West Nile Virus Pilot Screening in Field-Collected Aedes japonicus (Theobald, 1901): An Update of Species Distribution in Poland, 2025
Abstract
1. Introduction
2. Materials and Methods
2.1. Mosquito Collection and Location Characteristics
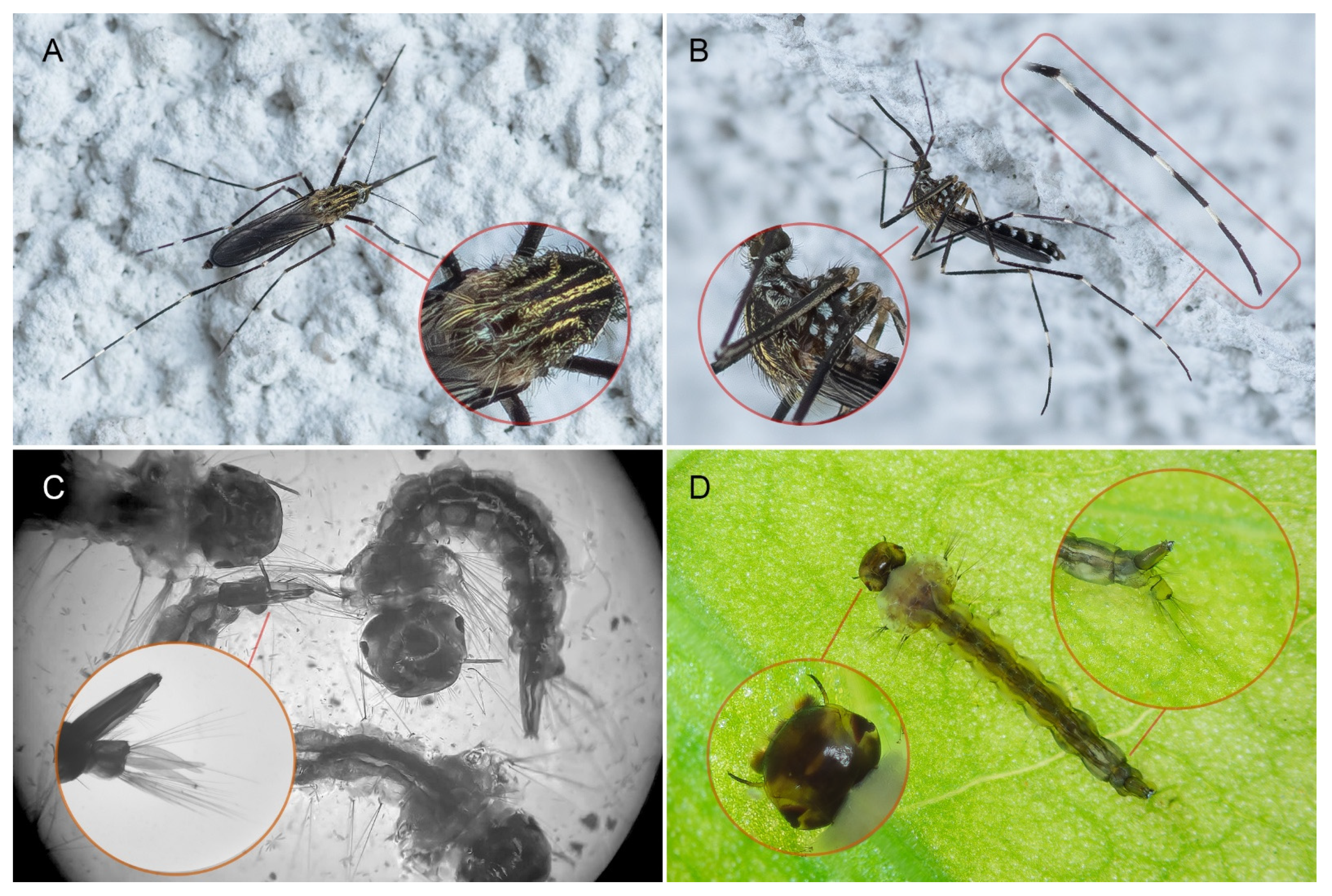
2.2. Aedes japonicus Species Identification
2.3. Screening for the Presence of WNV
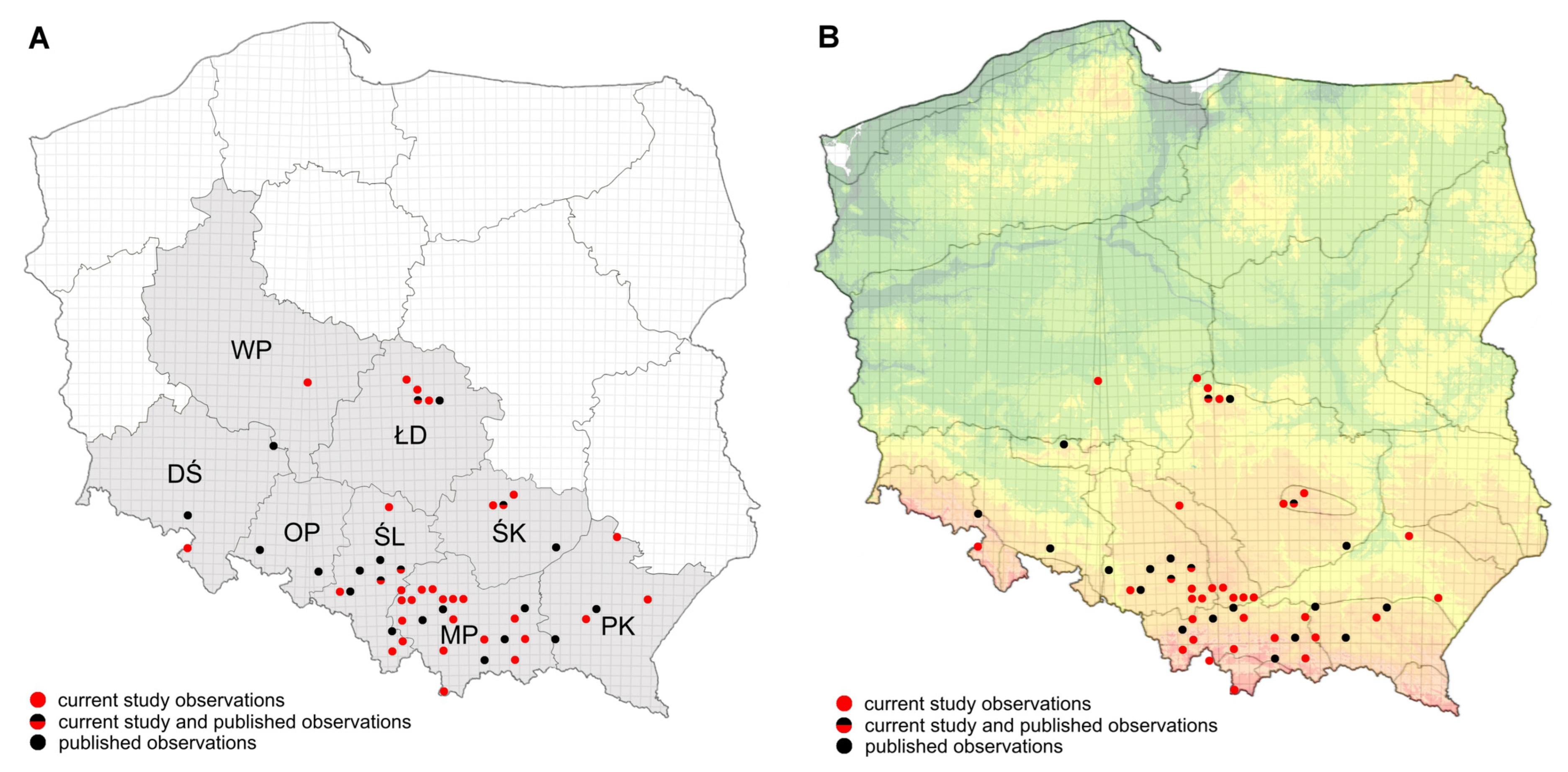
2.4. An Update on the Occurrence Range of Aedes japonicus in Poland
3. Results
3.1. Capture of Mosquitoes for WNV Screening
3.2. West Nile Virus Screening in Field-Collected Aedes japonicus
3.3. An Update of Aedes japonicus Distribution in Poland, 2025
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ae. | Aedes |
| ECDC | European Centre for Disease Prevention and Control |
| ELISA | Enzyme-linked immunosorbent assay |
| RNA | Ribonucleic acid |
| RT-PCR | Reverse Transcription Polymerase Chain Reaction |
| rRT-PCR | real-time Reverse Transcription Polymerase Chain Reaction |
| UTM | Universal Transverse Mercator |
| WNF | West Nile fever |
| WNV | West Nile virus |
References
- Tanaka, K. Studies on the pupal mosquitoes of Japan. (5) Four subspecies of Aedes (Finlaya) japonicus Theobald, including subsp. shintienensis from Taiwan (Diptera, Culicidae). Jpn. J. Syst. Entomol. 2002, 8, 63–77. [Google Scholar]
- Laird, M.; Calder, L.; Thornton, R.C.; Syme, R.; Holder, P.W.; Mogi, M. Japanese Aedes albopictus among four mosquito species reaching New Zealand in used tires. J. Am. Mosq. Control Assoc. 1994, 10, 14–23. [Google Scholar]
- Monath, T.P. Japanese Encephalitis: Risk of Emergence in the United States and the Resulting Impact. Viruses 2023, 16, 54. [Google Scholar] [CrossRef]
- Schaffner, F.; Chouin, S.; Guilloteau, J. First record of Ochlerotatus (Finlaya) japonicus japonicus (Theobald, 1901) in metropolitan France. J. Am. Mosq. Control Assoc. 2003, 19, 1–5. [Google Scholar]
