of possible HCV infection, prompting confirmatory testing. These tools help reduce delays in diagnosis and prevent loss to follow-up [118].
Furthermore, artificial intelligence algorithms applied to clinical, laboratory, and demographic data can identify individuals at higher risk of infection. This predictive approach may enhance the targeting of screening interventions and promote more efficient use of healthcare resources [119].
A concrete example is the integration of alert modules into EHR systems that identify patients with elevated liver enzymes, a history of transfusions, or other relevant risk factors. These tools have proven effective in increasing testing uptake and accelerating access to antiviral therapy [120].
13.3. Expanding Screening Programs for High-Risk Populations
One of the central strategic directions in the elimination of hepatitis C is the expansion of screening programs, particularly targeting high-risk populations and individuals with limited access to healthcare services. Future interventions should include universal screening in high-prevalence settings, such as hospitals, emergency departments, mental health institutions, and correctional facilities [74,121]. The deployment of mobile testing units and the implementation of community-based screening initiatives can significantly improve coverage in underserved or geographically isolated areas. Furthermore, the integration of digital algorithms and EHRs can enhance logistical efficiency and facilitate the early identification of high-risk individuals.
For such interventions to be effective and fair over the long term, they must align with the social and cultural context of the target communities. They should also uphold data confidentiality and promote stigma-free environments. Overcoming barriers like inadequate healthcare infrastructure, low health literacy, and interruptions in care demands an integrated response that brings together multiple sectors and engages local partners. Beyond the clinical benefits, early detection and timely treatment can generate substantial long-term cost savings by reducing the burden associated with advanced liver disease.
13.4. Public Awareness and Health Education Campaigns
Educational and awareness initiatives targeting the general public play an essential supporting role in efforts to eliminate hepatitis C. These campaigns contribute to increased awareness of transmission risks, the importance of early testing, and the availability of effective antiviral therapies. Communication strategies must be clear, accessible, and culturally sensitive, targeting both the general population and vulnerable high-risk groups.
Reducing hepatitis C-related stigma is a key objective. This can be achieved through the ongoing training of healthcare professionals, fostering empathetic and non-discriminatory behavior within healthcare settings, and incorporating hepatitis C-related content into medical education curricula. Targeted outreach campaigns, developed in partnership with community leaders and NGOs, can promote engagement and support the integration of affected individuals into healthcare systems.
13.5. Alignment with WHO Strategies and Recommendations
To ensure the effectiveness and sustainability of hepatitis C elimination efforts, national public health policies must be aligned with the strategic guidelines issued by the WHO. This framework involves ensuring universal access to testing and treatment, establishing strong harm reduction programs, especially for PWIDs, and enhancing surveillance systems to effectively track progress toward elimination goals.
An essential component of this alignment is the continuous training of healthcare professionals to provide non-discriminatory, patient-centered care. Public awareness and education campaigns must also be sustained, using digital platforms and social media to disseminate culturally appropriate messages. The success of these efforts depends on multisectoral collaboration involving public health authorities, NGOs, the private sector, and affected communities.
Such an integrated and coordinated approach represents a critical pillar in achieving global hepatitis C elimination goals.
13.6. Ensuring Universal Access to Antiviral Treatment
Building on this strategic framework, ensuring equitable and universal access to DAA therapies remains a central operational priority. Removing financial and administrative barriers is essential to increasing treatment uptake, particularly among vulnerable and hard-to-reach populations. This includes engaging in negotiations at the national or regional level with pharmaceutical manufacturers and supporting the production and distribution of generic medicines in LMICs [122,123].
The simplification of how treatment is initiated represents an equally critical aspect. Standardized treatment protocols applicable in both primary care and community settings can facilitate rapid diagnosis and early identification of patients eligible for therapy [71]. Coverage for essential diagnostic procedures—such as HCV RNA testing and viral genotyping—through reimbursement mechanisms further supports treatment adherence and patient engagement.
The elimination of unjustified administrative restrictions, such as treatment eligibility criteria based on liver disease stage or past substance use, is critical for promoting equitable access to care [124,125]. In parallel, scaling up early testing efforts among high-risk populations can accelerate case detection and reduce community-level transmission. Providing treatment in a supportive, respectful setting helps reduce stigma around HCV and reinforces the message that it is a curable disease and that affected individuals deserve care, dignity, and inclusion. Embedding these principles into national public health strategies is essential to achieving global hepatitis C elimination targets.
13.7. Supporting Collaborative Efforts Across Countries and the Adoption of Evidence-Based Practices
Enhancing international partnerships and facilitating the dissemination of effective intervention models are essential elements of the global strategy for eliminating HCV. Given the significant disparities in healthcare infrastructure across regions, the practical experience accumulated by countries such as Egypt and Australia—both of which have made notable progress in this area—can guide the implementation of adapted solutions in resource-limited settings [96,113].
Broad access to validated therapeutic protocols, clinical guidelines, educational materials, and modern technologies depends on close cooperation with leading international organizations, such as the WHO, the CDC, and the ECDC [19,126,127]. Such collaborations support standardized care while enabling adaptation to each country’s unique epidemiological and social context.
Furthermore, creating a unified international structure, possibly guided by WHO, to track progress in HCV elimination could allow for the systematic collection and comparison of data related to national plans and their practical application. Such a mechanism would support continuous evaluation of intervention effectiveness and encourage the use of tested models, promoting evidence-based approaches. Constant communication between countries, supported through such a structure, could accelerate global efforts and help reduce inequalities in regard to access to diagnosis and treatment.
13.8. Advancing Vaccine Development for Hepatitis C
Advancing vaccine development for HCV continues to represent a critical objective in ongoing biomedical research efforts. Although antiviral therapies have achieved remarkable progress, there is still a need for a preventive solution that reduces the risk of transmission and supports eradication efforts. The main challenges in this area arise from the virus’s high genetic diversity and its ability to evade the host immune response.
Future research is focused on identifying viral components with high immunogenic potential, capable of eliciting a durable and effective immune response. In parallel, innovative vaccination technologies—such as messenger RNA (mRNA) platforms and viral vectors—are being explored, offering the possibility to tailor immune responses and enhance protection.
A deeper understanding of how HCV interacts with the immune system is essential for the development of viable prophylactic strategies. Studies on viral persistence mechanisms and immunological correlates of protection are expected to contribute to the optimization of vaccine candidate design.
