Enteric Viruses in Turkeys: A Systematic Review and Comparative Data Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Meta-Analysis
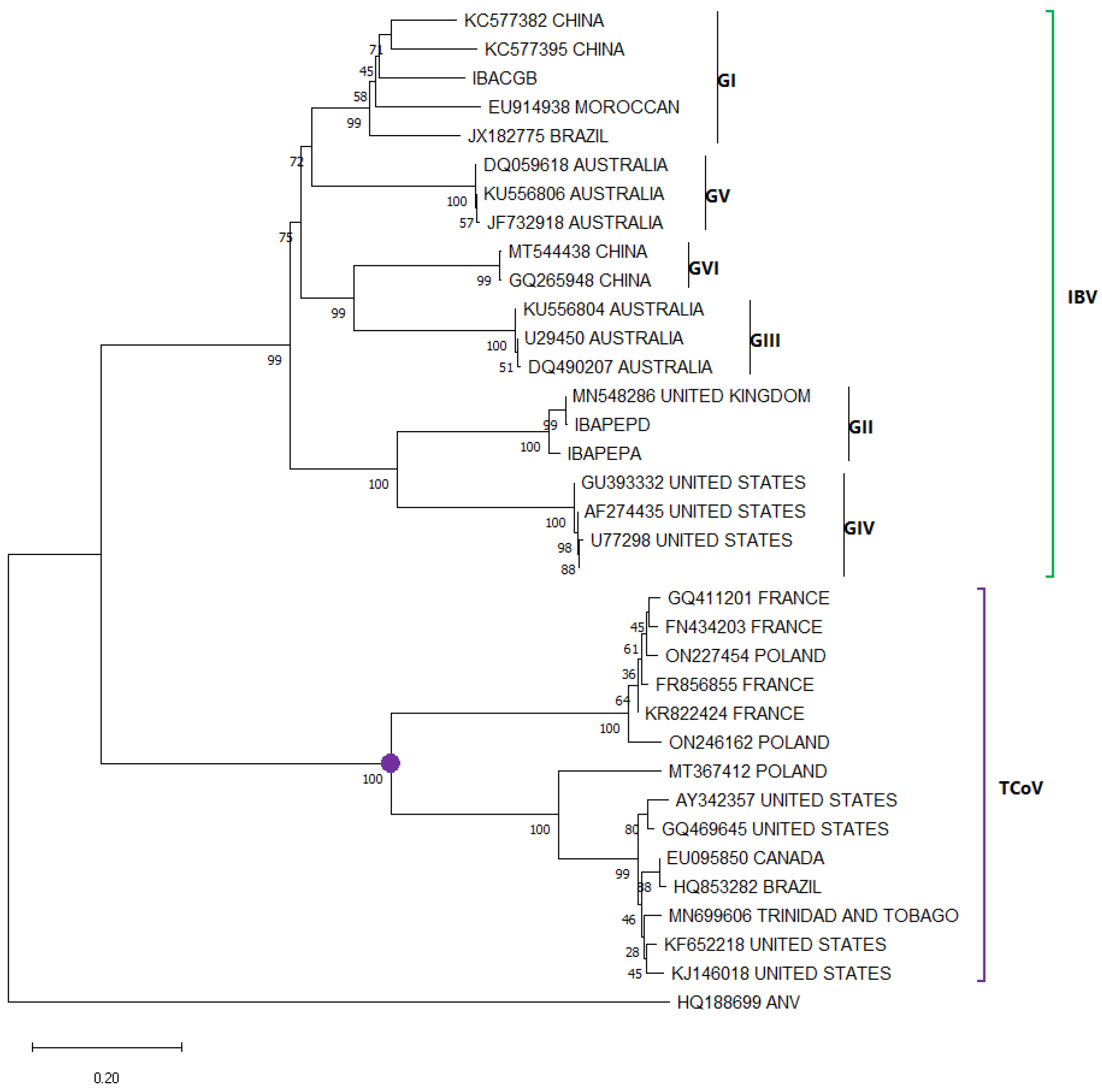
2.3. Phylogenetic Analysis
3. Main Enteric Viruses Associated with PEC
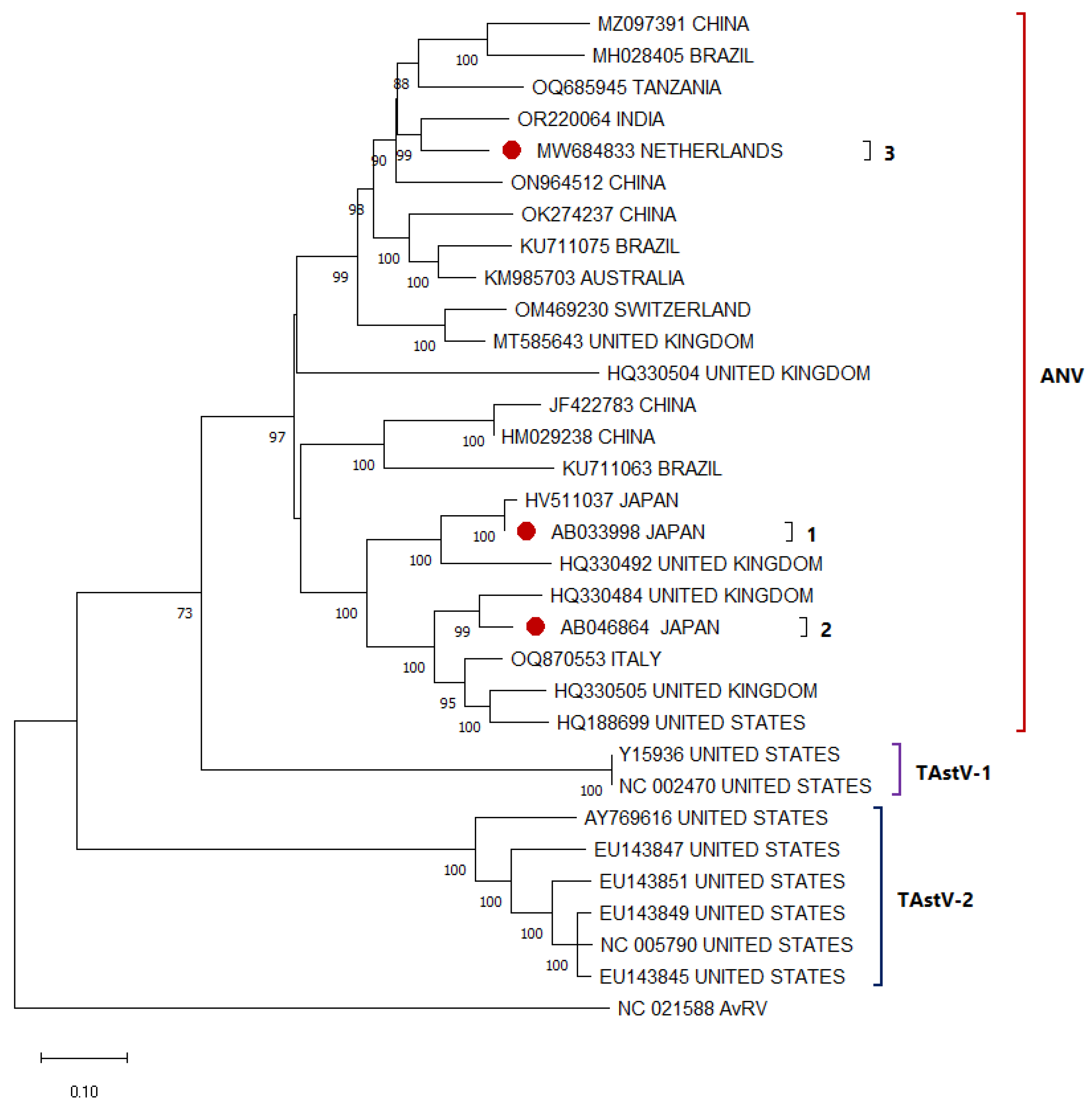
3.1. Avian Astrovirus
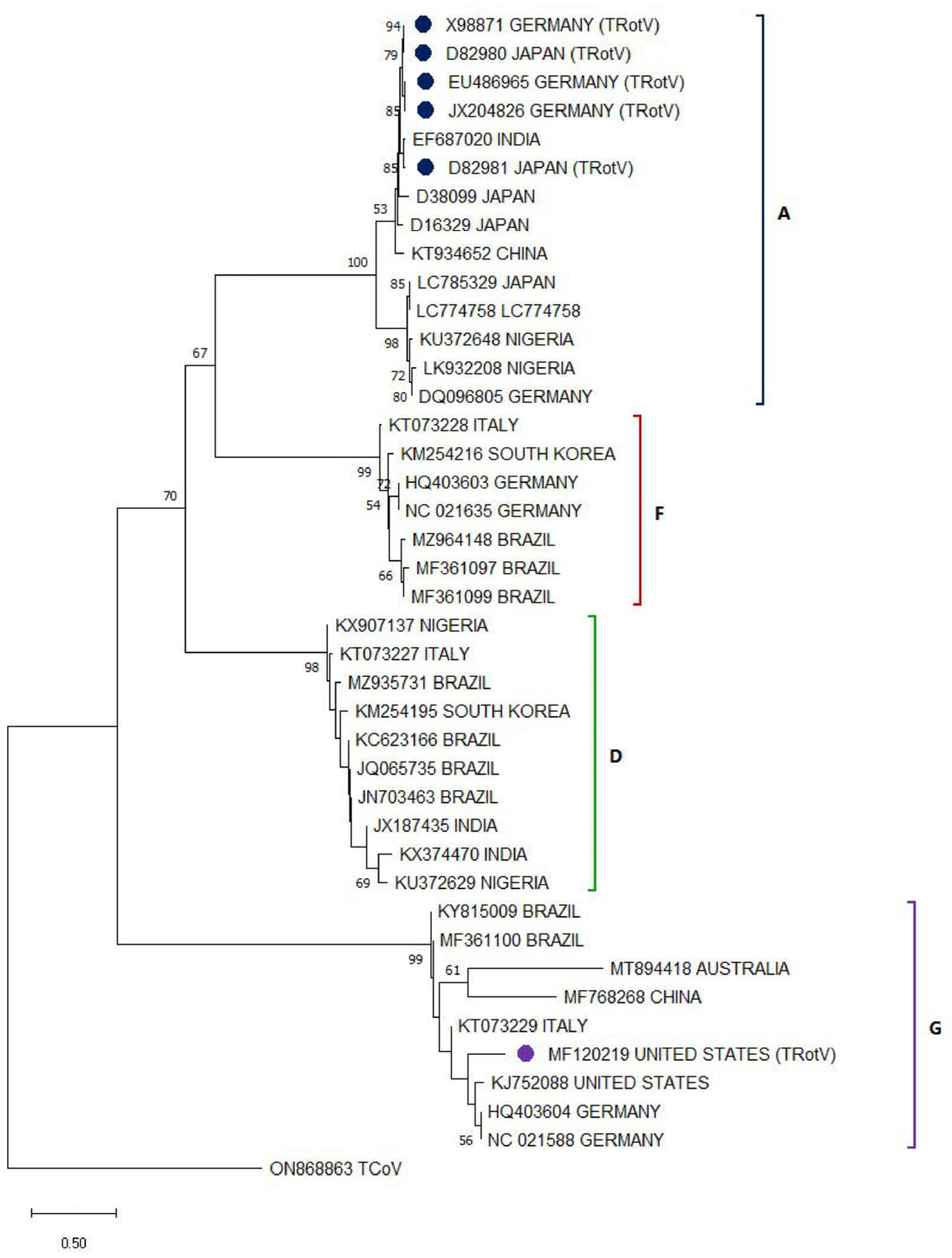
3.2. Avian Rotavirus
3.3. Avian Reovirus
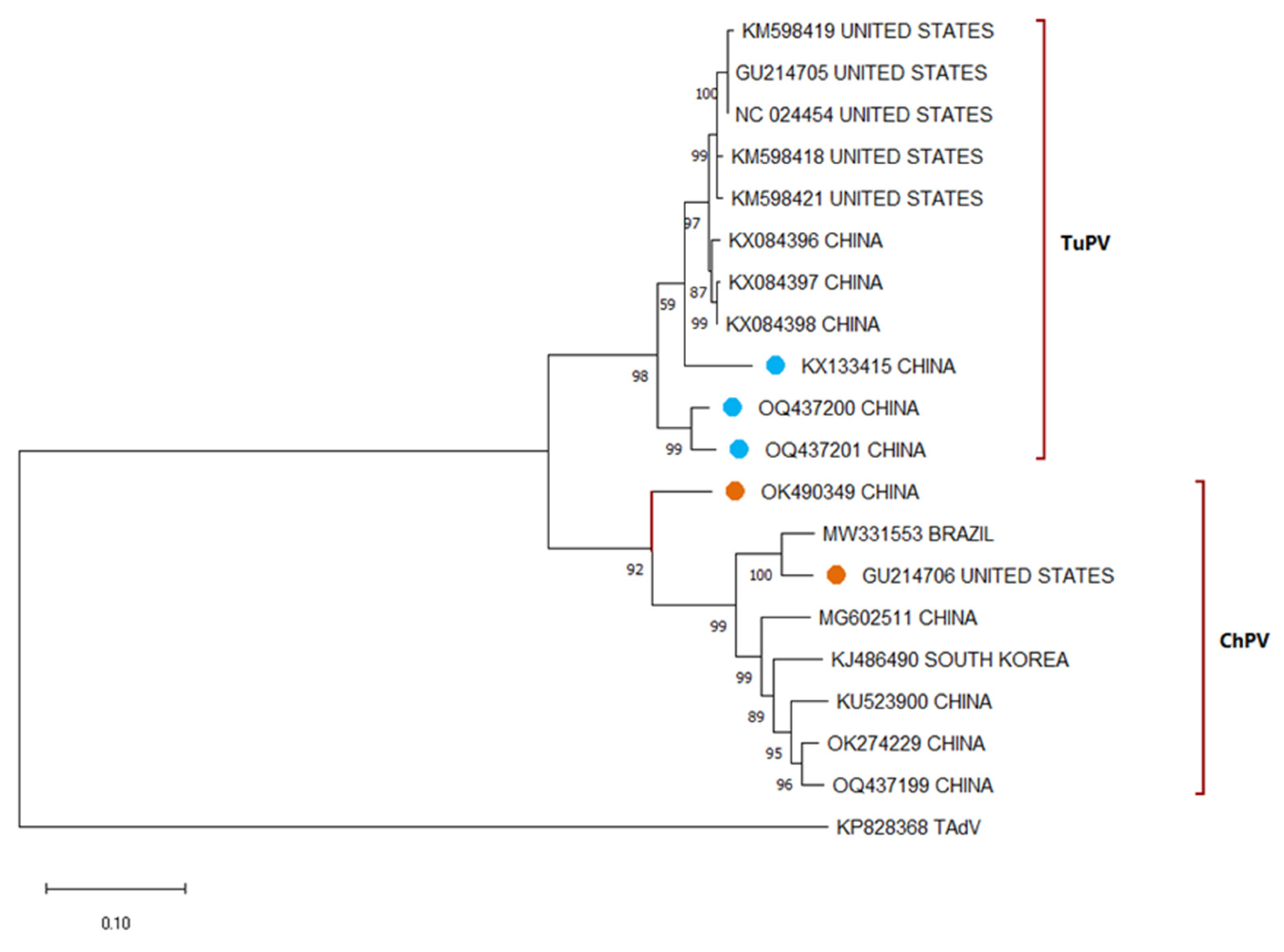
3.4. Avian Parvovirus
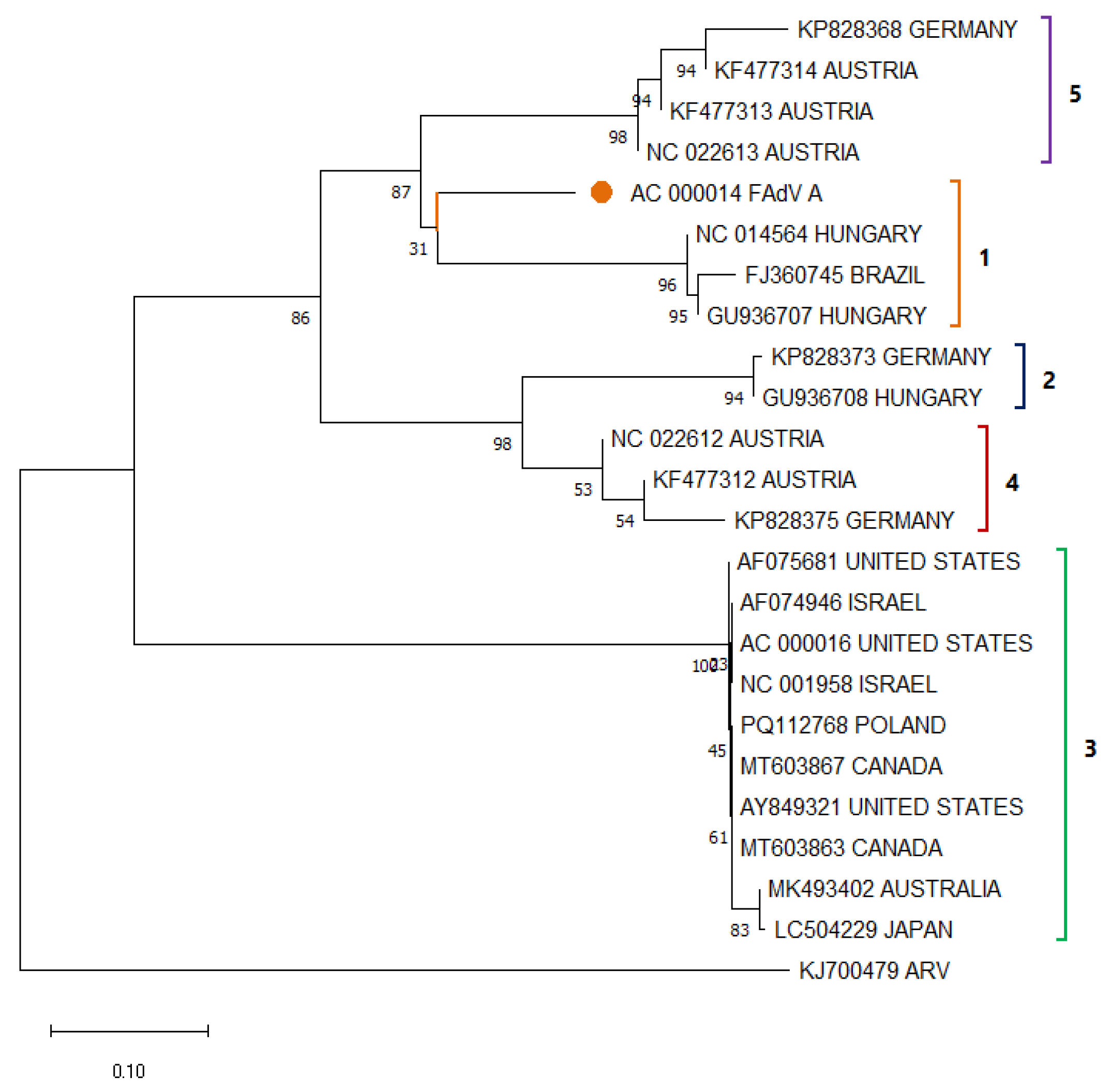
3.5. Avian Adenovirus
3.6. Turkey Coronavirus
| Locality | Enteric Viruses Prevalence | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TastV | TRotV | TRV | TuPV | TAdV | TCoV | ||||||||
| South America | Brazil | 30% (ANV) ● 10% (TAstV-1) ● 44.7% (TAstV-2) ● | [11,77] | 52.60% ● | [11] | 7.9% ● | [11] | N/A | N/A | 5.3% (TAdV-1) ● 0% (HEV) ● | [11] | 55.30% ● | [11] |
| North America | United States | 74.4%(UTA) □ 55.8% (TAstV-2) ● 9.3% (TAstV-1) ● | [78] | 51.2% ● | [79] | 53.5% □ | [80] | 2.3% ● | [12] | 63.15%(HEV) ● | [81] | 10.03% ● | [80] |
| Canada | UV | UV | UV | UV | UV | UV | UV | UV | 43.75%(HEV) ● | [63] | UV | UV | |
| Europe | Poland | 44.90% (UTA) ● 35.3% (TAstV-1) ● * 94.1% (TAstV-2) ● | [82,83] | 18.8% ● | [84] | 77.50% ◊ | [84] | 27.5% ● | [83] | 8.98% (UTAd) ● | [85] | 9.7% ● | [86] |
| Germany | UV | UV | * 88.15% ● | [87] | UV | UV | UV | UV | 30.43%(UTAd) ◊ | [88] | UV | UV | |
| Czech Republic | UV | UV | UV | UV | UV | UV | UV | UV | 65.07% (HEV) ▲ | [89] | UV | UV | |