- Versteirt, V.; Schaffner, F.; Garros, C.; Dekoninck, W.; Coosemans, M.; Van Bortel, W. Introduction and establishment of the exotic mosquito species Aedes japonicus japonicus (Diptera: Culicidae) in Belgium. J. Med. Entomol. 2009, 46, 1464–1467. [Google Scholar] [CrossRef] [PubMed]
- Schaffner, F.; Kaufmann, C.; Hegglin, D.; Mathis, A. The invasive mosquito Aedes japonicus in Central Europe. Med. Vet. Entomol. 2009, 23, 448–451. [Google Scholar] [CrossRef]
- Seidel, B.; Duh, D.; Nowotny, N.; Allerberger, F. First record of the mosquitoes Aedes (Ochlerotatus) japonicus japonicus (Theobald, 1901) in Austria and Slovenia 2011 and for Aedes (Stegomyia) albopictus (Skuse, 1895) in Austria. Entomol. Zeitschrift. 2012, 122, 223–226. [Google Scholar]
- Seidel, B.; Nowotny, N.; Bakonyi, T.; Allerberger, F.; Schaffner, F. Spread of Aedes japonicus japonicus (Theobald, 1901) in Austria, 2011–2015, and first records of the subspecies for Hungary, 2012, and the principality of Liechtenstein, 2015. Parasites Vectors 2016, 9, 356. [Google Scholar] [CrossRef]
- Ibáñez-Justicia, A.; Kampen, H.; Braks, M.; Schaffner, F.; Steeghs, M.; Werner, D.; Zielke, D.; den Hartog, W.; Brooks, M.; Dik, M.; et al. First report of established population of Aedes japonicus japonicus (Theobald, 1901) (Diptera: Culicidae) in the Netherlands. J. Eur. Mosq. Control Assoc. 2014, 32, 9–13. [Google Scholar]
- Klobučar, A.; Lipovac, I.; Žagar, N.; Mitrović-Hamzić, S.; Tešić, V.; Vilibić-Čavlek, T.; Merdić, E. First record and spreading of the invasive mosquito Aedes japonicus japonicus (Theobald, 1901) in Croatia. Med. Vet. Entomol. 2019, 33, 171–176. [Google Scholar] [CrossRef]
