HBV Infection Drives PSMB5-Dependent Proteasomal Activation in Humanized Mice and HBV-Associated HCC
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Sample Collection
2.2. Preparation of Human Liver Tissue Lysates
2.3. Assessment of Proteasome Activity in Patient Liver and Serum Samples
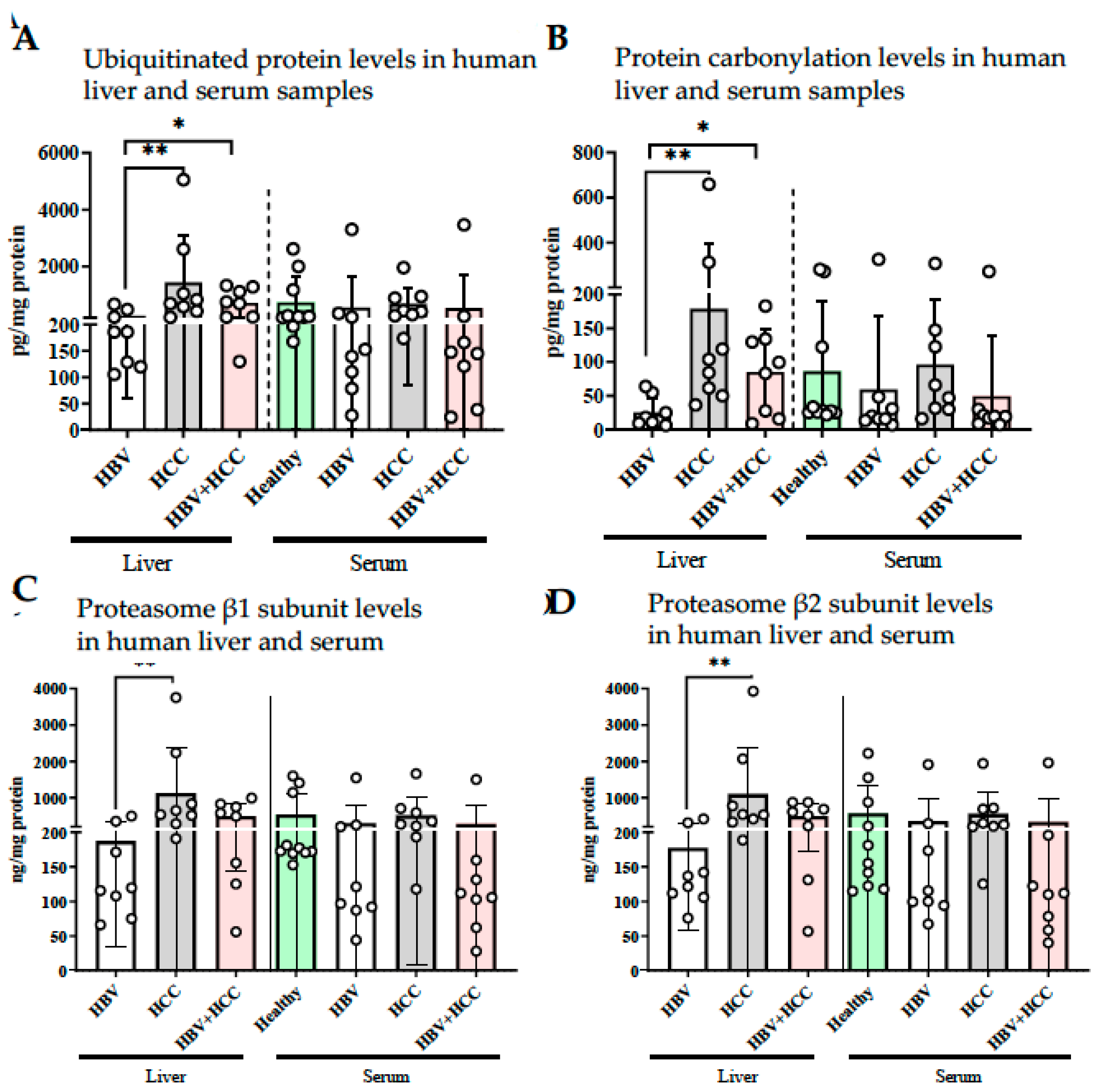
2.4. Ubiquitin, Protein Carbonyl, and Proteasome β1/β2/β5 Subunit Levels in Liver and Serum Samples
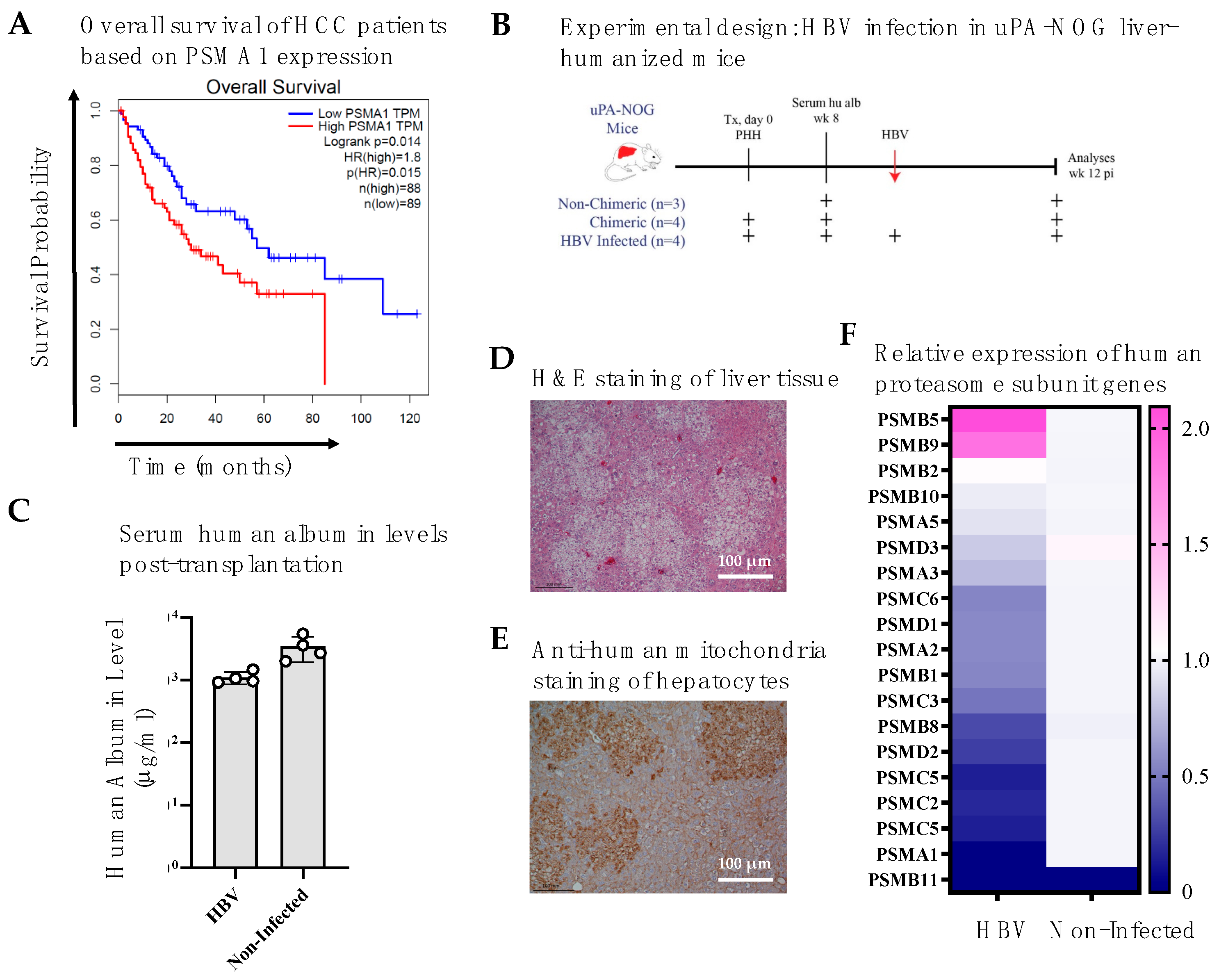
2.5. Mice, Transplantation, and HBV Infection
2.6. Immunohistochemical Analysis of Mouse Liver
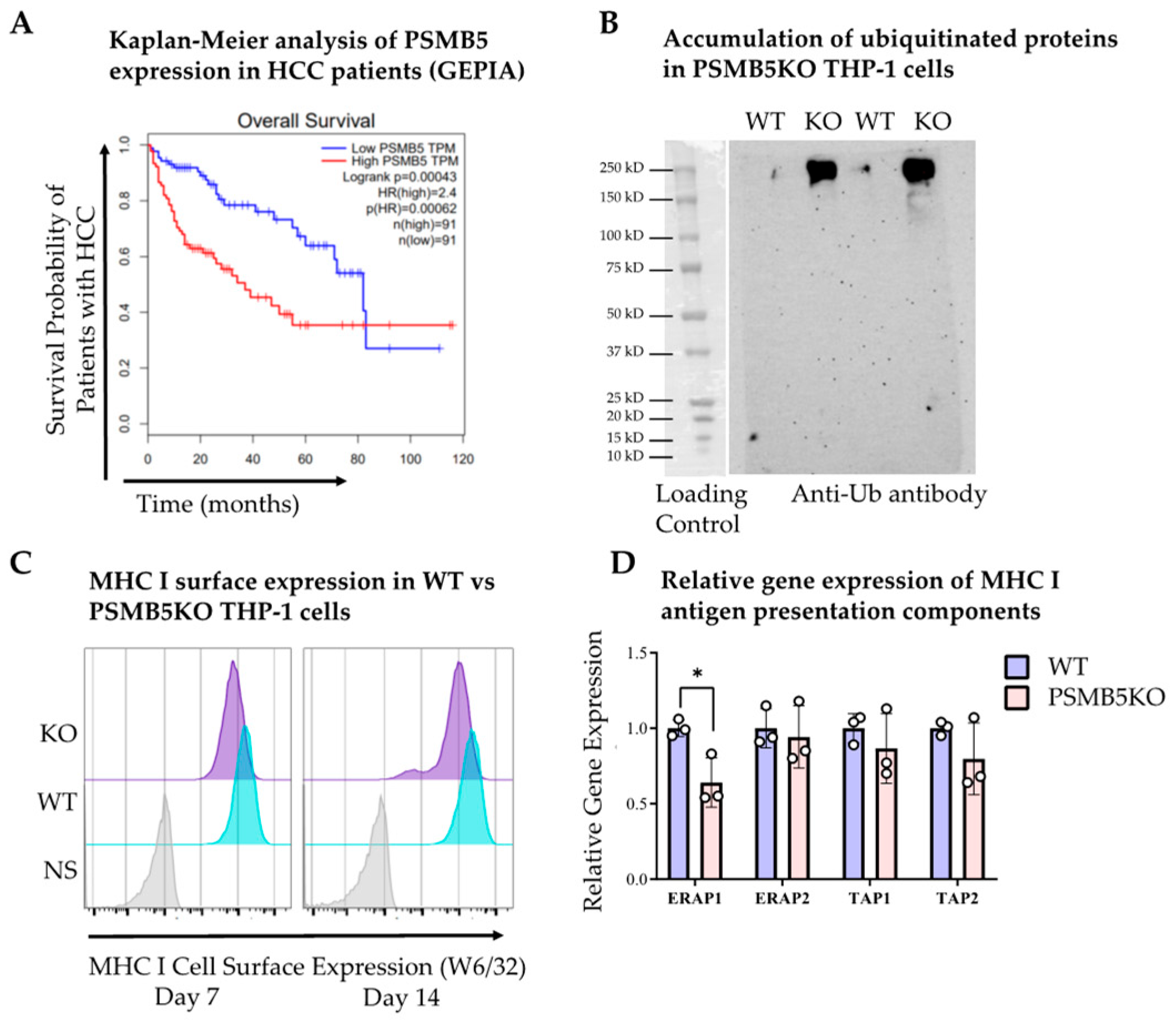
2.7. Cell Culture and CRISPR-Cas9 Knockout
2.8. RNA Extraction and Real-Time qPCR
2.9. Western Blot Analysis
2.10. Flow Cytometry of THP-1 Cell Surface Markers
2.11. Comparative Analysis of Gene Expression
2.12. Statistical Analyses
3. Results
