The Meandrous Route of Rilpivirine in the Search for the Miraculous Drug to Treat HIV Infections
Abstract
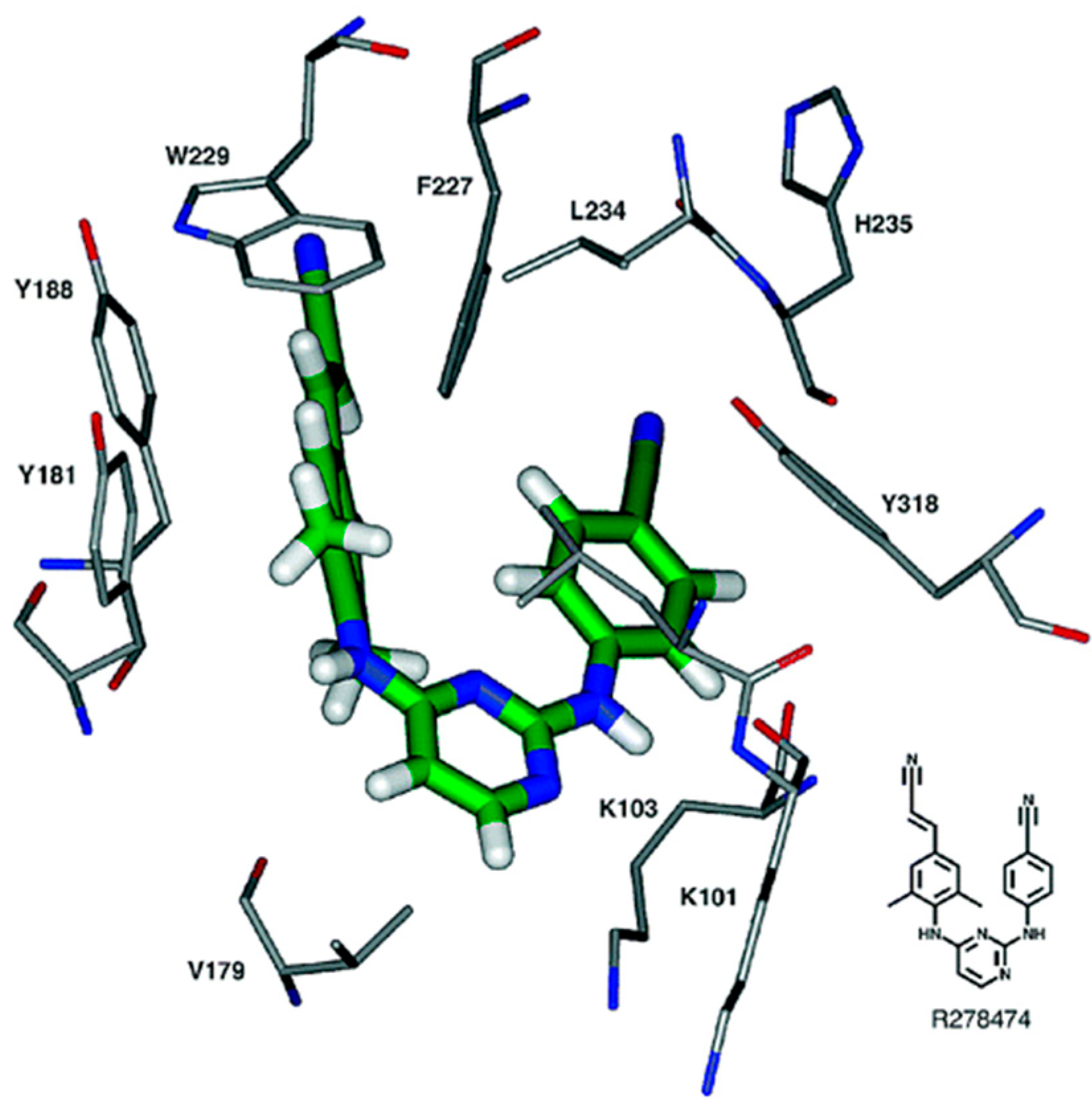
1. Introduction
2. Chemical Structure
3. Clinical Use of Rilpivirine
4. Combination of Rilpivirine (RPV) with Tenofovir Disoproxil Fumarate (TDF) or Tenofovir Alafenamide (TAF)
5. Combinations of Rilpivirine (RPV) with Darunavir (Boosted with Either Ritonavir or Cobicistat) or Dolutegravir
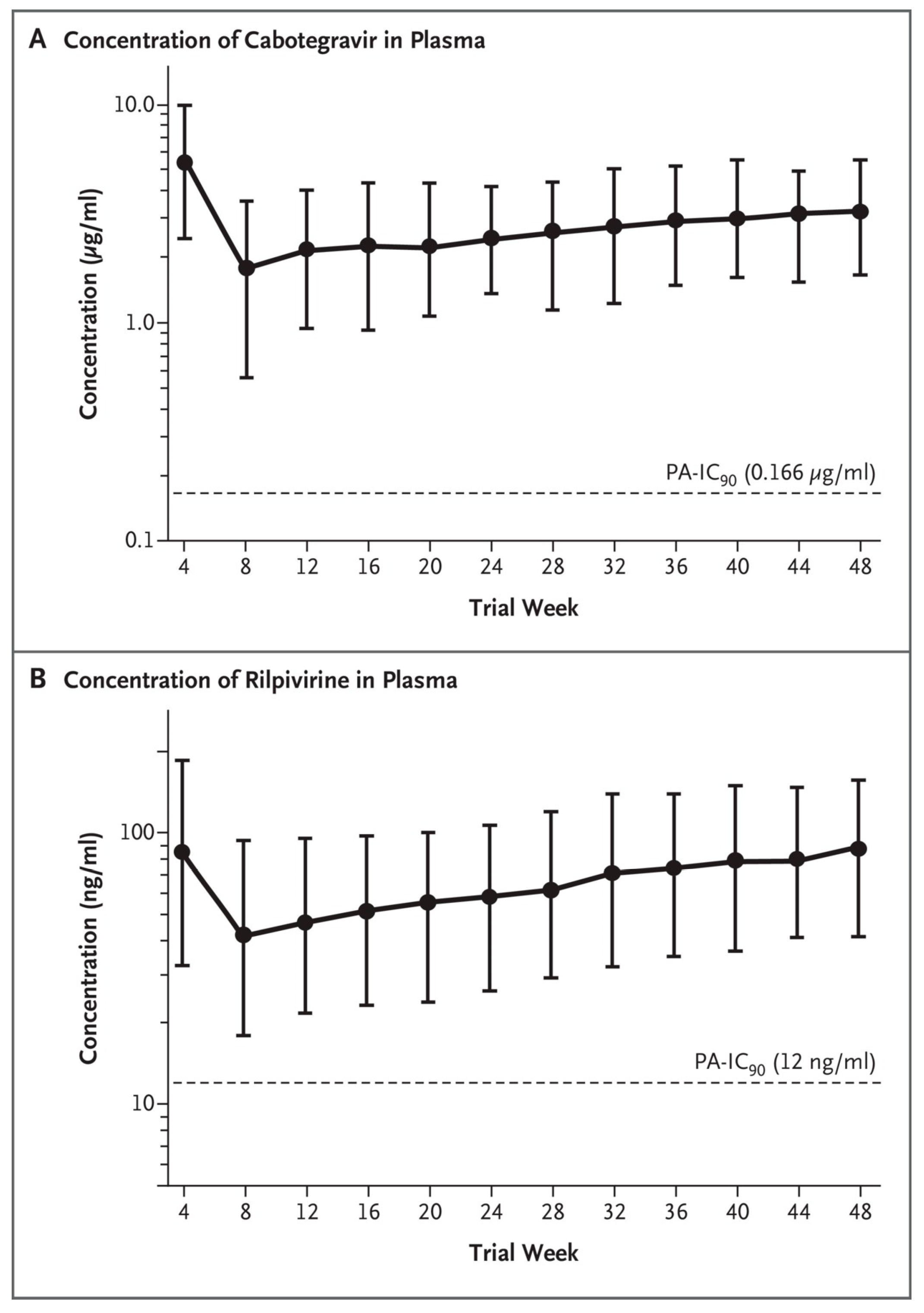
6. Combination of Rilpivirine (RPV) with Cabotegravir (CAB): 2019–2021
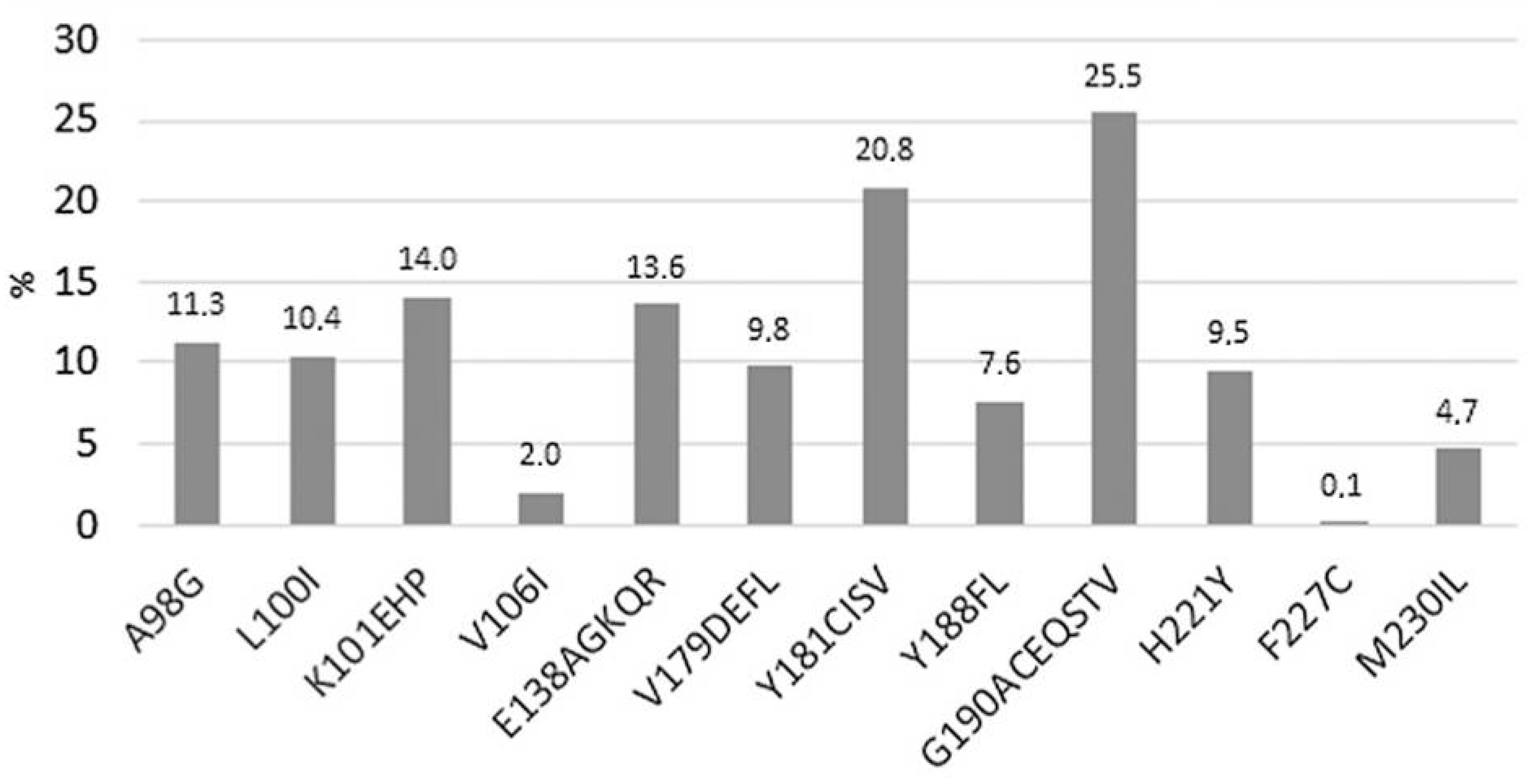
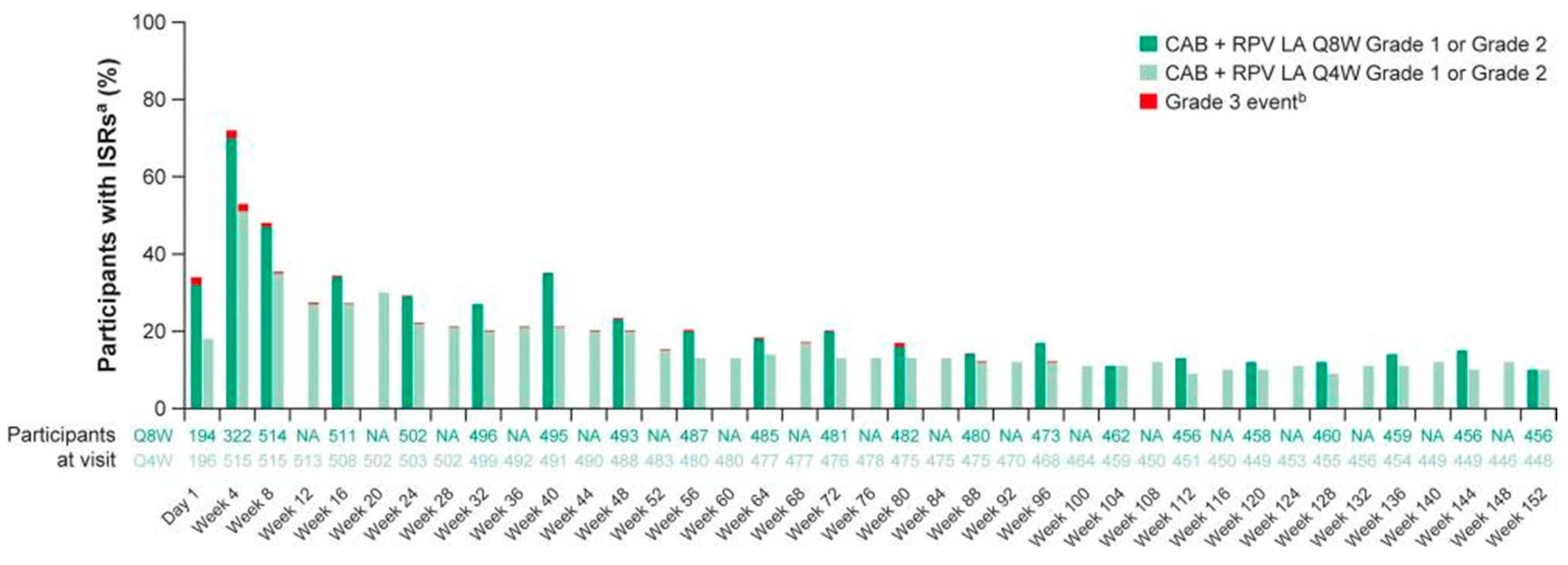
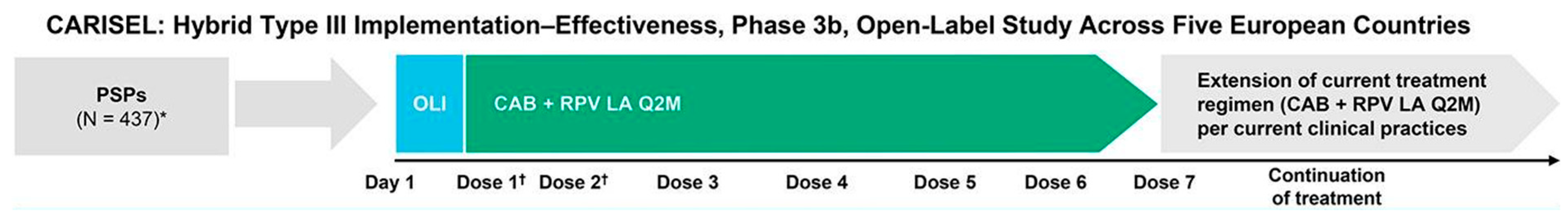
7. Combination of Rilpivirine (RPV) with Cabotegravir (CAB): 2021–2023
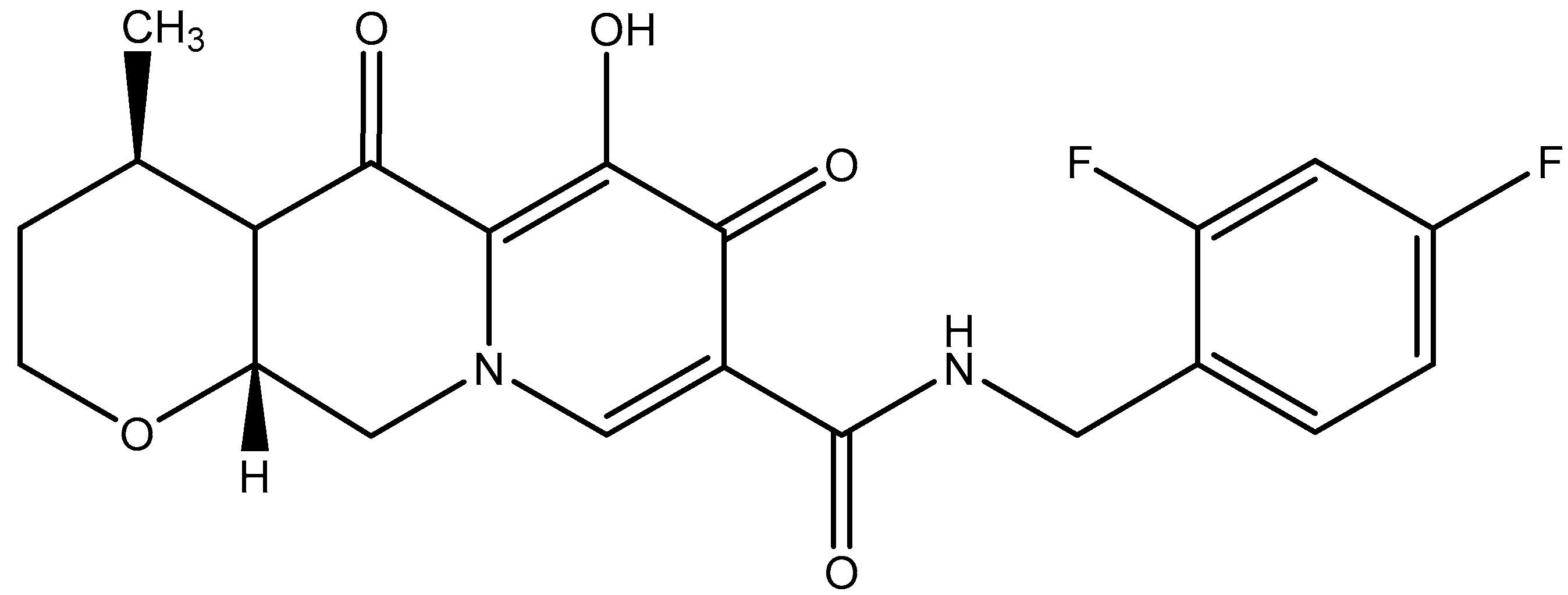
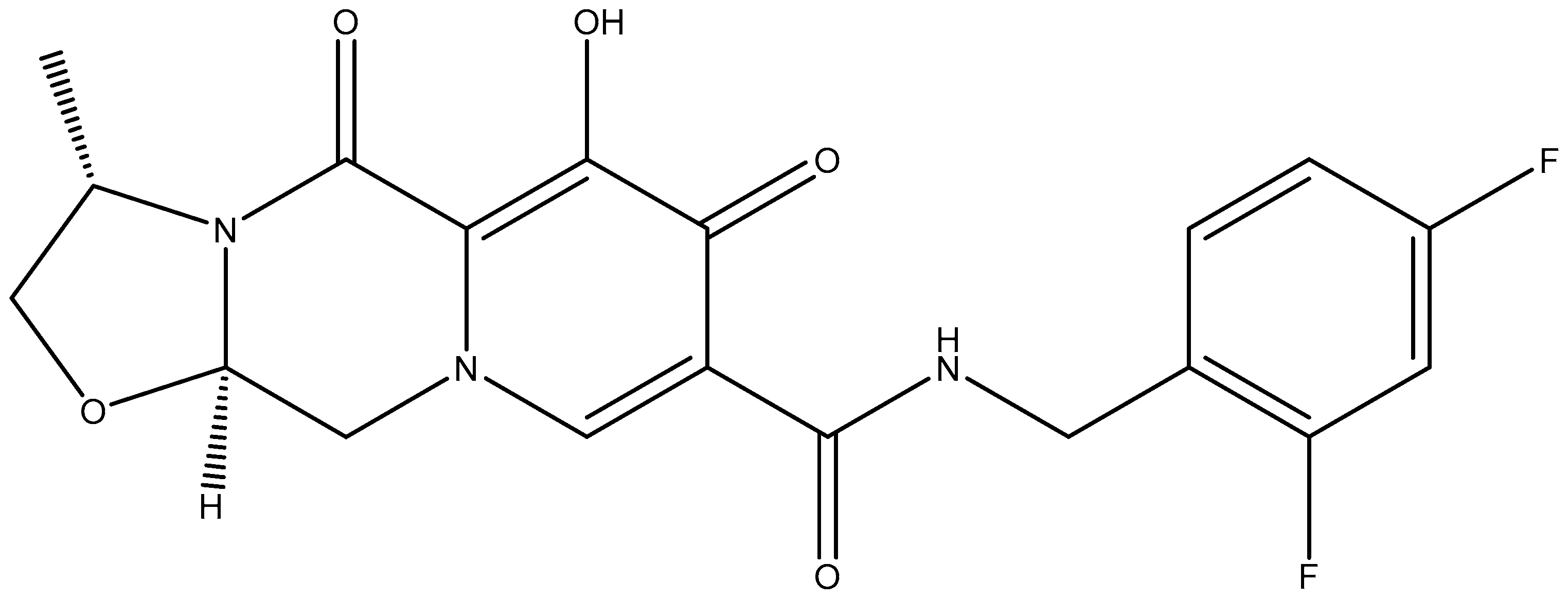
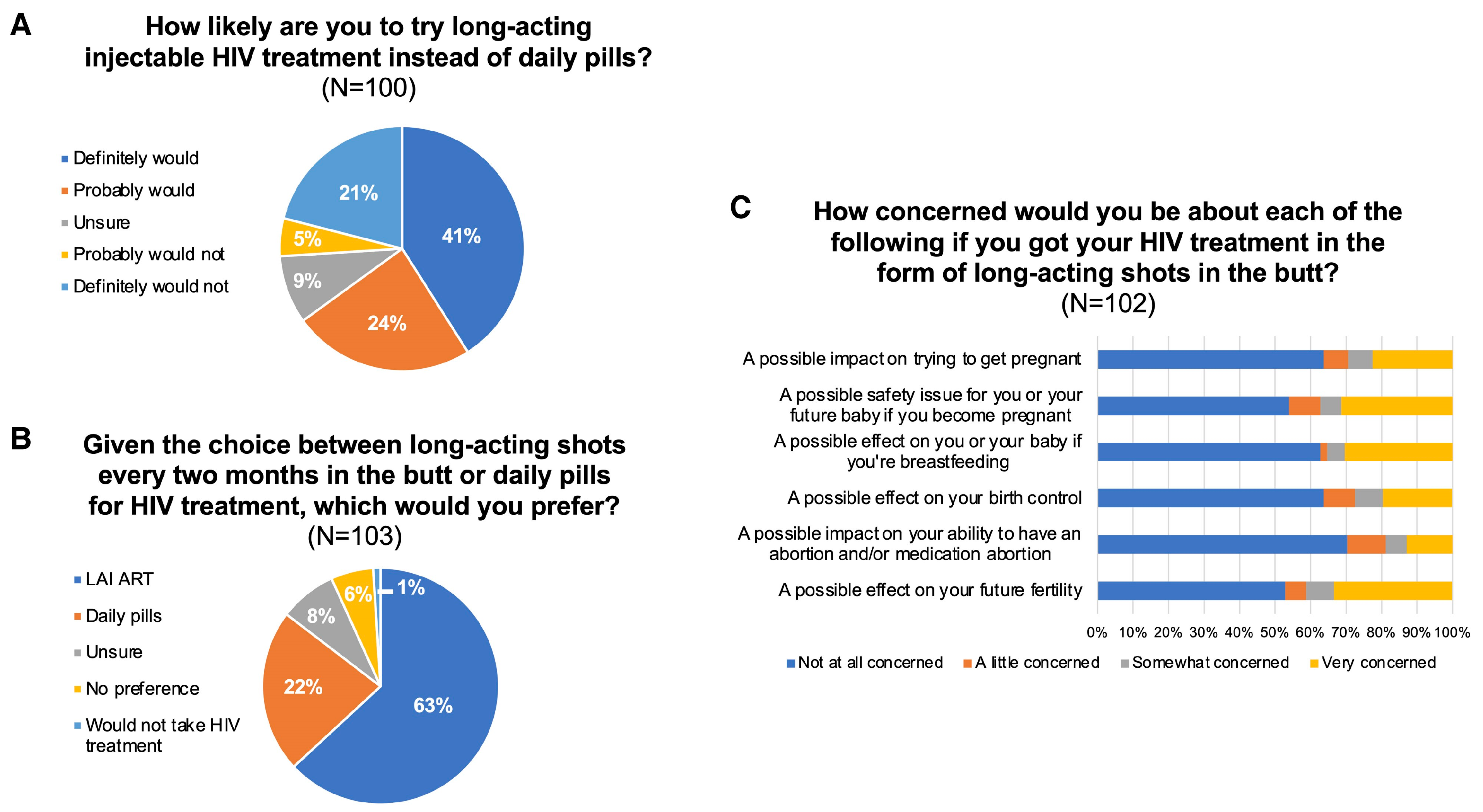
8. Combination of Rilpivirine (RPV) with Cabotegravir (CAB): 2024–2025
9. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Janssen, P.A.; Lewi, P.J.; Arnold, E.; Daeyaert, F.; de Jonge, M.; Heeres, J.; Koymans, L.; Vinkers, M.; Guillemont, J.; Pasquier, E.; et al. In search of a novel anti-HIV drug: Multidisciplinary coordination in the discovery of 4-[[4-[[4-[(1E)-2-cyanoethenyl]-2,6-dimethylphenyl]amino]-2-pyrimidinyl]amino]benzonitrile (R278474, rilpivirine). J. Med. Chem. 2005, 48, 1901–1909. [Google Scholar] [CrossRef]
- Pauwels, R.; Andries, K.; Desmyter, J.; Schols, D.; Kukla, M.J.; Breslin, H.J.; Raeymaeckers, A.; Van Gelder, J.; Woestenborghs, R.; Heykants, J.; et al. Potent and selective inhibition of HIV-1 replication in vitro by a novel series of TIBO derivatives. Nature 1990, 343, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Clark, A.D., Jr.; Lewi, P.J.; Heeres, J.; De Jonge, M.R.; Koymans, L.M.; Vinkers, H.M.; Daeyaert, F.; Ludovici, D.W.; Kukla, M.J.; et al. Roles of conformational and positional adaptability in structure-based design of TMC125-R165335 (etravirine) and related non-nucleoside reverse transcriptase inhibitors that are highly potent and effective against wild-type and drug-resistant HIV-1 variants. J. Med. Chem. 2004, 47, 2550–2560. [Google Scholar] [CrossRef]
- Andries, K.; Azijn, H.; Thielemans, T.; Ludovici, D.; Kukla, M.; Heeres, J.; Janssen, P.; De Corte, B.; Vingerhoets, J.; Pauwels, R.; et al. TMC125, a novel next-generation nonnucleoside reverse transcriptase inhibitor active against nonnucleoside reverse transcriptase inhibitor-resistant human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 2004, 48, 4680–4686. [Google Scholar] [CrossRef]
- Vingerhoets, J.; Azijn, H.; Fransen, E.; De Baere, I.; Smeulders, L.; Jochmans, D.; Andries, K.; Pauwels, R.; de Béthune, M.P. TMC125 displays a high genetic barrier to the development of resistance: Evidence from in vitro selection experiments. J. Virol. 2005, 79, 12773–12782. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, R. New non-nucleoside reverse transcriptase inhibitors (NNRTIs) in development for the treatment of HIV infections. Curr. Opin. Pharmacol. 2004, 4, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Guillemont, J.; Pasquier, E.; Palandjian, P.; Vernier, D.; Gaurrand, S.; Lewi, P.J.; Heeres, J.; de Jonge, M.R.; Koymans, L.M.; Daeyaert, F.F.; et al. Synthesis of novel diarylpyrimidine analogues and their antiviral activity against human immunodeficiency virus type 1. J. Med. Chem. 2005, 48, 2072–2079. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, J.; Zhou, Z.; Fu, Z.; Cherukupalli, S.; Kang, D.; Zhan, P.; Liu, X. The development of an effective synthetic route of rilpivirine. BMC Chem. 2021, 15, 22. [Google Scholar] [CrossRef]
- Mhaske, D.K.; Kumbhar, A.S. Simultaneous quantification of (E) and (Z) isomers of rilpivirine and four degradation products in bulk and tablets by reversed-phase ultra-high-performance liquid chromatography and confirmation of all by molecular weight. J. Sep. Sci. 2023, 46, e2201067. [Google Scholar] [CrossRef]
- Gatechompol, S.; Avihingsanon, A.; Apornpong, T.; Han, W.M.; Kerr, S.J.; Ruxrungtham, K. Efficacy and improvement of lipid profile after switching to rilpivirine in resource limited setting: Real life clinical practice. AIDS Res. Ther. 2019, 16, 7. [Google Scholar] [CrossRef]