- Seidel, B.; Montarsi, F.; Huemer, H.P.; Indra, A.; Capelli, G.; Allerberger, F.; Nowotny, N. First record of the Asian bush mosquito, Aedes japonicus japonicus, in Italy: Invasion from an established Austrian population. Parasites Vectors 2016, 9, 284. [Google Scholar] [CrossRef]
- Janssen, N.; Graovac, N.; Vignjević, G.; Bogojević, M.S.; Turić, N.; Klobučar, A.; Kavran, M.; Petrić, D.; Ćupina, A.I.; Fischer, S.; et al. Rapid spread and population genetics of Aedes japonicus japonicus (Diptera: Culicidae) in southeastern Europe (Croatia, Bosnia and Herzegovina, Serbia). PLoS ONE 2020, 15, e0241235. [Google Scholar] [CrossRef]
- Eritja, R.; Ruiz-Arrondo, I.; Delacour-Estrella, S.; Schaffner, F.; Álvarez-Chachero, J.; Bengoa, M.; Puig, M.Á.; Melero-Alcíbar, R.; Oltra, A.; Bartumeus, F. First detection of Aedes japonicus in Spain: An unexpected finding triggered by citizen science. Parasites Vectors 2019, 12, 53. [Google Scholar] [CrossRef]
- Čabanová, V.; Boršová, K.; Svitok, M.; Oboňa, J.; Svitková, I.; Barbušinová, E.; Derka, T.; Sláviková, M.; Klempa, B. An unwanted companion reaches the country: The first record of the alien mosquito Aedes japonicus japonicus (Theobald, 1901) in Slovakia. Parasites Vectors 2021, 14, 572. [Google Scholar] [CrossRef]
- Horváth, C.; Cazan, C.D.; Mihalca, A.D. Emergence of the invasive Asian bush mosquito, Aedes (Finlaya) japonicus japonicus, in an urban area, Romania. Parasites Vectors 2021, 14, 192. [Google Scholar] [CrossRef]
- Vojtíšek, J.; Janssen, N.; Šikutová, S.; Šebesta, O.; Kampen, H.; Rudolf, I. Emergence of the invasive Asian bush mosquito Aedes (Hulecoeteomyia) japonicus (Theobald, 1901) in the Czech Republic. Parasites Vectors 2022, 15, 250. [Google Scholar] [CrossRef]
- Citizen Science Platform iNaturalist. Available online: https://www.inaturalist.org/observations/145260810 (accessed on 10 September 2025).
