Spatiotemporal Distribution and Risk Factors of African Swine Fever Outbreak Cases in Uganda for the Period 2010–2023
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Collection
2.2. Geographical Spatial Analysis
2.3. Time Series and Cluster Analysis
3. Results
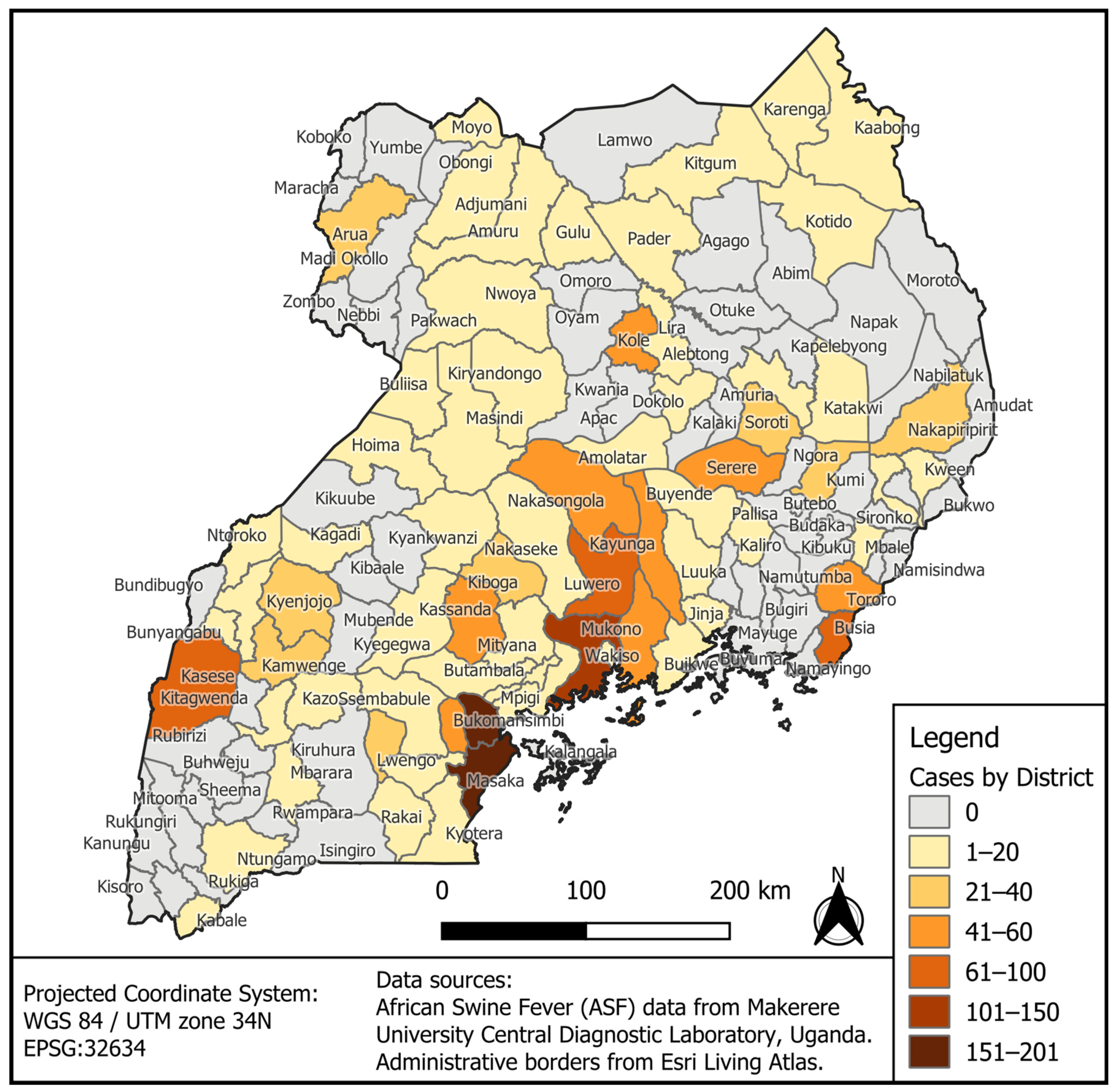
3.1. Geographical Distribution of ASF Outbreak Cases by District for the Period 2010–2023
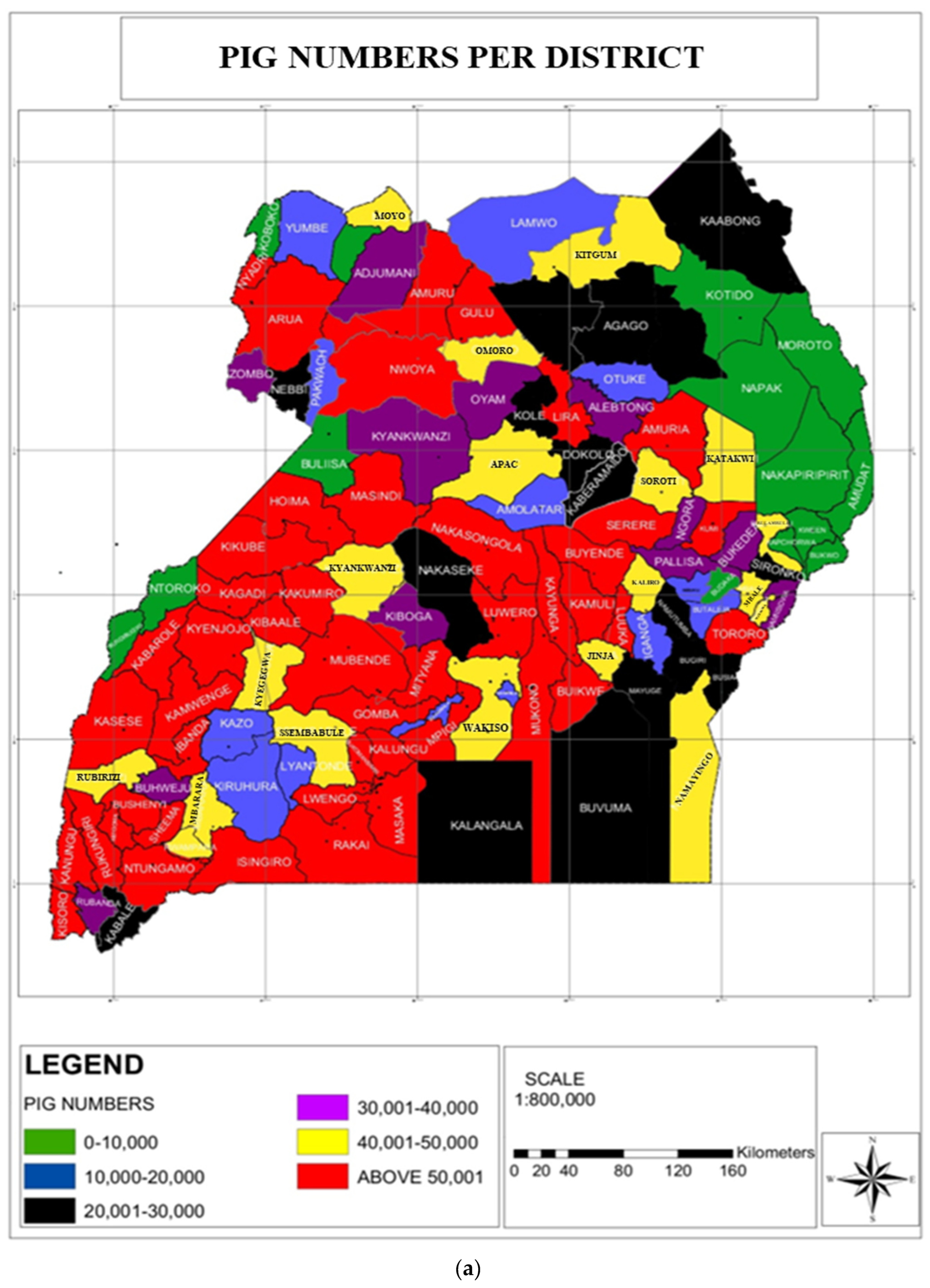
3.2. Pig Number Estimates vis-à-vis ASF Outbreaks in Uganda
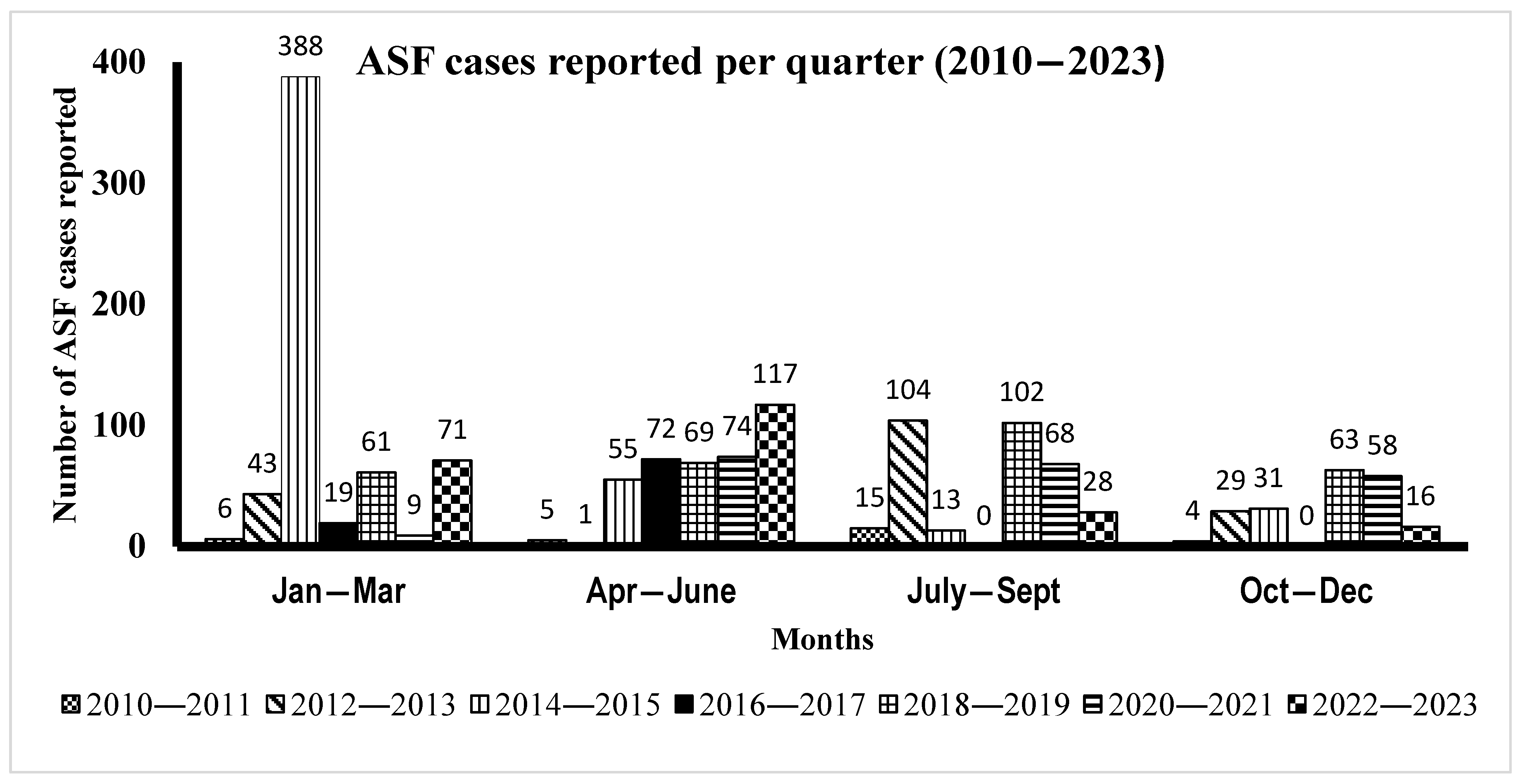
3.3. Time Series Distribution of ASF Cases by Month from 2010 to 2023
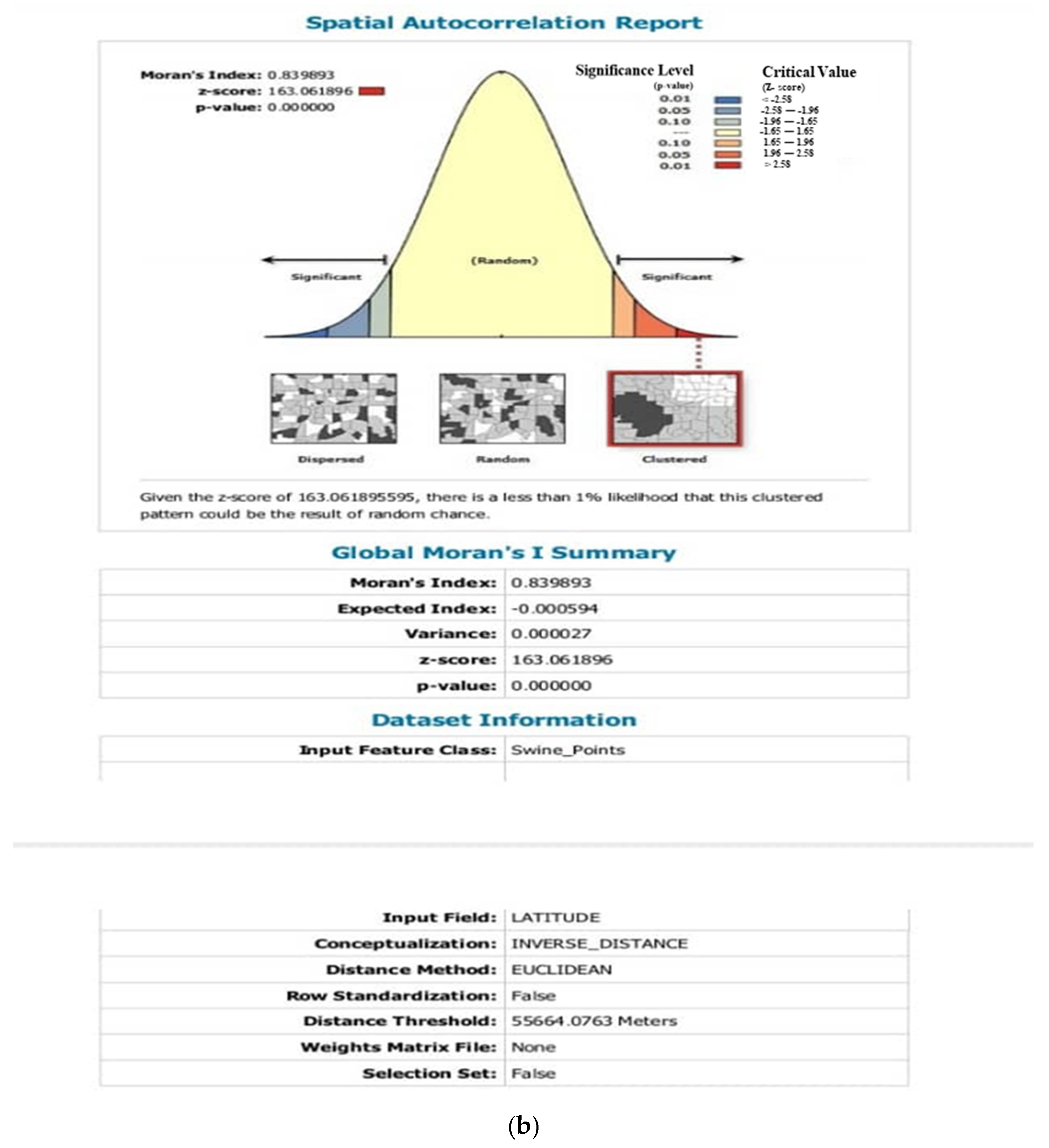
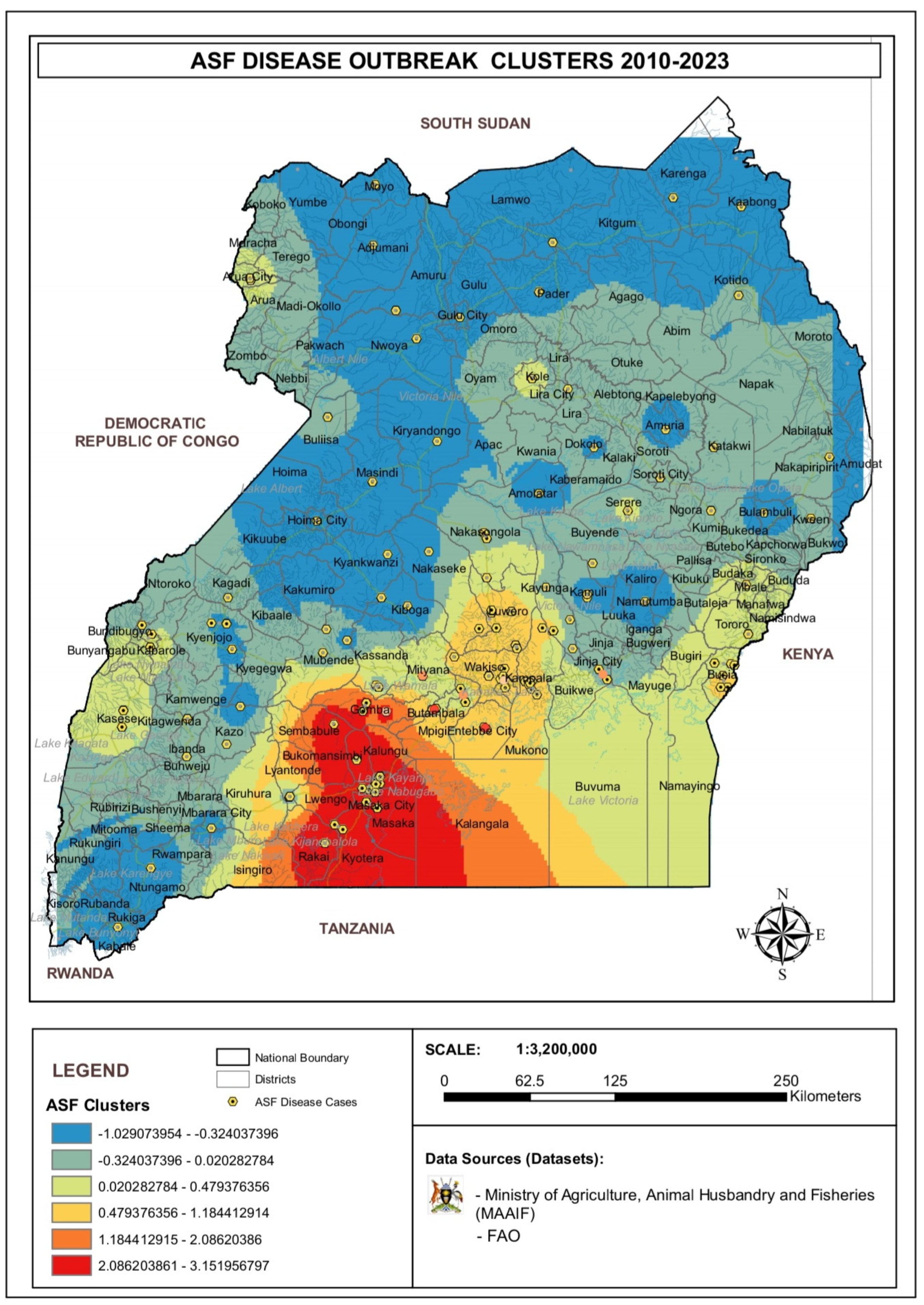
3.4. Analysis of ASF Disease Cluster
4. Discussion
5. Conclusions
6. Limitation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASF | African Swine Fever |
| ASFV | African Swine Fever Virus |
| DRC | Democratic Republic of Congo |
| MAAIF | Ministry of Agriculture, Animal Industry and Fisheries |
| FAO | Food Agriculture Organization |
| CDL | Central Diagnostic Lab |
Appendix A
Appendix B

Appendix C
| Year | January–March | April–June | July–September | October–December | Total |
|---|---|---|---|---|---|
| 2010–2011 | 6 | 5 | 15 | 4 | 30 |
| 2012–2013 | 43 | 1 | 104 | 29 | 177 |
| 2014–2015 | 388 | 55 | 13 | 31 | 487 |
| 2016–2017 | 19 | 72 | 0 | 0 | 91 |
| 2018–2019 | 61 | 69 | 102 | 63 | 295 |
| 2020–2021 | 9 | 74 | 68 | 58 | 209 |
| 2022–2023 | 71 | 117 | 28 | 16 | 232 |
References
- Alonso, C.; Borca, M.; Dixon, L.; Revilla, Y.; Rodriguez, F.; Escribano, J.M. ICTV Report Consortium. ICTV Virus Taxonomy Profile: Asfarviridae. J. Gen. Virol. 2018, 99, 613–614. [Google Scholar] [CrossRef] [PubMed]
- Eustace Montgomery, R. On A Form of Swine Fever Occurring in British East Africa (Kenya Colony). J. Comp. Pathol. Ther. 1921, 34, 159–191. [Google Scholar] [CrossRef]
