Genetic and Statistical Study of Anelloviruses and Gyroviruses in Diarrheic Cats and Their Co-Occurrence Patterns
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Polymerase Chain Reactions (PCRs)
2.3. Sequencing and Phylogenetic Analysis
2.4. Statistical Analyses
3. Results
3.1. Prevalence of Feline Panleukopenia Virus
3.2. Prevalence of the Anelloviridae Family
3.3. Genetic Profiling and Characteristics of Anelloviruses and Gyroviruses
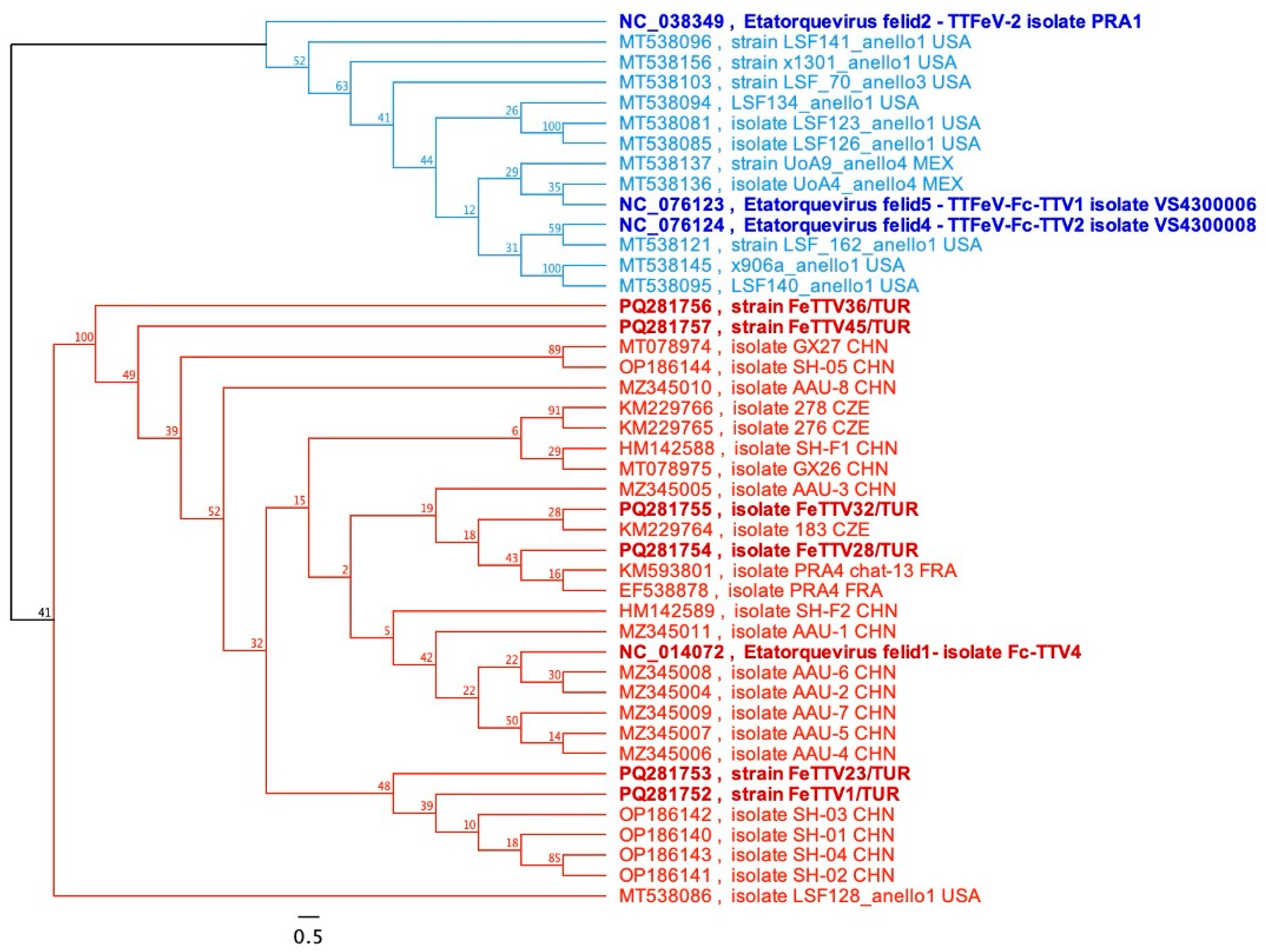
3.3.1. Molecular Analysis of TTFeV1
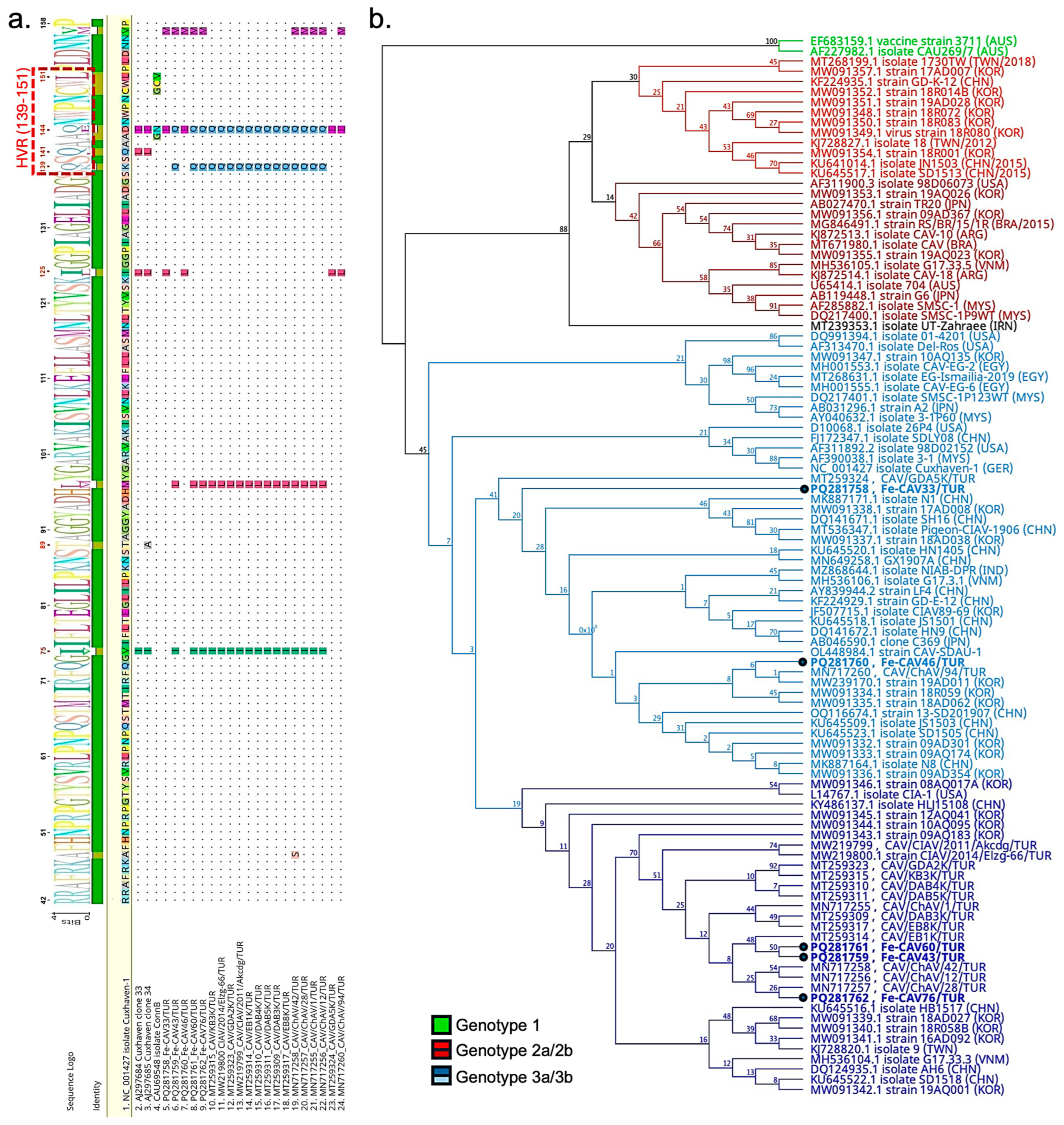
3.3.2. Molecular Analysis of Chicken Anemia Virus
3.3.3. Molecular Analyses of Avian gyrovirus 2, Avian gyrovirus 3 and Avian gyrovirus 4
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Varsani, A.; Opriessnig, T.; Celer, V.; Maggi, F.; Okamoto, H.; Blomstrom, A.L.; Cadar, D.; Harrach, B.; Biagini, P.; Kraberger, S. Taxonomic update for mammalian anelloviruses (family Anelloviridae). Arch. Virol. 2021, 166, 2943–2953. [Google Scholar] [CrossRef]
- Nishizawa, T.; Okamoto, H.; Konishi, K.; Yoshizawa, H.; Miyakawa, Y.; Mayumi, M. A novel DNA virus (TTV) associated with elevated transaminase levels in posttransfusion hepatitis of unknown etiology. Biochem. Biophys. Res. Commun. 1997, 241, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Mushahwar, I.K.; Erker, J.C.; Muerhoff, A.S.; Leary, T.P.; Simons, J.N.; Birkenmeyer, L.G.; Chalmers, M.L.; Pilot-Matias, T.J.; Dexai, S.M. Molecular and biophysical characterization of TT virus: Evidence for a new virus family infecting humans. Proc. Natl. Acad. Sci. USA 1999, 96, 3177–3182. [Google Scholar] [CrossRef]
- Okamoto, H.; Nishizawa, T.; Takahashi, M.; Tawara, A.; Peng, Y.; Kishimoto, J.; Wang, Y. Genomic and evolutionary characterization of TT virus (TTV) in tupaias and comparison with species-specific TTVs in humans and non-human primates. J. Gen. Virol. 2001, 82, 2041–2050. [Google Scholar] [CrossRef]
- Okamoto, H.; Takahashi, M.; Nishizawa, T.; Tawara, A.; Fukai, K.; Muramatsu, U.; Naito, Y.; Yoshikawa, A. Genomic characterization of TT viruses (TTVs) in pigs, cats and dogs and their relatedness with species-specific TTVs in primates and tupaias. J. Gen. Virol. 2002, 83, 1291–1297. [Google Scholar] [CrossRef]