3.1. Higher Proteasomal Subunit Expression in HCC Is Associated with Poorer Survival
3.2. HBV Infection in Liver-Humanized Mice Increases PSMB5 Transcript Levels
3.3. HBV-Infected Patient Livers Exhibit Elevated Chymotrypsin-like Proteolytic Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALT | Alanine transaminase |
| APC | Antigen presentation complex |
| HBV | Hepatitis B virus |
| HCC | Hepatocellular carcinoma |
| CHB | Chronic hepatitis B |
| MHC | Major histocompatibility complex |
| NA | Not applicable |
| PSMB5 | Proteasome 20S Subunit Beta 5 |
| UPS | Ubiquitin proteasomal system |
| sgRNA | Single guide RNA |
| uPA-NOG | urokinase-type plasminogen activator- NOD/Shi-scid IL2Rγnull mouse |
References
- Jung, T.; Catalgol, B.; Grune, T. The proteasomal system. Mol. Asp. Med. 2009, 30, 191–296. [Google Scholar] [CrossRef]
- Groll, M.; Ditzel, L.; Löwe, J.; Stock, D.; Bochtler, M.; Bartunik, H.D.; Huber, R. Structure of 20S proteasome from yeast at 2.4Å resolution. Nature 1997, 386, 463–471. [Google Scholar] [CrossRef]
- Breusing, N.; Grune, T. Regulation of proteasome-mediated protein degradation during oxidative stress and aging. Biol. Chem. 2008, 389, 203–209. [Google Scholar] [CrossRef]
- Tanaka, K. Molecular biology of proteasomes. Mol. Biol. Rep. 1995, 21, 21–26. [Google Scholar] [CrossRef]
- Ramachandran, K.V.; Fu, J.M.; Schaffer, T.B.; Na, C.H.; Delannoy, M.; Margolis, S.S. Activity-Dependent Degradation of the Nascentome by the Neuronal Membrane Proteasome. Mol. Cell 2018, 71, 169–177.e6. [Google Scholar] [CrossRef]
- Kammerl, I.E.; Meiners, S. Proteasome function shapes innate and adaptive immune responses. Am. J. Physiol. Cell. Mol. Physiol. 2016, 311, L328–L336. [Google Scholar] [CrossRef] [PubMed]
- Ubiquitin Proteasome System. Current Insights into Mechanism Cellular Regulation and Disease; IntechOpen: Rijeka, Croatia, 2019; ISBN 978-1-83881-0-0. Available online: https://www.intechopen.com/books/8301 (accessed on 29 September 2025).
- Cui, F.; Blach, S.; Mingiedi, C.M.; Gonzalez, M.A.; Alaama, A.S.; Mozalevskis, A.; Séguy, N.; Rewari, B.B.; Chan, P.-L.; Le, L.-V.; et al. Global reporting of progress towards elimination of hepatitis B and hepatitis C. Lancet Gastroenterol. Hepatol. 2023, 8, 332–342. [Google Scholar] [CrossRef]
- Osmani, Z.; Boonstra, A. Recent Insights into the Role of B Cells in Chronic Hepatitis B and C Infections. Pathogens 2023, 12, 815. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Health Organization. Fact Sheets. Hepatitis B. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b (accessed on 29 September 2025).