- Azijn, H.; Tirry, I.; Vingerhoets, J.; de Béthune, M.P.; Kraus, G.; Boven, K.; Jochmans, D.; Van Craenenbroeck, E.; Picchio, G.; Rimsky, L.T. TMC278, a next-generation nonnucleoside reverse transcriptase inhibitor (NNRTI), active against wild-type and NNRTI-resistant HIV-1. Antimicrob. Agents Chemother. 2010, 54, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.G.; Else, L.J.; Mesquita, P.M.; Egan, D.; Back, D.J.; Karolia, Z.; Ringner-Nackter, L.; Higgs, C.J.; Herold, B.C.; Gazzard, B.G.; et al. A compartmental pharmacokinetic evaluation of long-acting rilpivirine in HIV-negative volunteers for pre-exposure prophylaxis. Clin. Pharmacol. Ther. 2014, 96, 314–323. [Google Scholar] [CrossRef] [PubMed]
- McGowan, I.; Dezzutti, C.S.; Siegel, A.; Engstrom, J.; Nikiforov, A.; Duffill, K.; Shetler, C.; Richardson-Harman, N.; Abebe, K.; Back, D.; et al. Long-acting rilpivirine as potential pre-exposure prophylaxis for HIV-1 prevention (the MWRI-01 study): An open-label, phase 1, compartmental, pharmacokinetic and pharmacodynamic assessment. Lancet HIV 2016, 3, e569–e578. [Google Scholar] [CrossRef]
- Cranston, R.D.; Dezzutti, C.S.; Siegel, A.; Engstrom, J.; Shetler, C.; Richardson-Harman, N.; Abebe, K.Z.; Back, D.; Else, L.; Egan, D.; et al. A multiple dose phase 1 assessment of rilpivirine long acting in a model of preexposure prophylaxis against HIV. AIDS Res. Hum. Retroviruses 2019, 35, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, H.K.; Tillotson, J.; Lade, J.M.; Bekker, L.G.; Li, S.; Pathak, S.; Justman, J.; Mgodi, N.; Swaminathan, S.; Sista, N.; et al. Metabolism of long-acting rilpivirine after intramuscular injection: HIV prevention trials network study 076 (HPTN 076). AIDS Res. Hum. Retroviruses 2021, 37, 173–183. [Google Scholar] [CrossRef]
- Vannappagari, V.; Ragone, L.; Henegar, C.; van Wyk, J.; Brown, D.; Demarest, J.; Quercia, R.; St Clair, M.; Underwood, M.; Gatell, J.M.; et al. Prevalence of pretreatment and acquired HIV-1 mutations associated with resistance to lamivudine or rilpivirine: A systematic review. Antivir. Ther. 2019, 24, 393–404. [Google Scholar] [CrossRef]
- Sayan, M.; Sultanoglu, N.; Sanlidag, T. Dynamics of rilpivirine resistance-associated mutation: E138 in reverse transcriptase among antiretroviral-naive HIV-1-infected individuals in Turkey. AIDS Res. Hum. Retroviruses 2023, 39, 84–90. [Google Scholar] [CrossRef]
- Frange, P.; Tubiana, R.; Sibiude, J.; Canestri, A.; Arvieux, C.; Brunet-Cartier, C.; Cotte, L.; Reynes, J.; Mandelbrot, L.; Warszawski, J.; et al. Rilpivirine in HIV-1-positive women initiating pregnancy: To switch or not to switch? J. Antimicrob. Chemother. 2020, 75, 1324–1331. [Google Scholar] [CrossRef]
- Islam, S.; Rahaman, M.H.; Yu, M.; Noll, B.; Martin, J.H.; Wang, S.; Head, R. Anti-leukaemic activity of rilpivirine Is mediated by Aurora A kinase inhibition. Cancers 2023, 15, 1044. [Google Scholar] [CrossRef]
- Pereira, M.; Vale, N. Evolution of antiretroviral drug rilpivirine and approach to oncology. Int. J. Mol. Sci. 2023, 24, 2890. [Google Scholar] [CrossRef]
- Martí-Rodrigo, A.; Alegre, F.; Moragrega, Á.B.; García-García, F.; Martí-Rodrigo, P.; Fernández-Iglesias, A.; Gracia-Sancho, J.; Apostolova, N.; Esplugues, J.V.; Blas-García, A. Rilpivirine attenuates liver fibrosis through selective STAT1-mediated apoptosis in hepatic stellate cells. Gut 2020, 69, 920–932. [Google Scholar]
- Sariyer, I.K.; Gordon, J.; Burdo, T.H.; Wollebo, H.S.; Gianti, E.; Donadoni, M.; Bellizzi, A.; Cicalese, S.; Loomis, R.; Robinson, J.A.; et al. Suppression of Zika virus infection in the brain by the antiretroviral drug rilpivirine. Mol. Ther. 2019, 27, 2067–2079. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Hong, L.; Yang, C.; Zhang, S.; Zhang, Y.; Huang, S.; Xie, R.; Li, X.; Ma, Q.; Li, H. Effect of rilpivirine on the pharmacokinetics of methadone in HIV-Infected Chinese patients. Expert. Rev. Clin. Pharmacol. 2019, 12, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Grañana-Castillo, S.; Montanha, M.C.; Bearon, R.; Khoo, S.; Siccardi, M. Evaluation of drug-drug interaction between rilpivirine and rifapentine using PBPK modelling. Front. Pharmacol. 2022, 13, 1076266. [Google Scholar] [CrossRef]
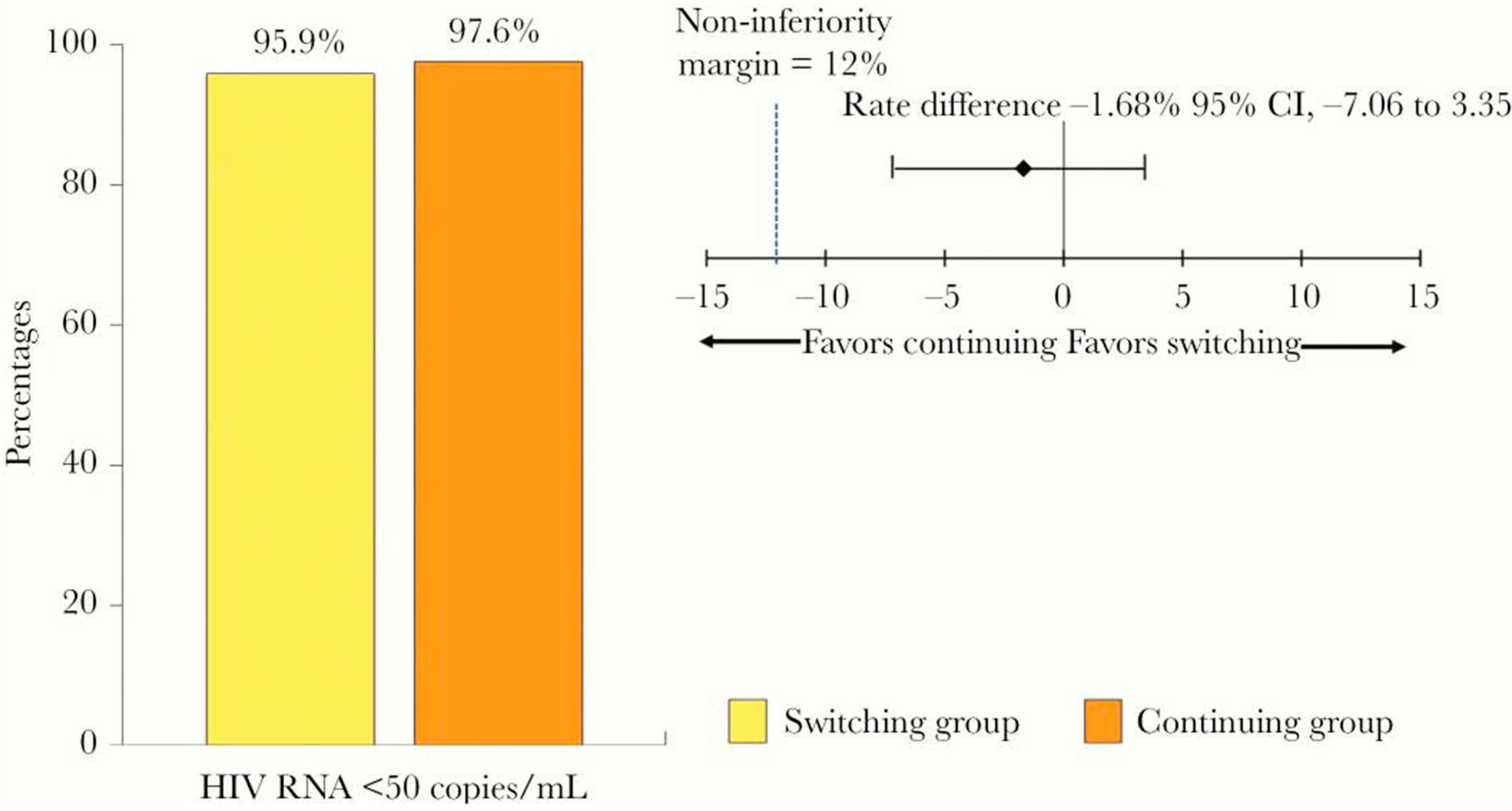
- Wiriyatanakorn, S.; Sungkanuparph, S. Switching tenofovir/emtricitabine/efavirenz (TDF/FTC/EFV) to TDF/FTC/rilpivirine vs continuing TDF/FTC/EFV in HIV-infected patients with virological suppression: A randomized controlled trial. Open Forum Infect. Dis. 2019, 6, ofz297. [Google Scholar] [CrossRef]
