Artificial Intelligence for Predicting Lung Immune Responses to Viral Infections: From Mechanistic Insights to Clinical Applications
Abstract
1. Introduction
1.1. Burden of Viral Respiratory Infections
1.2. Rationale and Aim of the Narrative Review
2. Mechanistic Basis of Lung Immune Responses to Viral Infections
2.1. Innate Immunity
2.2. Adaptive Responses
2.3. Dysregulated and Pathological Responses
2.4. Knowledge Gaps and Predictive Modeling
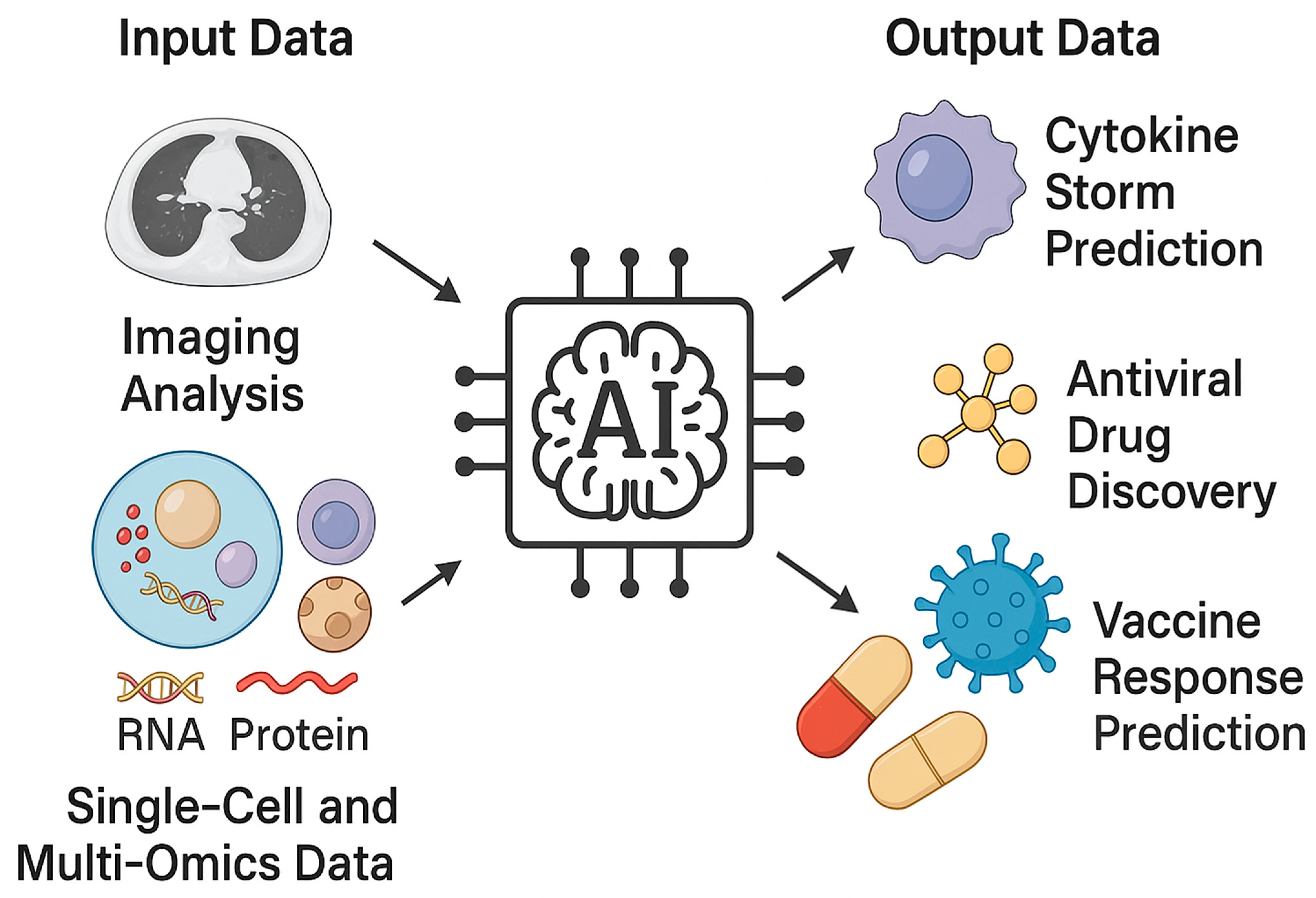
3. AI and Computational Approaches for Modeling Immune Responses
3.1. Machine Learning (ML) Methods: Regression, Random Forest, SVM
3.2. Deep Learning (DL): Neural Networks for Complex Non-Linear Relationships
3.3. Systems Biology and Network-Based AI: Modeling Host–Virus Interaction Networks
3.4. Multimodal Integration: Omics (Transcriptomics, Proteomics, Metabolomics), Imaging (HRCT, Radiomics), and Clinical Data
3.5. Explainable AI (XAI): Importance for Biological Interpretation and Clinical Trust
3.6. Critical Appraisal of Current AI Evidence and Biological Novelty
4. AI for Predicting Disease Severity and Outcomes
4.1. Predicting Cytokine Storm and ARDS Risk
4.2. Identifying Patients at Risk of Severe Influenza Pneumonia or RSV Bronchiolitis
4.3. AI Models for Mortality Prediction, ICU Admission, Ventilatory Support Needs
5. AI for Immune Response Modeling, Vaccine Prediction, and Antiviral Drug Repurposing
5.1. Identifying Immune Signatures Predictive of Antiviral or Immunomodulatory Therapy Response
5.2. Predicting Vaccine Responsiveness and Durability of Immune Protection
5.3. AI-Assisted Drug Repurposing for Viral Pneumonia
6. Conclusions—The Time for Translational Intelligence
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paget, J.; Danielle Iuliano, A.; Taylor, R.J.; Simonsen, L.; Viboud, C.; Spreeuwenberg, P.; Global Seasonal Influenza-Associated Mortality Collaborator Network and GLaMOR Collaborating Teams. Estimates of mortality associated with seasonal influenza for the European Union from the GLaMOR project. Vaccine 2022, 40, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Lucero-Obusan, C.; Schirmer, P.L.; Wendelboe, A.; Oda, G.; Holodniy, M. Epidemiology and burden of influenza in the U.S. Department of Veterans Affairs. Influenza Other Respir. Viruses 2018, 12, 293–298. [Google Scholar] [CrossRef]
- GBD 2017 Influenza Collaborators. Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: An analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2019, 7, 69–89. [Google Scholar] [CrossRef]
- Bergeron, H.C.; Tripp, R.A. Immunopathology of RSV: An Updated Review. Viruses 2021, 13, 2478. [Google Scholar] [CrossRef] [PubMed]
- Savic, M.; Penders, Y.; Shi, T.; Branche, A.; Pirçon, J.Y. Respiratory syncytial virus disease burden in adults aged 60 years and older in high-income countries: A systematic literature review and meta-analysis. Influenza Other Respir. Viruses 2023, 17, e13031. [Google Scholar] [CrossRef]
