Epidemiological Profiles of Human Rabies Cases in Tunisia Between 2000 and 2022
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
- −
- Protocol A1: Day 0 (D0): Rabies Immunoglobulin (RIG) by wound infiltration +1 dose Rabies Vaccine (RV) via IntraMuscular Route, D3: 1 RV, D7: depends on animal observation
- −
- Protocol A2: D0: 2 doses RV, D7: depends on animal observation
- −
- Protocol B1: D0: RIG + 1 RV, D3: 1 RV, D7: 1 RV, D14: 1 RV, D28: 1 RV
- −
- Protocol B2: D0: 2 RV, D7: 1 RV, D21: 1 RV
2.2. Data Analysis
2.3. Statistical Analysis
3. Results
3.1. Epidemiological Profile of Human Rabies in Tunisia (2000–2022)
3.1.1. Temporal Distribution of Human Rabies
- Incidence and Annual Distribution
- Seasonality
- Monthly Distribution
3.1.2. Geographic Distribution of Human Rabies
- By Governorates
- By Type of Environment
3.1.3. Sociodemographic Distribution of Human Rabies
- Gender
- Age
- Gender and Age
3.1.4. Distribution of Human Rabies by Characteristics of the Exposing Animal
- Animal Species
- Presence or Absence of an Owner
- Outcome of the Exposing Animal
3.2. Clinical Profile of Human Rabies During the Period 2000–2022
3.2.1. Distribution of Human Rabies According to Clinical Characteristics
- Nature and Site of Contact with the Exposing Animal
- Nature, Site of Exposure, and Age of Affected Patients
- Incubation Period of Human Rabies
- Incubation Period and Site of Contact with the Exposing Animal
- Clinical Manifestations of Human Rabies
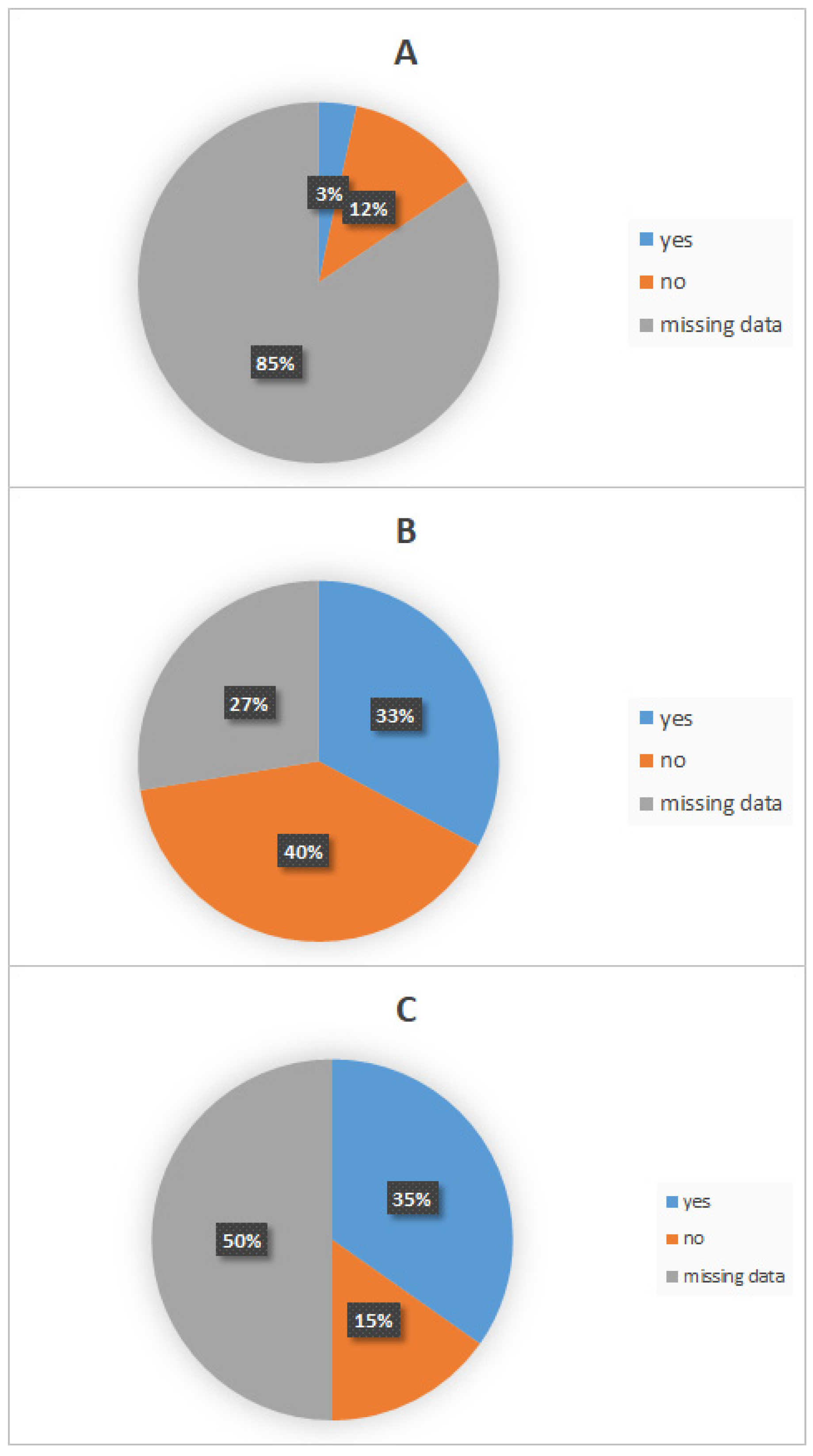
3.2.2. Post-Exposure Measures
- Wound Washing and Disinfection
- Healthcare Facility Consultation
- Utilization of Post-Exposure Prophylaxis (PEP)
- Time Interval Between Exposure and Post-Exposure Prophylaxis Administration
- Post-Exposure Prophylaxis Protocol