- Munderi, P.; Were, E.; Avihingsanon, A.; Mbida, P.A.M.; Mohapi, L.; Moussa, S.B.; Jansen, M.; Bicer, C.; Mohammed, P.; van Delft, Y. Switching at low HIV-1 RNA into fixed dose combinations: TDF/FTC/RPV is non-inferior to TDF/FTC/EFV in first-line suppressed patients living with HIV. S. Afr. J. HIV Med. 2019, 20, 949. [Google Scholar] [CrossRef]
- Chauveau, M.; Billaud, E.; Bonnet, B.; Merrien, D.; Hitoto, H.; Bouchez, S.; Michau, C.; Hall, N.; Perez, L.; Sécher, S.; et al. Tenofovir DF/emtricitabine/rilpivirine as HIV post-exposure prophylaxis: Results from a multicentre prospective study. J. Antimicrob. Chemother. 2019, 74, 1021–1027. [Google Scholar] [CrossRef]
- Taramasso, L.; Berruti, M.; Briano, F.; Di Biagio, A. The switch from tenofovir disoproxil fumarate to tenofovir alafenamide determines weight gain in patients on rilpivirine-based regimen. AIDS 2020, 34, 877–881. [Google Scholar]
- Mu, Y.; Pham, M.; Podany, A.T.; Cory, T.J. Evaluating emtricitabine + rilpivirine + tenofovir alafenamide in combination for the treatment of HIV-infection. Expert. Opin. Pharmacother. 2020, 21, 389–397. [Google Scholar] [CrossRef]
- Wu, P.F.; Lin, H.H.; Chen, H.P.; Huang, P.C.; Ke, M.Y. Metabolic outcomes of changing from rilpivirine/tenofovir disoproxil fumarate/emtricitabine to rilpivirine/tenofovir alafenamide/emtricitabine: A longitudinal study. Health Sci. Rep. 2024, 7, e70275. [Google Scholar] [CrossRef] [PubMed]
- Daly, M.B.; Dinh, C.; Holder, A.; Rudolph, D.; Ruone, S.; Swaims-Kohlmeier, A.; Khalil, G.; Sharma, S.; Mitchell, J.; Condrey, J.; et al. SHIV remission in macaques with early treatment initiation and ultra long-lasting antiviral activity. Nat. Commun. 2024, 15, 10550. [Google Scholar] [CrossRef] [PubMed]
- Pasquau, J.; de Jesus, S.E.; Arazo, P.; Crusells, M.J.; Ríos, M.J.; Lozano, F.; de la Torre, J.; Galindo, M.J.; Carmena, J.; Santos, J.; et al. Effectiveness and safety of dual therapy with rilpivirine and boosted darunavir in treatment-experienced patients with advanced HIV infection: A preliminary 24 week analysis (RIDAR study). BMC Infect. Dis. 2019, 19, 207. [Google Scholar] [CrossRef]
- Navarro, J.; González-Cordón, A.; Casado, J.L.; Bernardino, J.I.; Domingo, P.; Portilla, J.; Llibre, J.M.; Colomer, J.; Rial-Crestelo, D.; Vizcarra, P.; et al. Effectiveness of boosted darunavir plus rilpivirine in patients with long-lasting HIV-1 infection: DARIL study. J. Antimicrob. Chemother. 2020, 75, 1955–1960. [Google Scholar] [CrossRef]
- Di Cristo, V.; Adorni, F.; Maserati, R.; Annovazzi Lodi, M.; Bruno, G.; Maggi, P.; Volpe, A.; Vitiello, P.; Abeli, C.; Bonora, S.; et al. 96-week results of a dual therapy with darunavir/ritonavir plus rilpivirine once a day vs triple therapy in patients with suppressed viraemia: Virological success and non-HIV related morbidity evaluation. HIV Res. Clin. Pract. 2020, 21, 34–43. [Google Scholar] [CrossRef]
- Maggiolo, F.; Gianotti, N.; Comi, L.; Di Filippo, E.; Fumagalli, L.; Nozza, S.; Galli, L.; Valenti, D.; Rizzi, M.; Castagna, A. Rilpivirine plus cobicistat-boosted darunavir as a two-drug switch regimen in HIV-infected, virologically suppressed subjects on steady standard three-drug therapy: A randomized, controlled, non-inferiority trial (PROBE 2). J. Antimicrob. Chemother. 2020, 75, 1332–1337. [Google Scholar] [CrossRef]
- Ho, S.; Wong, J.G.; Ng, O.T.; Lee, C.C.; Leo, Y.S.; Lye, D.C.B.; Wong, C.S. Efficacy and safety of abacavir/lamivudine plus rilpivirine as a first-line regimen in treatment-naïve HIV-1 infected adults. AIDS Res. Ther. 2020, 17, 23. [Google Scholar] [CrossRef]
- Lim, Z.C.; Hoo, G.S.; Ang, J.H.; Teng, C.B.; Ang, L.W.; Lee, C.C.; Leo, Y.S.; Law, H.L.; Ng, O.T.; Wong, C.S. Safety and effectiveness of switching to Abacavir/Lamivudine plus rilpivirine for maintenance therapy in virologically suppressed HIV-1 individuals in Singapore (SEALS). AIDS Res. Ther. 2021, 18, 80. [Google Scholar] [CrossRef]
- Casado, J.L.; Monsalvo, M.; Fontecha, M.; Vizcarra, P.; Rodriguez, M.A.; Vivancos, M.J.; Moreno, S. Dolutegravir plus rilpivirine as dual regimen in virologically suppressed HIV-1 infected patients in a clinical setting. HIV Res. Clin. Pract. 2019, 20, 64–72. [Google Scholar] [CrossRef]
- Hester, E.K.; Astle, K. Dolutegravir-rilpivirine, dual antiretroviral therapy for the treatment of HIV-1 infection. Ann. Pharmacother. 2019, 53, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Aboud, M.; Orkin, C.; Podzamczer, D.; Bogner, J.R.; Baker, D.; Khuong-Josses, M.A.; Parks, D.; Angelis, K.; Kahl, L.P.; Blair, E.A.; et al. Efficacy and safety of dolutegravir-rilpivirine for maintenance of virological suppression in adults with HIV-1: 100-week data from the randomised, open-label, phase 3 SWORD-1 and SWORD-2 studies. Lancet HIV 2019, 6, e576–e587. [Google Scholar] [CrossRef] [PubMed]