Integrating these research directions outlines the possibility of developing a safe, effective, and broadly applicable vaccine. Such an innovation would complement existing therapeutic interventions and represent a valuable tool in the long-term control of HCV infection.
Author Contributions
Conceptualization, D.T. and L.A.; methodology, D.T. and L.A.; software, A.C. and D.P.; validation, L.A., A.C. and D.P.; formal analysis, D.T.; investigation, L.A.; resources, D.P.; data curation, D.T.; writing—original draft preparation, D.T.; writing—review and editing, D.T. and L.A.; visualization, D.T.; supervision, L.A.; project administration, A.C.; funding acquisition, D.T. All authors have read and agreed to the published version of the manuscript.
Funding
The payment of APC was supported by “Dunarea de Jos”, University of Galati.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| HCV | Hepatitis C virus |
| WHO | World Health Organization |
| DAAs | Direct-acting antiviral agents |
| HCC | Hepatocellular carcinoma |
| PEG-IFNα | PEGylated interferon alpha |
| RBV | Ribavirin |
| HCV RNA | Hepatitis C virus ribonucleic acid |
| SVR12 | Sustained virologic response at 12 weeks |
| SVR | Sustained virologic response |
| HBV | Hepatitis B virus |
| PWIDs | People who inject drugs |
| HIV | Human Immunodeficiency Virus |
| MSMs | Men who have sex with men |
| IDU | Injection drug use |
| ECDC | European Centre for Disease Prevention and Control |
| E1 | HCV envelope glycoprotein 1 |
| E2 | HCV envelope glycoprotein 2 |
| 3′UTR | 3′ untranslated region |
| 5′UTR | 5′ untranslated region |
| NAFLD | Non-alcoholic fatty liver disease |
| OAT | Opioid agonist therapy |
| LMICs | Low- and middle-income countries |
| ELISA | Enzyme-linked immunosorbent assay |
| CLIA | Chemiluminescence immunoassay |
| NAT | Nucleic acid amplification testing |
| HEOR | Health economics and outcomes research |
| USA | United States of America |
| UK | United Kingdom |
| TB | Tuberculosis |
| NGOs | Non-governmental organizations |
| DDT | Dry drop test |
| STIs | Sexually transmitted infections |
| BBV | Blood-borne Viral |
| EU | European Union |
| EHRs | Electronic health records |
| CDC | Centers for Disease Control and Prevention |
| mRNA | Messenger RNA |
References
- Hepatitis, C. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (accessed on 11 May 2025).
- Topi, S.; Gaxhja, E.; Charitos, I.A.; Colella, M.; Santacroce, L. Hepatitis C Virus: History and Current Knowledge. Gastroenterol. Insights 2024, 15, 676–707. [Google Scholar] [CrossRef]
- Sepúlveda-Crespo, D.; Volpi, C.; Amigot-Sánchez, R.; Yélamos, M.B.; Díez, C.; Gómez, J.; Hontañón, V.; Berenguer, J.; González-García, J.; Martín-Escolano, R.; et al. Sustained Long-Term Decline in Anti-HCV Neutralizing Antibodies in HIV/HCV-Coinfected Patients Five Years after HCV Therapy: A Retrospective Study. Pharmaceuticals 2024, 17, 1152. [Google Scholar] [CrossRef]
- Lombardi, A.; Mondelli, M.U.; ESCMID Study Group for Viral Hepatitis (ESGVH). Hepatitis C: Is Eradication Possible? Liver Int. Off. J. Int. Assoc. Study Liver 2019, 39, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.-C.; Chiang, H.-C.; Chiu, Y.-C.; Chien, S.-C.; Cheng, P.-N.; Chiu, H.-C. Chronic Hepatitis C Virus Infection: An Ongoing Challenge in Screening and Treatment. Life 2023, 13, 1964. [Google Scholar] [CrossRef] [PubMed]
- Sarrazin, C. Treatment Failure with DAA Therapy: Importance of Resistance. J. Hepatol. 2021, 74, 1472–1482. [Google Scholar] [CrossRef] [PubMed]
- Marculescu, A.D.; Gavat, C.-C.; Nechita, A.; Topor, G.; Vasilescu, L.V.; Debita, M.; Axente, E.R.; Trinca, C.L.; Anghel, L. Investigation of linearity, detection limit (LD) and quantitation limit(LQ) of active substance from pharmaceutical tablets. Rev. Chim. 2019, 70, 259–262. [Google Scholar] [CrossRef]
- Blach, S.; Terrault, N.A.; Tacke, F.; Gamkrelidze, I.; Craxi, A.; Tanaka, J.; Waked, I.; Dore, G.J.; Abbas, Z.; Abdallah, A.R.; et al. Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: A modelling study. Lancet Gastroenterol. Hepatol. 2022, 7, 396–415. [Google Scholar] [CrossRef] [PubMed]
- Falade-Nwulia, O.; Sulkowski, M.S.; Merkow, A.; Latkin, C.; Mehta, S.H. Understanding and addressing hepatitis C reinfection in the oral direct acting antiviral era. J. Viral Hepat. 2018, 25, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Pawlotsky, J.-M.; Negro, F.; Aghemo, A.; Berenguer, M.; Dalgard, O.; Dusheiko, G.; Marra, F.; Puoti, M.; Wedemeyer, H. EASL recommendations on treatment of hepatitis C: Final update of the series. J. Hepatol. 2020, 73, 1170–1218. [Google Scholar] [CrossRef] [PubMed]
- Stroffolini, T.; Stroffolini, G. Prevalence and Modes of Transmission of Hepatitis C Virus Infection: A Historical Worldwide Review. Viruses 2024, 16, 1115. [Google Scholar] [CrossRef]
- Cottrell, E.B.; Chou, R.; Wasson, N.; Rahman, B.; Guise, J.-M. Reducing risk for mother-to-infant transmission of hepatitis C virus: A systematic review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2013, 158, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Darvishi, N.; Hemmati, M.; Shohaimi, S.; Ghyasi, Y.; Hossaini, F.; Bazrafshan, M.-R.; Akbari, H.; Mohammadi, M. Global prevalence of hepatitis C in prisoners: A comprehensive systematic review and meta-analysis. Arch. Virol. 2022, 167, 1025–1039. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, L.; Peacock, A.; Colledge, S.; Leung, J.; Grebely, J.; Vickerman, P.; Stone, J.; Cunningham, E.B.; Trickey, A.; Dumchev, K.; et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: A multistage systematic review. Lancet Glob. Health 2017, 5, e1192–e1207. [Google Scholar] [CrossRef] [PubMed]
- Global Hepatitis Report. 2017. Available online: https://www.who.int/publications/i/item/9789241565455 (accessed on 14 May 2025).