- Li, L.; Giannitti, F.; Low, J.; Keyes, C.; Ullmann, L.S.; Deng, X.; Aleman, M.; Pesavento, P.A.; Pusterla, N.; Delwart, E. Exploring the virome of diseased horses. J. Gen. Virol. 2015, 96, 2721–2733. [Google Scholar] [CrossRef]
- Polster, S.; Lechmann, J.; Lienhard, J.; Peltzer, D.; Prahauser, B.; Bachofen, C.; Seehusen, F. First Report of TTSuV1 in Domestic Swiss Pigs. Viruses 2022, 14, 870. [Google Scholar] [CrossRef]
- Siahpoush, M.; Noorbazargan, H.; Kalantari, S.; Shayestehpour, M.; Yazdani, S. Coinfection of torque teno virus (TTV) and human papillomavirus (HPV) in cervical samples of women living in Tehran, Iran. Iran. J. Microbiol. 2022, 14, 181–185. [Google Scholar] [CrossRef]
- Zhu, C.X.; Shan, T.L.; Cui, L.; Luo, X.N.; Liu, Z.J.; Tang, S.D.; Liu, Z.W.; Yuan, C.L.; Lan, D.L.; Zhao, W.; et al. Molecular detection and sequence analysis of feline Torque teno virus (TTV) in China. Virus Res. 2011, 156, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Y.; Ji, J.; Chen, F.; Sun, B.; Xue, C.; Ma, J.; Bi, Y.; Xie, Q. Identification of a chicken anemia virus variant-related gyrovirus in stray cats in china, 2012. BioMed Res. Int. 2014, 2014, 313252. [Google Scholar] [CrossRef]
- Zhang, W.; Li, L.; Deng, X.; Kapusinszky, B.; Pesavento, P.A.; Delwart, E. Faecal virome of cats in an animal shelter. J. Gen. Virol. 2014, 95, 2553–2564. [Google Scholar] [CrossRef]
- Jarosova, V.; Hrazdilova, K.; Filipejova, Z.; Schanilec, P.; Celer, V. Whole genome sequencing and phylogenetic analysis of feline anelloviruses. Infect. Genet. Evol. 2015, 32, 130–134. [Google Scholar] [CrossRef]
- Niu, J.T.; Yi, S.S.; Dong, G.Y.; Guo, Y.B.; Zhao, Y.L.; Huang, H.L.; Wang, K.; Hu, G.X.; Dong, H. Genomic Characterization of Diverse Gyroviruses Identified in the Feces of Domestic Cats. Sci. Rep. 2019, 9, 13303. [Google Scholar] [CrossRef]
- Kim, H.R.; Kwon, Y.K.; Bae, Y.C.; Oem, J.K.; Lee, O.S. Molecular characterization of chicken infectious anemia viruses detected from breeder and broiler chickens in South Korea. Poult. Sci. 2010, 89, 2426–2431. [Google Scholar] [CrossRef]