- Moorhouse, M.A.; Cohen, K. The role of rilpivirine in Southern Africa. S. Afr. J. HIV Med. 2019, 20, 825. [Google Scholar] [CrossRef]
- van Wyk, J.; Orkin, C.; Rubio, R.; Bogner, J.; Baker, D.; Khuong-Josses, M.A.; Parks, D.; Angelis, K.; Kahl, L.P.; Matthews, J.; et al. Brief report: Durable suppression and low rate of virologic failure 3 years after switch to dolutegravir +rilpivirine 2-drug regimen: 148-week results from the SWORD-1 and SWORD-2 randomized clinical trials. J. Acquir. Immune Defic. Syndr. 2020, 85, 325–330. [Google Scholar] [CrossRef]
- Mehta, R.; Lagishetty, C.V.; Angelis, K.; Aylott, A.; Kahl, L.; Blair, L.; Matthews, J.; Wynne, B.; Crauwels, H.; Underwood, M.; et al. Pharmacokinetic and pharmacokinetic/pharmacodynamic characterization of the dolutegravir/rilpivirine two-drug regimen in SWORD-1/-2 phase 3 studies. Br. J. Clin. Pharmacol. 2023, 89, 2190–2200. [Google Scholar] [CrossRef]
- Hidalgo-Tenorio, C.; Vinuesa, D.; García-Vallecillos, C.; Muñoz-Medina, L.; Sequera, S.; Javier, R.; López-Ruz, M.; Sadyrbaeva-Dolgova, S.; Pasquau, J. Rildo: Real-world multicenter study on the effectiveness and safety of single-tablet regimen of dolutegravir plus rilpivirine in treatment-experienced people living with HIV. Viruses 2022, 14, 2626. [Google Scholar] [CrossRef]
- Palacios, R.; Gómez-Ayerbe, C.; Casado, J.L.; Tejerina, F.; Montes, M.L.; Castaño, M.; Ocampo, A.; Rial, D.; Ribera, E.; Galindo, M.J.; et al. Efficacy and safety of dolutegravir/rilpivirine in real-world clinical practice. GeSIDA study 1119. HIV Med. 2023, 24, 933–937. [Google Scholar] [CrossRef]
- Troya, J.; Dueñas, C.; Irazola, I.; de Los Santos, I.; de la Fuente, S.; Gil, D.; Hernández, C.; Galindo, M.J.; Gómez, J.; Delgado, E.; et al. Dolutegravir plus rilpivirine: Benefits beyond viral suppression: DORIPEX retrospective study. Medicine 2022, 101, e29252. [Google Scholar] [CrossRef]
- Llibre, J.M.; López Cortés, L.F.; Aylott, A.; Wynne, B.; Matthews, J.; Van Solingen-Ristea, R.; Vandermeulen, K.; van Wyk, J.; Kahl, L.P. Brief report: Evaluation of inflammation and atherogenesis biomarkers through 148 weeks postswitch to dolutegravir and rilpivirine in SWORD-1/SWORD-2. J. Acquir. Immune Defic. Syndr. 2022, 91, 73–78. [Google Scholar] [CrossRef]
- Dueñas-Gutiérrez, C.; Buzón, L.; Pedrero-Tomé, R.; Iribarren, J.A.; De Los Santos, I.; De la Fuente, S.; Pousada, G.; Moran, M.A.; Moreno, E.; Ferreira, E.; et al. Efficacy and safety of two-drug regimens with dolutegravir plus rilpivirine or lamivudine in HIV-1 virologically suppressed people living with HIV. Viruses 2023, 15, 936. [Google Scholar] [CrossRef]
- Ciccullo, A.; Baldin, G.; Borghi, V.; Cossu, M.V.; Giacomelli, A.; Lagi, F.; Farinacci, D.; Iannone, V.; Passerotto, R.A.; Capetti, A.; et al. Analysing the efficacy and tolerability of dolutegravir plus either rilpivirine or lamivudine in a multicentre cohort of virologically suppressed PLWHIV. J. Antimicrob. Chemother. 2022, 78, 117–121. [Google Scholar] [CrossRef]
- Lemaitre, F.; Lagoutte-Renosi, J.; Gagnieu, M.C.; Parant, F.; Venisse, N.; Grégoire, M.; Bouchet, S.; Garraffo, R.; Lê, M.P.; Muret, P.; et al. Therapeutic drug monitoring and virological response at week 48 in a cohort of HIV-1-infected patients switching to dolutegravir/rilpivirine dual maintenance therapy (ANRS-MIE-BIRIDER study). Br. J. Clin. Pharmacol. 2024, 90, 264–273. [Google Scholar] [CrossRef]
- Ramos-Ruperto, L.; Arcos-Rueda, M.D.M.; de Miguel-Buckley, R.; Busca-Arenzana, C.; Mican, R.; Montejano, R.; Delgado-Hierro, A.; Montes, M.L.; Valencia, M.E.; Serrano, L.; et al. Sex differences in the effectiveness and tolerability of dolutegravir plus rilpivirine as a switch strategy in people living with HIV. HIV Med. 2024, 25, 684–691. [Google Scholar] [CrossRef]
- Schneider, S.; Blick, G.; Burke, C.; Ward, D.; Benson, P.; Felizarta, F.; Green, D.; Donovan, C.; Harper, G.; Merrill, D.; et al. Two-drug regimens dolutegravir/lamivudine and dolutegravir/rilpivirine are effective with few discontinuations in US real-world settings: Results from the TANDEM study. Infect. Dis. Ther. 2024, 13, 891–906. [Google Scholar] [CrossRef]
- Fernandez, C.; van Halsema, C.L. Evaluating cabotegravir/rilpivirine long-acting, injectable in the treatment of HIV infection: Emerging data and therapeutic potential. HIV AIDS 2019, 11, 179–192. [Google Scholar] [CrossRef]