- WHO Publishes Updated Guidance on Hepatitis C Infection—With New Recommendations on Treatment of Adolescents and Children, Simplified Service Delivery and Diagnostics. Available online: https://www.who.int/news/item/24-06-2022-WHO-publishes-updated-guidance-on-hepatitis-C-infection (accessed on 14 May 2025).
- Winter, R.J.; Holmes, J.A.; Papaluca, T.J.; Thompson, A.J. The Importance of Prisons in Achieving Hepatitis C Elimination: Insights from the Australian Experience. Viruses 2022, 14, 497. [Google Scholar] [CrossRef]
- Busschots, D.; Kremer, C.; Bielen, R.; Koc, Ö.M.; Heyens, L.; Nevens, F.; Hens, N.; Robaeys, G. Hepatitis C prevalence in incarcerated settings between 2013–2021: A systematic review and meta-analysis. BMC Public Health 2022, 22, 2159. [Google Scholar] [CrossRef] [PubMed]
- Public Health Guidance on HIV, Hepatitis B and C Testing in the EU/EEA. Available online: https://www.ecdc.europa.eu/en/publications-data/public-health-guidance-hiv-hepatitis-b-and-c-testing-eueea (accessed on 14 July 2025).
- Global Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections. 2021. Available online: https://www.who.int/publications/i/item/9789240027077 (accessed on 14 July 2025).
- Oancea, C.N.; Butaru, A.E.; Streba, C.T.; Pirici, D.; Rogoveanu, I.; Diculescu, M.M.; Gheonea, D.I. Global hepatitis C elimination: History, evolution, revolutionary changes and barriers to overcome. Rom. J. Morphol. Embryol. 2020, 61, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhang, C.; Shi, J.-J.; Zhang, J.-Y.; Wang, F.-S. Hepatitis C: Milestones from Discovery to Clinical Cure. Mil. Med. Res. 2020, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Ghany, M.G.; Lok, A.S.F.; Dienstag, J.L.; Feinstone, S.M.; Hoofnagle, J.H.; Jake Liang, T.; Seeff, L.B.; Cohen, D.E.; Bezerra, J.A.; Chung, R.T. The 2020 Nobel Prize for Medicine or Physiology for the Discovery of Hepatitis C Virus: A Triumph of Curiosity and Persistence. Hepatology 2021, 74, 2813–2823. [Google Scholar] [CrossRef] [PubMed]
- The Nobel Prize in Physiology or Medicine 2020—Advanced Information. Available online: https://www.nobelprize.org/prizes/medicine/2020/advanced-information/ (accessed on 15 June 2025).
- Ansaldi, F.; Orsi, A.; Sticchi, L.; Bruzzone, B.; Icardi, G. Hepatitis C virus in the new era: Perspectives in epidemiology, prevention, diagnostics and predictors of response to therapy. World J. Gastroenterol. WJG 2014, 20, 9633–9652. [Google Scholar] [CrossRef] [PubMed]
- Gondeau, C.; Pageaux, G.P.; Larrey, D. Hepatitis C Virus Infection: Are There Still Specific Problems with Genotype 3? World J. Gastroenterol. 2015, 21, 12101–12113. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, N.; Moratorio, G.; Cristina, J.; Moreno, P. Hepatitis C Virus Genetic Variability and Evolution. World J. Hepatol. 2015, 7, 831–845. [Google Scholar] [CrossRef] [PubMed]
- Basit, H.; Tyagi, I.; Koirala, J. Hepatitis C. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Sayad, B.; Bozorgomid, A.; Sayad, N.; Azhdari, M.; Bahadori, M.; Rezaeian, S.; Gholizadeh, M. The prevalence of hepatitis C virus genotypes and factors associated with cirrhosis, fatty liver, and viral load: A registry-based cross-sectional cohort study in Western Iran during 1999–2023. Health Sci. Rep. 2024, 7, e70079. [Google Scholar] [CrossRef]
- Chan, A.; Patel, K.; Naggie, S. Genotype 3 Infection—The Last Stand of Hepatitis C Virus. Drugs 2017, 77, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Guidance on Prevention of Viral Hepatitis B and C Among People Who Inject Drugs. Available online: https://www.who.int/publications/i/item/9789241504041 (accessed on 9 June 2025).