- Sauvage, V.; Cheval, J.; Foulongne, V.; Gouilh, M.A.; Pariente, K.; Manuguerra, J.C.; Richardson, J.; Dereure, O.; Lecuit, M.; Burguiere, A.; et al. Identification of the First Human, a Virus Related to Chicken Anemia Virus. J. Virol. 2011, 85, 7948–7950. [Google Scholar] [CrossRef]
- Rijsewijk, F.A.M.; dos Santos, H.F.; Teixeira, T.F.; Cibulski, S.P.; Varela, A.P.M.; Dezen, D.; Franco, A.C.; Roehe, P.M. Discovery of a genome of a distant relative of chicken anemia virus reveals a new member of the genus Gyrovirus. Arch. Virol. 2011, 156, 1097–1100. [Google Scholar] [CrossRef]
- Feher, E.; Bali, K.; Kaszab, E.; Ihasz, K.; Jakab, S.; Nagy, B.; Ursu, K.; Farkas, S.L.; Banyai, K. A novel gyrovirus in a common pheasant (Phasianus colchicus) with poult enteritis and mortality syndrome. Arch. Virol. 2022, 167, 1349–1353. [Google Scholar] [CrossRef] [PubMed]
- Varsani, A.; Kraberger, S.; Opriessnig, T.; Maggi, F.; Celer, V.; Okamoto, H.; Biagini, P. Anelloviridae taxonomy update 2023. Arch. Virol. 2023, 168, 277. [Google Scholar] [CrossRef] [PubMed]
- Karki, M.; Bora, M.; Manu, M.; Kumar, A. An updated review on chicken infectious anaemia. World Poult. Sci. J. 2024, 81, 117–134. [Google Scholar] [CrossRef]
- Chu, D.K.; Poon, L.L.; Chiu, S.S.; Chan, K.H.; Ng, E.M.; Bauer, I.; Cheung, T.K.; Ng, I.H.; Guan, Y.; Wang, D.; et al. Characterization of a novel gyrovirus in human stool and chicken meat. J. Clin. Virol. 2012, 55, 209–213. [Google Scholar] [CrossRef]
- Lima, D.A.; Cibulski, S.P.; Finkler, F.; Teixeira, T.F.; Varela, A.P.M.; Cerva, C.; Loiko, M.R.; Scheffer, C.M.; dos Santos, H.F.; Mayer, F.Q.; et al. Faecal virome of healthy chickens reveals a large diversity of the eukaryote viral community, including novel circular ssDNA viruses. J. Gen. Virol. 2017, 98, 690–703. [Google Scholar] [CrossRef]
- Buonavoglia, C.; Martella, V.; Pratelli, A.; Tempesta, M.; Cavalli, A.; Buonavoglia, D.; Bozzo, G.; Elia, G.; Decaro, N.; Carmichael, L. Evidence for evolution of canine parvovirus type 2 in Italy. J. Gen. Virol. 2001, 82, 3021–3025. [Google Scholar] [CrossRef]
- Stecher, G.; Tamura, K.; Kumar, S. Molecular Evolutionary Genetics Analysis (MEGA) for macOS. Mol. Biol. Evol. 2020, 37, 1237–1239. [Google Scholar] [CrossRef]