- Kremsdorf, D.; Soussan, P.; Paterlini-Brechot, P.; Brechot, C. Hepatitis B virus-related hepatocellular carcinoma: Paradigms for viral-related human carcinogenesis. Oncogene 2006, 25, 3823–3833. [Google Scholar] [CrossRef]
- Tsukuda, S.; Watashi, K. Hepatitis B virus biology and life cycle. Antivir. Res. 2020, 182, 104925. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; You, H.; Kong, D.; Zheng, K.; Tang, R. The interaction of hepatitis B virus with the ubiquitin proteasome system in viral replication and associated pathogenesis. Virol. J. 2019, 16, 1–10. [Google Scholar] [CrossRef]
- Robek, M.D.; Garcia, M.L.; Boyd, B.S.; Chisari, F.V. Role of Immunoproteasome Catalytic Subunits in the Immune Response to Hepatitis B Virus. J. Virol. 2007, 81, 483–491. [Google Scholar] [CrossRef]
- Zhang, Z.; Torii, N.; Furusaka, A.; Malayaman, N.; Hu, Z.; Liang, T. Structural and Functional Characterization of Interaction between Hepatitis B Virus X Protein and the Proteasome Complex. J. Biol. Chem. 2000, 275, 15157–15165. [Google Scholar] [CrossRef]
- Bandi, P.; Garcia, M.L.; Booth, C.J.; Chisari, F.V.; Robek, M.D. Bortezomib Inhibits Hepatitis B Virus Replication in Transgenic Mice. Antimicrob. Agents Chemother. 2010, 54, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Sari, G.; van de Garde, M.D.; van Schoonhoven, A.; Voermans, J.J.; van der Eijk, A.A.; de Man, R.A.; Boonstra, A.; Vanwolleghem, T.; Pas, S.D. Hepatitis E Virus Shows More Genomic Alterations in Cell Culture than In Vivo. Pathogens 2019, 8, 255. [Google Scholar] [CrossRef] [PubMed]
- Sari, G.; Mulders, C.E.; Zhu, J.; van Oord, G.W.; Feng, Z.; Kreeft-Voermans, J.J.; Boonstra, A.; Vanwolleghem, T. Treatment induced clearance of hepatitis E viruses by interferon-lambda in liver-humanized mice. Liver Int. 2021, 41, 2866–2873. [Google Scholar] [CrossRef]
- Sagan, D.; Eckardt-Schupp, F.; Eichholtz-Wirth, H. Reduced expression of SRC family kinases decreases PI3K activity in NBS1 lymphoblasts. Biochem. Biophys. Res. Commun. 2008, 377, 181–186. [Google Scholar] [CrossRef]
- van de Garde, M.D.B.; Pas, S.D.; van der Net, G.; de Man, R.A.; Osterhaus, A.D.M.E.; Haagmans, B.L.; van der Eijk, A.A. Hepatitis E virus genotype 3 infection of human liver chimeric mice as a model for chronic HEV infection. J. Virol. 2016, 90, 4394–4401. Available online: https://pubmed.ncbi.nlm.nih.gov/26889028/ (accessed on 29 September 2025). [CrossRef]
- Jamet, E. An eye-tracking study of cueing effects in multimedia learning. Comput. Human Behav. 2014, 32, 47–53. [Google Scholar] [CrossRef]