- Meteliuk, A.; Sazonova, Y.; Goldmann, E.; Xu, S.; Liutyi, V.; Liakh, T.; Spirina, T.; Lekholetova, M.; Islam, Z.; Ompad, D.C. The impact of the 2014 military conflict in the east of Ukraine and the Autonomous Republic of the Crimea among patients receiving opioid agonist therapies. J. Subst. Use Addict. Treat. 2024, 160, 209312. [Google Scholar] [CrossRef] [PubMed]
- Bashir, H.H.; Kamani, L.; Usman, M.; Kishwar, K. Awareness and safe practices of Hepatitis-B and C prevention and transmission among workers of women beauty salons. Pak. J. Med. Sci. 2022, 38, 2156–2162. [Google Scholar] [CrossRef] [PubMed]
- Caminada, S.; Mele, A.; Ferrigno, L.; Alfonsi, V.; Crateri, S.; Iantosca, G.; Sabato, M.; Tosti, M.E. Risk of parenterally transmitted hepatitis following exposure to invasive procedures in Italy: SEIEVA surveillance 2000–2021. J. Hepatol. 2023, 79, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Epstein, R.L.; Sabharwal, V.; Wachman, E.M.; Saia, K.A.; Vellozzi, C.; Hariri, S.; Linas, B.P. Perinatal Transmission of Hepatitis C Virus: Defining the Cascade of Care. J. Pediatr. 2018, 203, 34–40.e1. [Google Scholar] [CrossRef] [PubMed]
- Newsum, A.M.; Matser, A.; Schinkel, J.; van der Valk, M.; Brinkman, K.; van Eeden, A.; Lauw, F.N.; Rijnders, B.J.A.; van de Laar, T.J.W.; van de Kerkhof, M.; et al. Incidence of HCV Reinfection Among HIV-Positive MSM and Its Association With Sexual Risk Behavior: A Longitudinal Analysis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021, 73, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Monin, M.B.; Ingiliz, P.; Lutz, T.; Scholten, S.; Cordes, C.; Martínez-Rebollar, M.; Spinner, C.D.; Nelson, M.; Rausch, M.; Bhagani, S.; et al. Low Spontaneous Clearance Rates of Recently Acquired Hepatitis C Virus in Human Immunodeficiency Virus-Positive Men Who Have Sex With Men (PROBE-C Study). Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2023, 76, e607–e612. [Google Scholar] [CrossRef] [PubMed]
- Terrault, N.A.; Dodge, J.L.; Murphy, E.L.; Tavis, J.E.; Kiss, A.; Levin, T.R.; Gish, R.G.; Busch, M.P.; Reingold, A.L.; Alter, M.J. Sexual transmission of hepatitis C virus among monogamous heterosexual couples: The HCV partners study. Hepatology 2013, 57, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Maugard, C.; Relave, J.; Klinkicht, M.; Fabra, C. Clinical performance evaluation of Elecsys HIV Duo, Anti-HCV II, HBsAg II, Anti-HBc II, and Syphilis assays for routine screening of first-time blood donor samples at a French blood donation center. Transfus. Clin. Biol. 2022, 29, 79–83. [Google Scholar] [CrossRef]
- Daw, M.A.; El-Bouzedi, A.A.; Ahmed, M.O.; Dau, A.A.; Agnan, M.M.; Drah, A.M. Geographic Integration of Hepatitis C Virus: A Global Threat. World J. Virol. 2016, 5, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Ruta, S.; Cernescu, C. Injecting drug use: A vector for the introduction of new hepatitis C virus genotypes. World J. Gastroenterol. WJG 2015, 21, 10811–10823. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, H.; Hossain, M.F.; Shrestha, P.; Mahmud, S.; Husain, M.; Ahmed, R. Prevalence and associated risk factors of current hepatitis C infection among U.S. general population and injection drug users aged 20–59 years: NHANES 2009–2018. PLoS ONE 2024, 19, e0309345. [Google Scholar] [CrossRef] [PubMed]
- Walters, S.M.; Frank, D.; Felsher, M.; Jaiswal, J.; Fletcher, S.; Bennett, A.S.; Friedman, S.R.; Ouellet, L.J.; Ompad, D.C.; Jenkins, W.; et al. How the rural risk environment underpins hepatitis C risk: Qualitative findings from rural southern Illinois, United States. Int. J. Drug Policy 2023, 112, 103930. [Google Scholar] [CrossRef] [PubMed]
- Tohme, R.A.; Holmberg, S.D. Is Sexual Contact a Major Mode of Hepatitis C Virus Transmission? Hepatol. Baltim. Md 2010, 52, 1497–1505. [Google Scholar] [CrossRef] [PubMed]
- Hagan, H.; Jordan, A.E.; Neurer, J.; Cleland, C.M. Incidence of sexually-transmitted hepatitis C virus infection in HIV-positive men who have sex with men: A systematic review and meta-analysis. AIDS Lond. Engl. 2015, 29, 2335–2345. [Google Scholar] [CrossRef] [PubMed]
- Hepatitis C—Annual Epidemiological Report for 2020. Available online: https://www.ecdc.europa.eu/en/publications-data/hepatitis-c-annual-epidemiological-report-2020 (accessed on 19 July 2025).
- Panagiotakopoulos, L.; Sandul, A.L.; Conners, E.E.; Foster, M.A.; Nelson, N.P.; Wester, C.; Barnett, E.; Jhaveri, R.; Lazenby, G.; Lee, C.; et al. CDC Recommendations for Hepatitis C Testing Among Perinatally Exposed Infants and Children—United States, 2023. MMWR Recomm. Rep. 2023, 72, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.H.; Kam, L.; Yeo, Y.H.; Huang, D.Q.; Henry, L.; Cheung, R.; Nguyen, M.H. Characteristics and Treatment Rate of Patients With Hepatitis C Virus Infection in the Direct-Acting Antiviral Era and During the COVID-19 Pandemic in the United States. JAMA Netw. Open 2022, 5, e2245424. [Google Scholar] [CrossRef] [PubMed]
- Feier, R.D.; Topor, G.; Anghel, L.; Aungurencei, A.E.; Negraia, M.R. Rehabilitation and Creation of Favorable Conditions for the Improvement of the Comfort and Quality of Acrylates Used in the Sphere of Removable Dentures. Rev. Chim. 2019, 70, 3188–3192. [Google Scholar] [CrossRef]
- Stefanopol, I.A.; Baroiu, L.; Constantin, G.B.; Danila, D.M.; Anghel, L.; Nechifor, A.; Tatu, A.L. Diagnostic and Management of Undescended Ovary—A Preoperative Dilemma: A Case-Based Systematic Review. Int. J. Womens Health 2022, 14, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Kronfli, N.; Cox, J. Care for People with Hepatitis C in Provincial and Territorial Prisons. CMAJ Can. Med. Assoc. J. 2018, 190, E93–E94. [Google Scholar] [CrossRef] [PubMed]
- Hayes, C.N.; Imamura, M.; Tanaka, J.; Chayama, K. Road to elimination of HCV: Clinical challenges in HCV management. Liver Int. 2022, 42, 1935–1944. [Google Scholar] [CrossRef]
- Hepatitis C Guidance 2019 Update: American Association for the Study of Liver Diseases–Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection—Ghany—2020—Hepatology—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/hep.31060 (accessed on 11 May 2025).