- Guindon, S.; Lethiec, F.; Duroux, P.; Gascuel, O. PHYML Online—A web server for fast maximum likelihood-based phylogenetic inference. Nucleic Acids Res. 2005, 33, W557–W559. [Google Scholar] [CrossRef]
- Martin, D.P.; Murrell, B.; Golden, M.; Khoosal, A.; Muhire, B. RDP4: Detection and analysis of recombination patterns in virus genomes. Virus Evol. 2015, 1, vev003. [Google Scholar] [CrossRef]
- Dolan, E.D.; Doyle, E.; Tran, H.R.; Slater, M.R. Pre-mortem risk factors for mortality in kittens less than 8 weeks old at a dedicated kitten nursery. J. Feline Med. Surg. 2021, 23, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Watson, V.E.; Jacob, M.E.; Flowers, J.R.; Strong, S.J.; DebRoy, C.; Gookin, J.L. Association of Atypical Enteropathogenic Escherichia coli with Diarrhea and Related Mortality in Kittens. J. Clin. Microbiol. 2017, 55, 2719–2735. [Google Scholar] [CrossRef] [PubMed]
- Stuetzer, B.; Hartmann, K. Feline parvovirus infection and associated diseases. Vet. J. 2014, 201, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Rubini, S.; Rubini, R.; Bertocchi, S.; Zordan, S.; Magri, A.; Barsi, F.; Sampieri, M.; Locatelli, C.A.; Baldini, E.; Manfredini, S.; et al. A case of severe benzalkonium chloride intoxication in a cat. Acta Vet. Scand. 2024, 66, 18. [Google Scholar] [CrossRef] [PubMed]
- Guilford, W.G.; Jones, B.R.; Markwell, P.J.; Arthur, D.G.; Collett, M.G.; Harte, J.G. Food sensitivity in cats with chronic idiopathic gastrointestinal problems. J. Vet. Intern. Med. 2001, 15, 7–13. [Google Scholar] [CrossRef]
- Aydin, H.; Timurkan, M.O. A pilot study on feline astrovirus and feline panleukopenia virus in shelter cats in Erzurum, Turkey. Rev. Med. Vet.-Toulouse 2018, 169, 52–57. [Google Scholar]
- Hasircioglu, S.; Aslim, H.P.; Kale, M.; Bulut, O.; Koçlu, O.; Orta, Y.S. Molecular characterization of carnivore protoparvovirus strains circulating in cats in Turkey. Pesq. Vet. Bras. 2023, 43, e07178. [Google Scholar] [CrossRef]
- Wang, J.Y.; Yan, Z.R.; Liu, H.R.; Wang, W.J.; Liu, Y.K.; Zhu, X.; Tian, L.L.; Zhao, J.J.; Peng, Q.S.; Bi, Z.W. Prevalence and Molecular Evolution of Parvovirus in Cats in Eastern Shandong, China, between 2021 and 2022. Transbound. Emerg. Dis. 2024, 2024, 5514806. [Google Scholar] [CrossRef]