- Rajoli, R.K.R.; Curley, P.; Chiong, J.; Back, D.; Flexner, C.; Owen, A.; Siccardi, M. Predicting drug-drug interactions between rifampicin and long-acting cabotegravir and rilpivirine using physiologically based pharmacokinetic modeling. J. Infect. Dis. 2019, 219, 1735–1742. [Google Scholar] [CrossRef]
- Swindells, S.; Andrade-Villanueva, J.F.; Richmond, G.J.; Rizzardini, G.; Baumgarten, A.; Masiá, M.; Latiff, G.; Pokrovsky, V.; Bredeek, F.; Smith, G.; et al. Long-acting cabotegravir and rilpivirine for maintenance of HIV-1 suppression. N. Engl. J. Med. 2020, 382, 1112–1123. [Google Scholar] [CrossRef]
- Orkin, C.; Arasteh, K.; Górgolas Hernández-Mora, M.; Pokrovsky, V.; Overton, E.T.; Girard, P.M.; Oka, S.; Walmsley, S.; Bettacchi, C.; Brinson, C.; et al. Long-acting cabotegravir and rilpivirine after oral induction for HIV-1 infection. N. Engl. J. Med. 2020, 382, 1124–1135. [Google Scholar] [CrossRef]
- Currier, J.S. Monthly injectable antiretroviral therapy—Version 1.0 of a new treatment approach. N. Engl. J. Med. 2020, 382, 1164–1165. [Google Scholar] [CrossRef]
- Murray, M.; Antela, A.; Mills, A.; Huang, J.; Jäger, H.; Bernal, E.; Lombaard, J.; Katner, H.; Walmsley, S.; Khuong-Josses, M.A.; et al. Patient-reported outcomes in ATLAS and FLAIR participants on long-acting regimens of cabotegravir and rilpivirine over 48 weeks. AIDS Behav. 2020, 24, 3533–3544. [Google Scholar] [CrossRef]
- Rizzardini, G.; Overton, E.T.; Orkin, C.; Swindells, S.; Arasteh, K.; Górgolas Hernández-Mora, M.; Pokrovsky, V.; Girard, P.M.; Oka, S.; Andrade-Villanueva, J.F.; et al. Long-acting injectable cabotegravir + rilpivirine for HIV maintenance therapy: Week 48 pooled analysis of phase 3 ATLAS and FLAIR trials. J. Acquir. Immune Defic. Syndr. 2020, 85, 498–506. [Google Scholar] [CrossRef]
- Markham, A. Cabotegravir plus rilpivirine: First approval. Drugs 2020, 80, 915–922. [Google Scholar] [CrossRef]
- Cabotegravir and Rilpivirine. Am. J. Health Syst. Pharm. 2021, 78, 925–931. [CrossRef] [PubMed]
- Letendre, S.L.; Mills, A.; Hagins, D.; Swindells, S.; Felizarta, F.; Devente, J.; Bettacchi, C.; Lou, Y.; Ford, S.; Sutton, K.; et al. Pharmacokinetics and antiviral activity of cabotegravir and rilpivirine in cerebrospinal fluid following long-acting injectable administration in HIV-infected adults. J. Antimicrob. Chemother. 2020, 75, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Cutrell, A.G.; Schapiro, J.M.; Perno, C.F.; Kuritzkes, D.R.; Quercia, R.; Patel, P.; Polli, J.W.; Dorey, D.; Wang, Y.; Wu, S.; et al. Exploring predictors of HIV-1 virologic failure to long-acting cabotegravir and rilpivirine: A multivariable analysis. AIDS 2021, 35, 1333–1342. [Google Scholar] [CrossRef]
- Durham, S.H.; Chahine, E.B. Cabotegravir-rilpivirine: The first complete long-acting injectable regimen for the treatment of HIV-1 infection. Ann. Pharmacother. 2021, 55, 1397–1409. [Google Scholar] [CrossRef] [PubMed]
- Mills, A.; Richmond, G.J.; Newman, C.; Osiyemi, O.; Cade, J.; Brinson, C.; De Vente, J.; Margolis, D.A.; Sutton, K.C.; Wilches, V.; et al. Long-acting cabotegravir and rilpivirine for HIV-1 suppression: Switch to 2-monthly dosing after 5 years of daily oral therapy. AIDS 2022, 36, 195–203. [Google Scholar] [CrossRef]
- Orkin, C.; Oka, S.; Philibert, P.; Brinson, C.; Bassa, A.; Gusev, D.; Degen, O.; García, J.G.; Morell, E.B.; Tan, D.H.S.; et al. Long-acting cabotegravir plus rilpivirine for treatment in adults with HIV-1 infection: 96-week results of the randomised, open-label, phase 3 FLAIR study. Lancet HIV 2021, 8, e185–e196. [Google Scholar] [CrossRef]
- Jaeger, H.; Overton, E.T.; Richmond, G.; Rizzardini, G.; Andrade-Villanueva, J.F.; Mngqibisa, R.; Hermida, A.O.; Thalme, A.; Belonosova, E.; Ajana, F.; et al. Long-acting cabotegravir and rilpivirine dosed every 2 months in adults with HIV-1 infection (ATLAS-2M), 96-week results: A randomised, multicentre, open-label, phase 3b, non-inferiority study. Lancet HIV 2021, 8, e679–e689. [Google Scholar] [CrossRef]
- Hodge, D.; Back, D.J.; Gibbons, S.; Khoo, S.H.; Marzolini, C. Pharmacokinetics and drug-drug interactions of long-acting intramuscular cabotegravir and rilpivirine. Clin. Pharmacokinet. 2021, 60, 835–853. [Google Scholar] [CrossRef]
- Zeuli, J.D.; Rivera, C.G.; Smith, B.L.; Otto, A.; Temesgen, Z. Cabotegravir: A novel HIV integrase inhibitor combined with rilpivirine as the first long-acting injectable program for the treatment of HIV infection. Drugs Today 2022, 58, 555–576. [Google Scholar] [CrossRef]