| Asia | Turkiye | 43.4% (TAstV-2) ● | [90] | UV | UV | UV | UV | 70% ● | [17] | UV | UV | UV | UV |
| Iran | UV | UV | UV | UV | UV | UV | UV | UV | UV | UV | 44.4% ● | [91] | |
| Hungary | 4.08% (ANV) ● 83.67% (TAstV-1) ● 26.53% (TAstV-2) ● | [14] | 28.57% ● | [14] | 14.28% ● | [14] | 73.46% ● | [14] | 0%(HEV) ● | [14] | 14.28% ● | [14] | |
| China | UV | UV | UV | UV | UV | UV | 85.71% ● | [53] | UV | UV | UV | UV | |
| Oceania | Australia | UV | UV | UV | UV | UV | UV | UV | UV | 80% ▲ | [92] | UV | UV |
| Africa | Nigeria | 0% ● | [93] | 0.00% ● | [93] | 0.00% ● | [93] | UV | UV | 0.00% ● | [93] | 0% ● | [93] |
3.7. Last Reported Prevalence Within the Last 15 Years
3.8. Coinfections Involving Turkey Enteric Viruses
3.9. Other Viruses
3.10. Cross-Species Infections of Enteric Viruses
3.11. Clinical Features of Enteric Viral Infections in Turkeys
4. Diagnostic Methods
4.1. PCR/qPCR and NGS
4.2. ELISA and Serological Techniques
4.3. Electron Microscopy
5. Control and Prevention Strategies
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PEC | Poult Enteric Complex |
| PEMS | Poult Enteritis and Mortality Syndrome |
| TMS | Turkey Mortality Syndrome |
| RSS | Runting-Stunting Syndrome |
| LTS | Light Turkey Syndrome |
| PES | Poult Enteritis Syndrome |
| AAstV | Avian Astrovirus |
| TAstV | Turkey Astrovirus |
| ANV | Avian Nephritis Virus |
| AvRV | Avian Rotavirus |
| TRotV | Turkey Rotavirus |
| AvRVA | Avian Rotavirus Group A |
| ARV | Avian Reovirus |