- Tang, Y.S.; Tang, N.; Zhu, J.R.; Wang, M.; Liu, Y.; Lyu, Y.L. Molecular characteristics and genetic evolutionary analyses of circulating parvoviruses derived from cats in Beijing. BMC Vet.-Res. 2022, 18, 195. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, H.K.; Nookala, M.; Thangamani, N.R.K.; Sivaprakasam, A.; Antony, P.X.; Thanislass, J.; Srinivas, M.V.; Pillai, R.M. Molecular characterisation of parvoviruses from domestic cats reveals emergence of newer variants in India. J. Feline Med. Surg. 2017, 19, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.; Habib, T.; Chouhan, C.S.; Hassan, J.; Rahman, A.K.M.A.; Nazir, K.H.M.N.H. Epidemiology and molecular characterization of Feline panleukopenia virus from suspected domestic cats in selected Bangladesh regions. PLoS ONE 2023, 18, e0282559. [Google Scholar] [CrossRef]
- Abdel-Baky, M.M.M.; El-Khabaz, K.A.S.; Abdelbaset, A.E.; Hamed, M.I. Clinico-epidemiological survey of feline parvovirus circulating in three Egyptian provinces from 2020 to 2021. Arch. Virol. 2023, 168, 126. [Google Scholar] [CrossRef]
- Rehme, T.; Hartmann, K.; Truyen, U.; Zablotski, Y.; Bergmann, M. Feline Panleukopenia Outbreaks and Risk Factors in Cats in Animal Shelters. Viruses 2022, 14, 1248. [Google Scholar] [CrossRef]
- Truyen, U.; Addie, D.; Belak, S.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Gruffydd-Jones, T.; Hartmann, K.; Hosie, M.J.; Lloret, A.; et al. Feline panleukopenia. ABCD guidelines on prevention and management. J. Feline Med. Surg. 2009, 11, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Brussel, K.V.; Wang, X.; Shi, M.; Carrai, M.; Li, J.; Martella, V.; Beatty, J.A.; Holmes, E.C.; Barrs, V.R. Identification of novel astroviruses in the gastrointestinal tract of domestic cats. Viruses 2020, 12, 1301. [Google Scholar] [CrossRef]
- Castro, T.X.; Garcia, R.d.C.N.C.; Fumian, T.M.; Costa, E.M.; Mello, R.; White, P.A.; Leite, J.P. Detection and molecular characterization of caliciviruses (vesivirus and norovirus) in an outbreak of acute diarrhea in kittens from Brazil. Vet. J. 2015, 206, 115–117. [Google Scholar] [CrossRef]