- Bares, S.H.; Scarsi, K.K. A new paradigm for antiretroviral delivery: Long-acting cabotegravir and rilpivirine for the treatment and prevention of HIV. Curr. Opin. HIV AIDS 2022, 17, 22–31. [Google Scholar] [CrossRef]
- Rusconi, S.; Santoro, M.M.; Capetti, A.F.; Gianotti, N.; Zazzi, M. The future of long-acting cabotegravir plus rilpivirine therapy: Deeds and misconceptions. Int. J. Antimicrob. Agents 2022, 60, 106627. [Google Scholar] [CrossRef]
- Steegen, K.; Chandiwana, N.; Sokhela, S.; Venter, W.D.F.; Hans, L. Impact of rilpivirine cross-resistance on long-acting cabotegravir-rilpivirine in low and middle-income countries. AIDS 2023, 37, 1009–1011. [Google Scholar] [CrossRef]
- Jeffrey, J.L.; St Clair, M.; Wang, P.; Wang, C.; Li, Z.; Beloor, J.; Talarico, C.; Fridell, R.; Krystal, M.; White, C.T.; et al. Impact of Integrase Sequences from HIV-1 Subtypes A6/A1 on the In Vitro Potency of Cabotegravir or Rilpivirine. Antimicrob. Agents Chemother. 2022, 66, e0170221. [Google Scholar] [CrossRef] [PubMed]
- Maruapula, D.; Moraka, N.O.; Bareng, O.T.; Mokgethi, P.T.; Choga, W.T.; Seatla, K.K.; Kelentse, N.; Koofhethille, C.K.; Zuze, B.J.L.; Gaolathe, T.; et al. Archived rilpivirine-associated resistance mutations among ART-naive and virologically suppressed people living with HIV-1 subtype C in Botswana: Implications for cabotegravir/rilpivirine use. J. Antimicrob. Chemother. 2023, 78, 2489–2495. [Google Scholar] [CrossRef] [PubMed]
- Masich, A.M.; Gomes, D.; Higginson, R.T.; Morgan, Z.; Nixon, D.; Tran, M.; Winthrop, E.; Fulco, P.P. HIV virologic response and baseline genotypic resistance in a long-acting cabotegravir/rilpivirine initiation program. AIDS 2023, 37, 1641–1642. [Google Scholar] [CrossRef] [PubMed]
- Overton, E.T.; Richmond, G.; Rizzardini, G.; Thalme, A.; Girard, P.M.; Wong, A.; Porteiro, N.; Swindells, S.; Reynes, J.; Noe, S.; et al. Long-acting cabotegravir and rilpivirine dosed every 2 months in adults with human immunodeficiency virus 1 type 1 infection: 152-week results from ATLAS-2M, a randomized, open-label, phase 3b, noninferiority study. Clin. Infect. Dis. 2023, 76, 1646–1654. [Google Scholar] [CrossRef]
- Qazzaz, H.; Parganas, C.; Cory, T.J. An evaluation of long-acting cabotegravir + rilpivirine for the treatment of virologically suppressed adults living with HIV. Expert. Opin. Pharmacother. 2022, 23, 1485–1495. [Google Scholar] [CrossRef]
- Rakhmanina, N.; Richards, K.; Adeline Koay, W.L. Transient viremia in young adults with HIV after the switch to long-acting cabotegravir and rilpivirine: Considerations for dosing schedule and monitoring. J. Acquir. Immune Defic. Syndr. 2023, 92, e14–e17. [Google Scholar] [CrossRef]
- Patel, P.; Ford, S.L.; Baker, M.; Meyer, C.; Garside, L.; D’Amico, R.; Van Solingen-Ristea, R.; Crauwels, H.; Polli, J.W.; Seal, C.; et al. Pregnancy outcomes and pharmacokinetics in pregnant women living with HIV exposed to long-acting cabotegravir and rilpivirine in clinical trials. HIV Med. 2023, 24, 568–579. [Google Scholar] [CrossRef]
- Moffatt, K.; Tekko, I.A.; Vora, L.; Volpe-Zanutto, F.; Hutton, A.R.J.; Mistilis, J.; Jarrahian, C.; Akhavein, N.; Weber, A.D.; McCarthy, H.O.; et al. Development and evaluation of dissolving microarray patches for co-administered and repeated intradermal delivery of long-acting rilpivirine and cabotegravir nanosuspensions for paediatric HIV antiretroviral therapy. Pharm. Res. 2023, 40, 1673–1696. [Google Scholar] [CrossRef]
- Bettonte, S.; Berton, M.; Stader, F.; Battegay, M.; Marzolini, C. Management of drug-drug interactions between long-acting cabotegravir and rilpivirine and comedications with inducing properties: A modeling study. Clin. Infect. Dis. 2023, 76, 1225–1236. [Google Scholar] [CrossRef]
- Llibre, J.M.; Kuritzkes, D.A.R. Long-acting cabotegravir and rilpivirine: Innovation, new challenges, and opportunities. Clin. Infect. Dis. 2023, 76, 1655–1657. [Google Scholar] [CrossRef]
- Parker, B.; Ward, T.; Hayward, O.; Jacob, I.; Arthurs, E.; Becker, D.; Anderson, S.J.; Chounta, V.; Van de Velde, N. Cost-effectiveness of the long-acting regimen cabotegravir plus rilpivirine for the treatment of HIV-1 and its potential impact on adherence and viral transmission: A modelling study. PLoS ONE 2021, 16, e0245955. [Google Scholar] [CrossRef]