| TRV | Turkey Reovirus |
| TARV | Turkey Arthritis Reovirus |
| TERV | Turkey Enteric Reovirus |
| APV | Avian Parvovirus |
| TuPV | Turkey Parvovirus |
| ChPV | Chicken Parvovirus |
| TAdV | Turkey Adenovirus |
| FAdV | Fowl Adenovirus |
| HEV | Hemorrhagic Enteritis Virus |
| TCoV | Turkey Coronavirus |
| AvCoV | Avian Coronavirus |
| IBV | Infectious Bronchitis Virus |
| AvPiV | Avian Picornavirus |
| ACV | Avian Calicivirus |
| PCR | Polymerase Chain Reaction |
| qPCR | Quantitative PCR |
| RT-PCR | Reverse Transcriptase PCR |
| NGS | Next-Generation Sequencing |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| IFA | Immunofluorescence Assay |
| TEM | Transmission Electron Microscopy |
| ORF | Open Reading Frame |
| RdRp | RNA-dependent RNA Polymerase |
| VP | Viral Protein |
| NSP | Non-Structural Protein |
References
- Day, J.M.; Zsak, L. Recent Progress in the Characterization of Avian Enteric Viruses. Avian Dis. 2013, 57, 573–580. [Google Scholar] [CrossRef]
- BARNES, H.J.; GUY, J.S.; VAILLANCOURT, J.P. Poult Enteritis Complex. Rev. Sci. Tech. L’oie 2000, 19, 565–588. [Google Scholar] [CrossRef] [PubMed]
- Barnes, H.J.; Guy, J.S.; Brow, T.P.; Edens, F.W. Poult Enteritis and Mortality Syndrome (“Spiking Mortality in Turkeys”) and Related Disorders: An Update. In College of Veterinary Medicine, North Carolina State University, Raleigh, NC 27695. 6 October 1996; Volume 100, pp. 564–575. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/19972213138 (accessed on 13 July 2025).