- Van Brussel, K.; Wang, X.; Shi, M.; Carrai, M.; Feng, S.; Li, J.; Holmes, E.C.; Beatty, J.A.; Barrs, V.R. The enteric virome of cats with feline panleukopenia differs in abundance and diversity from healthy cats. Transbound. Emerg. Dis. 2022, 69, e2952–e2966. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liu, C.; Yi, J.; Shi, Y.; Li, H.; Liu, H. Genomic Characteristics of Feline Anelloviruses Isolated from Domestic Cats in Shanghai, China. Vet. Sci. 2023, 10, 444. [Google Scholar] [CrossRef]
- Biagini, P.; Uch, R.; Belhouchet, M.; Attoui, H.; Cantaloube, J.F.; Brisbarre, N.; de Micco, P. Circular genomes related to anelloviruses identified in human and animal samples by using a combined rolling-circle amplification/sequence-independent single primer amplification approach. J. Gen. Virol. 2007, 88, 2696–2701. [Google Scholar] [CrossRef]
- Kraberger, S.; Serieys, L.E.; Richet, C.; Fountain-Jones, N.M.; Baele, G.; Bishop, J.M.; Nehring, M.; Ivan, J.S.; Newkirk, E.S.; Squires, J.R.; et al. Complex evolutionary history of felid anelloviruses. Virology 2021, 562, 176–189. [Google Scholar] [CrossRef]
- Phan, T.G.; Li, L.; O’Ryan, M.G.; Cortes, H.; Mamani, N.; Bonkoungou, I.J.O.; Wang, C.; Leutenegger, C.M.; Delwart, E. A third gyrovirus species in human faeces. J. Gen. Virol. 2012, 93, 1356–1361. [Google Scholar] [CrossRef]
- Nishiyama, S.; Dutia, B.M.; Stewart, J.P.; Meredith, A.L.; Shaw, D.J.; Simmonds, P.; Sharp, C.P. Identification of novel anelloviruses with broad diversity in UK rodents. J. Gen. Virol. 2014, 95, 1544–1553. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, L.; Tu, Z.; Sun, S.; Sun, Y.; Yi, L.; Tu, C.; He, B. Virome Profiling of an Amur leopard cat Reveals Multiple Anelloviruses and a Bocaparvovirus. Vet. Sci. 2022, 9, 640. [Google Scholar] [CrossRef] [PubMed]
- Borkosky, S.S.; Whitley, C.; Kopp-Schneider, A.; zur Hausen, H.; de Villiers, E.M. Epstein-Barr virus stimulates torque teno virus replication: A possible relationship to multiple sclerosis. PLoS ONE 2012, 7, e32160. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.S.; Araujo, O.C.; Savassi-Ribas, F.; Fernandes, C.A.; Coelho, H.S.; Niel, C.; Villela-Nogueira, C.A.; Araujo, N.M. Prevalence of occult hepatitis B virus infection and Torque teno virus infection and their association with hepatocellular carcinoma in chronic hepatitis C patients. Virus Res. 2017, 242, 166–172. [Google Scholar] [CrossRef]