- Chapman, D.A.G.; Darby, A.C.; Da Silva, M.; Upton, C.; Radford, A.D.; Dixon, L.K. Genomic Analysis of Highly Virulent Georgia 2007/1 Isolate of African Swine Fever Virus. Emerg. Infect. Dis. 2011, 17, 599–605. [Google Scholar] [CrossRef]
- Li, Z.; Chen, W.; Qiu, Z.; Li, Y.; Fan, J.; Wu, K.; Li, X.; Zhao, M.; Ding, H.; Fan, S.; et al. African Swine Fever Virus: A Review. Life 2022, 12, 1255. [Google Scholar] [CrossRef]
- Penrith, M.-L.; Vosloo, W.; Jori, F.; Bastos, A.D.S. African swine fever virus eradication in Africa. Virus Res. 2013, 173, 228–246. [Google Scholar] [CrossRef]
- Muwonge, A.; Munang’andu, H.M.; Kankya, C.; Biffa, D.; Oura, C.; Skjerve, E.; Oloya, J. African swine fever among slaughter pigs in Mubende district, Uganda. Trop. Anim. Health Prod. 2012, 44, 1593–1598. [Google Scholar] [CrossRef]
- Jori, F.; Bastos, A.D.S. Role of Wild Suids in the Epidemiology of African Swine Fever. EcoHealth 2009, 6, 296–310. [Google Scholar] [CrossRef]
- Korennoy, F.I.; Gulenkin, V.M.; Malone, J.B.; Mores, C.N.; Dudnikov, S.A.; Stevenson, M.A. Spatio-temporal modeling of the African swine fever epidemic in the Russian Federation, 2007–2012. Spat. Spatio-Temporal Epidemiol. 2014, 11, 135–141. [Google Scholar] [CrossRef]
- Chenais, E.; Boqvist, S.; Emanuelson, U.; Von Brömssen, C.; Ouma, E.; Aliro, T.; Masembe, C.; Ståhl, K.; Sternberg-Lewerin, S. Quantitative assessment of social and economic impact of African swine fever outbreaks in northern Uganda. Prev. Vet. Med. 2017, 144, 134–148. [Google Scholar] [CrossRef]
- Aliro, T.; Odongo, W.; Ståhl, K.; Dione, M.M.; Okello, D.M.; Masembe, C.; Chenais, E. Actions and perceived impact of African swine fever control measures along the smallholder pig value chain in Uganda. Trop. Anim. Health Prod. 2023, 55, 410. [Google Scholar] [CrossRef] [PubMed]