- Sari, G.; van Oord, G.W.; van de Garde, M.D.; Voermans, J.J.; Boonstra, A.; Vanwolleghem, T. Sexual Dimorphism in Hepatocyte Xenograft Models. Cell Transplant. 2021, 30, 09636897211006132. [Google Scholar] [CrossRef]
- Pas, S.D.; Niesters, H.G.M. Detection of HBV DNA using real time analysis. J. Clin. Virol. 2002, 25, 93–94. Available online: https://pubmed.ncbi.nlm.nih.gov/12126725/ (accessed on 29 September 2025). [CrossRef] [PubMed]
- Pas, S.D.; Fries, E.; De Man, R.A.; Osterhaus, A.D.M.E.; Niesters, H.G.M. Development of a Quantitative Real-Time Detection Assay for Hepatitis B Virus DNA and Comparison with Two Commercial Assays. J. Clin. Microbiol. 2000, 38, 2897–2901. [Google Scholar] [CrossRef]
- Dandri, M.; Petersen, J. Animal models of HBV infection. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 265–275. Available online: https://pubmed.ncbi.nlm.nih.gov/28774409/ (accessed on 29 September 2025). [CrossRef]
- The Cancer Genome Atlas Research Network. Comprehensive and Integrative Genomic Characterization of Hepatocellular Carcinoma. Cell 2017, 169, 1327–1341.e23. [Google Scholar] [CrossRef]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef]
- Rana, P.S.; Ignatz-Hoover, J.J.; Driscoll, J.J. Targeting Proteasomes and the MHC Class I Antigen Presentation Machinery to Treat Cancer, Infections and Age-Related Diseases. Cancers 2023, 15, 5632. [Google Scholar] [CrossRef]
- Sari, G.; Rock, K.L. Tumor immune evasion through loss of MHC class-I antigen presentation. Curr. Opin. Immunol. 2023, 83, 102329. [Google Scholar] [CrossRef]
- Levrero, M.; Zucman-Rossi, J. Mechanisms of HBV-induced hepatocellular carcinoma. J. Hepatol. 2016, 64, S84–S101. [Google Scholar] [CrossRef]
- Suemizu, H.; Hasegawa, M.; Kawai, K.; Taniguchi, K.; Monnai, M.; Wakui, M.; Suematsu, M.; Ito, M.; Peltz, G.; Nakamura, M. Establishment of a humanized model of liver using NOD/Shi-scid IL2Rgnull mice. Biochem. Biophys. Res. Commun. 2008, 377, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Saura-Esteller, J.; de Jong, M.; King, L.A.; Ensing, E.; Winograd, B.; de Gruijl, T.D.; Parren, P.W.H.I.; van der Vliet, H.J. Gamma Delta T-Cell Based Cancer Immunotherapy: Past-Present-Future. Front. Immunol. 2022, 13, 915837. [Google Scholar] [CrossRef] [PubMed]
- Qian, G.H.; Pan, C.; Zhao, J.; Wang, H.; Xie, G.; Xie, J.; Wang, Y. NIRF, a novel ubiquitin ligase, interacts with hepatitis B virus core protein and promotes its degradation. Biotechnol. Lett. 2012, 34, 29–36. Available online: https://pubmed.ncbi.nlm.nih.gov/22072112/ (accessed on 29 September 2025). [CrossRef] [PubMed]