- Brown, T.P.; Garcia, A.; Kelley, L. Spiking Mortality of Turkey Poults: 1. Experimental Reproduction in Isolation Facilities. Avian Dis. 1997, 41, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Smart, I.J.; Barr, D.A.; Reece, R.L.; Forsyth, W.M.; Ewing, I. Experimental Reproduction of the Runting-stunting Syndrome of Broiler Chickens. Avian Pathol. 1988, 17, 617–627. [Google Scholar] [CrossRef]
- Mor, S.K.; Sharafeldin, T.A.; Abin, M.; Kromm, M.; Porter, R.E.; Goyal, S.M.; Patnayak, D.P. The Occurrence of Enteric Viruses in Light Turkey Syndrome. Avian Pathol. 2013, 42, 497–501. [Google Scholar] [CrossRef]
- Jindal, N.; Patnayak, D.P.; Ziegler, A.F.; Lago, A.; Goyal, S.M. Experimental Reproduction of Poult Enteritis Syndrome: Clinical Findings, Growth Response, and Microbiology. Poult. Sci. 2009, 88, 949–958. [Google Scholar] [CrossRef]
- Shehata, A.A.; Basiouni, S.; Sting, R.; Akimkin, V.; Hoferer, M.; Hafez, H.M. Poult Enteritis and Mortality Syndrome in Turkey Poults: Causes, Diagnosis and Preventive Measures. Animals 2021, 11, 2063. [Google Scholar] [CrossRef]
- Moura-Alvarez, J.; Nuñez, L.F.N.; Astolfi-Ferreira, C.S.; Knöbl, T.; Chacón, J.L.; Moreno, A.M.; Jones, R.C.; Ferreira, A.J.P. Detection of Enteric Pathogens in Turkey Flocks Affected with Severe Enteritis, in Brazil. Trop. Anim. Health Prod. 2014, 46, 1051–1058. [Google Scholar] [CrossRef]
- Jindal, N.; Mor, S.K.; Goyal, S.M. Enteric Viruses in Turkey Enteritis. Virusdisease 2014, 25, 173–185. [Google Scholar] [CrossRef]
- Moura-Alvarez, J.; Chacon, J.V.; Scanavini, L.S.; Nuñez, L.F.N.; Astolfi-Ferreira, C.S.; Jones, R.C.; Piantino Ferreira, A.J. Enteric Viruses in Brazilian Turkey Flocks: Single and Multiple Virus Infection Frequency According to Age and Clinical Signs of Intestinal Disease. Poult. Sci. 2013, 92, 945–955. [Google Scholar] [CrossRef]
- Sharafeldin, T.A.; Singh, A.; Abdel-Glil, M.Y.; Mor, S.K.; Porter, R.E.; Goyal, S.M. Prevalence of Parvovirus in Minnesota Turkeys. Poult. Sci. 2017, 96, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Verma, H.; Mor, S.K.; Erber, J.; Goyal, S.M. Prevalence and Complete Genome Characterization of Turkey Picobirnaviruses. Infect. Genet. Evol. 2015, 30, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Palade, E.A.; Demeter, Z.; Hornyák, Á.; Nemes, C.; Kisary, J.; Rusvai, M. High Prevalence of Turkey Parvovirus in Turkey Flocks from Hungary Experiencing Enteric Disease Syndromes. Avian Dis. 2011, 55, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Murgia, M.V.; Rauf, A.; Tang, Y.; Gingerich, E.; Lee, C.-W.; Saif, Y.M. Prevalence of Parvoviruses in Commercial Turkey Flocks. Avian Dis. 2012, 56, 744–749. [Google Scholar] [CrossRef]
- Domanska-Blicharz, K.; Jacukowicz, A.; Lisowska, A.; Minta, Z. Genetic Characterization of Parvoviruses Circulating in Turkey and Chicken Flocks in Poland. Arch. Virol. 2012, 157, 2425–2430. [Google Scholar] [CrossRef]
- Abayli, H.; Aslan, A.N.; Tonbak, S.; Ongor, H.; Akan, M. Molecular Epidemiology of Chicken and Turkey Parvovirus in Turkiye: High Risk, Genetic Recombination Signals, and First Complete Genome Analysis. Arch. Virol. 2025, 170, 42. [Google Scholar] [CrossRef]
- Mor, S.K.; Marthaler, D.; Verma, H.; Sharafeldin, T.A.; Jindal, N.; Porter, R.E.; Goyal, S.M. Phylogenetic Analysis, Genomic Diversity and Classification of M Class Gene Segments of Turkey Reoviruses. Vet. Microbiol. 2015, 176, 70–82. [Google Scholar] [CrossRef]
- Quaglia, G.; Di Francesco, A.; Catelli, E.; Mescolini, G.; Lupini, C. Turkey Adenovirus 3: ORF1 Gene Sequence Comparison between Vaccine-like and Field Strains. Vet. Res. Commun. 2023, 47, 2307–2313. [Google Scholar] [CrossRef]
- Domanska-Blicharz, K.; Sajewicz-Krukowska, J. Recombinant Turkey Coronavirus: Are Some S Gene Structures of Gammacoronaviruses Especially Prone to Exchange? Poult. Sci. 2021, 100, 101018. [Google Scholar] [CrossRef]
- Flageul, A.; Courtillon, C.; Allée, C.; Leroux, A.; Blanchard, Y.; Deleforterie, Y.; Grasland, B.; Brown, P.A. Extracting Turkey Coronaviruses from the Intestinal Lumen of Infected Turkey Embryos Yields Full Genome Data with Good Coverage by NGS. Avian Pathol. 2022, 51, 291–294. [Google Scholar] [CrossRef]
- Jindal, N.; Patnayak, D.P.; Chander, Y.; Ziegler, A.F.; Goyal, S.M. Comparison of Capsid Gene Sequences of Turkey Astrovirus-2 from Poult-Enteritis-Syndrome-Affected and Apparently Healthy Turkeys. Arch. Virol. 2011, 156, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, J.B.; Lee, T.W. Astroviruses: Human and Animal. In Novartis Foundation Symposia; Wiley: Hoboken, NJ, USA, 2007; pp. 92–107. [Google Scholar]
- McNulty, M.; Curran, W.; McFerran, J. Detection of Astroviruses in Turkey Faeces by Direct Electron Microscopy. Vet. Record. 1980, 106, 561. [Google Scholar] [CrossRef]
- Reynolds, D.L.; Saif, Y.M. Astrovirus: A Cause of an Enteric Disease in Turkey Poults. Avian Dis. 1986, 30, 728–735. [Google Scholar] [CrossRef]
- Imada, T.; Yamaguchi, S.; Mase, M.; Tsukamoto, K.; Kubo, M.; Morooka, A. Avian Nephritis Virus (ANV) as a New Member of the Family Astroviridae and Construction of Infectious ANV CDNA. J. Virol. 2000, 74, 8487–8493. [Google Scholar] [CrossRef]
- Jonassen, C.M.; Jonassen T, T.Ø.; Sveen, T.M.; Grinde, B. Complete Genomic Sequences of Astroviruses from Sheep and Turkey: Comparison with Related Viruses. Virus Res. 2003, 91, 195–201. [Google Scholar] [CrossRef]
- Koci, M.D.; Seal, B.S.; Schultz-Cherry, S. Molecular Characterization of an Avian Astrovirus. J. Virol. 2000, 74, 6173–6177. [Google Scholar] [CrossRef]
- Raji, A.A.; Ideris, A.; Bejo, M.H.; Omar, A.R. Molecular Characterization and Pathogenicity of Novel Malaysian Chicken Astrovirus Isolates. Avian Pathol. 2022, 51, 51–65. [Google Scholar] [CrossRef]
- Yin, D.; Tian, J.; Yang, J.; Tang, Y.; Diao, Y. Pathogenicity of Novel Goose-Origin Astrovirus Causing Gout in Goslings. BMC Vet. Res. 2021, 17, 40. [Google Scholar] [CrossRef]