- Gulenkin, V.M.; Korennoy, F.I.; Karaulov, A.K.; Dudnikov, S.A. Cartographical analysis of African swine fever outbreaks in the territory of the Russian Federation and computer modeling of the basic reproduction ratio. Prev. Vet. Med. 2011, 102, 167–174. [Google Scholar] [CrossRef]
- Oganesyan, A.S.; Petrova, O.N.; Korennoy, F.I.; Bardina, N.S.; Gogin, A.E.; Dudnikov, S.A. African swine fever in the Russian Federation: Spatio-temporal analysis and epidemiological overview. Virus Res. 2013, 173, 204–211. [Google Scholar] [CrossRef]
- Perez, A.; AlKhamis, M.; Carlsson, U.; Brito, B.; Carrasco-Medanic, R.; Whedbee, Z.; Willeberg, P. Global animal disease surveillance. Spat. Spatio-Temporal Epidemiol. 2011, 2, 135–145. [Google Scholar] [CrossRef]
- Kimi, R.; Beegum, M.; Nandi, S.; Dubal, Z.B.; Sinha, D.K.; Singh, B.R.; Vinodhkumar, O.R. Spatio-temporal dynamics and distributional trend analysis of African swine fever outbreaks (2020–2021) in North-East India. Trop. Anim. Health Prod. 2024, 56, 39. [Google Scholar] [CrossRef]
- Ma, J.; Chen, H.; Gao, X.; Xiao, J.; Wang, H. African swine fever emerging in China: Distribution characteristics and high-risk areas. Prev. Vet. Med. 2020, 175, 104861. [Google Scholar] [CrossRef]
- Franch-Pardo, I.; Napoletano, B.M.; Rosete-Verges, F.; Billa, L. Spatial analysis and GIS in the study of COVID-19. A review. Sci. Total Environ. 2020, 739, 140033. [Google Scholar] [CrossRef]
- Shao, Q.; Li, R.; Han, Y.; Han, D.; Qiu, J. Temporal and Spatial Evolution of the African Swine Fever Epidemic in Vietnam. Int. J. Environ. Res. Public Health 2022, 19, 8001. [Google Scholar] [CrossRef] [PubMed]
