Interactions Between Hantavirus Nucleoprotein and Glycoproteins: A Quantitative Fluorescence Microscopy Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. NP-NP Interactions in Human Epithelial Cells Are Stronger than in Rodent Epithelial Cells
3.2. NP Interacts with Gc Within Few Small Intracellular Regions
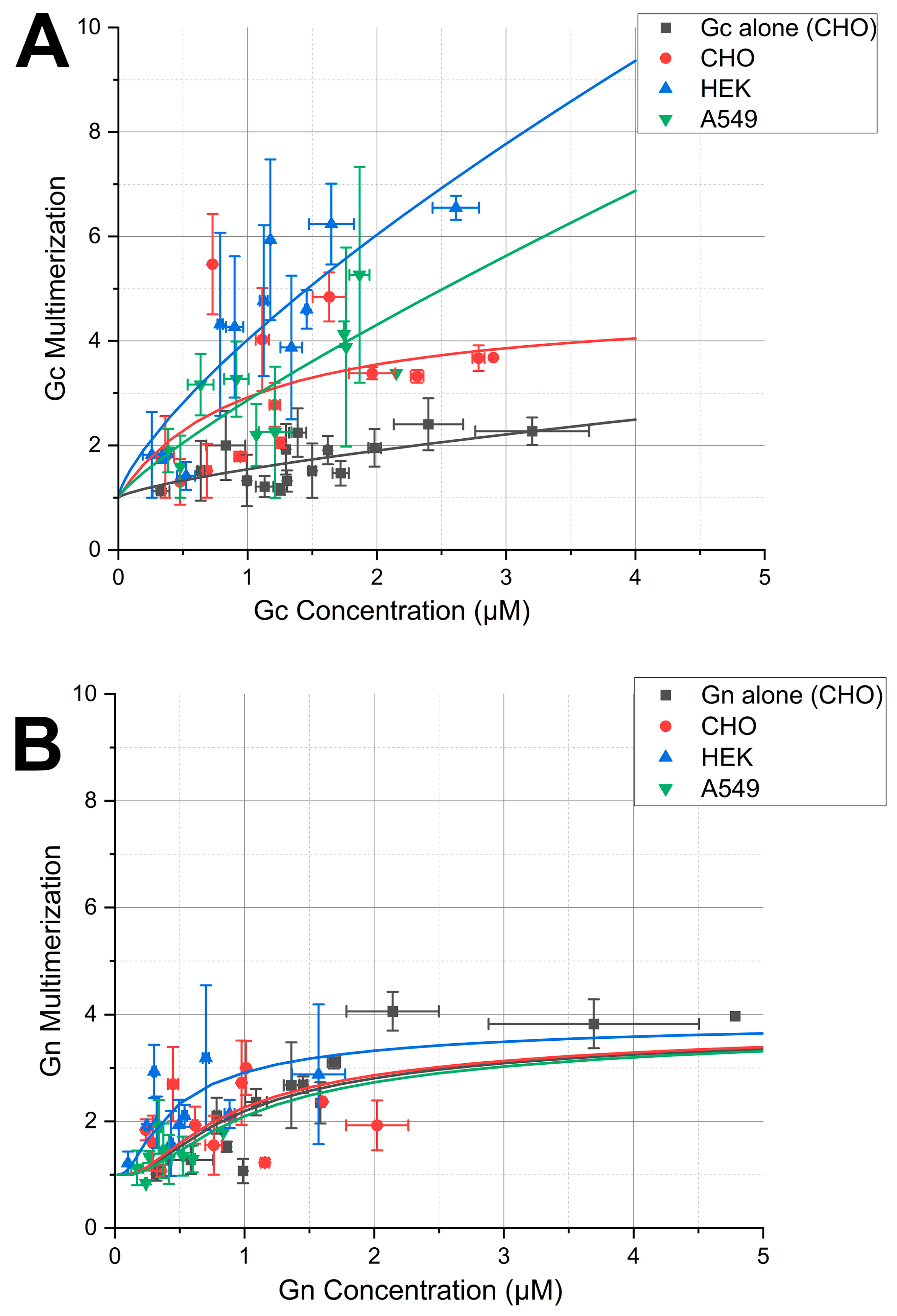
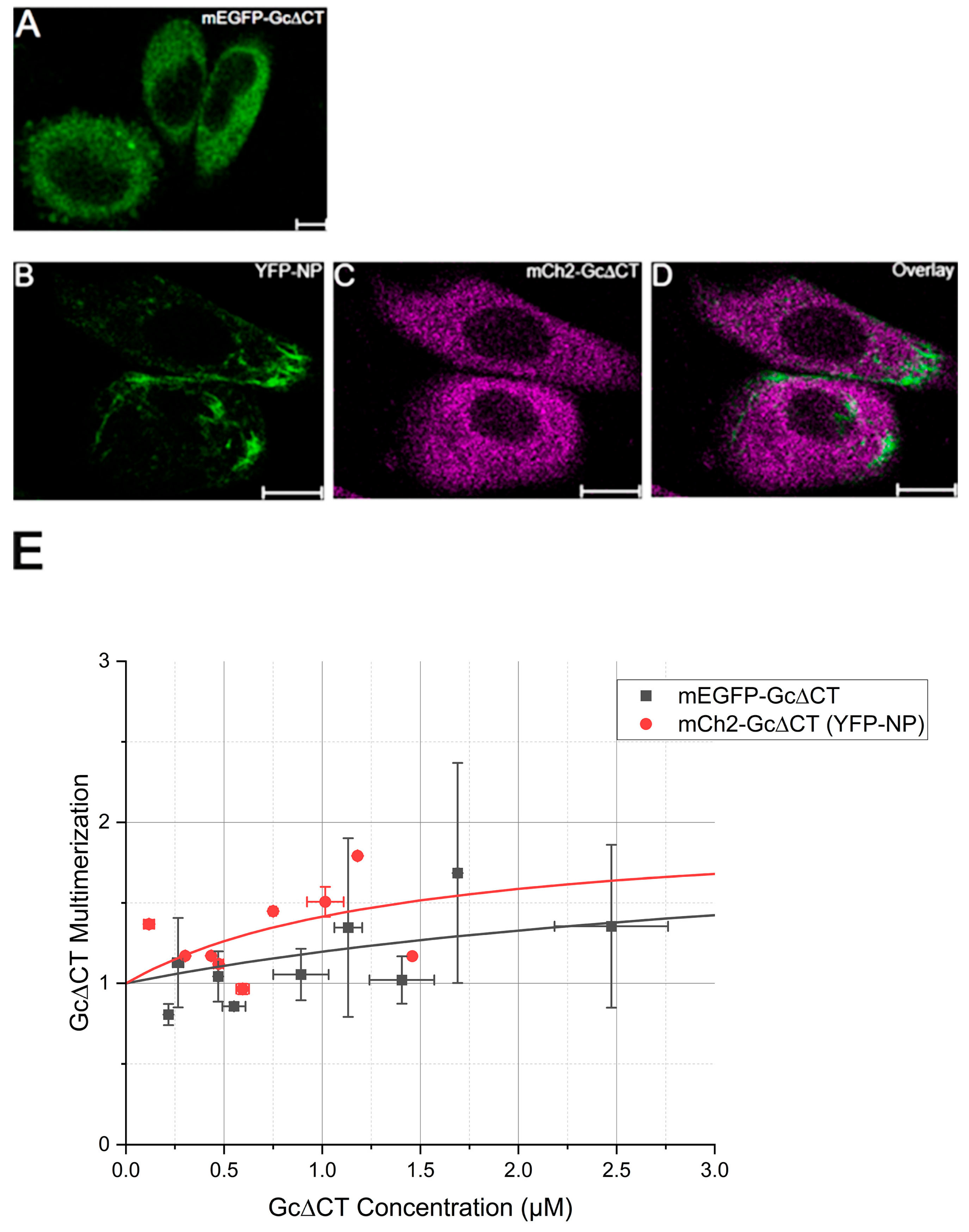
3.3. Apparent Gc Multimerization Increases in the Presence of NP
3.4. Gn Remains in a Monomer–Tetramer Equilibrium, Independent of the Presence of NP
3.5. Deletion of Gc-CT Domain Abolishes the Enhancement of Gc-Gc Interactions Induced by NP
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HVs | Orthohantaviruses |
| GPs | Glycoproteins |
| RdRp | RNA-dependent RNA polymerase |
| RNPs | Ribonucleoproteins |
| NP | Nucleoprotein |
| CT | Cytoplasmic tail |
| PUUV | Puumala orthohantavirus |
| N&B | Number and Brightness |
| ccN&B | Cross-correlation N&B |
| CHO | Chinese hamster ovary |
| HEK | Human embryonic kidney |
| A549 | Adenocarcinomic human alveolar basal epithelial |
| SP | Signal peptide |
| GPC | Glycoprotein precursor |
| FP | Fluorescent protein |
| YFP | Yellow fluorescent protein |
| mTurq2 | Monomeric Turqoise2 |
| mEGFP | Monomeric enhanced green FP |
| ∆CT | devoid of cytoplasmic tail |
| SD | standard deviation |
References
- Kim, W.K.; Cho, S.; Lee, S.H.; No, J.S.; Lee, G.Y.; Park, K.; Lee, D.; Jeong, S.T.; Song, J.W. Genomic Epidemiology and Active Surveillance to Investigate Outbreaks of Hantaviruses. Front. Cell Infect. Microbiol. 2020, 10, 532388. [Google Scholar] [CrossRef] [PubMed]
- Hepojoki, J.; Strandin, T.; Lankinen, H.; Vaheri, A. Hantavirus structure--molecular interactions behind the scene. J. Gen. Virol. 2012, 93, 1631–1644. [Google Scholar] [CrossRef]
- Bignon, E.A.; Albornoz, A.; Guardado-Calvo, P.; Rey, F.A.; Tischler, N.D. Molecular organization and dynamics of the fusion protein Gc at the hantavirus surface. Elife 2019, 8, e46028. [Google Scholar] [CrossRef]