- Thouvenelle, M.L.; Haynes, J.S.; Sell, J.L.; Reynolds, D.L. Astrovirus Infection in Hatchling Turkeys: Alterations in Intestinal Maltase Activity. Avian Dis. 1995, 39, 343–348. [Google Scholar] [CrossRef]
- Da Silva, S.E.L.; Bonetti, A.M.; Petrocelli, A.T.M.; Ferrari, H.F.; Luvizotto, M.C.R.; Cardoso, T.C. Detection of Turkey Astrovirus in Young Poults Affected with Poult Enteritis Complex in Brazil. J. Vet. Med. Sci. 2008, 70, 629–631. [Google Scholar] [CrossRef]
- Kaboudi, K. Virus-Induced Immunosuppression in Turkeys (Meleagris gallopavo): A Review. Open Vet. J. 2020, 9, 349. [Google Scholar] [CrossRef]
- Todd, D.; Trudgett, J.; Smyth, V.J.; Donnelly, B.; McBride, N.; Welsh, M.D. Capsid Protein Sequence Diversity of Avian Nephritis Virus. Avian Pathol. 2011, 40, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Mor, S.K.; Jindal, N.; Patnayak, D.; Sobhy, N.M.; Luong, N.T.; Goyal, S.M. Detection and Molecular Characterization of Astroviruses in Turkeys. Arch. Virol. 2016, 161, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Strain, E.; Kelley, L.A.; Schultz-Cherry, S.; Muse, S.V.; Koci, M.D. Genomic Analysis of Closely Related Astroviruses. J. Virol. 2008, 82, 5099–5103. [Google Scholar] [CrossRef] [PubMed]
- Patton, J.T.; Desselberger, U. Rotaviruses and Rotavirus Vaccines: Special Issue Editorial. Viruses 2024, 16, 1665. [Google Scholar] [CrossRef]
- Le, X.; Tao, Y.; Wang, B.; Hou, Y.; Ning, Y.; Hou, J.; Wang, R.; Li, Q.; Xia, X. Diversity and Potential Cross-Species Transmission of Rotavirus A in Wild Animals in Yunnan, China. Microorganisms 2025, 13, 145. [Google Scholar] [CrossRef]
- Beserra, L.A.R.; Barbosa, C.M.; Berg, M.; Brandão, P.E.; Soares, R.M.; Gregori, F. Genome Constellations of Rotavirus a Isolated from Avian Species in Brazil, 2008–2015. Braz. J. Microbiol. 2020, 51, 1363–1375. [Google Scholar] [CrossRef]
- Kindler, E.; Trojnar, E.; Heckel, G.; Otto, P.H.; Johne, R. Analysis of Rotavirus Species Diversity and Evolution Including the Newly Determined Full-Length Genome Sequences of Rotavirus F and G. Infect. Genet. Evol. 2013, 14, 58–67. [Google Scholar] [CrossRef]
- Falcone, E.; Busi, C.; Lavazza, A.; Monini, M.; Bertoletti, M.; Canelli, E.; Vignolo, E.; Ruggeri, F.M.; Boniotti, M.B. Molecular Characterization of Avian Rotaviruses Circulating in Italian Poultry Flocks. Avian Pathol. 2015, 44, 509–515. [Google Scholar] [CrossRef]
- McNulty, M.S.; Allan, G.M.; Stuart, J.C. Rotavirus Infection in Avian Species. Vet. Rec. 1978, 103, 319–320. [Google Scholar] [CrossRef] [PubMed]
- Dhama, K.; Saminathan, M.; Karthik, K.; Tiwari, R.; Shabbir, M.Z.; Kumar, N.; Malik, Y.S.; Singh, R.K. Avian Rotavirus Enteritis—An Updated Review. Vet. Q. 2015, 35, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Knutson, T.P.; Porter, R.E.; Ciarlet, M.; Mor, S.K.; Marthaler, D.G. Genome Characterization of Turkey Rotavirus G Strains from the United States Identifies Potential Recombination Events with Human Rotavirus B Strains. J. Gen. Virol. 2017, 98, 2931–2936. [Google Scholar] [CrossRef]
- Kumar, R.; Sharafeldin, T.A.; Sobhy, N.M.; Goyal, S.M.; Porter, R.E.; Mor, S.K. Comparative Pathogenesis of Turkey Reoviruses. Avian Pathol. 2022, 51, 435–444. [Google Scholar] [CrossRef]
- Mor, S.K.; Verma, H.; Sharafeldin, T.A.; Porter, R.E.; Ziegler, A.F.; Noll, S.L.; Goyal, S.M. Survival of Turkey Arthritis Reovirus in Poultry Litter and Drinking Water. Poult. Sci. 2015, 94, 639–642. [Google Scholar] [CrossRef]
- Goldenberg, D.; Pasmanik-Chor, M.; Pirak, M.; Kass, N.; Lublin, A.; Yeheskel, A.; Heller, D.; Pitcovski, J. Genetic and Antigenic Characterization of Sigma C Protein from Avian Reovirus. Avian Pathol. 2010, 39, 189–199. [Google Scholar] [CrossRef]
- Souza, S.O.; De Carli, S.; Lunge, V.R.; Ikuta, N.; Canal, C.W.; Pavarini, S.P.; Driemeier, D. Pathological and Molecular Findings of Avian Reoviruses from Clinical Cases of Tenosynovitis in Poultry Flocks from Brazil. Poult. Sci. 2018, 97, 3550–3555. [Google Scholar] [CrossRef]
- Kapgate, S.S.; Kumanan, K.; Vijayarani, K.; Barbuddhe, S.B. Avian Parvovirus: Classification, Phylogeny, Pathogenesis and Diagnosis. Avian Pathol. 2018, 47, 536–545. [Google Scholar] [CrossRef]
- Trampel, D.W.; Kinden, D.A.; Solorzano, R.F.; Stogsdill, P.L. Parvovirus-like Enteropathy in Missouri Turkeys. Avian Dis. 1983, 27, 49–54. [Google Scholar] [CrossRef]
- Zsak, L.; Strother, K.O.; Kisary, J. Partial Genome Sequence Analysis of Parvoviruses Associated with Enteric Disease in Poultry. Avian Pathol. 2008, 37, 435–441. [Google Scholar] [CrossRef]
- Zsak, L.; Cha, R.M.; Li, F.; Day, J.M. Host Specificity and Phylogenetic Relationships of Chicken and Turkey Parvoviruses. Avian Dis. 2015, 59, 157–161. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, B.; Xie, Z.; Zhang, M.; Fan, Q.; Deng, X.; Xie, Z.; Li, M.; Zeng, T.; Xie, L.; et al. Molecular Characterization of Emerging Chicken and Turkey Parvovirus Variants and Novel Strains in Guangxi, China. Sci. Rep. 2023, 13, 13083. [Google Scholar] [CrossRef]
- Biđin, M.; Lojkić, I.; Biđin, Z.; Tišljar, M.; Majnarić, D. Identification and Phylogenetic Diversity of Parvovirus Circulating in Commercial Chicken and Turkey Flocks in Croatia. Avian Dis. 2011, 55, 693–696. [Google Scholar] [CrossRef]
- Aslan, A.N.; Abayli, H.; Tonbak, S.; Ongor, H.; Unal, A.; Akan, M.; Yalcinkaya, E. First Detection and Molecular Characterization of Chaphamaparvovirus Galliform in Broiler and Turkey Flocks in Türkiye. BMC Vet. Res. 2025, 21, 153. [Google Scholar] [CrossRef]
- Fitzgerald, S.D.; Rautenschlein, S.; Mahsoub, H.M.; Pierson, F.W.; Reed, W.M.; Jack, S.W. Adenovirus Infections. In Diseases of Poultry, 14th ed.; Swayne, D.E., Boulianne, M., Logue, C.M., McDougald, L.R., Nair, V., Suarez, D.L., Wit, S., Grimes, T., Johnson, D., Kromm, M., et al., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 321–363. [Google Scholar] [CrossRef]