- Ekakoro, J.E.; Lubega, A.; Kayaga, E.B.; Ndoboli, D.; Bluhm, A.P.; Wampande, E.M.; Blackburn, J.K.; Havas, K.A.; Norris, M.H. An Investigation of Burkholderia pseudomallei Seroprevalence in Market Pigs Slaughtered at Selected Pig Abattoirs in Uganda. Pathogens 2022, 11, 1363. [Google Scholar] [CrossRef] [PubMed]
- Adedeji, A.J.; Atai, R.B.; Gyang, H.E.; Gambo, P.; Habib, M.A.; Weka, R.; Muwanika, V.B.; Masembe, C.; Luka, P.D. Live pig markets are hotspots for spread of African swine fever virus in Nigeria. Transbound. Emerg. Dis. 2022, 69, E1526–E1540. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Li, R.; Xiao, Y.; Xia, J.; Zhu, H.; Niu, Y.; Huang, D.; Shao, Q.; Cui, Y.; Wang, Y. Spatiotemporal Heterogeneity in Human Schistosoma japonicum Infection at Village Level in Hubei Province, China. Int. J. Environ. Res. Public Health 2019, 16, 2198. [Google Scholar] [CrossRef]
- Okwasiimire, R.; Kayaga, E.B.; Ekakoro, J.E.; Ndoboli, D.; Schumann, K.; Faburay, B.; Nassali, A.; Hauser, C.; Ochoa, K.; Wampande, E.M.; et al. Spatiotemporal description of African swine fever virus nucleic acid and antibodies detected in pigs sampled at abattoirs in the greater Kampala metropolitan area, Uganda from May 2021 through June 2022. Porc. Health Manag. 2023, 9, 51. [Google Scholar] [CrossRef]
- Kalenzi Atuhaire, D.; Ochwo, S.; Afayoa, M.; Norbert Mwiine, F.; Kokas, I.; Arinaitwe, E.; Ademun-Okurut, R.A.; Boniface Okuni, J.; Nanteza, A.; Ayebazibwe, C.; et al. Epidemiological Overview of African Swine Fever in Uganda (2001–2012). J. Vet. Med. 2013, 2013, 949638. [Google Scholar] [CrossRef]
- Ogweng, P.; Bowden, C.F.; Smyser, T.J.; Muwanika, V.B.; Piaggio, A.J.; Masembe, C. Ancestry and genome-wide association study of domestic pigs that survive African swine fever in Uganda. Trop. Anim. Health Prod. 2024, 56, 366. [Google Scholar] [CrossRef]