- Gierek, M.; Ochała-Gierek, G.; Woźnica, A.J.; Zaleśny, G.; Jarosz, A.; Niemiec, P. Winged Threat on the Offensive: A Literature Review Due to the First Identification of Aedes japonicus in Poland. Viruses 2024, 16, 703. [Google Scholar] [CrossRef] [PubMed]
- Schaffner, F.; Kwaśnik, M.; Myczko, Ł.; Rożek, W.; Eritja, R.; Lippert, S.; Wint, G.R.W.; Leszczyńska, J. A Survey Targeting Aedes invasive mosquito species in central Europe, Summer 2023, reveals the extensive occurrence of Aedes japonicus in Poland. J. Eur. Mosq. Control Assoc. 2024, 1–15. [Google Scholar] [CrossRef]
- Andreadis, T.G.; Anderson, J.F.; Munstermann, L.E.; Wolfe, R.J.; Florin, D.A. Discovery, distribution, and abundance of the newly introduced mosquito Ochlerotatus japonicus (Diptera: Culicidae) in Connecticut, USA. J. Med. Entomol. 2001, 38, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Yee, D.A. Tires as habitats for mosquitoes: A review of studies within the eastern United States. J. Med. Entomol. 2008, 45, 581–593. [Google Scholar]
- Kaufman, M.G.; Fonseca, D.M. Invasion biology of Aedes japonicus japonicus (Diptera: Culicidae). Annu. Rev. Entomol. 2014, 59, 31–49. [Google Scholar] [CrossRef]
- Hardstone, M.C.; Andreadis, T.G. Weak larval competition between the invasive mosquito Aedes japonicus japonicus (Diptera: Culicidae) and three resident container-inhabiting mosquitoes in the laboratory. J. Med. Entomol. 2012, 49, 277–285. [Google Scholar] [CrossRef]
- Bernard, K.A.; Maffei, J.G.; Jones, S.A.; Kauffman, E.B.; Ebel, G.; Dupuis, A.P., 2nd; Ngo, K.A.; Nicholas, D.C.; Young, D.M.; Shi, P.Y.; et al. West Nile virus infection in birds and mosquitoes, New York State, 2000. Emerg. Infect. Dis. 2021, 7, 679–685. [Google Scholar] [CrossRef]
- DeCarlo, C.H.; Campbell, S.R.; Bigler, L.L.; Mohammed, H.O. Aedes japonicus and West Nile Virus in New York. J. Am. Mosq. Control Assoc. 2020, 36, 261–263. [Google Scholar] [CrossRef]
- Lühken, R.; Brattig, N.; Becker, N. Introduction of invasive mosquito species into Europe and prospects for arbovirus transmission and vector control in an era of globalization. Infect. Dis. Poverty 2023, 12, 109. [Google Scholar] [CrossRef] [PubMed]
- Aedes jaonicus distribution in Europe, European Centre for Disease Prevention and Control. Available online: https://www.ecdc.europa.eu/en/publications-data/aedes-japonicus-current-known-distribution-october-2023 (accessed on 10 September 2025).