- Zocca, E.; Seraceni, S.; Cafaro, T.; Cervone, T.E.; Cardarelli, L.; Valisi, M.; Polidori, I.; Pieri, M.; Tomassetti, F.; Broccolo, F. Evaluation of Two-Assay Serological Testing Strategies for Anti-HCV Screening in Italian Populations: A Dual Screening Approach. Diagnostics 2024, 14, 570. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.J.; Wang, L.L.; Ning, H.-C.; Tao, C.M.; Hirankarn, N.; Kuakarn, S.; Yang, R.; Han, T.H.; Chan, R.C.; Hussain, B.M.; et al. Evaluarea testului Elecsys® Anti-HCV II pentru screeningul de rutină cu virusul hepatitei C a diferitelor populații din Asia Pacific și detectarea infecției precoce. J. Clin. Virol. 2015, 64, 20–27. [Google Scholar] [CrossRef]
- Cuomo, G.; Franconi, I.; Riva, N.; Bianchi, A.; Digaetano, M.; Santoro, A.; Codeluppi, M.; Bedini, A.; Guaraldi, G.; Mussini, C. Migration and health: A retrospective study about the prevalence of HBV, HIV, HCV, tuberculosis and syphilis infections amongst newly arrived migrants screened at the Infectious Diseases Unit of Modena, Italy. J. Infect. Public Health 2019, 12, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Evans, H.; Balasegaram, S.; Douthwaite, S.; Hunter, L.; Kulasegaram, R.; Wong, T.; Querol-Rubiera, A.; Nebbia, G. An innovative approach to increase viral hepatitis diagnoses and linkage to care using opt-out testing and an integrated care pathway in a London Emergency Department. PLoS ONE 2018, 13, e0198520. [Google Scholar] [CrossRef] [PubMed]
- Monti, M.; Caruso, T.; Castellaccio, A.; De Giorgi, I.; Cavallini, G.; Manca, M.L.; Lorini, S.; Marri, S.; Petraccia, L.; Madia, F.; et al. HBV and HCV testing outcomes among marginalized communities in Italy, 2019–2024: A prospective study. Lancet Reg. Health Eur. 2025, 49, 101172. [Google Scholar] [CrossRef] [PubMed]
- Pinazo-Bandera, J.M.; Aranda, J.; García-García, A.M.; Alcántara, R.; Ortega-Alonso, A.; Del Campo-Herrera, E.; Clavijo, E.; García-Escaño, M.D.; Ruiz Ruiz, J.J.; Morales-Herrera, M.; et al. Hepatitis C virus point-of-care microelimination approach in a vulnerable population in the South of Spain. Gastroenterol. Rep. 2024, 12, goad077. [Google Scholar] [CrossRef]
- Barror, S.; Avramovic, G.; Oprea, C.; Surey, J.; Story, A.; Macías, J.; Cullen, W.; Crowley, D.; Horan, A.; Naughton, A.M.; et al. HepCare Europe: A service innovation project. HepCheck: Enhancing HCV identification and linkage to care for vulnerable populations through intensified outreach screening. A prospective multisite feasibility study. J. Antimicrob. Chemother. 2019, 74, v39–v46. [Google Scholar] [CrossRef] [PubMed]
- Strehlow, A.J.; Robertson, M.J.; Zerger, S.; Rongey, C.; Arangua, L.; Farrell, E.; O’Sullivan, A.; Gelberg, L. Hepatitis C among Clients of Health Care for the Homeless Primary Care Clinics. J. Health Care Poor Underserved 2012, 23, 811–833. [Google Scholar] [CrossRef] [PubMed]
- Gelberg, L.; Robertson, M.J.; Arangua, L.; Leake, B.D.; Sumner, G.; Moe, A.; Andersen, R.M.; Morgenstern, H.; Nyamathi, A. Prevalence, distribution, and correlates of hepatitis C virus infection among homeless adults in Los Angeles. Public Health Rep. Wash. DC 1974 2012, 127, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Khalili, M.; Powell, J.; Park, H.H.; Bush, D.; Naugle, J.; Ricco, M.; Magee, C.; Braimoh, G.; Zevin, B.; Fokuo, J.K.; et al. Shelter-Based Integrated Model Is Effective in Scaling Up Hepatitis C Testing and Treatment in Persons Experiencing Homelessness. Hepatol. Commun. 2022, 6, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.M.M.B.; Martelli, C.M.T.; Moreira, R.C.; Merchan-Hamman, E.; Stein, A.T.; Cardoso, M.R.A.; Figueiredo, G.M.; Montarroyos, U.R.; Braga, C.; Turchi, M.D.; et al. Prevalence and risk factors of Hepatitis C virus infection in Brazil, 2005 through 2009: A cross-sectional study. BMC Infect. Dis. 2013, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Coppola, N.; Monari, C.; Alessio, L.; Onorato, L.; Gualdieri, L.; Sagnelli, C.; Minichini, C.; Sagnelli, E.; Di Caprio, G.; Surace, L.; et al. Blood-borne chronic viral infections in a large cohort of immigrants in southern Italy: A seven-centre, prospective, screening study. Travel Med. Infect. Dis. 2020, 35, 101551. [Google Scholar] [CrossRef]
- Segala, F.V.; Novara, R.; Panico, G.; Laforgia, R.; Raho, L.; Schiavone, M.; Civile, G.; Laforgia, N.; Di Gregorio, S.; Guido, G.; et al. Prevalence of Sexually Transmitted Infections and Predictors for Loss to Follow Up among Marginalized Homeless and Migrant Communities: A Cross-Sectional Study. Ann. Glob. Health 90 2024, 90, 25. [Google Scholar] [CrossRef] [PubMed]
- Buti, M.; Vaz-Pinto, I.; Magno Pereira, V.; Casado, M.; Llaneras, J.; Barreira, A.; Esteves, C.; Guimarães, M.; Gorgulho, A.; Mourão, T.; et al. Emergency Department Contribution to HCV Elimination in the Iberian Peninsula. Int. J. Emerg. Med. 2024, 17, 5. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.A.; Umashankar, K.; Maheswaran, A.; Martin, M.T.; Lee, J.; Odishoo, M.; Lin, J.Y.; Touchette, D.R. Cost-effectiveness analysis of emergency department-based hepatitis C screening and linkage-to-care program. BMC Health Serv. Res. 2024, 24, 1308. [Google Scholar] [CrossRef] [PubMed]
- Cambianica, A.; Marchese, V.; Pennati, F.; Faustinelli, A.; Migliorati, M.; Roda, F.; Spinetti, A.; Zaltron, S.; Fiorentini, S.; Caruso, A.; et al. Chronic Hepatitis C Cascade of Care in Prisoners—Is There Still Some Work to Do? Analysis of Two Large Penitentiaries in Northern Italy. Int. J. Environ. Res. Public. Health 2024, 21, 104. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, F.-D.; Li, L.-Q.; Chen, E.-Q. Management of in- and out-of-Hospital Screening for Hepatitis C. Front. Public Health 2022, 10, 984810. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, A.; Cooke, G.S.; Nayagam, S.; Thursz, M.; Hallett, T.B. Scaling up prevention and treatment towards the elimination of hepatitis C: A global mathematical model. Lancet Lond. Engl. 2019, 393, 1319–1329. [Google Scholar] [CrossRef] [PubMed]