- Polverino, F.; Stern, D.A.; Ruocco, G.; Balestro, E.; Bassetti, M.; Candelli, M.; Cirillo, B.; Contoli, M.; Corsico, A.; D’Amico, F.; et al. Comorbidities, Cardiovascular Therapies, and COVID-19 Mortality: A Nationwide, Italian Observational Study (ItaliCO). Front. Cardiovasc. Med. 2020, 7, 585866. [Google Scholar] [CrossRef]
- D’Ambrosio, F.; Lomazzi, M.; Moore, M.; Maida, A.; Ricciardi, R.; Munno, L.; Lettieri, M.; De Vito, E.; Ricciardi, W.; Calabrò, G.E. Addressing the Underestimated Burden of RSV in Older Adults in Europe: Epidemiology, Surveillance Gaps, and Public Health Implications. Vaccines 2025, 13, 510. [Google Scholar] [CrossRef]
- Tana, C. Editorial: Frailty in older patients during the COVID-19 era. Front. Med. 2024, 10, 1348468. [Google Scholar] [CrossRef]
- Michalski, J.E.; Kurche, J.S.; Schwartz, D.A. From ARDS to pulmonary fibrosis: The next phase of the COVID-19 pandemic? Transl. Res. 2022, 241, 13–24. [Google Scholar] [CrossRef]
- Buttia, C.; Llanaj, E.; Raeisi-Dehkordi, H.; Kastrati, L.; Amiri, M.; Meçani, R.; Taneri, P.E.; Ochoa, S.A.G.; Raguindin, P.F.; Wehrli, F.; et al. Prognostic models in COVID-19 infection that predict severity: A systematic review. Eur. J. Epidemiol. 2023, 38, 355–372. [Google Scholar] [CrossRef] [PubMed]
- Moffa, L.; Tana, C. Healthcare-Associated Infections (HAI) in the Elderly: Molecular Mechanisms of Immunosenescence and Clinical, Nutritional and Therapeutic Implications. Int. J. Mol. Sci. 2025, 26, 9649. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, R.R.; Shaw, A.C. Paradoxical changes in innate immunity in aging: Recent progress and new directions. J. Leukoc. Biol. 2015, 98, 937–943. [Google Scholar] [CrossRef]
- Jing, Y.; Shaheen, E.; Drake, R.R.; Chen, N.; Gravenstein, S.; Deng, Y. Aging is associated with a numerical and functional decline in plasmacytoid dendritic cells, whereas myeloid dendritic cells are relatively unaltered in human peripheral blood. Hum. Immunol. 2009, 70, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Zhang, Y.; Zhang, K.; Yuan, Y.; Jia, S.; Liu, J. The Complement System, Aging, and Aging-Related Diseases. Int. J. Mol. Sci. 2022, 23, 8689. [Google Scholar] [CrossRef]
- Kaech, S.M.; Cui, W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat. Rev. Immunol. 2012, 12, 749–761. [Google Scholar] [CrossRef]
- Flores, K.G.; Li, J.; Sempowski, G.D.; Haynes, B.F.; Hale, L.P. Analysis of the human thymic perivascular space during aging. J. Clin. Investig. 1999, 104, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Rocamora-Reverte, L.; Melzer, F.L.; Würzner, R.; Weinberger, B. The Complex Role of Regulatory T Cells in Immunity and Aging. Front. Immunol. 2021, 11, 616949. [Google Scholar] [CrossRef]
- Quiros-Roldan, E.; Sottini, A.; Natali, P.G.; Imberti, L. The Impact of Immune System Aging on Infectious Diseases. Microorganisms 2024, 12, 775. [Google Scholar] [CrossRef]