- Niczyporuk, J.S.; Samorek-Salamonowicz, E.; Lecollinet, S.; Pancewicz, S.A.; Kozdruń, W.; Czekaj, H. Occurrence of West Nile virus antibodies in wild birds, horses, and humans in Poland. BioMed Res. Int. 2015, 2015, 234181. [Google Scholar] [CrossRef]
- Niczyporuk, J.S.; Kozdrun, W.; Czujkowska, A.; Blanchard, Y.; Helle, M.; Dheilly, N.M.; Gonzalez, G. West Nile Virus Lineage 2 in Free-Living Corvus cornix Birds in Poland. Trop. Med. Infect. Dis. 2023, 8, 417. [Google Scholar] [CrossRef] [PubMed]
- Bażanów, B.; Jansen van Vuren, P.; Szymański, P.; Stygar, D.; Frącka, A.; Twardoń, J.; Kozdrowski, R.; Pawęska, J.T. A Survey on West Nile and Usutu Viruses in Horses and Birds in Poland. Viruses 2018, 10, 87. [Google Scholar] [CrossRef] [PubMed]
- Kondrusik, M.; Ferenczi, E.; Zajkowska, J.; Pancewicz, S.; Grygorczuk, S.; Świerzbińska, R.; Hermanowska-Szpakowicz, T. The evaluation of serum presence of antibodies reacting with West Nile Fever virus (WNV) antigens among inhabitants from Podlaskie and Świętokrzyskie region. Przegl. Epidemiol. 2007, 61, 409–416. (In Polish) [Google Scholar]
- Hermanowska-Szpakowicz, T.; Grygorczuk, S.; Kondrusik, M.; Zajkowska, J.; Pancewicz, S. West Nile Virus Infection. Przegl. Epidemiol. 2006, 60, 93–98. (In Polish) [Google Scholar]
- West Nile fever in Europe in 2024, European Centre for Disease Prevention and Control. Available online: https://wnv-monthly.ecdc.europa.eu/archive/wnv-2024.html (accessed on 10 September 2025).
- Tanaka, K.; Mizusawa, K.; Saugstad, E.S. A revision of the adult and larval mosquitoes of Japan (including the Ryukyu Archipelago and the Ogasawara islands) and Korea (Diptera: Culicidae). Am. Entomoll. Inst. 1979, 16, 1–987. [Google Scholar]
- Mosquito surveillance guidelines, European Centre for Disease Prevention and Control. Available online: https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/TER-Mosquito-surveillance-guidelines.pdf (accessed on 12 September 2025).
- Becker, N.; Petrić, D.; Zgomba, M.; Boase, C.; Madon, M.B.; Dahl, C.; Kaiser, A. Mosquitoes; Fascinating Life Sciences; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Niczyporuk, J.S.; Samorek-Salamonowicz, E.; Kozdruń, W.; Mizak, Z. Attempts to detect West Nile Virus in wild birds in Poland. Acta Vet. Hung. 2011, 59, 405–408. [Google Scholar] [CrossRef]
- Eiden, M.; Vina-Rodriguez, A.; Hoffmann, B.; Ziegler, U.; Groschup, M.H. Two new real-time quantitative reverse transcription polymerase chain reaction assays with unique target sites for the specific and sensitive detection of lineages 1 and 2 West Nile virus strains. J. Vet. Diagn. Investig. 2010, 22, 748–753. [Google Scholar] [CrossRef]
- MapaUTM Software Website. Available online: https://www.heteroptera.us.edu.pl/mapautm.html (accessed on 25 September 2025).
- Hayes, E.B.; Komar, N.; Nasci, R.S.; Montgomery, S.P.; O’Leary, D.R.; Campbell, G.L. Epidemiology and transmission dynamics of West Nile virus disease. Emerg. Infect. Dis. 2005, 11, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Kampen, H.; Holicki, C.M.; Ziegler, U.; Groschup, M.H.; Tews, B.A.; Werner, D. West Nile Virus Mosquito Vectors (Diptera: Culicidae) in Germany. Viruses 2020, 12, 493. [Google Scholar] [CrossRef] [PubMed]
- Turell, M.J.; O’Guinn, M.L.; Dohm, D.J.; Jones, J.W. Vector competence of North American mosquitoes (Diptera: Culicidae) for West Nile virus. J. Med. Entomol. 2001, 38, 130–134. [Google Scholar] [CrossRef]
- Molaei, G.; Farajollahi, A.; Scott, J.J.; Gaugler, R.; Andreadis, T.G. Human bloodfeeding by the recently introduced mosquito, Aedes japonicus japonicus, and public health implications. J. Am. Mosq. Control Assoc. 2009, 25, 210–214. [Google Scholar] [CrossRef]