3.2.3. Disease Progression and Outcome
- Time from Symptom Onset to Death
- Laboratory Diagnosis
- Time from Clinical Diagnosis to Sample Collection
- Time from Sample Collection to Laboratory Confirmation
4. Discussion
4.1. Temporal Distribution
4.2. Geographical Distribution
4.3. Characteristics of Exposed Patients
4.4. Characteristics of the Exposing Animal
4.5. Clinical Characteristics
4.6. Post-Exposure Prophylaxis (PEP)
4.7. Response and Diagnostic Challenges
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CSF | CerebroSpinal Fluid |
| FAO | Food and Agriculture Organization |
| FAT | Fluorescent Antibody Test |
| FAVN | Fluorescent Antibody Virus Neutralization test |
| GARC | Global Alliance for Rabies Control |
| IBCM | Integrated Bite Case Management |
| IPT | Pasteur Institute in Tunis |
| PEP | Post-Exposure Prophylaxis |
| RFFIT | Rapid Fluorescent Focus Inhibition Test |
| RIG | Rabies ImmunoGlobulin |
| RTCIT | Rabies Tissue Culture Infection Test |
| RT-PCR | Reverse-Transcription Polymerase Chain Reaction |
| RV | Rabies Vaccine |
| WHO | World Health Organization |
| WOAH | World Organization of Animal Health |
References
- Brunker, K.; Mollentze, N. Rabies Virus. Trends Microbiol. 2018, 26, 886–887. [Google Scholar] [CrossRef] [PubMed]
- Hampson, K.; Coudeville, L.; Lembo, T.; Sambo, M.; Kieffer, A.; Attlan, M.; Barrat, J.; Blanton, J.D.; Briggs, D.J.; Cleaveland, S.; et al. Estimating the global burden of endemic canine rabies. PLoS Negl. Trop. Dis. 2015, 9, e0003709. [Google Scholar]
- World Health Organization. WHO Expert Consultation on Rabies: Third Report; World Health Organization: Geneva, Switzerland, 2018; 183p, Available online: https://apps.who.int/iris/handle/10665/272364 (accessed on 1 April 2025).