- Petazzi, R.A.; Koikkarah, A.A.; Tischler, N.D.; Chiantia, S. Detection of Envelope Glycoprotein Assembly from Old-World Hantaviruses in the Golgi Apparatus of Living Cells. J. Virol. 2021, 95, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Olal, D.; Daumke, O. Structure of the Hantavirus Nucleoprotein Provides Insights into the Mechanism of RNA Encapsidation. Cell Rep. 2016, 14, 2092–2099. [Google Scholar] [CrossRef] [PubMed]
- Hepojoki, J.; Strandin, T.; Wang, H.; Vapalahti, O.; Vaheri, A.; Lankinen, H. Cytoplasmic tails of hantavirus glycoproteins interact with the nucleocapsid protein. J. Gen. Virol. 2010, 91, 2341–2350. [Google Scholar] [CrossRef]
- Wang, H.; Alminaite, A.; Vaheri, A.; Plyusnin, A. Interaction between hantaviral nucleocapsid protein and the cytoplasmic tail of surface glycoprotein Gn. Virus Res. 2010, 151, 205–212. [Google Scholar] [CrossRef]
- Strandin, T.; Hepojoki, J.; Wang, H.; Vaheri, A.; Lankinen, H. The cytoplasmic tail of hantavirus Gn glycoprotein interacts with RNA. Virology 2011, 418, 12–20. [Google Scholar] [CrossRef]
- Shimizu, K.; Yoshimatsu, K.; Koma, T.; Yasuda, S.P.; Arikawa, J. Role of nucleocapsid protein of hantaviruses in intracellular traffic of viral glycoproteins. Virus Res. 2013, 178, 349–356. [Google Scholar] [CrossRef]
- Strandin, T.; Hepojoki, J.; Vaheri, A. Cytoplasmic tails of bunyavirus Gn glycoproteins-Could they act as matrix protein surrogates? Virology 2013, 437, 73–80. [Google Scholar] [CrossRef]
- Welke, R.W.; Sperber, H.S.; Bergmann, R.; Koikkarah, A.; Menke, L.; Sieben, C.; Kruger, D.H.; Chiantia, S.; Herrmann, A.; Schwarzer, R. Characterization of Hantavirus N Protein Intracellular Dynamics and Localization. Viruses 2022, 14, 457. [Google Scholar] [CrossRef]
- Wohland, T.; Maiti, S.; Macháň, R. An Introduction to Fluorescence Correlation Spectroscopy; IOP Publishing Ltd.: Bristol, UK, 2020. [Google Scholar]
- Petrich, A.; Dunsing, V.; Bobone, S.; Chiantia, S. Influenza A M2 recruits M1 to the plasma membrane: A fluorescence fluctuation microscopy study. Biophys. J. 2021, 120, 5478–5490. [Google Scholar] [CrossRef]