- Apperson, C.S.; Hassan, H.K.; Harrison, B.A.; Savage, H.M.; Aspen, S.E.; Farajollahi, A.; Crans, W.; Daniels, T.J.; Falco, R.C.; Benedict, M.; et al. Host feeding patterns of established and potential mosquito vectors of West Nile virus in the eastern United States. Vector Borne Zoonotic Dis. 2004, 4, 71–82. [Google Scholar] [CrossRef]
- Williges, E.; Farajollahi, A.; Scott, J.J.; McCuiston, L.J.; Crans, W.J.; Gaugler, R. Laboratory colonization of Aedes japonicus japonicus. J. Am. Mosq. Control Assoc. 2008, 24, 591–593. [Google Scholar] [CrossRef] [PubMed]
- Linthout, C.; Martins, A.D.; de Wit, M.; Delecroix, C.; Abbo, S.R.; Pijlman, G.P.; Koenraadt, C.J.M. The potential role of the Asian bush mosquito Aedes japonicus as spillover vector for West Nile virus in the Netherlands. Parasites Vectors 2024, 17, 262. [Google Scholar] [CrossRef]
- Cebrián-Camisón, S.; Martínez-de la Puente, J.; Figuerola, J. A Literature Review of Host Feeding Patterns of Invasive Aedes Mosquitoes in Europe. Insects 2020, 11, 848. [Google Scholar] [CrossRef]
- Schönenberger, A.C.; Wagner, S.; Tuten, H.C.; Schaffner, F.; Torgerson, P.; Furrer, S.; Mathis, A.; Silaghi, C. Host preferences in host-seeking and blood-fed mosquitoes in Switzerland. Med. Vet. Entomol. 2016, 30, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Bartlett-Healy, K.; Unlu, I.; Obenauer, P.; Hughes, T.; Healy, S.; Crepeau, T.; Farajollahi, A.; Kesavaraju, B.; Fonseca, D.; Schoeler, G.; et al. Larval mosquito habitat utilization and community dynamics of Aedes albopictus and Aedes japonicus (Diptera: Culicidae). J. Med. Entomol. 2012, 49, 813–824. [Google Scholar] [CrossRef]
- Chaves, L.F.; Friberg, M.D.; Moji, K. Synchrony of globally invasive Aedes spp. immature mosquitoes along an urban altitudinal gradient in their native range. Sci. Total Environ. 2020, 734, 139365. [Google Scholar] [CrossRef] [PubMed]
- Garamszegi, L.Z.; Soltész, Z.; Kurucz, K.; Szentiványi, T. Using community science data to assess the association between urbanization and the presence of invasive Aedes species in Hungary. Parasites Vectors 2023, 16, 158. [Google Scholar] [CrossRef]
- Klobučar, A.; Kavran, M.; Petrinić, S.; Curman Posavec, M. Temporal Activity and Distribution of the Invasive Mosquitoes Aedes albopictus and Aedes japonicus in the Zagreb Area, Croatia. Trop. Med. Infect. Dis. 2024, 9, 263. [Google Scholar] [CrossRef]
- Nanfack-Minkeu, F.; Delong, A.; Luri, M.; Poelstra, J.W. Invasive Aedes japonicus Mosquitoes Dominate the Aedes Fauna Collected with Gravid Traps in Wooster, Northeastern Ohio, USA. Insects 2023, 14, 56. [Google Scholar] [CrossRef]
- Koban, M.B.; Kampen, H.; Scheuch, D.E.; Frueh, L.; Kuhlisch, C.; Janssen, N.; Steidle, J.L.M.; Schaub, G.A.; Werner, D. The Asian bush mosquito Aedes japonicus japonicus (Diptera: Culicidae) in Europe, 17 years after its first detection, with a focus on monitoring methods. Parasites Vectors 2019, 12, 109. [Google Scholar] [CrossRef]
- Ibañez-Justicia, A.; Teekema, S.; den Hartog, W.; Jacobs, F.; Dik, M.; Stroo, A. The Effectiveness of Asian Bush Mosquito (Aedes japonicus japonicus) Control Actions in Colonised Peri-urban Areas in the Netherlands. J. Med. Entomol. 2018, 55, 673–680. [Google Scholar] [CrossRef]
- Dussault, C.; Nelder, M.P.; Russell, C.; Johnson, S.; Vrbova, L. Evaluating the impact of Aedes japonicus invasion on the mosquito community in the Greater Golden Horseshoe region (Ontario, Canada). PLoS ONE 2018, 13, e0208911. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Available online: https://www.ecdc.europa.eu/en/news-events/epidemiological-update-west-nile-virus-transmission-season-europe-2018 (accessed on 30 October 2025).