- WHO; OIE; FAO; GARC. Zero by 30: The Global Strategic Plan to End Human Deaths from Dog-Mediated Rabies by 2030; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Ripani, A.; Mérot, J.; Bouguedour, R.; Zrelli, M. Review of rabies situation and control in the North African region with a focus on Tunisia. Rev. Sci. Tech. 2017, 36, 831–838. [Google Scholar] [CrossRef]
- Institut Pasteur de Tunis. Rapports Des Laboratoires et Services de L’Institut Pasteur de Tunis; Institut Pasteur de Tunis: Tunis, Tunisia, 2012. [Google Scholar]
- Rupprecht, C.E.; Fooks, A.R.; Abela-Ridder, B. Laboratory Techniques in Rabies, 5th ed.; LYSSA LLC: Atlanta, GA, USA, 2018. [Google Scholar]
- OIE. Rabies (Infection with Rabies Virus and Other Lyssaviruses)—Chapter 3.1.17. In Terrestrial Manual; OIE: Paris, France, 2018. [Google Scholar]
- Asma, B.H.; Amal, E.; Hela, F.; Khaled, F.; Mariem, H.; Lamia, G.; Abdelmajid, M. A Child Surviving Rabies in Tunisia: A Case Report. Indian J. Pediatr. 2024, 91, 308. [Google Scholar] [CrossRef] [PubMed]
- Knobel, D.L.; Cleaveland, S.; Coleman, P.G.; Fèvre, E.M.; I Meltzer, M.; Miranda, M.E.G.; Shaw, A.; Zinsstag, J.; Meslin, F.-X. Re-evaluating the burden of rabies in Africa and Asia. Bull. World Health Organ. 2005, 83, 360–368. [Google Scholar]
- Taylor, L.H.; Hampson, K.; Fahrion, A.; Abela Ridder, B.; Nel, L.H. Difficulties in estimating the human burden of canine rabies. Acta Trop. 2017, 165, 133–140. [Google Scholar] [CrossRef]
- Institut Pasteur de Tunis. Rapport 2020 Institut Pasteur De Tunis. 2022. Available online: https://www.slideshare.net/Pasteur_Tunis/rapport-2020-institut-pasteur-de-tunis (accessed on 15 March 2025).
- Zhou, H.; Vong, S.; Liu, K.; Li, Y.; Mu, D.; Wang, L.; Yin, W.; Yu, H. Human rabies in China, 1960–2014: A descriptive epidemiological study. PLoS Negl. Trop. Dis. 2016, 10, e0004874. [Google Scholar] [CrossRef]
- The Changing Landscape of Rabies Epidemiology and Control—PMC. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC7612516/?utm_source=chatgpt.com (accessed on 1 April 2025).
- Susilawathi, N.M.; E Darwinata, A.; Dwija, I.B.; Budayanti, N.S.; Wirasandhi, G.A.; Subrata, K.; Susilarini, N.K.; Sudewi, R.A.; Wignall, F.S.; Mahardika, G.N. Epidemiological and clinical features of human rabies cases in Bali 2008–2010. BMC Infect. Dis. 2012, 12, 81. [Google Scholar] [CrossRef]
- Talbi, C.; Lemey, P.; Suchard, M.A.; Abdelatif, E.; Elharrak, M.; Jalal, N.; Faouzi, A.; Echevarría, J.E.; Morón, S.V.; Rambaut, A.; et al. Phylodynamics and human-mediated dispersal of a zoonotic virus. PLoS Pathog. 2010, 6, e1001166. [Google Scholar] [CrossRef]
- Amouri, I.K.; Kharmachi, H.; Djebbi, A.; Saadi, M.; Hogga, N.; Zakour, L.B.; Ghram, A. Molecular Characterization of Rabies Virus Isolated from Dogs in Tunisia: Evidence of Two Phylogenetic Variants. Virus Res. 2011, 158, 246–250. [Google Scholar] [CrossRef]
- Sofeu, C.L.; Broban, A.; Njimah, A.N.; Momo, J.B.; Sadeuh-Mba, S.A.; Druelles, S.; L’azou, M.; Tejiokem, M.C. Improving systematic rabies surveillance in Cameroon: A pilot initiative and results for 2014–2016. PLoS Negl. Trop. Dis. 2018, 12, e0006597. [Google Scholar] [CrossRef] [PubMed]
- Weyer, J.; Dermaux-Msimang, V.; Grobbelaar, A.; Le Roux, C.; Moolla, N.; Paweska, J.; Blumberg, L. Epidemiology of human rabies in south Africa, 2008–2018. South Afr. Med. J. 2020, 110, 877–881. [Google Scholar] [CrossRef]
- Mohtasham Amiri, Z.; Pourmarzi, D.; Razi, M. Epidemiology of dog bite, a potential source of rabies in Guilan, north of Iran. Asian Pac. J. Trop. Dis. 2015, 5, 104–108. [Google Scholar] [CrossRef]
- World Health Organization. Rabies. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/rabies (accessed on 30 September 2023).