- Schachner, A.; Matos, M.; Grafl, B.; Hess, M. Fowl Adenovirus-Induced Diseases and Strategies for Their Control—A Review on the Current Global Situation. Avian Pathol. 2018, 47, 111–126. [Google Scholar] [CrossRef] [PubMed]
- MASE, M.; NAKAMURA, K. Phylogenetic Analysis of Fowl Adenoviruses Isolated from Chickens with Gizzard Erosion in Japan. J. Vet. Med. Sci. 2014, 76, 1535–1538. [Google Scholar] [CrossRef] [PubMed]
- Mo, J. Historical Investigation of Fowl Adenovirus Outbreaks in South Korea from 2007 to 2021: A Comprehensive Review. Viruses 2021, 13, 2256. [Google Scholar] [CrossRef] [PubMed]
- Quaye, A.; Pickett, B.E.; Griffitts, J.S.; Berges, B.K.; Poole, B.D. Characterizing the Splice Map of Turkey Hemorrhagic Enteritis Virus. Virol. J. 2024, 21, 175. [Google Scholar] [CrossRef]
- Ramsubeik, S.; Jerry, C.; Uzal, F.A.; Stoute, S. Necrotic Enteritis in a Commercial Turkey Flock Coinfected with Hemorrhagic Enteritis Virus. J. Vet. Diagn. Investig. 2023, 35, 317–321. [Google Scholar] [CrossRef]
- Durairaj, V.; Nezworski, J.; Drozd, M.; Clark, S.; Veen, R. Vander Concurrent Histomonas Meleagridis and Hemorrhagic Enteritis Virus Infection in a Turkey Flock with Recurrent History of Blackhead Disease. Avian Dis. 2024, 68, 56–64. [Google Scholar] [CrossRef]
- Palomino-Tapia, V.; Mitevski, D.; Inglis, T.; van der Meer, F.; Abdul-Careem, M.F. Molecular Characterization of Hemorrhagic Enteritis Virus (HEV) Obtained from Clinical Samples in Western Canada 2017–2018. Viruses 2020, 12, 941. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, S.; Awadin, W.; Karam, R.; Salem, S.; El-Shaieb, A. Pathological and Phylogenetic Characterization of a Rare Fowl Adenovirus (FAdV-8b) Associated with Inclusion Body Hepatitis in Naturally Infected Meleagris Gallopavo. Arch. Virol. 2024, 169, 146. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.A.; Courtillon, C.; Weerts, E.A.W.S.; Andraud, M.; Allée, C.; Vendembeuche, A.; Amelot, M.; Rose, N.; Verheije, M.H.; Eterradossi, N. Transmission Kinetics and Histopathology Induced by European Turkey Coronavirus during Experimental Infection of Specific Pathogen Free Turkeys. Transbound. Emerg. Dis. 2019, 66, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Hemida, M.G.; Barta, J.R.; Ojkic, D.; Yoo, D. Complete Genomic Sequence of Turkey Coronavirus. Virus Res. 2008, 135, 237–246. [Google Scholar] [CrossRef]
- Verbeek, A.; Tijssen, P. Sequence Analysis of the Turkey Enteric Coronavirus Nucleocapsid and Membrane Protein Genes: A Close Genomic Relationship with Bovine Coronavirus. J. Gen. Virol. 1991, 72, 1659–1666. [Google Scholar] [CrossRef]
- Ritchie, A.E.; Deshmukh, D.R.; Larsen, C.T.; Pomeroy, B.S. Electron Microscopy of Coronavirus-like Particles Characteristic of Turkey Bluecomb Disease. Avian Dis. 1973, 17, 546–558. [Google Scholar] [CrossRef]
- Guy, J.S. Isolation and Propagation of Coronaviruses in Embryonated Eggs. Methods Mol. Biol. 2008, 454, 109–117. [Google Scholar]
- Carver, D.K.; Vaillancourt, J.P.; Stringham, M.; Guy, J.S.; Barnes, H.J. Mortality Patterns Associated with Poult Enteritis Mortality Syndrome (PEMS) and Coronaviral Enteritis in Turkey Flocks Raised in PEMS-Affected Regions. Avian Dis. 2001, 45, 985–991. [Google Scholar] [CrossRef]
- Guy, J.S.; Smith, L.G.; Breslin, J.J.; Vaillancourt, J.P.; Barnes, H.J. High Mortality and Growth Depression Experimentally Produced in Young Turkeys by Dual Infection with Enteropathogenic Escherichia Coli and Turkey Coronavirus. Avian Dis. 2000, 44, 105–113. [Google Scholar] [CrossRef]
- Ambepitiya Wickramasinghe, I.N.; de Vries, R.P.; Weerts, E.A.W.S.; van Beurden, S.J.; Peng, W.; McBride, R.; Ducatez, M.; Guy, J.; Brown, P.; Eterradossi, N.; et al. Novel Receptor Specificity of Avian Gammacoronaviruses That Cause Enteritis. J. Virol. 2015, 89, 8783–8792. [Google Scholar] [CrossRef]
- Valastro, V.; Holmes, E.C.; Britton, P.; Fusaro, A.; Jackwood, M.W.; Cattoli, G.; Monne, I. S1 Gene-Based Phylogeny of Infectious Bronchitis Virus: An Attempt to Harmonize Virus Classification. Infect. Genet. Evol. 2016, 39, 349–364. [Google Scholar] [CrossRef]
- Kang, K.; Day, J.M.; Eldemery, F.; Yu, Q. Pathogenic Evaluation of a Turkey Coronavirus Isolate (TCoV NC1743) in Turkey Poults for Establishing a TCoV Disease Model. Vet. Microbiol. 2021, 259, 109155. [Google Scholar] [CrossRef]
- Chen, Y.-N.; Loa, C.C.; Ababneh, M.M.-K.; Wu, C.C.; Lin, T.L. Genotyping of Turkey Coronavirus Field Isolates from Various Geographic Locations in the Unites States Based on the Spike Gene. Arch. Virol. 2015, 160, 2719–2726. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, L.L.; Beserra, L.A.R.; Soares, R.M.; Gregori, F. Turkey Astrovirus Type 1 (TAstV-1) and Chicken Astrovirus (CAstV) Detection in Brazilian Chicken Flocks. Avian Dis. 2016, 60, 681–687. [Google Scholar] [CrossRef]
- Ramsubeik, S.; Jerry, C.; Crossley, B.; Armién, A.G.; Rejmanek, D.; Pitesky, M.; Shivaprasad, H.L.; Stoute, S. Analysis of Diagnostic Cases of Turkey Viral Enteritis in Commercial Turkey Poults in California. J. Appl. Poult. Res. 2022, 31, 100238. [Google Scholar] [CrossRef]
- Jindal, N.; Chander, Y.; Patnayak, D.P.; Mor, S.K.; Ziegler, A.F.; Goyal, S.M. A Multiplex RT-PCR for the Detection of Astrovirus, Rotavirus, and Reovirus in Turkeys. Avian Dis. 2012, 56, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Mor, S.K.; Kumar, R.; Sobhy, N.M.; Singh, A.; Kakrudi, N.; Marusak, R.A.; Goyal, S.M.; Porter, R.E. Enteric Viruses Associated with Mid-Growth Turkey Enteritis. Avian Dis. 2020, 64, 471–477. [Google Scholar] [CrossRef]
- Shah, J.D.; Scharber, S.K.; Cardona, C.J. Development and Application of Quantitative Real-Time PCR for the Rapid Detection of Hemorrhagic Enteritis Virus in Tissue Samples. Avian Dis. 2013, 57, 300–302. [Google Scholar] [CrossRef]
- Domańska-Blicharz, K.; Jacukowicz, A.; Bocian, L.; Minta, Z. Astroviruses in Polish Commercial Turkey Farms in 2009–2012. Avian Dis. 2014, 58, 158–164. [Google Scholar] [CrossRef]