- Erazo, D.; Grant, L.; Ghisbain, G.; Marini, G.; Colón-González, F.J.; Wint, W.; Rizzoli, A.; Van Bortel, W.; Vogels, C.B.F.; Grubaugh, N.D.; et al. Contribution of climate change to the spatial expansion of West Nile virus in Europe. Nat. Commun. 2024, 15, 1196. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Khatib, M.N.; Ballal, S.; Kaur, M.; Nathiya, D.; Sharma, S.; Prasad, G.V.S.; Sinha, A.; Gaidhane, A.M.; Mohapatra, P.; et al. West Nile Virus in a changing climate: Epidemiology, pathology, advances in diagnosis and treatment, vaccine designing and control strategies, emerging public health challenges—A comprehensive review. Emerg. Microbes Infect. 2025, 14, 2437244. [Google Scholar] [CrossRef] [PubMed]
- Ruberto, I.; Kretschmer, M.; Zabel, K.; Sunenshine, R.; Smith, K.; Townsend, J.; Richard, D.; Erhart, L.M.; Staab, N.; Komatsu, K.; et al. Notes from the Field: An Outbreak of West Nile Virus—Arizona, 2019. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 123–124. [Google Scholar] [CrossRef]
- Levine, R.S.; Mead, D.G.; Hamer, G.L.; Brosi, B.J.; Hedeen, D.L.; Hedeen, M.W.; McMillan, J.R.; Bisanzio, D.; Kitron, U.D. Supersuppression: Reservoir Competency and Timing of Mosquito Host Shifts Combine to Reduce Spillover of West Nile Virus. Am. J. Trop. Med. Hyg. 2016, 95, 1174–1184. [Google Scholar] [CrossRef]
- Poh, K.C.; Chaves, L.F.; Reyna-Nava, M.; Roberts, C.M.; Fredregill, C.; Bueno, R., Jr.; Debboun, M.; Hamer, G.L. The influence of weather and weather variability on mosquito abundance and infection with West Nile virus in Harris County, Texas, USA. Sci. Total Environ. 2019, 675, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Reisen, W.K.; Thiemann, T.; Barker, C.M.; Lu, H.; Carroll, B.; Fang, Y.; Lothrop, H.D. Effects of warm winter temperature on the abundance and gonotrophic activity of Culex (Diptera: Culicidae) in California. J. Med. Entomol. 2010, 47, 230–237. [Google Scholar] [CrossRef]
- Wimberly, M.C.; Lamsal, A.; Giacomo, P.; Chuang, T.W. Regional variation of climatic influences on West Nile virus outbreaks in the United States. Am. J. Trop. Med. Hyg. 2014, 91, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk, A.M.; Bednorz, E. Climate Atlas of Poland (1991–2020); Bogucki Scientific Publishing House: Poznań, Poland, 2022. [Google Scholar]
- Climate in Poland in 2024, Institute of Meteorology and Water Management—National Research Institute (IMGW-PIB, Poland). Available online: https://imgw.pl/charakterystyka-wybranych-elementow-klimatu-w-polsce-w-2024-roku-podsumowanie/ (accessed on 30 October 2025).
- Climate in Poland in January 2024, Institute of Meteorology and Water Management—National Research Institute (IMGW-PIB, Poland). Available online: https://www.imgw.pl/wydarzenia/charakterystyka-wybranych-elementow-klimatu-w-polsce-w-styczniu-2024-roku (accessed on 30 October 2025).
- Climate in Poland in February 2024, Institute of Meteorology and Water Management—National Research Institute (IMGW-PIB, Poland). Available online: https://www.imgw.pl/wydarzenia/charakterystyka-wybranych-elementow-klimatu-w-polsce-w-lutym-2024-roku (accessed on 30 October 2025).
- Climate in Poland in March 2024, Institute of Meteorology and Water Management—National Research Institute (IMGW-PIB, Poland). Available online: https://www.imgw.pl/wydarzenia/charakterystyka-wybranych-elementow-klimatu-w-polsce-w-marcu-2024-roku (accessed on 30 October 2025).