- Ponsich, A.; Goutard, F.; Sorn, S.; Tarantola, A. A prospective study on the incidence of dog bites and management in a rural Cambodian, rabies-endemic setting. Acta Trop. 2016, 160, 62–67. [Google Scholar] [CrossRef]
- Tiembré, I.; Dagnan, S.; Douba, A.; Adjogoua, E.V.; Bourhy, H.; Dacheux, L.; Kouassi, L.; Dosso, M.; Odehouri, K.P. Surveillance épidémiologique de la rage humaine dans un contexte d’endémie de rage canine en Cote d’Ivoire. Med. Mal. Infect. 2010, 40, 398–403. [Google Scholar] [CrossRef]
- Pham, Q.D.; Phan, L.T.; Nguyen, T.P.T.; Doan, Q.M.N.; Nguyen, H.D.; Luong, Q.C.; Nguyen, T.V. An evaluation of the rabies surveillance in southern Vietnam. Front. Public Health 2021, 9, 610905. [Google Scholar] [CrossRef]
- Teklu, G.G.; Hailu, T.G.; Eshetu, G.R. High incidence of human rabies exposure in northwestern Tigray, Ethiopia: A four-year retrospective study. PLoS Negl. Trop. Dis. 2017, 11, e0005271. [Google Scholar] [CrossRef]
- Oukaili, K. Stratégie De Lutte Contre LA rage En Tunisie Situation Actuelle Perspectives. 2017. Available online: https://rr-africa.woah.org/wp-content/uploads/2017/05/presentation_municipalite_tunis.pdf (accessed on 1 September 2023).
- Ben Hassine, T.; Ben Ali, M.; Ghodhbane, I.; Ben Said, Z.; Hammami, S. Rabies in Tunisia: A spatio-temporal analysis in the region of CapBon-Nabeul. Acta Trop. 2021, 216, 105822. [Google Scholar] [CrossRef]
- Bengoumi, M.; Mansouri, R.; Ghram, B.; Mérot, J. Rabies in north Africa and the Middle East: Current situation, strategies and outlook. Rev. Sci. Tech. 2018, 37, 497–510. [Google Scholar] [CrossRef]
- Bouslama, Z.; Kharmachi, H.; Basdouri, N.; Ben Salem, J.; Ben Maiez, S.; Handous, M.; Saadi, M.; Ghram, A.; Turki, I. Molecular Epidemiology of Rabies in Wild Canidae in Tunisia. Viruses 2021, 13, 2473. [Google Scholar] [CrossRef]
- Zinsstag, J.; Lechenne, M.; Laager, M.; Mindekem, R.; Naïssengar, S.; Oussiguéré, A.; Bidjeh, K.; Rives, G.; Tessier, J.; Madjaninan, S.; et al. Vaccination of dogs in an African city interrupts rabies transmission and reduces human exposure. Sci. Transl. Med. 2017, 9, 6984. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Vaccinating Against Rabies to Save Lives. 2023. Available online: https://www.who.int/activities/vaccinating-against-rabies-to-save-lives (accessed on 1 September 2023).
- Kalthoum, S.; Ben Salah, C.; Rzeigui, H.; Gharbi, R.; Guesmi, K.; Ben Salem, A.; Ferchichi, S.; Zammel, F.; Fatnassi, N.; Bahloul, C.; et al. Owned and free-roaming dogs in the north west of Tunisia: Estimation, characteristics and application for the control of dog rabies. Heliyon 2021, 7, e08347. [Google Scholar] [CrossRef] [PubMed]
- Organisation Mondiale de la Santé Animale. Code Terrestre. 2023. Available online: https://www.woah.org/fr/ce-que-nous-faisons/normes/codes-et-manuels/acces-en-ligne-au-code-terrestre/ (accessed on 1 September 2023).