- Sperber, H.S.; Welke, R.W.; Petazzi, R.A.; Bergmann, R.; Schade, M.; Shai, Y.; Chiantia, S.; Herrmann, A.; Schwarzer, R. Self-association and subcellular localization of Puumala hantavirus envelope proteins. Sci. Rep. 2019, 9, 707. [Google Scholar] [CrossRef] [PubMed]
- Guardado-Calvo, P.; Bignon, E.A.; Stettner, E.; Jeffers, S.A.; Pérez-Vargas, J.; Pehau-Arnaudet, G.; Tortorici, M.A.; Jestin, J.-L.; England, P.; Tischler, N.D.; et al. Mechanistic Insight into Bunyavirus-Induced Membrane Fusion from Structure-Function Analyses of the Hantavirus Envelope Glycoprotein Gc. PLoS Pathog. 2016, 12, e1005813. [Google Scholar] [CrossRef] [PubMed]
- Noack, D.; Goeijenbier, M.; Reusken, C.; Koopmans, M.P.G.; Rockx, B.H.G. Orthohantavirus Pathogenesis and Cell Tropism. Front. Cell Infect. Microbiol. 2020, 10, 399. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Rissanen, I.; Zeltina, A.; Hepojoki, J.; Raghwani, J.; Harlos, K.; Pybus, O.G.; Huiskonen, J.T.; Bowden, T.A. A Molecular-Level Account of the Antigenic Hantaviral Surface. Cell Rep. 2016, 16, 278. [Google Scholar] [CrossRef]
- Ravkov, E.V.; Compans, R.W. Hantavirus nucleocapsid protein is expressed as a membrane-associated protein in the perinuclear region. J. Virol. 2001, 75, 1808–1815. [Google Scholar] [CrossRef]
- Engdahl, T.B.; Kuzmina, N.A.; Ronk, A.J.; Mire, C.E.; Hyde, M.A.; Kose, N.; Josleyn, M.D.; Sutton, R.E.; Mehta, A.; Wolters, R.M.; et al. Broad and potently neutralizing monoclonal antibodies isolated from human survivors of New World hantavirus infection. Cell Rep. 2021, 36, 109453. [Google Scholar] [CrossRef]
- Wang, Z.; Ren, S.; Li, Q.; Royster, A.D.; Lin, L.; Liu, S.; Ganaie, S.S.; Qiu, J.; Mir, S.; Mir, M.A. Hantaviruses use the endogenous host factor P58IPK to combat the PKR antiviral response. PLoS Pathog. 2021, 17, e1010007. [Google Scholar] [CrossRef]