- Sanz, I.; Wei, C.; Jenks, S.A.; Cashman, K.S.; Tipton, C.; Woodruff, M.C.; Hom, J.; Lee, F.E. Challenges and Opportunities for Consistent Classification of Human B Cell and Plasma Cell Populations. Front. Immunol. 2019, 10, 2458. [Google Scholar] [CrossRef]
- Chung, H.Y.; Kim, D.H.; Lee, E.K.; Chung, K.W.; Chung, S.; Lee, B.; Seo, A.Y.; Chung, J.H.; Jung, Y.S.; Im, E.; et al. Redefining Chronic Inflammation in Aging and Age-Related Diseases: Proposal of the Senoinflammation Concept. Aging Dis. 2019, 10, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, C.; Marchi, S.; Simoes, I.C.M.; Ren, Z.; Morciano, G.; Perrone, M.; Patalas-Krawczyk, P.; Borchard, S.; Jędrak, P.; Pierzynowska, K.; et al. Mitochondria and Reactive Oxygen Species in Aging and Age-Related Diseases. Int. Rev. Cell Mol. Biol. 2018, 340, 209–344. [Google Scholar]
- Wiley, C.D.; Schaum, N.; Alimirah, F.; Lopez-Dominguez, J.A.; Orjalo, A.V.; Scott, G.; Desprez, P.Y.; Benz, C.; Davalos, A.R.; Campisi, J. Small-molecule MDM2 antagonists attenuate the senescence-associated secretory phenotype. Sci. Rep. 2018, 8, 2410. [Google Scholar] [CrossRef]
- McDonald, L.T. Healing after COVID-19: Are survivors at risk for pulmonary fibrosis? Am. J. Physiol. Lung Cell Mol. Physiol. 2021, 320, L257–L265. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Martínez, G.; Jiménez-Álvarez, L.A.; Cruz-Lagunas, A.; Ignacio-Cortés, S.; Gómez-García, I.A.; Rodríguez-Reyna, T.S.; Choreño-Parra, J.A.; Zúñiga, J. Possible Role of Matrix Metalloproteinases and TGF-β in COVID-19 Severity and Sequelae. J. Interferon Cytokine Res. 2022, 42, 352–368. [Google Scholar] [CrossRef]
- López-Gil, L.; Pascual-Ahuir, A.; Proft, M. Genomic Instability and Epigenetic Changes during Aging. Int. J. Mol. Sci. 2023, 24, 14279. [Google Scholar] [CrossRef]
- Mekov, E.; Miravitlles, M.; Petkov, R. Artificial intelligence and machine learning in respiratory medicine. Expert. Rev. Respir. Med. 2020, 14, 559–564. [Google Scholar] [CrossRef]
- Zaslavsky, M.E.; Craig, E.; Michuda, J.K.; Sehgal, N.; Ram-Mohan, N.; Lee, J.Y.; Nguyen, K.D.; Hoh, R.A.; Pham, T.D.; Röltgen, K.; et al. Disease diagnostics using machine learning of B cell and T cell receptor sequences. Science. 2025, 387, eadp2407. [Google Scholar] [CrossRef] [PubMed]
- Tur, K. Multi-Modal Machine Learning Approach for COVID-19 Detection Using Biomarkers and X-Ray Imaging. Diagnostics 2024, 14, 2800. [Google Scholar] [CrossRef]
- Rahman, T.; Chowdhury, M.E.H.; Khandakar, A.; Bin Mahbub, Z.; Hossain, S.A.; Alhatou, A.; Abdalla, E.; Muthiyal, S.; Islam, K.F.; Kashem, S.B.A.; et al. BIO-CXRNET: A Robust Multimodal Stacking Machine Learning Technique for Mortality Risk Prediction of COVID-19 Patients using Chest X-Ray Images and Clinical Data. arXiv 2022, arXiv:2206.07595. [Google Scholar] [CrossRef]