- Hammami, S.; Schumacher, C.; Cliquet, F.; Tlatli, A.; Aubert, A.; Aubert, M. Vaccination of Tunisian dogs with the lyophilised SAG2 oral rabies vaccine incorporated into the DBL2 dog bait. Vet. Res. 1999, 30, 607–613. [Google Scholar] [PubMed]
- Hemachudha, T.; Ugolini, G.; Wacharapluesadee, S.; Sungkarat, W.; Shuangshoti, S.; Laothamatas, J. Human rabies: Neuropathogenesis, diagnosis, and management. Lancet Neurol. 2013, 12, 498–513. [Google Scholar] [CrossRef]
- Maier, T.; Schwarting, A.; Mauer, D.; Ross, R.S.; Martens, A.; Kliem, V.; Wahl, J.; Panning, M.; Baumgarte, S.; Müller, T.; et al. Management and outcomes after multiple corneal and solid organ transplantations from a donor infected with rabies virus. Clin. Infect. Dis. 2010, 50, 1112–1119. [Google Scholar] [CrossRef]
- Burton, E.C.; Burns, D.K.; Opatowsky, M.J.; El-Feky, W.H.; Fischbach, B.; Melton, L.; Sanchez, E.; Randall, H.; Watkins, D.L.; Chang, J.; et al. Rabies encephalomyelitis: Clinical, neuroradiological, and pathological findings in 4 transplant recipients. Arch. Neurol. 2005, 62, 873–882. [Google Scholar] [CrossRef]
- Simani, S.; Fayaz, A.; Rahimi, P.; Eslami, N.; Howeizi, N.; Biglari, P. Six fatal cases of classical rabies virus without biting incidents, Iran 1990–2010. J. Clin. Virol. 2012, 54, 251–254. [Google Scholar] [CrossRef]
- Hemachudha, T.; Laothamatas, J.; Rupprecht, C.E. Human rabies: A disease of complex neuropathogenetic mechanisms and diagnostic challenges. Lancet Neurol. 2002, 1, 101–109. [Google Scholar] [CrossRef]
- Khemaissia, F.; Amor, S.; Gharbi, R.; Nehdi, K.; Letaief, H.; Ben Alaya, N.; Sakkouhi, S.; Boughanmi, M. Epidemiologic profile and the quality of care management of people exposed to rabies; Bousalem district, Jendouba, Tunisia; 2020. Int. J. Infect. Dis. 2023, 130, 72. [Google Scholar] [CrossRef]
- Dimaano, E.M.; Scholand, S.J.; Alera, M.P.; Belandres, D.B. Clinical and epidemiological features of human rabies cases in the Philippines: A review from 1987 to 2006. Int. J. Infect. Dis. 2011, 15, 495–499. [Google Scholar] [CrossRef]
- Ghosh, S.; Rana, S.; Islam, K.; Chowdhury, S.; Haider, N.; Kafi, M.A.H.; Ullah, S.M.; Shah, R.A.; Jahan, A.A.; Mursalin, H.S.; et al. Trends and clinico-epidemiological features of human rabies cases in Bangladesh 2006–2018. Sci. Rep. 2020, 10, 2410. [Google Scholar] [CrossRef]
- Qi, L.; Su, K.; Shen, T.; Tang, W.; Xiao, B.; Long, J.; Zhao, H.; Chen, X.; Xia, Y.; Xiong, Y.; et al. Epidemiological characteristics and post-exposure prophylaxis of human rabies in Chongqing, China, 2007–2016. BMC Infect. Dis. 2018, 18, 6. [Google Scholar] [CrossRef]
- Diop, S.A.; Manga, N.M.; Dia, N.M.; Ndour, C.T.; Seydi, M.; Soumare, M.; Diop, B.M.; Sow, P.S. Le point sur la rage humaine au Sénégal de 1986 à 2005. Med. Mal. Infect. 2007, 37, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Lakhanpal, U.; Sharma, R.C. An epidemiological study of 177 cases of human rabies. Int. J. Epidemiol. 1985, 14, 614–617. [Google Scholar] [CrossRef]
- Charlton, K.M.; Nadin Davis, S.; Casey, G.A.; Wandeler, A.I. The long incubation period in rabies: Delayed progression of infection in muscle at the site of exposure. Acta Neuropathol. 1997, 94, 73–77. [Google Scholar] [CrossRef]