- Roperto, S.; Paciello, O.; Paolini, F.; Pagnini, U.; Palma, E.; Di Palo, R.; Russo, V.; Roperto, F.; Venuti, A. Short communication: Detection of human Torque teno virus in the milk of water buffaloes (Bubalus bubalis). J. Dairy Sci. 2009, 92, 5928–5932. [Google Scholar] [CrossRef]
- Chan, P.K.; Tam, W.H.; Yeo, W.; Cheung, J.L.; Zhong, S.; Cheng, A.F. High carriage rate of TT virus in the cervices of pregnant women. Clin. Infect. Dis. 2001, 32, 1376–1377. [Google Scholar] [CrossRef]
- Canuti, M.; Buka, S.; Jazaeri Farsani, S.M.; Oude Munnink, B.B.; Jebbink, M.F.; van Beveren, N.J.; de Haan, L.; Goldstein, J.; Seidman, L.J.; Tsuang, M.T.; et al. Reduced maternal levels of common viruses during pregnancy predict offspring psychosis: Potential role of enhanced maternal immune activity? Schizophr. Res. 2015, 166, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yang, Q.; Bi, X.; Shi, H.; Yang, J.; Cheng, X.; Yan, T.; Zhang, H.; Cheng, Z. The Synergy of Chicken Anemia Virus and Gyrovirus Homsa 1 in Chickens. Viruses 2023, 15, 515. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Kim, H.; Kwon, Y.; Kim, H. Genetic characterization of chicken infectious anaemia viruses isolated in Korea and their pathogenicity in chicks. Front. Cell Infect. Microbiol. 2024, 14, 1333596. [Google Scholar] [CrossRef] [PubMed]
- Noteborn, M.H.M.; Verschueren, C.A.J.; Koch, G.; Van der Eb, A.J. Simultaneous expression of recombinant baculovirus-encoded chicken anaemia virus (CAV) proteins VP1 and VP2 is required for formation of the CAV-specific neutralizing epitope. J. Gen. Virol. 1998, 79, 3073–3077. [Google Scholar] [CrossRef][Green Version]
- Fang, L.C.; Zhen, Y.; Su, Q.; Zhu, H.F.; Guo, X.Y.; Zhao, P. Efficacy of CpG-ODN and Freund’s immune adjuvants on antibody responses induced by chicken infectious anemia virus VP1, VP2, and VP3 subunit proteins. Poult. Sci. 2019, 98, 1121–1126. [Google Scholar] [CrossRef]