- Ungur, A.; Cazan, C.D.; Panait, L.-C.; Coroian, M.; Cătoi, C. What Is the Real Influence of Climatic and Environmental Factors in the Outbreaks of African Swine Fever? Animals 2022, 12, 781. [Google Scholar] [CrossRef]
- Costard, S.; Wieland, B.; De Glanville, W.; Jori, F.; Rowlands, R.; Vosloo, W.; Roger, F.; Pfeiffer, D.U.; Dixon, L.K. African swine fever: How can global spread be prevented? Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2683–2696. [Google Scholar] [CrossRef]
- Penrith, M.-L.; Vosloo, W. Review of African swine fever: Transmission, spread and control. J. S. Afr. Vet. Assoc. 2009, 80, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, C.; Ademun, A.R.; Nieto, R.; Nantima, N.; Arias, M.; Martín, E.; Pelayo, V.; Bishop, R.P. Genotyping of African swine fever virus (ASFV) isolates associated with disease outbreaks in Uganda in 2007. Afr. J. Biotechnol. 2011, 10, 3488–3497. [Google Scholar] [CrossRef]
- Ekakoro, J.E.; Nawatti, M.; Singler, D.F.; Ochoa, K.; Kizza, R.; Ndoboli, D.; Ndumu, D.B.; Wampande, E.M.; Havas, K.A. A survey of biosecurity practices of pig farmers in selected districts affected by African swine fever in Uganda. Front. Vet. Sci. 2023, 10, 1245754. [Google Scholar] [CrossRef] [PubMed]
- Chenais, E.; Lewerin, S.S.; Boqvist, S.; Ståhl, K.; Alike, S.; Nokorach, B.; Emanuelson, U. Smallholders’ perceptions on biosecurity and disease control in relation to African swine fever in an endemically infected area in Northern Uganda. BMC Vet. Res. 2019, 15, 279. [Google Scholar] [CrossRef]
- Wartenberg, D. Investigating disease clusters: Why, when and how? J. R. Stat. Soc. Ser. A Stat. Soc. 2001, 164, 13–22. [Google Scholar] [CrossRef]