- NHS England. Blood-Borne Viruses Opt-out Testing in Emergency Departments in Areas of High and Extremely High HIV Prevalence: Good Practice Guidance; NHS England: London, UK, 2024. [Google Scholar]
- Zhu, L.; Furukawa, N.W.; Thompson, W.W.; Reitsma, M.B.; Randall, L.M.; Van Handel, M.; Asher, A.K.; Valverde, E.; Linas, B.P.; Salomon, J.A. Health and Economic Impact of Periodic Hepatitis C Virus Testing Among People Who Inject Drugs. JAMA Health Forum 2025, 6, e251870. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Health Sector Strategy on Viral Hepatitis 2016–2021. Towards Ending Viral Hepatitis; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Gomaa, A.; Gomaa, M.; Allam, N.; Waked, I. Hepatitis C Elimination in Egypt: Story of Success. Pathogens 2024, 13, 681. [Google Scholar] [CrossRef] [PubMed]
- Avramovic, G.; O’Doherty, L.; McHugh, T.; Remy, A.J.; Happiette, A.; Bouchkira, H.; Murat, P.; Scemama, O.; Esclade, A.; Farfan Camacho, M.I.; et al. Benchmarking of an Intervention Aiming at the Micro-Elimination of Hepatitis C in Vulnerable Populations in Perpignan, France, to Inform Scale-Up and Elimination on the French Territory. Viruses 2024, 16, 1645. [Google Scholar] [CrossRef]
- Saludes, V.; Bordoy, A.E.; Yela, E.; Turú, E.; Not, A.; López-Corbeto, E.; Egea-Cortés, L.; González-Candelas, F.; Casabona, J.; Group for the Study and Control of Infectious Diseases in Prison (GRUMIP); et al. Incidence and molecular epidemiology of hepatitis C virus reinfection in prisons in Catalonia, Spain (Re-HCV study). Sci. Rep. 2023, 13, 16012. [Google Scholar] [CrossRef] [PubMed]
- Interim Guidance for Country Validation of Viral Hepatitis Elimination. Available online: https://www.who.int/publications/i/item/9789240028395 (accessed on 12 May 2025).
- Crespo, J.; Llerena, S.; Cobo, C.; Cabezas, J. Is HCV Elimination Possible in Prison? Rev. Esp. Sanid. Penit. 2017, 19, 70–73. [Google Scholar] [PubMed]
- Stöver, H.; Meroueh, F.; Marco, A.; Keppler, K.; Saiz de la Hoya, P.; Littlewood, R.; Wright, N.; Nava, F.; Alam, F.; Walcher, S.; et al. Offering HCV treatment to prisoners is an important opportunity: Key principles based on policy and practice assessment in Europe. BMC Public Health 2019, 19, 30. [Google Scholar] [CrossRef] [PubMed]
- Hajarizadeh, B.; Cunningham, E.B.; Valerio, H.; Martinello, M.; Law, M.; Janjua, N.Z.; Midgard, H.; Dalgard, O.; Dillon, J.; Hickman, M.; et al. Hepatitis C reinfection after successful antiviral treatment among people who inject drugs: A meta-analysis. J. Hepatol. 2020, 72, 643–657. [Google Scholar] [CrossRef] [PubMed]
- Mambro, A.; Mortazhejri, S.; Ortiz-Paredes, D.; Patey, A.; Fontaine, G.; Dussault, C.; Cox, J.; Grimshaw, J.M.; Presseau, J.; Kronfli, N. Understanding Perceptions of Hepatitis C and Its Management Among People with Experience of Incarceration in Quebec, Canada: A Qualitative Study Guided by the Common Sense Self-Regulation Model. Viruses 2024, 16, 1910. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M.J.; Kronfli, N.; Cabezas, J.; Sheehan, Y.; Scheibe, A.; Brahni, T.; Naik, K.; Mashabela, P.; Chan, P.; Luhmann, N.; et al. The role of low-income and middle-income country prisons in eliminating hepatitis C. Lancet Public Health 2022, 7, e578–e579. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.J.; Shrestha, L.B.; Lloyd, A.R.; Dawson, O.; Sheehan, Y.; Sheehan, J.; Maduka, N.B.C.; Cabezas, J.; Akiyama, M.J.; Kronfli, N. Barriers and advocacy needs for hepatitis C services in prisons: Informing the prisons hepatitis C advocacy toolkit. Int. J. Drug Policy 2024, 126, 104386. [Google Scholar] [CrossRef]
- Kronfli, N.; Dussault, C.; Chalifoux, S.; Kavoukian, H.; Klein, M.B.; Cox, J. A randomized pilot study assessing the acceptability of rapid point-of-care hepatitis C virus (HCV) testing among male inmates in Montreal, Canada. Int. J. Drug Policy 2020, 85, 102921. [Google Scholar] [CrossRef]
- Kronfli, N.; Buxton, J.A.; Jennings, L.; Kouyoumdjian, F.; Wong, A. Hepatitis C virus (HCV) care in Canadian correctional facilities: Where are we and where do we need to be? Can. Liver J. 2019, 2, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Kronfli, N.; Linthwaite, B.; Kouyoumdjian, F.; Klein, M.B.; Lebouché, B.; Sebastiani, G.; Cox, J. Interventions to increase testing, linkage to care and treatment of hepatitis C virus (HCV) infection among people in prisons: A systematic review. Int. J. Drug Policy 2018, 57, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Cheema, J.S.; Suckow, S.; Ramers, C.; Loose, P.; Tomada, A.; Tweeten, S.; Stamos-Buesig, T.; Abramovitz, D.; Eger, W.H.; Strathdee, S.A.; et al. Is San Diego California on Track to Reach HCV Elimination? A Modeling Analysis of Combination Prevention Strategies. Viruses 2024, 16, 1819. [Google Scholar] [CrossRef] [PubMed]
- Wynn, A.; Tweeten, S.; McDonald, E.; Wooten, W.; Lucas, K.; Cyr, C.L.; Hernandez, M.; Ramirez, F.; VanWormer, C.; Suckow, S.; et al. The estimated hepatitis C seroprevalence and key population sizes in San Diego in 2018. PLoS ONE 2021, 16, e0251635. [Google Scholar] [CrossRef] [PubMed]
- Wedemeyer, H.; Duberg, A.S.; Buti, M.; Rosenberg, W.M.; Frankova, S.; Esmat, G.; Örmeci, N.; Van Vlierberghe, H.; Gschwantler, M.; Akarca, U.; et al. Strategies to manage hepatitis C virus (HCV) disease burden. J. Viral Hepat. 2014, 21, 60–89. [Google Scholar] [CrossRef]
- Jose-Abrego, A.; Trujillo-Trujillo, M.E.; Laguna-Meraz, S.; Roman, S.; Panduro, A. Epidemiology of Hepatitis C Virus in HIV Patients from West Mexico: Implications for Controlling and Preventing Viral Hepatitis. Pathog. Basel Switz. 2024, 13, 360. [Google Scholar] [CrossRef] [PubMed]