- Moreno, S.; Rivero, A.; Ventayol, P.; Falcó, V.; Torralba, M.; Schroeder, M.; Neches, V.; Vallejo-Aparicio, L.A.; Mackenzie, I.; Turner, M.; et al. Cabotegravir and rilpivirine long-acting antiretroviral therapy administered every 2 months is cost-effective for the treatment of HIV-1 in Spain. Infect. Dis. Ther. 2023, 12, 2039–2055. [Google Scholar] [CrossRef]
- Nachega, J.B.; Scarsi, K.K.; Gandhi, M.; Scott, R.K.; Mofenson, L.M.; Archary, M.; Nachman, S.; Decloedt, E.; Geng, E.H.; Wilson, L.; et al. Long-acting antiretrovirals and HIV treatment adherence. Lancet HIV 2023, 10, e332–e342. [Google Scholar] [CrossRef]
- Patel, P.; Teichner, P.; Elliot, E.; Boffito, M.; Murray, M.; Polli, J.W.; Baker, M.; Ford, S.L.; Han, K.; Russu, A.; et al. Practical dosing guidance for the management of clinician-administered injections of long-acting cabotegravir and rilpivirine. Ther. Adv. Infect. Dis. 2023, 10, 20499361231214626. [Google Scholar] [CrossRef]
- Orkin, C.; Hayes, R.; Haviland, J.; Wong, Y.L.; Ring, K.; Apea, V.; Kasadha, B.; Clarke, E.; Byrne, R.; Fox, J.; et al. Perspectives of people with HIV on implementing long acting cabotegravir plus rilpivirine in clinics and community settings in the UK: Results from the anti-sexist, anti-racist, anti-ageist ILANA study. Clin. Infect. Dis. 2025, 80, 1103–1113. [Google Scholar] [CrossRef]
- Hickey, M.D.; Gistand, N.; Grochowski, J.; Mayorga-Munoz, F.; Imbert, E.; Szumowski, J.D.; Oskarsson, J.; Shiels, M.; Dilworth, S.; Appa, A.; et al. 48-week viral suppression rates in people with HIV starting long-acting CAB/RPV with initial viremia. Clin. Infect. Dis. 2025, 80, 864–870. [Google Scholar] [CrossRef]
- Serris, A.; Ferre, V.M.; Le Hingrat, Q.; Bachelard, A.; Charpentier, C.; Exarchopoulos, M.; Damond, F.; Phung, B.C.; Landman, R.; Yazdanpanah, Y.; et al. Real-world data on long-acting intramuscular maintenance therapy with cabotegravir and rilpivirine mirror Phase 3 results. J. Antimicrob. Chemother. 2024, 79, 2932–2938. [Google Scholar] [CrossRef]
- Davis, J.M.; Rana, A.; Sax, P.E.; Bares, S.H. Long-acting cabotegravir plus rilpivirine in people with HIV with adherence challenges and viremia: Current data and future directions. Clin. Infect. Dis. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Muccini, C.; Gianotti, N.; Lolatto, R.; Nozza, S.; Diotallevi, S.; Castagna, A. CD4+/CD8+ improvement after switch from a second-generation integrase inhibitor regimen to long-acting cabotegravir and rilpivirine. AIDS 2024, 38, 1890–1892. [Google Scholar] [CrossRef]
- Kirk, S.E.; Young, C.; Berry, H.; Hanson, R.; Moreland, A.; Fonner, V.; Gebregziabher, M.; Williams, J.; Meissner, E.G. Comparison of at-home versus in-clinic receipt of long-acting injectable cabotegravir/rilpivirine. Clin. Infect. Dis. 2025, 80, 613–617. [Google Scholar] [CrossRef]
- Han, K.; Gevorkyan, H.; Sadik Shaik, J.; Crauwels, H.; Leemereise, C.; Bontempo, G.; Win, B.; Chounta, V.; Seal, C.; DeMoor, R.; et al. Pharmacokinetics and tolerability of cabotegravir and rilpivirine long-acting intramuscular injections to the vastus lateralis (lateral thigh) muscles of healthy adult participants. Antimicrob. Agents Chemother. 2024, 68, e0078123. [Google Scholar] [CrossRef] [PubMed]
- Cossu, M.V.; D’Avolio, A.; Gervasoni, C.; Giacomelli, A.; Cattaneo, D.; Moschese, D. Switching to deltoid intramuscular injections maintains therapeutic trough concentrations of rilpivirine and cabotegravir in people with HIV. Antimicrob. Agents Chemother. 2024, 68, e0017524. [Google Scholar] [CrossRef] [PubMed]
- Maguire, C.; Rueve, K.; Farmer, E.; Huesgen, E.; Karaj, A.; Binkley, A.; Mounzer, K.; Brizzi, M.; Chary, P.; Sung, P.; et al. Real world virologic outcomes in patients with elevated body mass index receiving long acting cabotegravir/rilpivirine. Clin. Infect. Dis. 2024; online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Elliot, E.R.; Polli, J.W.; Patel, P.; Garside, L.; Grove, R.; Barnett, V.; Roberts, J.; Byrapuneni, S.; Crauwels, H.; Ford, S.L.; et al. Efficacy, safety, and pharmacokinetics by body mass index category in phase 3/3b long-acting cabotegravir plus rilpivirine trials. J. Infect. Dis. 2024, 230, e34–e42. [Google Scholar] [CrossRef]
- Gaur, A.H.; Capparelli, E.V.; Calabrese, K.; Baltrusaitis, K.; Marzinke, M.A.; McCoig, C.; Van Solingen-Ristea, R.M.; Mathiba, S.R.; Adeyeye, A.; Moye, J.H.; et al. Safety and pharmacokinetics of oral and long-acting injectable cabotegravir or long-acting injectable rilpivirine in virologically suppressed adolescents with HIV (IMPAACT 2017/MOCHA): A phase 1/2, multicentre, open-label, non-comparative, dose-finding study. Lancet HIV 2024, 11, e211–e221. [Google Scholar] [CrossRef]