- Elliott, P.; Wakefield, J. Disease clusters: Should they be investigated, and, if so, when and how? J. R. Stat. Soc. Ser. A Stat. Soc. 2001, 164, 3–12. [Google Scholar] [CrossRef]
- Okwasiimire, R.; Flint, J.F.; Kayaga, E.B.; Lakin, S.; Pierce, J.; Barrette, R.W.; Faburay, B.; Ndoboli, D.; Ekakoro, J.E.; Wampande, E.M.; et al. Whole Genome Sequencing Shows that African Swine Fever Virus Genotype IX Is Still Circulating in Domestic Pigs in All Regions of Uganda. Pathogens 2023, 12, 912. [Google Scholar] [CrossRef]
- Aliro, T.; Chenais, E.; Odongo, W.; Okello, D.M.; Masembe, C.; Ståhl, K. Prevention and Control of African Swine Fever in the Smallholder Pig Value Chain in Northern Uganda: Thematic Analysis of Stakeholders’ Perceptions. Front. Vet. Sci. 2022, 8, 707819. [Google Scholar] [CrossRef] [PubMed]
- Lichoti, J.K.; Davies, J.; Kitala, P.M.; Githigia, S.M.; Okoth, E.; Maru, Y.; Bukachi, S.A.; Bishop, R.P. Social network analysis provides insights into African swine fever epidemiology. Prev. Vet. Med. 2016, 126, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nantima, N.; Ocaido, M.; Ouma, E.; Davies, J.; Dione, M.; Okoth, E.; Mugisha, A.; Bishop, R. Risk factors associated with occurrence of African swine fever outbreaks in smallholder pig farms in four districts along the Uganda-Kenya border. Trop. Anim. Health Prod. 2015, 47, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Chenais, E.; Sternberg-Lewerin, S.; Boqvist, S.; Emanuelson, U.; Aliro, T.; Tejler, E.; Cocca, G.; Masembe, C.; Ståhl, K. African Swine Fever in Uganda: Qualitative Evaluation of Three Surveillance Methods with Implications for Other Resource-Poor Settings. Front. Vet. Sci. 2015, 2, 51. [Google Scholar] [CrossRef]
- Penrith, M.-L.; Van Emmenes, J.; Hakizimana, J.N.; Heath, L.; Kabuuka, T.; Misinzo, G.; Odoom, T.; Wade, A.; Zerbo, H.L.; Luka, P.D. African Swine Fever Diagnosis in Africa: Challenges and Opportunities. Pathogens 2024, 13, 296. [Google Scholar] [CrossRef]