- Domańska-Blicharz, K.; Bocian, Ł.; Lisowska, A.; Jacukowicz, A.; Pikuła, A.; Minta, Z. Cross-Sectional Survey of Selected Enteric Viruses in Polish Turkey Flocks between 2008 and 2011. BMC Vet. Res. 2017, 13, 108. [Google Scholar] [CrossRef]
- Czekaj, H.; Kozdruń, W.; Styś-Fijoł, N.; Niczyporuk, J.S.; Piekarska, K. Occurrence of Reovirus (ARV) Infections in Poultry Flocks in Poland in 2010–2017. J. Vet. Res. 2018, 62, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Niczyporuk, J.S.; Samorek-Salamonowicz, E.; Czekaj, H. Occurrence of Adenovirus Field Strains in Birds Infected with Marek’S Disease Virus. Bull. Vet. Inst. Pulawy 2012, 56, 435–440. [Google Scholar] [CrossRef]
- Domańska-Blicharz, K.; Lisowska, A.; Opolska, J.; Pikuła, A.; Sajewicz-Krukowska, J. Molecular Epidemiology of Turkey Coronaviruses in Poland. Viruses 2022, 14, 1023. [Google Scholar] [CrossRef] [PubMed]
- Akimkin, V.; Bindel, F.; Hoferer, M.; Sting, R.; Polley, B.; Hänel, A.; Hafez, H.M. One-Step RT-QPCR with an Internal Control System for the Detection of Turkey Rotaviruses in Faecal Samples. J. Virol. Methods 2011, 177, 112–117. [Google Scholar] [CrossRef]
- Kleine, A.; Hafez, H.M.; Lüschow, D. Investigations on Aviadenoviruses Isolated from Turkey Flocks in Germany. Avian Pathol. 2017, 46, 181–187. [Google Scholar] [CrossRef]
- Lobová, D.; Celer, V. Expression and Serological Reactivity of Hemorrhagic Enteritis Virus Hexon Protein. Folia Microbiol. 2016, 61, 227–232. [Google Scholar] [CrossRef]
- Jindal, N.; Patnayak, D.P.; Chander, Y.; Ziegler, A.F.; Goyal, S.M. Detection and Molecular Characterization of Enteric Viruses from Poult Enteritis Syndrome in Turkeys. Poult. Sci. 2010, 89, 217–226. [Google Scholar] [CrossRef]
- Kashi, F.; Madani, S.A.; Ghalyanchilangeroudi, A.; Najafi, H. Diagnosis of Poult Enteritis Complex (PEC) and Molecular Detection of Avian Coronaviruses in Some Commercial Turkey Flocks in Iran. Iran J. Vet. Res. 2021, 22, 342–346. [Google Scholar] [CrossRef]
- Gerber, P.F.; Spatz, S.; Gray, P.; Alfirevich, S.; Walkden-Brown, S.W. Circulation and Molecular Characterization of Hemorrhagic Enteritis Virus in Commercial Turkey and Meat Chicken Flocks in Australia. Avian Dis. 2022, 66, 53–59. [Google Scholar] [CrossRef]
- Adebiyi, A.I.; Mcilwaine, K.; Oluwayelu, D.O.; Smyth, V.J. Detection and Characterization of Chicken Astrovirus Associated with Hatchery Disease in Commercial Day-Old Turkeys in Southwestern Nigeria. Arch. Virol. 2021, 166, 1607–1614. [Google Scholar] [CrossRef]
- Ongor, H.; Bulut, H.; Cetinkaya, B.; Akan, M.; Tonbak, S.; Mor, S.K.; Goyal, S.M. Detection of Astrovirus, Coronavirus and Haemorrhagic Enteritis Virus in Turkeys with Poult Enteritis Mortality Syndrome in Turkey. J. Poult. Sci. 2015, 52, 232–237. [Google Scholar] [CrossRef]
- Domanska-Blicharz, K.; Seroka, A.; Minta, Z. One-Year Molecular Survey of Astrovirus Infection in Turkeys in Poland. Arch. Virol. 2011, 156, 1065–1072. [Google Scholar] [CrossRef]
- Giovanardi, D.; Lupini, C.; Pesente, P.; Rossi, G.; Ortali, G.; Catelli, E. Longitudinal Field Studies of Avian Metapneumovirus and Turkey Hemorrhagic Enteritis Virus in Turkeys Suffering from Colibacillosis Associated Mortality. Vet. Res. Commun. 2014, 38, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Saif, Y.M.; Guy, J.S.; Day, J.M.; Cattoli, G.; Hayhow, C.S. Viral Enteric Infections. In Diseases of Poultry; Wiley: Hoboken, NJ, USA, 2020; pp. 401–445. [Google Scholar]
- Grafl, B.; Gaußmann, B.; Bilic, I.; Folkertsma, R.; Hess, M. Influence of Biosecurity on the Occurrence of Various Enteric Viruses in Broiler Flocks. Avian Pathol. 2025, 54, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Feng, B.; Xie, Z.; Deng, X.; Zhang, M.; Xie, Z.; Xie, L.; Fan, Q.; Luo, S.; Zeng, T.; et al. Epidemiological Surveillance of Parvoviruses in Commercial Chicken and Turkey Farms in Guangxi, Southern China, During 2014–2019. Front. Vet. Sci. 2020, 7, 561371. [Google Scholar] [CrossRef] [PubMed]
- Day, J.M.; Zsak, L. Investigating Turkey Enteric Picornavirus and Its Association with Enteric Disease in Poults. Avian Dis. 2015, 59, 138–142. [Google Scholar] [CrossRef]
- Day, J.M.; Zsak, L. Molecular Characterization of Enteric Picornaviruses in Archived Turkey and Chicken Samples from the United States. Avian Dis. 2016, 60, 500–505. [Google Scholar] [CrossRef]
- Pantin-Jackwood, M.J.; Spackman, E.; Day, J.M. Pathology and Virus Tissue Distribution of Turkey Origin Reoviruses in Experimentally Infected Turkey Poults. Vet. Pathol. 2007, 44, 185–195. [Google Scholar] [CrossRef]
- Koci, M.D.; Schultz-Cherry, S. Avian Astroviruses. Avian Pathol. 2002, 31, 213–227. [Google Scholar] [CrossRef]
- Pantin-Jackwood, M.J.; Day, J.M.; Jackwood, M.W.; Spackman, E. Enteric Viruses Detected by Molecular Methods in Commercial Chicken and Turkey Flocks in the United States between 2005 and 2006. Avian Dis. 2008, 52, 235–244. [Google Scholar] [CrossRef]
- Pantin-Jackwood, M.J.; Spackman, E.; Michael Day, J. Pathogenesis of Type 2 Turkey Astroviruses with Variant Capsid Genes in 2-Day-Old Specific Pathogen Free Poults. Avian Pathol. 2008, 37, 193–201. [Google Scholar] [CrossRef]
- Spackman, E.; Day, J.M.; Pantin-Jackwood, M.J. Astrovirus, Reovirus, and Rotavirus Concomitant Infection Causes Decreased Weight Gain in Broad-Breasted White Poults. Avian Dis. 2010, 54, 16–21. [Google Scholar] [CrossRef]
- Guy, J.S. Virally Induced Gastrointestinal Diseases of Chickens and Turkeys. In Avian Gut Function in Health and Disease; CABI: Wallingford, UK, 2006; pp. 227–243. [Google Scholar]
- Ngunjiri, J.M.; Ghorbani, A.; Jang, H.; Waliullah, S.; Elaish, M.; Abundo, M.C.; KC, M.; Taylor, K.J.M.; Porter, R.E.; Lee, C.-W. Specific-Pathogen-Free Turkey Model for Reoviral Arthritis. Vet. Microbiol. 2019, 235, 170–179. [Google Scholar] [CrossRef]