- Maslova, A.; Ramirez, R.N.; Ma, K.; Schmutz, H.; Wang, C.; Fox, C.; Ng, B.; Benoist, C.; Mostafavi, S. Immunological Genome Project Deep learning of immune cell differentiation. Proc. Natl. Acad. Sci. USA 2020, 117, 25655–25666. [Google Scholar] [CrossRef] [PubMed]
- Mason, D.M.; Reddy, S.T. Predicting adaptive immune receptor specificities by machine learning is a data generation problem. Cell Syst. 2024, 15, 1190–1197. [Google Scholar] [CrossRef]
- Durmuş Tekir, S.; Çakır, T.; Ardıç, E.; Sayılırbaş, A.S.; Konuk, G.; Konuk, M.; Sarıyer, H.; Uğurlu, A.; Karadeniz, İ.; Özgür, A.; et al. PHISTO: Pathogen–host interaction search tool. Bioinformatics 2013, 29, 1357–1358. [Google Scholar] [CrossRef]
- Madan, S.; Demina, V.; Stapf, M.; Ernst, O.; Fröhlich, H. Accurate prediction of virus-host protein-protein interactions via a Siamese neural network using deep protein sequence embeddings. Patterns 2022, 3, 100551. [Google Scholar] [CrossRef]
- Curion, F.; Theis, F.J. Machine learning integrative approaches to advance computational immunology. Genome Med. 2024, 16, 80. [Google Scholar] [CrossRef] [PubMed]
- Shiri, I.; Mostafaei, S.; Haddadi Avval, A.; Salimi, Y.; Sanaat, A.; Akhavanallaf, A.; Arabi, H.; Rahmim, A.; Zaidi, H. High-dimensional multinomial multiclass severity scoring of COVID-19 pneumonia using CT radiomics features and machine learning algorithms. Sci. Rep. 2022, 12, 14817. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Mao, L.; Chen, Y.; Yuan, L.; Wang, F.; Li, X.; Cai, Q.; Qiu, J.; Chen, F. Machine learning-based CT radiomics model distinguishes COVID-19 from non-COVID-19 pneumonia. BMC Infect. Dis. 2021, 21, 931. [Google Scholar] [CrossRef] [PubMed]
- Hardy-Werbin, M.; Maiques, J.M.; Busto, M.; Cirera, I.; Aguirre, A.; Garcia-Gisbert, N.; Zuccarino, F.; Carbullanca, S.; Del Carpio, L.A.; Ramal, D.; et al. MultiCOVID: A multi modal deep learning approach for COVID-19 diagnosis. Sci. Rep. 2023, 13, 18761. [Google Scholar] [CrossRef]
- Miglietta, L.; Rawson, T.M.; Galiwango, R.; Tasker, A.; Ming, D.K.; Akogo, D.; Ferreyra, C.; Aboagye, E.O.; Gordon, N.C.; Garcia-Vidal, C.; et al. Artificial intelligence and infectious disease diagnostics: State of the art and future perspectives. Lancet Infect. Dis. 2025, 16, S1473-3099(25)00354-8. [Google Scholar] [CrossRef]
- Abbas, Q.; Jeong, W.; Lee, S.W. Explainable AI in Clinical Decision Support Systems: A Meta-Analysis of Methods, Applications, and Usability Challenges. Healthcare 2025, 13, 2154. [Google Scholar] [CrossRef]
- Lin, J.; Gu, C.; Sun, Z.; Zhang, S.; Nie, S. Machine learning-based model for predicting theoccurrence and mortality of nonpulmonary sepsis-associated ARDS. Sci. Rep. 2024, 14, 28240. [Google Scholar]
- Sjoding, M.W.; Taylor, D.; Motyka, J.; Lee, E.; Co, I.; Claar, D.; McSparron, J.I.; Ansari, S.; Kerlin, M.P.; Reilly, J.P.; et al. Deep learning to detect acute respiratory distress syndrome on chest radiographs: A retrospective study with external validation. Lancet Digit. Health 2021, 3, e340–e348. [Google Scholar] [CrossRef]