- Mutua, F.; Dione, M. The Context of Application of Biosecurity for Control of African Swine Fever in Smallholder Pig Systems: Current Gaps and Recommendations. Front. Vet. Sci. 2021, 8, 689811. [Google Scholar] [CrossRef]
- Dione, M.M.; Dohoo, I.; Ndiwa, N.; Poole, J.; Ouma, E.; Amia, W.C.; Wieland, B. Impact of participatory training of smallholder pig farmers on knowledge, attitudes and practices regarding biosecurity for the control of African swine fever in Uganda. Transbound. Emerg. Dis. 2020, 67, 2482–2493. [Google Scholar] [CrossRef]
- Mwaura, F.M.; Okoboi, G. Climate Variability and Crop Production in Uganda. J. Sustain. Dev. 2014, 7, p159. [Google Scholar] [CrossRef]
- Kilama Luwa, J.; Majaliwa, J.-G.M.; Bamutaze, Y.; Kabenge, I.; Pilesjo, P.; Oriangi, G.; Bagula Mukengere, E. Variabilities and Trends of Rainfall, Temperature, and River Flow in Sipi Sub-Catchment on the Slopes of Mt. Elgon, Uganda. Water 2021, 13, 1834. [Google Scholar] [CrossRef]
- Vial, L. Biological and ecological characteristics of soft ticks (Ixodida: Argasidae) and their impact for predicting tick and associated disease distribution. Parasite 2009, 16, 191–202. [Google Scholar] [CrossRef]
- Mazur-Panasiuk, N.; Żmudzki, J.; Woźniakowski, G. African swine fever virus—Persistence in different environmental conditions and the possibility of its indirect transmission. J. Vet. Res. 2019, 63, 303–310. [Google Scholar] [CrossRef]
- Gallardo, C.; Okoth, E.; Pelayo, V.; Anchuelo, R.; Martín, E.; Simón, A.; Llorente, A.; Nieto, R.; Soler, A.; Martín, R.; et al. African swine fever viruses with two different genotypes, both of which occur in domestic pigs, are associated with ticks and adult warthogs, respectively, at a single geographical site. J. Gen. Virol. 2011, 92 Pt. 2, 432–444. [Google Scholar] [CrossRef]