- Johnson, N.; Fooks, A.; McColl, K. Reexamination of human rabies case with long incubation, Australia. Emerg. Infect. Dis. 2008, 14, 1950–1951. [Google Scholar] [CrossRef]
- Shankar, S.K.; Mahadevan, A.; Sapico, S.D.; Ghodkirekar, M.G.; Pinto, R.W.; Madhusudana, S.N. Rabies viral encephalitis with proable 25 year incubation period. Ann. Indian. Acad. Neurol. 2012, 15, 221–223. [Google Scholar] [CrossRef]
- Smith, J.S.; Fishbein, D.B.; Rupprecht, C.E.; Clark, K. Unexplained rabies in three immigrants in the United States—A virologic investigation. N. Engl. J. Med. 1991, 324, 205–211. [Google Scholar] [CrossRef]
- Fisher, C.R.; Streicker, D.G.; Schnell, M.J. The spread and evolution of rabies virus: Conquering new frontiers. Nat. Rev. Microbiol. 2018, 16, 241–255. [Google Scholar] [CrossRef]
- Madhusudana, S.N.; Sukumaran, S.M. Antemortem diagnosis and prevention of human rabies. Ann. Indian. Acad. Neurol. 2008, 11, 3–12. [Google Scholar] [CrossRef]
- Jackson, A.C. Human rabies: A 2016 update. Curr. Infect. Dis. Rep. 2016, 18, 38. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.M.; Cohen, D.; Koprowski, H.; Dean, D.; Ferrigan, L. Studies on the local treatment of wounds for the prevention of rabies. Bull. World Health Organ. 1962, 26, 765–775. [Google Scholar] [PubMed]
- Liu, Q.; Wang, X.; Liu, B.; Gong, Y.; Mkandawire, N.; Li, W.; Fu, W.; Li, L.; Gan, Y.; Shi, J.; et al. Improper wound treatment and delay of rabies post-exposure prophylaxis of animal bite victims in China: Prevalence and determinants. PLoS Negl. Trop. Dis. 2017, 11, e0005663. [Google Scholar] [CrossRef] [PubMed]
- Dodet, B.; Goswami, A.; Gunasekera, A.; de Guzman, F.; Jamali, S.; Montalban, C.; Purba, W.; Quiambao, B.; Salahuddin, N.; Sampath, G.; et al. Rabies awareness in eight Asian countries. Vaccine 2008, 26, 6344–6348. [Google Scholar] [CrossRef]
- Hampson, K.; Dobson, A.; Kaare, M.; Dushoff, J.; Magoto, M.; Sindoya, E.; Cleaveland, S. Rabies exposures, post-exposure prophylaxis and deaths in a region of endemic canine rabies. PLoS Negl. Trop. Dis. 2008, 2, 339. [Google Scholar] [CrossRef]
- Wilde, H. Failures of post-exposure rabies prophylaxis. Vaccine 2007, 25, 7605–7609. [Google Scholar] [CrossRef]
- Khalsi, F.; Ayari, A.; Romdhane, M.B.; Tinsa, F.; Boussetta, K. Rabies encephalitis in children: A resurgence of a fatal anthropozoonosis. Afr. Health Sci. 2018, 18, 539–541. [Google Scholar] [CrossRef]
- Dacheux, L.; Wacharapluesadee, S.; Hemachudha, T.; Meslin, F.-X.; Buchy, P.; Reynes, J.-M.; Bourhy, H. More accurate insight into the incidence of human rabies in developing countries through validated laboratory techniques. PLoS Negl. Trop. Dis. 2010, 4, e765. [Google Scholar] [CrossRef]
- Shantavasinkul, P.; Tantawichien, T.; Wacharapluesadee, S.; Jeamanukoolkit, A.; Udomchaisakul, P.; Chattranukulchai, P.; Wongsaroj, P.; Khawplod, P.; Wilde, H.; Hemachudha, T. Failure of rabies postexposure prophylaxis in patients presenting with unusual manifestations. Clin. Infect. Dis. 2010, 50, 77–79. [Google Scholar] [CrossRef]
- Mani, R.S.; Madhusudana, S.N. Laboratory diagnosis of human rabies: Recent advances. Sci. World J. 2013, 2013, 569712. [Google Scholar] [CrossRef]