- Samsó Jofra, L.; Puig, T.; Solà, I.; Trujols, J. Interim opioid agonist treatment for opioid addiction: A systematic review. Harm. Reduct. J. 2022, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Bregenzer, A.; Krismer, C.; Wendel, S.; Roser, P.; Fux, C.A. HCV elimination in a Swiss opioid agonist therapy programme—A cohort study. Swiss Med. Wkly. 2022, 152, 40009. [Google Scholar] [CrossRef] [PubMed]
- Romo, E.; Bianchet, E.; Dowd, P.; Mazor, K.M.; Stopka, T.J.; Friedmann, P.D. Syringe Access, Syringe Sharing, and Perceptions of HCV: A Qualitative Study Exploring the HCV Risk Environment in Rural Northern New England, United States. Viruses 2024, 16, 1364. [Google Scholar] [CrossRef] [PubMed]
- Midgard, H.; Malme, K.B.; Pihl, C.M.; Berg-Pedersen, R.M.; Tanum, L.; Klundby, I.; Haug, A.; Tveter, I.; Bjørnestad, R.; Olsen, I.C.; et al. Opportunistic Treatment of Hepatitis C Infection Among Hospitalized People Who Inject Drugs (OPPORTUNI-C): A Stepped Wedge Cluster Randomized Trial. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2024, 78, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Scott, N.; Sacks-Davis, R.; Wade, A.J.; Stoove, M.; Pedrana, A.; Doyle, J.S.; Thompson, A.J.; Wilson, D.P.; Hellard, M.E. Australia needs to increase testing to achieve hepatitis C elimination. Med. J. Aust. 2020, 212, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Gountas, I.; Sypsa, V.; Papatheodoridis, G.; Souliotis, K.; Athanasakis, K.; Razavi, H.; Hatzakis, A. Economic evaluation of the hepatitis C elimination strategy in Greece in the era of affordable direct-acting antivirals. World J. Gastroenterol. 2019, 25, 1327–1340. [Google Scholar] [CrossRef] [PubMed]
- Calleja, J.L.; Espin, J.; Kaushik, A.; Hernandez-Guerra, M.; Blissett, R.; Yehoshua, A.; Igloi-Nagy, A. The Efficiency of Increased HCV Testing and Treatment Strategies in Spain to Achieve Elimination Goals. PharmacoEconomics—Open 2024, 8, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Feld, J.J.; Klein, M.B.; Rahal, Y.; Lee, S.S.; Mohammed, S.; King, A.; Smyth, D.; Gonzalez, Y.S.; Nugent, A.; Janjua, N.Z. Timing of Elimination of Hepatitis C Virus in Canada’s Provinces. Can. Liver J. 2022, 5, 493–506. [Google Scholar] [CrossRef]
- Snell, G.; Marshall, A.D.; van Gennip, J.; Bonn, M.; Butler-McPhee, J.; Cooper, C.L.; Kronfli, N.; Williams, S.; Bruneau, J.; Feld, J.J.; et al. Public reimbursement policies in Canada for direct-acting antiviral treatment of hepatitis C virus infection: A descriptive study. Can. Liver J. 2023, 6, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Brouard, C.; Saboni, L.; Gautier, A.; Chevaliez, S.; Rahib, D.; Richard, J.-B.; Barin, F.; Larsen, C.; Sommen, C.; Pillonel, J.; et al. HCV and HBV prevalence based on home blood self-sampling and screening history in the general population in 2016: Contribution to the new French screening strategy. BMC Infect. Dis. 2019, 19, 896. [Google Scholar] [CrossRef] [PubMed]
- Brouard, C.; Pillonel, J.; Boussac, M.; de Lédinghen, V.; Rachas, A.; Silvain, C.; Lydié, N.; Chevaliez, S.; Pioche, C.; Durand, J.; et al. French hepatitis C care cascade: Substantial impact of direct-acting antivirals, but the road to elimination is still long. BMC Infect. Dis. 2020, 20, 759. [Google Scholar] [CrossRef] [PubMed]
- Safreed-Harmon, K.; Hetherington, K.L.; Aleman, S.; Alho, H.; Dalgard, O.; Frisch, T.; Gottfredsson, M.; Weis, N.; Lazarus, J.V. Hep-Nordic Study Group Policy responses to hepatitis C in the Nordic countries: Gaps and discrepant reporting in the Hep-Nordic study. PLoS ONE 2018, 13, e0190146. [Google Scholar] [CrossRef] [PubMed]
- Dröse, S.; Øvrehus, A.L.H.; Holm, D.K.; Madsen, L.W.; Mössner, B.K.; Søholm, J.; Hansen, J.F.; Røge, B.T.; Christensen, P.B. A multi-level intervention to eliminate hepatitis C from the Region of Southern Denmark: The C-Free-South project. BMC Infect. Dis. 2022, 22, 202. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, R.; Midtbø, J.E.; Kløvstad, H. Monitoring Progress Towards the Elimination of Hepatitis C as a Public Health Threat in Norway: A Modelling Study Among People Who Inject Drugs and Immigrants. J. Infect. Dis. 2024, 230, e700–e711. [Google Scholar] [CrossRef]
- Kronfli, N.; Dussault, C.; Bartlett, S.; Fuchs, D.; Kaita, K.; Harland, K.; Martin, B.; Whitten-Nagle, C.; Cox, J. Disparities in hepatitis C care across Canadian provincial prisons: Implications for hepatitis C micro-elimination. Can. Liver J. 2021, 4, 292–310. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.-H.; Fang, Y.-J.; Hsu, S.-J.; Lee, J.-Y.; Chiu, M.-C.; Yu, J.-J.; Kuo, C.-C.; Chen, C.-H. Microelimination of Chronic Hepatitis C by Universal Screening Plus Direct-Acting Antivirals for Incarcerated Persons in Taiwan. Open Forum Infect. Dis. 2020, 7, ofaa301. [Google Scholar] [CrossRef] [PubMed]
- Schillie, S. CDC Recommendations for Hepatitis C Screening Among Adults—United States, 2020. MMWR Recomm. Rep. 2020, 69, 1–17. [Google Scholar] [CrossRef]
- Norton, B.L.; Southern, W.N.; Steinman, M.; Smith, B.D.; Deluca, J.; Rosner, Z.; Litwin, A.H. No Differences in Achieving Hepatitis C Virus Care Milestones Between Patients Identified by Birth Cohort or Risk-Based Screening. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2016, 14, 1356–1360. [Google Scholar] [CrossRef] [PubMed]
- Martinello, M.; Hajarizadeh, B.; Dore, G.J. Hepatitis C Elimination in Australia: Progress and Challenges. Med. J. Aust. 2020, 212, 362–363. [Google Scholar] [CrossRef]
- Global Health Sector Strategies. Available online: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/strategies/global-health-sector-strategies (accessed on 19 July 2025).