- Broecker, S.; Adams, J.Y.; Kumar, G.; Callcut, R.A.; Ni, Y.; Strohmer, T. Multimodal Deep Learning for ARDS Detection. medRxiv 2025. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.; Wang, F.; Peng, Z. Advances in Biomarkers for Diagnosis and Treatment of ARDS. Diagnostics 2023, 13, 3296. [Google Scholar] [CrossRef]
- Zhou, K.; Qin, L.; Chen, Y.; Gao, H.; Ling, Y.; Qin, Q.; Mou, C.; Qin, T.; Lu, J. A machine learning model for predicting acute respiratory distress syndrome risk in patients with sepsis using circulating immune cell parameters: A retrospective study. BMC Infect. Dis. 2025, 25, 568. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.H.; Hsu, C.C.; Chen, C.J.; Hsu, S.L.; Liu, T.L.; Lin, H.J.; Wang, J.J.; Liu, C.F.; Huang, C.C. Predicting outcomes in older ED patients with influenza in real time using a big data-driven and machine learning approach to the hospital information system. BMC Geriatr. 2021, 21, 280. [Google Scholar] [CrossRef]
- Kieke, B.A.; Belongia, E.A.; McClure, D.L.; Shinde, V. Prediction of serious RSV-related outcomes in older adults with outpatient RSV respiratory illness during 12 consecutive seasons. Influenza Other Respir. Viruses 2020, 14, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ashrafi, N.; Kang, C.; Zhao, G.; Chen, Y.; Pishgar, M. A machine learning-based prediction of hospital mortality in mechanically ventilated ICU patients. PLoS ONE 2024, 19, e0309383. [Google Scholar] [CrossRef]
- Gao, J.; Lu, Y.; Ashrafi, N.; Domingo, I.; Alaei, K.; Pishgar, M. Prediction of sepsis mortality in ICU patients using machine learning methods. BMC Med. Inform. Decis. Mak. 2024, 24, 228. [Google Scholar] [CrossRef]
- Viderman, D.; Ayazbay, A.; Kalzhan, B.; Bayakhmetova, S.; Tungushpayev, M.; Abdildin, Y. Artificial Intelligence in the Management of Patients with Respiratory Failure Requiring Mechanical Ventilation: A Scoping Review. J. Clin. Med. 2024, 13, 7535. [Google Scholar] [CrossRef]
- Bermejo-Peláez, D.; San José Estépar, R.; Fernández-Velilla, M.; Palacios Miras, C.; Gallardo Madueño, G.; Benegas, M.; Gotera Rivera, C.; Cuerpo, S.; Luengo-Oroz, M.; Sellarés, J.; et al. Deep Learning-Based Lesion Subtyping and Prediction of Clinical Outcomes in COVID-19 Pneumonia Using Chest CT. Sci. Rep. 2022, 12, 9387. [Google Scholar] [CrossRef]
- Viderman, D.; Abdildin, Y.; Batkuldinova, K.; Badenes, R.; Bilotta, F. Artificial Intelligence in Resuscitation: A Scoping Review. J. Clin. Med. 2023, 12, 2254. [Google Scholar] [CrossRef]
- Sepúlveda, P.; Gallardo, A.; Arriagada, R.; Souza, B.; Patroniti, N.; Battaglini, D. Weaning failure from mechanical ventilation: A scoping review of the utility of ultrasonography in the weaning process. Br. J. Anaesth. 2025, 135, 1441–1455. [Google Scholar] [CrossRef]