- Vera-Otarola, J.; Castillo-Vargas, E.; Angulo, J.; Barriga, F.M.; Batlle, E.; Lopez-Lastra, M. The viral nucleocapsid protein and the human RNA-binding protein Mex3A promote translation of the Andes orthohantavirus small mRNA. PLoS Pathog. 2021, 17, e1009931. [Google Scholar] [CrossRef]
- Gallo, G.; Kotlik, P.; Roingeard, P.; Monot, M.; Chevreux, G.; Ulrich, R.G.; Tordo, N.; Ermonval, M. Diverse susceptibilities and responses of human and rodent cells to orthohantavirus infection reveal different levels of cellular restriction. PLoS Negl. Trop. Dis. 2022, 16, e0010844. [Google Scholar] [CrossRef]
- Sola-Riera, C.; Garcia, M.; Ljunggren, H.G.; Klingstrom, J. Hantavirus inhibits apoptosis by preventing mitochondrial membrane potential loss through up-regulation of the pro-survival factor BCL-2. PLoS Pathog. 2020, 16, e1008297. [Google Scholar] [CrossRef] [PubMed]
- Muyangwa, M.; Garanina, E.E.; Martynova, E.V.; Rizvanov, A.A. Lentivirus Expression of Hantavirus Nucleocapsid Proteins. BioNanoScience 2016, 6, 403–406. [Google Scholar] [CrossRef]
- Linkert, M.; Rueden, C.T.; Allan, C.; Burel, J.-M.; Moore, W.; Patterson, A.; Loranger, B.; Moore, J.; Neves, C.; MacDonald, D.; et al. Metadata matters: Access to image data in the real world. J. Cell Biol. 2010, 189, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Digman, M.A.; Dalal, R.; Horwitz, A.F.; Gratton, E. Mapping the number of molecules and brightness in the laser scanning microscope. Biophys. J. 2008, 94, 2320–2332. [Google Scholar] [CrossRef]
- Dunsing, V.; Luckner, M.; Zühlke, B.; Petazzi, R.A.; Herrmann, A.; Chiantia, S. Optimal fluorescent protein tags for quantifying protein oligomerization in living cells. Sci. Rep. 2018, 8, 10634. [Google Scholar] [CrossRef]
- Digman, M.A.; Wiseman, P.W.; Choi, C.; Horwitz, A.R.; Gratton, E. Stoichiometry of molecular complexes at adhesions in living cells. Proc. Natl. Acad. Sci. USA 2009, 106, 2170–2175. [Google Scholar] [CrossRef]
- Hagele, S.; Muller, A.; Nusshag, C.; Reiser, J.; Zeier, M.; Krautkramer, E. Virus- and cell type-specific effects in orthohantavirus infection. Virus Res. 2019, 260, 102–113. [Google Scholar] [CrossRef]