- Collins, L.F.; Sheth, A.N.; Tisdale, T.; Mehta, C.C.; Daniel, G.; Westreich, D.; Kassaye, S.; Topper, E.F.; Konkle-Parker, D.; Rana, A.; et al. Interest in and preference for long-acting injectable antiretroviral therapy in the era of approved cabotegravir/rilpivirine among reproductive-aged women in the U.S. south. Clin. Infect. Dis. 2025, 80, 164–167. [Google Scholar] [CrossRef]
- Gutner, C.A.; Hocqueloux, L.; Jonsson-Oldenbüttel, C.; Vandekerckhove, L.; van Welzen, B.J.; Slama, L.; Crusells-Canales, M.; Sierra, J.O.; DeMoor, R.; Scherzer, J.; et al. Implementation of long-acting cabotegravir and rilpivirine: Primary results from the perspective of staff study participants in the Cabotegravir and Rilpivirine Implementation Study in European Locations. J. Int. AIDS Soc. 2024, 27, e26243. [Google Scholar] [CrossRef]
- Hickey, M.D.; Grochowski, J.; Mayorga-Munoz, F.; Oskarsson, J.; Imbert, E.; Spinelli, M.; Szumowski, J.D.; Appa, A.; Koester, K.; Dauria, E.F.; et al. Identifying implementation determinants and strategies for long-acting injectable cabotegravir-rilpivirine in people with HIV who are virally unsuppressed. J. Acquir. Immune Defic. Syndr. 2024, 96, 280–289. [Google Scholar] [CrossRef]
- van Welzen, B.J.; Van Lelyveld, S.F.L.; Ter Beest, G.; Gisolf, J.H.; Geerlings, S.E.; Prins, J.M.; Van Twillert, G.; Van Nieuwkoop, C.; Van der Valk, M.; Burger, D.; et al. Virological failure after switch to long-acting cabotegravir and rilpivirine injectable therapy: An in-depth analysis. Clin. Infect. Dis. 2024, 79, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Ring, K.; Smuk, M.; Shongwe, M.; Okonta, L.; Mackie, N.E.; Ayres, S.; Barber, T.J.; Akodu, J.; Ferro, F.; Chilton, D.; et al. Multicentre service evaluation of injectable cabotegravir and rilpivirine delivery and outcomes across 12 UK clinics (SHARE LAI-net). HIV Med. 2024, 25, 1125–1134. [Google Scholar] [CrossRef]
- Kityo, C.; Mambule, I.K.; Musaazi, J.; Sokhela, S.; Mugerwa, H.; Ategeka, G.; Cresswell, F.; Siika, A.; Kosgei, J.; Shah, R.; et al. Switch to long-acting cabotegravir and rilpivirine in virologically suppressed adults with HIV in Africa (CARES): Week 48 results from a randomised, multicentre, open-label, non-inferiority trial. Lancet Infect. Dis. 2024, 24, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Orkin, C.; Ring, K. Implementing long-acting injectable cabotegravir and rilpivirine in Africa. Lancet Infect. Dis. 2024, 24, 1060–1061. [Google Scholar] [CrossRef]
- Jonsson-Oldenbüttel, C.; Ghosn, J.; van der Valk, M.; Florence, E.; Vera, F.; De Wit, S.; Rami, A.; Bonnet, F.; Hocqueloux, L.; Hove, K.; et al. Safety and effectiveness from the cabotegravir and rilpivirine implementation study in European locations study: Phase 3b hybrid type III implementation study integrating cabotegravir + rilpivirine long-acting into European clinical settings. J. Acquir. Immune Defic. Syndr. 2024, 96, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Luc, C.M.; Max, B.; Pérez, S.; Herrera, K.; Huhn, G.; Dworkin, M.S. Acceptability of long-acting cabotegravir + rilpivirine in a large, urban, ambulatory HIV clinic. J. Acquir. Immune Defic. Syndr. 2024, 97, 409–415. [Google Scholar] [CrossRef]
- Ring, K.; Orkin, C. Long-acting antiretroviral therapy in the context of viral suppression. Curr. Opin. HIV AIDS 2025, 20, 4–10. [Google Scholar] [CrossRef]
- Christopoulos, K.A.; Hickey, M.D.; Rana, A. Use of long-acting injectable cabotegravir/rilpivirine in people with HIV and adherence challenges. Curr. Opin. HIV AIDS 2025, 20, 11–18. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Clercq, E. The Meandrous Route of Rilpivirine in the Search for the Miraculous Drug to Treat HIV Infections. Viruses 2025, 17, 959. https://doi.org/10.3390/v17070959
De Clercq E. The Meandrous Route of Rilpivirine in the Search for the Miraculous Drug to Treat HIV Infections. Viruses. 2025; 17(7):959. https://doi.org/10.3390/v17070959
Chicago/Turabian StyleDe Clercq, Erik. 2025. "The Meandrous Route of Rilpivirine in the Search for the Miraculous Drug to Treat HIV Infections" Viruses 17, no. 7: 959. https://doi.org/10.3390/v17070959
APA StyleDe Clercq, E. (2025). The Meandrous Route of Rilpivirine in the Search for the Miraculous Drug to Treat HIV Infections. Viruses, 17(7), 959. https://doi.org/10.3390/v17070959