- Xu, H.; Ma, Y.; Zhuang, Y.; Zheng, Y.; Du, Z.; Zhou, X. Machine learning-based risk prediction model construction of difficult weaning in ICU patients with mechanical ventilation. Sci. Rep. 2024, 14, 20875. [Google Scholar] [CrossRef]
- Nakaya, H.I.; Hagan, T.; Duraisingham, S.S.; Lee, E.K.; Kwissa, M.; Rouphael, N.; Frasca, D.; Gersten, M.; Mehta, A.K.; Gaujoux, R.; et al. Systems Analysis of Immunity to Influenza Vaccination across Multiple Years and in Diverse Populations Reveals Shared Molecular Signatures. Immunity 2015, 43, 1186–1198. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Dias, P.; Lee, E.K.; Sorgi, S.; de Lima, D.S.; Urbanski, A.H.; Silveira, E.L.; Nakaya, H.I. Methods for predicting vaccine immunogenicity and reactogenicity. Hum. Vaccin. Immunother. 2020, 16, 269–276. [Google Scholar] [CrossRef]
- Hsieh, K.; Wang, Y.; Chen, L.; Zhao, Z.; Savitz, S.; Jiang, X.; Tang, J.; Kim, Y. Drug repurposing for COVID-19 using graph neural network and harmonizing multiple evidence. Sci. Rep. 2021, 11, 23179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Hu, X.; Li, G.; Deng, L. AntiViralDL: Computational Antiviral Drug Repurposing Using Graph Neural Network and Self-Supervised Learning. IEEE J. Biomed. Health Inform. 2023, 28, 548–556. [Google Scholar] [CrossRef]
- Basubrin, O. Current Status and Future of Artificial Intelligence in Medicine. Cureus 2025, 17, e77561. [Google Scholar] [CrossRef] [PubMed]
- Abu-El-Ruz, R.; Hasan, A.; Hijazi, D.; Masoud, O.; Abdallah, A.M.; Zughaier, S.M.; Al-Asmakh, M. Artificial Intelligence in Biomedical Sciences: A Scoping Review. Br. J. Biomed. Sci. 2025, 82, 14362. [Google Scholar] [CrossRef]
- Tana, C.; Siniscalchi, C.; Cerundolo, N.; Meschi, T.; Martelletti, P.; Tana, M.; Moffa, L.; Wells-Gatnik, W.; Cipollone, F.; Giamberardino, M.A. Smart Aging: Integrating AI into Elderly Healthcare. BMC Geriatr. 2025, in press. [Google Scholar]
- Aboy, M.; Minssen, T.; Vayena, E. Navigating the EU AI Act: Implications for regulated digital medical products. NPJ Digit. Med. 2024, 7, 237. [Google Scholar] [CrossRef] [PubMed]
| Domain | AI Application/Data Sources | Main Objectives | Key Achievements/Examples | Translational Potential |
|---|---|---|---|---|
| Prediction of cytokine storm and ARDS Clinical data (vitals, labs), imaging (CT/X-ray), biomarkers (IL-6, CRP, D-dimer) Integration of radiomics and omics Chest CT, proteomic/transcriptomic data Explainable AI (XAI) Multimodal (omics + imaging + clinical) | Early identification of hyperinflammatory deterioration Severity stratification and molecular-imaging correlation Interpretability and clinician trust | ML and DL models predicting ARDS onset with AUC > 0.8; integration of EHR and imaging features improves near-term risk prediction [40,41,42] Multimodal models (BIO-CXRNET, MultiCO- VID) achieve > 90% accuracy for mortality prediction [29,37] SHAP, Grad-CAM, and LIME frameworks enhance transparency and regulatory compliance [38,39] | High—supports real-time triage and early intervention High—enables precision diagnostics and monitoring Essential—prerequisite for adoption in clinical workflows |