- Trickey, A.; Fraser, H.; Lim, A.G.; Peacock, A.; Colledge, S.; Walker, J.G.; Leung, J.; Grebely, J.; Larney, S.; Martin, N.K.; et al. The contribution of injection drug use to hepatitis C virus transmission globally, regionally, and at country level: A modelling study. Lancet Gastroenterol. Hepatol. 2019, 4, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Waked, I.; Esmat, G.; Elsharkawy, A.; El-Serafy, M.; Abdel-Razek, W.; Ghalab, R.; Elshishiney, G.; Salah, A.; Abdel Megid, S.; Kabil, K.; et al. Screening and Treatment Program to Eliminate Hepatitis C in Egypt. N. Engl. J. Med. 2020, 382, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Buti, M.; Domínguez-Hernández, R.; Casado, M.Á.; Sabater, E.; Esteban, R. Healthcare value of implementing hepatitis C screening in the adult general population in Spain. PLoS ONE 2018, 13, e0208036. [Google Scholar] [CrossRef] [PubMed]
- Alavi, M.; Law, M.G.; Valerio, H.; Grebely, J.; Amin, J.; Hajarizadeh, B.; Selvey, C.; George, J.; Dore, G.J. Declining hepatitis C virus-related liver disease burden in the direct-acting antiviral therapy era in New South Wales, Australia. J. Hepatol. 2019, 71, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Beste, L.A.; Glorioso, T.J.; Ho, P.M.; Au, D.H.; Kirsh, S.R.; Todd-Stenberg, J.; Chang, M.F.; Dominitz, J.A.; Barón, A.E.; Ross, D. Telemedicine Specialty Support Promotes Hepatitis C Treatment by Primary Care Providers in the Department of Veterans Affairs. Am. J. Med. 2017, 130, 432–438.e3. [Google Scholar] [CrossRef] [PubMed]
- Kasting, M.L.; Giuliano, A.R.; Reich, R.R.; Duong, L.M.; Rathwell, J.; Roetzheim, R.G.; Vadaparampil, S.T. Electronic medical record-verified hepatitis C virus screening in a large health system. Cancer Med. 2019, 8, 4555–4564. [Google Scholar] [CrossRef] [PubMed]
- Federman, A.D.; Kil, N.; Kannry, J.; Andreopolous, E.M.; Toribio, W.; Lyons, J.M.; Singer, M.D.; Yartel, A.; Smith, B.D.; Rein, D.B.; et al. An Electronic Health Record-based Intervention to Promote Hepatitis C Virus Testing Among Adults Born Between 1945 and 1965: A Cluster-randomized Trial. Med. Care 2017, 55, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.-Y.; Huang, C.-F.; Hung, C.-H.; Tai, C.-M.; Mo, L.-R.; Kuo, H.-T.; Tseng, K.-C.; Lo, C.-C.; Bair, M.-J.; Wang, S.-J.; et al. Artificial intelligence predicts direct-acting antivirals failure among hepatitis C virus patients: A nationwide hepatitis C virus registry program. Clin. Mol. Hepatol. 2024, 30, 64–79. [Google Scholar] [CrossRef] [PubMed]
- García-Herola, A.; Domínguez-Hernández, R.; Casado, M.Á. Clinical and economic impact of an alert system in primary care for the detection of patients with chronic hepatitis C. PLoS ONE 2021, 16, e0260608. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, J.V.; Safreed-Harmon, K.; Thursz, M.R.; Dillon, J.F.; El-Sayed, M.H.; Elsharkawy, A.M.; Hatzakis, A.; Jadoul, M.; Prestileo, T.; Razavi, H.; et al. The Micro-Elimination Approach to Eliminating Hepatitis C: Strategic and Operational Considerations. Semin. Liver Dis. 2018, 38, 181–192. [Google Scholar] [CrossRef]
- Hill, A.; Simmons, B.; Gotham, D.; Fortunak, J. Rapid reductions in prices for generic sofosbuvir and daclatasvir to treat hepatitis C. J. Virus Erad. 2 2016, 2, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Andrieux-Meyer, I.; Cohn, J.; de Araújo, E.S.A.; Hamid, S.S. Disparity in Market Prices for hepatitis C Virus Direct-Acting Drugs. Lancet Glob. Health 2015, 3, e676–e677. [Google Scholar] [CrossRef]
- Brener, L.; Von Hippel, W.; Kippax, S.; Preacher, K.J. The role of physician and nurse attitudes in the health care of injecting drug users. Subst. Use Misuse 2010, 45, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Izzat, A.S.A.H.; Anghel, L.; Stefanescu, B.; Kantor, C.; Ciubara, A. Prevention of psychoactive substance use. Arch. Euromedica 2021, 11, 59–61. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Available online: https://www.who.int (accessed on 29 July 2025).[Green Version]
- CDC Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/index.html (accessed on 29 July 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).