- Niczyporuk, J.S.; Kozdruń, W.; Stolarek, A.; Piekarska, K.; Taraisuk, K.; Wyrostek, K.; Kycko, A.; Sell, B.; Janek, E.; Czujkowska, A.; et al. Threat to bird health in the context of West Nile virus. In Proceedings of the International Scientific Conference—Current and Future Threats to the Health of Poultry and Free-Living Birds, Puławy, Poland, 26–27 June 2025; pp. 52–55. [Google Scholar]
- Balatsos, G.; Beleri, S.; Tegos, N.; Bisia, M.; Karras, V.; Zavitsanou, E.; Papachristos, D.P.; Papadopoulos, N.T.; Michaelakis, A.; Patsoula, E. Overwintering West Nile virus in active Culex pipiens mosquito populations in Greece. Parasites Vectors 2024, 17, 286. [Google Scholar] [CrossRef]
- Kampen, H.; Werner, D. Out of the bush: The Asian bush mosquito Aedes japonicus japonicus (Theobald, 1901) (Diptera, Culicidae) becomes invasive. Parasites Vectors 2014, 7, 59. [Google Scholar] [CrossRef]
- Andreadis, T.G.; Wolfe, R.J. Evidence for reduction of native mosquitoes with increased expansion of invasive Ochlerotatus japonicus japonicus (Diptera: Culicidae) in the northeastern United States. J. Med. Entomol. 2010, 47, 43–52. [Google Scholar] [CrossRef]
- Cull, B. Online Crowdsourced Data from iNaturalist Can Assist Monitoring of Invasive Mosquitoes. Insects 2025, 16, 128. [Google Scholar] [CrossRef] [PubMed]
- Cull, B.; Vo, B.N.; Webb, C.; Williams, C.R. iNaturalist community observations provide valuable data on human-mosquito encounters. J. Vector Ecol. J. Soc. Vector Ecol. 2024, 49, R12–R26. [Google Scholar] [CrossRef] [PubMed]

| Date | Number of Ae. japonicus Specimens Caught (Collectors) | |||
|---|---|---|---|---|
| Kielce | Mikołów | Barcza | Kraków | |
| 1 September 2024 | 72 (P.N., K.B.) | |||
| 4 September 2024 | 9 (P.N., M.G.) | |||
| 11 September 2024 | 18 (P.N., M.G.) | |||
| 12 September 2024 | 20 (K.B.) | |||
| 18 September 2024 | 6 (M.G.) | |||
| 24 September 2024 | 12 (Ł.M.) | |||
| Voivodeship | Number of Observations | Observations (n) of Larvae/Adults in the Context of Land Use | |||||
|---|---|---|---|---|---|---|---|
| All | Larvae | Adults | Forest/Shrub | Rural | Suburban | Urban | |
| Dolnośląskie | 1 | 0 | 1 | 0 | 0 | 0/1 | 0 |
| Łódzkie | 14 | 3 | 11 | 0/1 | 0 | 2/8 | 1/2 |
| Małopolskie | 21 | 3 | 20 | 1/7 | 2/3 | 0/3 | 0/7 |
| Podkarpackie | 7 | 2 | 7 | 0 | 2/5 | 0/2 | 0 |
| Śląskie | 17 | 1 | 16 | 1/2 | 0 | 0/13 | 0/1 |
| Świętokrzyskie | 6 | 0 | 6 | 0/5 | 0 | 0/1 | 0 |
| Wielkopolskie | 1 | 0 | 1 | 0 | 0/1 | 0 | 0 |
| Total | 67 | 9 | 62 | 2/15 | 4/9 | 2/28 | 1/10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niemiec, P.; Niczyporuk, J.S.; Kozdruń, W.; Stolarek, A.; Mielczarek, Ł.; Słomczyński, K.; Barszcz, K.; Kuziora, P.; Jarosiewicz, G.; Jarosz, A.; et al. West Nile Virus Pilot Screening in Field-Collected Aedes japonicus (Theobald, 1901): An Update of Species Distribution in Poland, 2025. Viruses 2025, 17, 1515. https://doi.org/10.3390/v17111515
Niemiec P, Niczyporuk JS, Kozdruń W, Stolarek A, Mielczarek Ł, Słomczyński K, Barszcz K, Kuziora P, Jarosiewicz G, Jarosz A, et al. West Nile Virus Pilot Screening in Field-Collected Aedes japonicus (Theobald, 1901): An Update of Species Distribution in Poland, 2025. Viruses. 2025; 17(11):1515. https://doi.org/10.3390/v17111515
Chicago/Turabian StyleNiemiec, Paweł, Jowita Samanta Niczyporuk, Wojciech Kozdruń, Agnieszka Stolarek, Łukasz Mielczarek, Kamil Słomczyński, Kacper Barszcz, Paweł Kuziora, Grzegorz Jarosiewicz, Alicja Jarosz, and et al. 2025. "West Nile Virus Pilot Screening in Field-Collected Aedes japonicus (Theobald, 1901): An Update of Species Distribution in Poland, 2025" Viruses 17, no. 11: 1515. https://doi.org/10.3390/v17111515
APA StyleNiemiec, P., Niczyporuk, J. S., Kozdruń, W., Stolarek, A., Mielczarek, Ł., Słomczyński, K., Barszcz, K., Kuziora, P., Jarosiewicz, G., Jarosz, A., Woźnica, A. J., Zaleśny, G., Gwardjan, M., Ochała-Gierek, G., & Gierek, M. (2025). West Nile Virus Pilot Screening in Field-Collected Aedes japonicus (Theobald, 1901): An Update of Species Distribution in Poland, 2025. Viruses, 17(11), 1515. https://doi.org/10.3390/v17111515