- Davies, K.A.; Chadwick, B.; Hewson, R.; Fontana, J.; Mankouri, J.; Barr, J.N. The RNA Replication Site of Tula Orthohantavirus Resides within a Remodelled Golgi Network. Cells 2020, 9, 1569. [Google Scholar] [CrossRef] [PubMed]
- Merten, J.A.; Schultz, K.M.; Klug, C.S. Concentration-dependent oligomerization and oligomeric arrangement of LptA. Protein Sci. 2012, 21, 211–218. [Google Scholar] [CrossRef]
- Paul, M.D.; Rainwater, R.; Zuo, Y.; Gu, L.; Hristova, K. Probing Membrane Protein Association Using Concentration-Dependent Number and Brightness. Angew. Chem. Int. Ed. Engl. 2021, 60, 6503–6508. [Google Scholar] [CrossRef] [PubMed]
- Willensky, S.; Bar-Rogovsky, H.; Bignon, E.A.; Tischler, N.D.; Modis, Y.; Dessau, M. Crystal Structure of Glycoprotein C from a Hantavirus in the Post-fusion Conformation. PLoS Pathog. 2016, 12, e1005948. [Google Scholar] [CrossRef] [PubMed]
- Reuter, M.; Kruger, D.H. The nucleocapsid protein of hantaviruses: Much more than a genome-wrapping protein. Virus Genes. 2018, 54, 5–16. [Google Scholar] [CrossRef]
- Ramanathan, H.N.; Jonsson, C.B. New and Old World hantaviruses differentially utilize host cytoskeletal components during their life cycles. Virology 2008, 374, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.J.; Lee, Y.T.; Chu, H.; Son, S.Y.; Kim, M. Phosphorylation of the nucleocapsid protein of Hantaan virus by casein kinase II. J. Microbiol. 2015, 53, 343–347. [Google Scholar] [CrossRef]
- Gorbunova, E.E.; Mackow, E.R. Binding of the Andes Virus Nucleocapsid Protein to RhoGDI Induces the Release and Activation of the Permeability Factor RhoA. J. Virol. 2021, 95, e0039621. [Google Scholar] [CrossRef]
- Kaukinen, P.; Vaheri, A.; Plyusnin, A. Non-covalent interaction between nucleocapsid protein of Tula hantavirus and small ubiquitin-related modifier-1, SUMO-1. Virus Res. 2003, 92, 37–45. [Google Scholar] [CrossRef]
- Lin, Y.; Zhu, Y.; Jing, L.; Lei, X.; Xie, Z. Regulation of viral replication by host restriction factors. Front. Immunol. 2025, 16, 1484119. [Google Scholar] [CrossRef]