- Gomaa, M.H.; Yoo, D.; Ojkic, D.; Barta, J.R. Infection with a Pathogenic Turkey Coronavirus Isolate Negatively Affects Growth Performance and Intestinal Morphology of Young Turkey Poults in Canada. Avian Pathol. 2009, 38, 279–286. [Google Scholar] [CrossRef]
- Guy, J.S.; Schaeffer, J.L.; Barnes, H.J. Inclusion-Body Hepatitis in Day-Old Turkeys. Avian Dis. 1988, 32, 587–590. [Google Scholar] [CrossRef]
- Wilkes, R.P.; Chan, A.; Wooming, B. Targeted Detection and Molecular Epidemiology of Turkey Coronavirus Spike Gene Variants in Turkeys and Chickens. J. Vet. Diagn. Investig. 2022, 34, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Day, J.M.; Spackman, E.; Pantin-Jackwood, M. A Multiplex RT-PCR Test for the Differential Identification of Turkey Astrovirus Type 1, Turkey Astrovirus Type 2, Chicken Astrovirus, Avian Nephritis Virus, and Avian Rotavirus. Avian Dis. 2007, 51, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, K.; Pei, Y.; Xue, J.; Ruan, S.; Zhang, G. Development and Application of an MRT-QPCR Assay for Detecting Coinfection of Six Vertically Transmitted or Immunosuppressive Avian Viruses. Front. Microbiol. 2020, 11, 1581. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.K.W.; Poon, L.L.M.; Guan, Y.; Peiris, J.S.M. Novel Astroviruses in Insectivorous Bats. J. Virol. 2008, 82, 9107–9114. [Google Scholar] [CrossRef]
- Escutenaire, S.; Mohamed, N.; Isaksson, M.; Thorén, P.; Klingeborn, B.; Belák, S.; Berg, M.; Blomberg, J. SYBR Green Real-Time Reverse Transcription-Polymerase Chain Reaction Assay for the Generic Detection of Coronaviruses. Arch. Virol. 2007, 152, 41–58. [Google Scholar] [CrossRef]
- Tang, Y.; Yu, H.; Jiang, X.; Bao, E.; Wang, D.; Lu, H. Genetic Characterization of a Novel Pheasant-Origin Orthoreovirus Using Next-Generation Sequencing. PLoS ONE 2022, 17, e0277411. [Google Scholar] [CrossRef]
- Tang, Y.; Lin, L.; Sebastian, A.; Lu, H. Detection and Characterization of Two Co-Infection Variant Strains of Avian Orthoreovirus (ARV) in Young Layer Chickens Using next-Generation Sequencing (NGS). Sci. Rep. 2016, 6, 24519. [Google Scholar] [CrossRef]
- Gomaa, M.H.; Yoo, D.; Ojkic, D.; Barta, J.R. Seroprevalence of Turkey Coronavirus in North American Turkeys Determined by a Newly Developed Enzyme-Linked Immunosorbent Assay Based on Recombinant Antigen. Clin. Vaccine Immunol. 2008, 15, 1839–1844. [Google Scholar] [CrossRef]
- Liu, H.J.; Kuo, L.C.; Hu, Y.C.; Liao, M.H.; Lien, Y.Y. Development of an ELISA for Detection of Antibodies to Avian Reovirus in Chickens. J. Virol. Methods 2002, 102, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Myers, T.J.; Schat, K.A.; Mockett, A.P.A. Development of Immunoglobulin Class-Specific Enzyme-Linked Immunosorbent Assays for Measuring Antibodies against Avian Rotavirus. Avian Dis. 1989, 33, 53. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, M.; Loa, C.C.; Wu, C.C.; Lin, T.L. Recombinant Nucleocapsid Protein-Based Enzyme-Linked Immunosorbent Assay for Detection of Antibody to Turkey Coronavirus. J. Virol. Methods 2015, 217, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Nazerian, K.; Lee, L.F.; Payne, W.S. A Double-Antibody Enzyme-Linked Immunosorbent Assay for the Detection of Turkey Hemorrhagic Enteritis Virus Antibody and Antigen. Avian Dis. 1990, 34, 425–432. [Google Scholar] [CrossRef]
- Meliopoulos, V.A.; Kayali, G.; Burnham, A.; Oshansky, C.M.; Thomas, P.G.; Gray, G.C.; Beck, M.A.; Schultz-Cherry, S. Detection of Antibodies against Turkey Astrovirus in Humans. PLoS ONE 2014, 9, e96934. [Google Scholar] [CrossRef]
- Decaesstecker, M.; Charlier, G.; Meulemans, G. Epidemiological Study of Enteric Viruses in Broiler Chickens: Comparison of Tissue Culture and Direct Electron Microscopy. Avian Pathol. 1988, 17, 477–486. [Google Scholar] [CrossRef]
- Singh, A.K.; Berbís, M.Á.; Ballmann, M.Z.; Kilcoyne, M.; Menéndez, M.; Nguyen, T.H.; Joshi, L.; Cañada, F.J.; Jiménez-Barbero, J.; Benkő, M.; et al. Structure and Sialyllactose Binding of the Carboxy-Terminal Head Domain of the Fibre from a Siadenovirus, Turkey Adenovirus 3. PLoS ONE 2015, 10, e0139339. [Google Scholar] [CrossRef]
- Alkie, T.N.; Guenther, R.; Rautenschlein, S. Molecular Characterization of Hemorrhagic Enteritis Viruses (HEV) Detected in HEV-Vaccinated Commercial Turkey Flocks in Germany. Avian Dis. 2017, 61, 96–101. [Google Scholar] [CrossRef]
- Awe, O.O.; Kang, K.; Ibrahim, M.; Ali, A.; Elaish, M.; Saif, Y.M.; Lee, C.-W. Age-Related Susceptibility of Turkeys to Enteric Viruses. Avian Dis. 2015, 59, 207–212. [Google Scholar] [CrossRef]
- Jankowski, J.; Tykałowski, B.; Ognik, K.; Koncicki, A.; Kubińska, M.; Zduńczyk, Z. The Effect of Different Dietary Levels of DL-Methionine and DL-Hydroxy Analogue on the Antioxidant Status of Young Turkeys Infected with the Haemorrhagic Enteritis Virus. BMC Vet. Res. 2018, 14, 404. [Google Scholar] [CrossRef]
- Fasina, Y.O.; Suarez, D.L.; Ritter, G.D.; Gerken, E.C.; Farnell, Y.Z.; Wolfenden, R.; Hargis, B. Unraveling Frontiers in Poultry Health (Part 1)—Mitigating Economically Important Viral and Bacterial Diseases in Commercial Chicken and Turkey Production. Poult. Sci. 2024, 103, 103500. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loor-Giler, A.; Galdo-Novo, S.; Nuñez, L. Enteric Viruses in Turkeys: A Systematic Review and Comparative Data Analysis. Viruses 2025, 17, 1037. https://doi.org/10.3390/v17081037
Loor-Giler A, Galdo-Novo S, Nuñez L. Enteric Viruses in Turkeys: A Systematic Review and Comparative Data Analysis. Viruses. 2025; 17(8):1037. https://doi.org/10.3390/v17081037
Chicago/Turabian StyleLoor-Giler, Anthony, Sabrina Galdo-Novo, and Luis Nuñez. 2025. "Enteric Viruses in Turkeys: A Systematic Review and Comparative Data Analysis" Viruses 17, no. 8: 1037. https://doi.org/10.3390/v17081037
APA StyleLoor-Giler, A., Galdo-Novo, S., & Nuñez, L. (2025). Enteric Viruses in Turkeys: A Systematic Review and Comparative Data Analysis. Viruses, 17(8), 1037. https://doi.org/10.3390/v17081037