- Kayaga, E.B.; Wampande, E.M.; Ekakoro, J.E.; Okwasiimire, R.; Nassali, A.; Ochoa, K.; Hauser, C.; Ndoboli, D.; Havas, K.A. Detection of antibodies against Ornithodoros moubata salivary antigens and their association with detection of African swine fever virus in pigs slaughtered in central Uganda. Front. Vet. Sci. 2024, 11, 1328040. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wampande, E.M.; Opio, R.; Angeki, S.P.; Brown, C.; Faburay, B.; Ademun, R.O.; Ssekatawa, K.; South, D.D.; Waiswa, C.; Waiswa, P. Spatiotemporal Distribution and Risk Factors of African Swine Fever Outbreak Cases in Uganda for the Period 2010–2023. Viruses 2025, 17, 998. https://doi.org/10.3390/v17070998
Wampande EM, Opio R, Angeki SP, Brown C, Faburay B, Ademun RO, Ssekatawa K, South DD, Waiswa C, Waiswa P. Spatiotemporal Distribution and Risk Factors of African Swine Fever Outbreak Cases in Uganda for the Period 2010–2023. Viruses. 2025; 17(7):998. https://doi.org/10.3390/v17070998
Chicago/Turabian StyleWampande, Eddie M., Robert Opio, Simon P. Angeki, Corrie Brown, Bonto Faburay, Rose O. Ademun, Kenneth Ssekatawa, David D. South, Charles Waiswa, and Peter Waiswa. 2025. "Spatiotemporal Distribution and Risk Factors of African Swine Fever Outbreak Cases in Uganda for the Period 2010–2023" Viruses 17, no. 7: 998. https://doi.org/10.3390/v17070998
APA StyleWampande, E. M., Opio, R., Angeki, S. P., Brown, C., Faburay, B., Ademun, R. O., Ssekatawa, K., South, D. D., Waiswa, C., & Waiswa, P. (2025). Spatiotemporal Distribution and Risk Factors of African Swine Fever Outbreak Cases in Uganda for the Period 2010–2023. Viruses, 17(7), 998. https://doi.org/10.3390/v17070998