| Immune repertoire and multi-omics modeling BCR/TCR sequencing, single-cell RNA-seq, proteomics Ventilatory support and weaning prediction ICU physiological data, ventilator parameters AI models for severity, mortality, and ICU admission EHR, biomarkers, respiratory indices | Identification of immune signatures of protective vs. maladaptive responses Predicting need for MV and difficult weaning Early risk stratification and clinical decision support | ML on receptor repertoires (Mal-ID) achieves AUROC ≈ 0.9 for immune condition classification [27] Random Forest AUC ≈ 0.80; DL for ventilator optimization [53] RF and XGBoost models outperform conventional severity scores [45,48] | Moderate–High informs vaccine and therapeutic design Moderate–High—improves ICU resource allocation High—supports real-time prognosis and prioritization |
| Vaccine response prediction Transcriptomics, proteomics, cytokine profiling AI-assisted drug repurposing for viral pneumonia Knowledge graphs, virus–host–drug networks, clinical data | Forecasting immunogenicity, durability, and reactogenicity Prioritization of repurposable antivirals and immunomodulators | AI models predict antibody magnitude and durability from early innate signatures [54,55] GNN pipelines identify > 20 candidate drugs with validated synergistic effects (e.g., azithromycin, atorvastatin) [56,57] | High—guides personalized immunization and booster strategies High—accelerates therapeutic translation and reduces R&D cost |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tana, C.; Soloperto, M.; Giuliano, G.; Erroi, G.; Di Maggio, A.; Tortorella, C.; Moffa, L. Artificial Intelligence for Predicting Lung Immune Responses to Viral Infections: From Mechanistic Insights to Clinical Applications. Viruses 2025, 17, 1482. https://doi.org/10.3390/v17111482
Tana C, Soloperto M, Giuliano G, Erroi G, Di Maggio A, Tortorella C, Moffa L. Artificial Intelligence for Predicting Lung Immune Responses to Viral Infections: From Mechanistic Insights to Clinical Applications. Viruses. 2025; 17(11):1482. https://doi.org/10.3390/v17111482
Chicago/Turabian StyleTana, Claudio, Massimo Soloperto, Giampiero Giuliano, Giorgio Erroi, Antonio Di Maggio, Cosimo Tortorella, and Livia Moffa. 2025. "Artificial Intelligence for Predicting Lung Immune Responses to Viral Infections: From Mechanistic Insights to Clinical Applications" Viruses 17, no. 11: 1482. https://doi.org/10.3390/v17111482
APA StyleTana, C., Soloperto, M., Giuliano, G., Erroi, G., Di Maggio, A., Tortorella, C., & Moffa, L. (2025). Artificial Intelligence for Predicting Lung Immune Responses to Viral Infections: From Mechanistic Insights to Clinical Applications. Viruses, 17(11), 1482. https://doi.org/10.3390/v17111482