- Snippe, M.; Willem Borst, J.; Goldbach, R.; Kormelink, R. Tomato spotted wilt virus Gc and N proteins interact in vivo. Virology 2007, 357, 115–123. [Google Scholar] [CrossRef]
- Ribeiro, D.; Borst, J.W.; Goldbach, R.; Kormelink, R. Tomato spotted wilt virus nucleocapsid protein interacts with both viral glycoproteins Gn and Gc in planta. Virology 2009, 383, 121–130. [Google Scholar] [CrossRef]
- Dunsing, V.; Petrich, A.; Chiantia, S. Multicolor fluorescence fluctuation spectroscopy in living cells via spectral detection. Elife 2021, 10, e69687. [Google Scholar] [CrossRef] [PubMed]
- Götzke, H.; Kilisch, M.; Martínez-Carranza, M.; Sograte-Idrissi, S.; Rajavel, A.; Schlichthaerle, T.; Engels, N.; Jungmann, R.; Stenmark, P.; Opazo, F.; et al. The ALFA-tag is a highly versatile tool for nanobody-based bioscience applications. Nat. Commun. 2019, 10, 4403. [Google Scholar] [CrossRef] [PubMed]
- Arsic, A.; Hagemann, C.; Stajkovic, N.; Schubert, T.; Nikic-Spiegel, I. Minimal genetically encoded tags for fluorescent protein labeling in living neurons. Nat. Commun. 2022, 13, 314. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aji, A.K.; Mandal, T.; Chiantia, S. Interactions Between Hantavirus Nucleoprotein and Glycoproteins: A Quantitative Fluorescence Microscopy Study. Viruses 2025, 17, 940. https://doi.org/10.3390/v17070940
Aji AK, Mandal T, Chiantia S. Interactions Between Hantavirus Nucleoprotein and Glycoproteins: A Quantitative Fluorescence Microscopy Study. Viruses. 2025; 17(7):940. https://doi.org/10.3390/v17070940
Chicago/Turabian StyleAji, Amit Koikkarah, Titas Mandal, and Salvatore Chiantia. 2025. "Interactions Between Hantavirus Nucleoprotein and Glycoproteins: A Quantitative Fluorescence Microscopy Study" Viruses 17, no. 7: 940. https://doi.org/10.3390/v17070940
APA StyleAji, A. K., Mandal, T., & Chiantia, S. (2025). Interactions Between Hantavirus Nucleoprotein and Glycoproteins: A Quantitative Fluorescence Microscopy Study. Viruses, 17(7), 940. https://doi.org/10.3390/v17070940