- Guzmán, M.G. Announcement. J. Med. Virol. 2007, 79, 1032. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, Z.; Doo, E.; Coux, O.; Goldberg, A.L.; Liang, T.J. Hepatitis B Virus X Protein Is both a Substrate and a Potential Inhibitor of the Proteasome Complex. J. Virol. 1999, 73, 7231–7240. [Google Scholar] [CrossRef]
- Xie, Z.; Chen, Y.; Liao, E.-Y.; Jiang, Y.; Liu, F.-Y.; Pennypacker, S.D. Phospholipase C-γ1 is required for the epidermal growth factor receptor-induced squamous cell carcinoma cell mitogenesis. Biochem. Biophys. Res. Commun. 2010, 397, 296–300. [Google Scholar] [CrossRef]
- Mukhtar, E.; Adhami, V.M.; Sechi, M.; Mukhtar, H. Dietary flavonoid fisetin binds to β-tubulin and disrupts microtubule dynamics in prostate cancer cells. Cancer Lett. 2015, 367, 173–183. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | HBV (n = 8) | Non-HBV + HCC (n = 8) | HBV + HCC (n = 8) | Healthy Controls (n = 10) |
|---|---|---|---|---|
| Male/Female n (%) | 6/2 (75.0)/(25.0) | 6/2 (75.0)/(25.0) | 8/0 (100.0/0.0) | 5/5 (50.00/50.00) |
| Age (±) | 52.4 ± 7.5 | 53.8 ± 19.3 | 61.8 ± 6.0 | 47.6 ± 9.6 |
| Cirrhosis n (%) | Yes 8 (100.0) | Yes 4 (75.00) | Yes 5 (62.5) | NA |
| ALT (±) | 31.3 ± 30.3 | 39.1 ± 39.9 | 31.6 ± 7.3 | 20.8 ± 4.05 |
| Ethnicity n (%) | White 8 (100.0) | White 8 (100.0) | White 8 (100.0) | White 10 (100.00) |
| Fibrosis Parameters | ||||
| FIB-4 Index (±) | 6.8 ± 3.9 | 5.4 ± 2.2 | 3.9 ± 2.8 | NA |
| ISHAK Score (%) | 6 (100) | NA | NA | NA |
| Fibrosis Stage n (%) * | NA | |||
| F0–2 | NA | 1 (12.5) | ||
| F3 | NA | 1 (12.5) | 1 (12.5) | |
| F4 | NA | 4 (50.0) | 5 (62.5) |
| Target | Sequence | Target | Sequence |
|---|---|---|---|
| PSMA1-F | ATACTTTCGGCAGCACCTCC | PSMB10-R | CTGCGGTCCAGTCAGGTCTA |
| PSMA1-R | AGACCAACTGTGGCTGAACC | PSMB11-F | CGTGGCTATCGCTACGACAT |
| PSMA2-F | GTGCTTTGGCTCTTCGGGTA | PSMB11_R | TGACACATGCTCCCATCCAC |
| PSMA2-R | GCTTTAATTCCCACGGACGG | PSMC2-F | ACAGCCTTTACAGGTTGCCA |
| PSMA3-F | GGCTGCAGTTTCATGTTAGGG | PSMC2_R | CTATCCACGCCCACTCTCATC |
| PSMA3-R | GATGGCACAGCCCCAATAAC | PSMC3_F | ATTGGGGGTTTGGACAAGCA |
| PSMA5-F | GTACGACAGGGGCGTGAATA | PSMC3_R | ATCAGCACCCCTTTTGGAGG |
| PSMA5-R | GCACACACCCTCTGATGTCT | PSMC4_F | TCTGGAGGCTGTGGATCAGA |
| PSMB1-F | CCCTTTGCAGCTGCGATTTT | PSMC4_R | AGTGCATTGCTGTGCTTGTG |
| PSMB1-R | GGGCTATCCCGCGTATGAAT | PSMC5_F | AGAGAAGATGGCGCTTGACG |
| PSMB2-F | CTCATCGGTATCCAAGGCCC | PSMC5_R | CTCCGGAGGTTTTGGCTCTT |
| PSMB2-R | GTCTCCAGCCTCTCCAACAC | PSMC6_F | AACACAAGGAGATCGACGGC |
| PSMB5-F | GCTACCGGTGAACCAGCG | PSMC6_R | CGATCTGCCCAACACTCTGTA |