- Madhusudana, S.N.; Nagaraj, D.; Uday, M.; Ratnavalli, E.; Kumar, M.V. Partial recovery from rabies in a six-year-old girl. Int. J. Infect. Dis. 2002, 6, 85–86. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, R.E.J.; Tieves, K.S.; Hoffman, G.M.; Ghanayem, N.S.; Amlie-Lefond, C.M.; Schwabe, M.J.; Chusid, M.J.; Rupprecht, C.E. Survival after treatment of rabies with induction of coma. N. Engl. J. Med. 2005, 352, 2508–2514. [Google Scholar] [CrossRef]
- Ayachi, A.; Benabdallah, R.; Handous, M. Profil Épidémiologique Des Cas De Rage Humaine En TUNISIE Entre 2000 Et 2022. In Proceedings of the Congrès De La SFMTSI-Santé En Méditerranée Pathologies-Migrations-Environnement, Saint-Raphaël, France, 22–24 May 2024. [Google Scholar]




| Nature of Exposure | Cases Number (%) |
|---|---|
| Single bite | 32/58 (55%) |
| Bites and scratches | 12/58 (20%) |
| Scratches | 5/58 (9%) |
| Multiple bites | 3/58 (5%) |
| Handling of a sick animal | 1/58 (2%) |
| Unknown (not apparent or not reported) | 5/58 (9%) |
| Site of Exposure | Cases Number (%) |
| Head, neck | 11/58 (19%) |
| Upper limbs | 9/58 (16%) |
| Lower limbs | 2/58 (3%) |
| Multiple sites | 1/58 (2%) |
| Unknown (not apparent or not reported) | 35/58 (60%) |
| Clinical Sign | Cases Number (%) |
|---|---|
| Hydrophobia | 34/51 (67%) |
| Behavioral disturbances | 33/51 (65%) |
| Fever | 18/51 (35%) |
| Altered consciousness, coma | 11/51 (22%) |
| Vomiting | 11/51 (22%) |
| Aerophobia | 9/51(18%) |
| Psychiatric symptoms (hallucinations, anxiety disorders, insomnia) | 9/51 (18%) |
| Hypersalivation | 7/51 (14%) |
| Paresthesia, paralysis, pain at exposure site | 7/51 (14%) |
| Dysphagia | 6/51 (12%) |
| Dyspnea | 5/51 (10%) |
| Asthenia | 4/51 (8%) |
| Chest–abdominal pain | 4/51 (8%) |
| Headache | 3/51 (6%) |
| Arthralgia, myalgia | 3/51 (6%) |
| Photophobia | 3/51 (6%) |
| Tremors | 2/51 (3%) |
| Cutaneous hyperesthesia | 1/51 (2%) |
| Convulsions | 1/51 (2%) |
| Conjunctival hyperemia | 1/51 (2%) |
| Hematemesis | 1/51 (2%) |
| Vertigo | 1/51 (2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayachi, A.; Benabdallah, R.; Bouratbine, A.; Aoun, K.; Bensalem, J.; Basdouri, N.; Benmaiz, S.; Bassalah, F.; Nouioui, C.; Soltani, M.; et al. Epidemiological Profiles of Human Rabies Cases in Tunisia Between 2000 and 2022. Viruses 2025, 17, 966. https://doi.org/10.3390/v17070966
Ayachi A, Benabdallah R, Bouratbine A, Aoun K, Bensalem J, Basdouri N, Benmaiz S, Bassalah F, Nouioui C, Soltani M, et al. Epidemiological Profiles of Human Rabies Cases in Tunisia Between 2000 and 2022. Viruses. 2025; 17(7):966. https://doi.org/10.3390/v17070966
Chicago/Turabian StyleAyachi, Amal, Rym Benabdallah, Aida Bouratbine, Karim Aoun, Jihen Bensalem, Nourhen Basdouri, Samia Benmaiz, Farah Bassalah, Chaima Nouioui, Mohamed Soltani, and et al. 2025. "Epidemiological Profiles of Human Rabies Cases in Tunisia Between 2000 and 2022" Viruses 17, no. 7: 966. https://doi.org/10.3390/v17070966
APA StyleAyachi, A., Benabdallah, R., Bouratbine, A., Aoun, K., Bensalem, J., Basdouri, N., Benmaiz, S., Bassalah, F., Nouioui, C., Soltani, M., Ghouili, K., Bouslema, Z., Kharmechi, H., & Handous, M. (2025). Epidemiological Profiles of Human Rabies Cases in Tunisia Between 2000 and 2022. Viruses, 17(7), 966. https://doi.org/10.3390/v17070966