| PSMB5-R | CAACTATGACTCCATGGCGGA | PSMD1_F | ATGGGAGGATGGAAGAGGCT |
| PSMB8-F | GCTCCTGGCTGACTTCTAGT | PSMD1_R | AACATGTAGCAGGCGTCGAA |
| PSMB8-R | TGAACGTTCCTTTCTCCGTCC | PSMD2_F | CATGACTTCAGTGCCCAAGC |
| PSMB9-F | GGCGTTGTGATGGGTTCTGA | PSMD2_R | CGGAGATGATGTCAGCAGCA |
| PSMB9-R | AGAGAGTGCACAGTAGATGCG | PSMD3_F | GCGAATCAAAGCCATCCAGC |
| PSMB10-F | AGCTACACGCGTTATCTACGG | PSMD3_R | CAGCTCCACCACGATGAGAA |
| Gene Name | 5-Year Survival of High Expressers (%) | 5-Year Survival of Low Expressers (%) | p-Value $ |
|---|---|---|---|
| PSMA1 | 36 | 56 | 0.000038 |
| PSMA2 | 32 | 56 | 0.00013 |
| PSMA3 | 37 | 57 | 0.002 |
| PSMA5 | 42 | 57 | 0.00023 |
| PSMA6 | 44 | 49 | 0.075 |
| PSMA7 | 32 | 59 | 0.000025 |
| PSMB1 | 39 | 51 | 0.024 |
| PSMB2 | 31 | 56 | 0.00000035 |
| PSMB3 | 43 | 59 | 0.055 |
| PSMB4 | 39 | 55 | 0.00075 |
| PSMB5 | 31 | 57 | 0.000029 |
| PSMB6 | 43 | 52 | 0.27 |
| PSMB7 | 44 | 50 | 0.02 |
| PSMB8 | 34 | 55 | 0.078 |
| PSMB9 | 40 | 56 | 0.057 |
| PSMB10 | 50 | 43 | 0.072 |
| PSMC1 | 42 | 53 | 0.012 |
| PSMC2 | 41 | 51 | 0.021 |
| PSMC5 | 43 | 52 | 0.0083 |
| PSMC6 | 38 | 51 | 0.0031 |
| PSMD1 | 19 | 58 | 8.6 × 10−12 |
| PSMD2 | 32 | 53 | 0.00000066 |
| PSMD3 | 43 | 51 | 0.0057 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jannuzzi, A.T.; Sari, G.; Arslan-Eseryel, S.; Zeybel, M.; Yilmaz, Y.; Dayangac, M.; Yigit, B.; Arga, K.Y.; Boonstra, A.; Eren, F.; et al. HBV Infection Drives PSMB5-Dependent Proteasomal Activation in Humanized Mice and HBV-Associated HCC. Viruses 2025, 17, 1454. https://doi.org/10.3390/v17111454
Jannuzzi AT, Sari G, Arslan-Eseryel S, Zeybel M, Yilmaz Y, Dayangac M, Yigit B, Arga KY, Boonstra A, Eren F, et al. HBV Infection Drives PSMB5-Dependent Proteasomal Activation in Humanized Mice and HBV-Associated HCC. Viruses. 2025; 17(11):1454. https://doi.org/10.3390/v17111454
Chicago/Turabian StyleJannuzzi, Ayse Tarbin, Gulce Sari, Sema Arslan-Eseryel, Mujdat Zeybel, Yusuf Yilmaz, Murat Dayangac, Buket Yigit, Kazim Yalcin Arga, Andre Boonstra, Fatih Eren, and et al. 2025. "HBV Infection Drives PSMB5-Dependent Proteasomal Activation in Humanized Mice and HBV-Associated HCC" Viruses 17, no. 11: 1454. https://doi.org/10.3390/v17111454
APA StyleJannuzzi, A. T., Sari, G., Arslan-Eseryel, S., Zeybel, M., Yilmaz, Y., Dayangac, M., Yigit, B., Arga, K. Y., Boonstra, A., Eren, F., & Karademir-Yilmaz, B. (2025). HBV Infection Drives PSMB5-Dependent Proteasomal Activation in Humanized Mice and HBV-Associated HCC. Viruses, 17(11), 1454. https://doi.org/10.3390/v17111454