- Renshaw, R.W.; Soine, C.; Weinkle, T.; OConnell, P.H.; Ohashi, K.; Watson, S.; Lucio, B.; Harrington, S.; Schat, K.A. A hypervariable region in VP1 of chicken infectious anemia virus mediates rate of spread and cell tropism in tissue culture. J. Virol. 1996, 70, 8872–8878. [Google Scholar] [CrossRef]
- Todd, D.; Scott, A.N.J.; Ball, N.W.; Borghmans, B.J.; Adair, B.M. Molecular basis of the attenuation exhibited by molecularly cloned highly passaged chicken anemia virus isolates. J. Virol. 2002, 76, 8472–8474. [Google Scholar] [CrossRef]
- Abayli, H.; Tonbak, S.; Ozbek, R.; Karabulut, B. Viral analysis of tumor-bearing chicken flocks in Turkey over the last decade (2011–2020). J. Hell. Vet. Med. Soc. 2023, 74, 5267–5276. [Google Scholar] [CrossRef]
- Okay, S.; Askar, S. Molecular characterization of VP2 and VP3 proteins of chicken anemia virus isolates in Turkey. Turk. J. Vet. Anim. Sci. 2021, 45, 336–345. [Google Scholar] [CrossRef]
- Yao, S.; Gao, X.; Tuo, T.; Han, C.; Gao, Y.; Qi, X.; Zhang, Y.; Liu, C.; Gao, H.; Wang, Y.; et al. Novel characteristics of the avian gyrovirus 2 genome. Sci. Rep. 2017, 7, 41068. [Google Scholar] [CrossRef] [PubMed]
- Maggi, F.; Macera, L.; Focosi, D.; Vatteroni, M.L.; Boggi, U.; Antonelli, G.; Eloit, M.; Pistello, M. Human gyrovirus DNA in human blood, Italy. Emerg. Infect. Dis. 2012, 18, 956–959. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, Q.; Li, Y.; Yu, Z.; Huang, H.; Lan, T.; Wang, W.; Cao, L.; Shi, Y.; Sun, W.; et al. Cross-species transmission potential of chicken anemia virus and avian gyrovirus 2. Infect. Genet. Evol. 2022, 99, 105249. [Google Scholar] [CrossRef]
- Fehér, E.; Pazár, P.; Kovács, E.; Farkas, S.L.; Lengyel, G.; Jakab, F.; Martella, V.; Bányai, K. Molecular detection and characterization of human gyroviruses identified in the ferret fecal virome. Arch. Virol. 2014, 159, 3401–3406. [Google Scholar] [CrossRef]
- Xu, S.; Man, Y.; Yu, Z.; Xu, X.; Ji, J.; Kan, Y.; Bi, Y.; Xie, Q.; Yao, L. Molecular analysis of Gyrovirus galga1 variants identified from the sera of dogs and cats in China. Vet. Q. 2024, 44, 1–8. [Google Scholar] [CrossRef]
- Duarte, M.A.; Silva, J.M.F.; Brito, C.R.; Teixeira, D.S.; Melo, F.L.; Ribeiro, B.M.; Nagata, T.; Campos, F.S. Faecal Virome Analysis of Wild Animals from Brazil. Viruses 2019, 11, 803. [Google Scholar] [CrossRef]
- Yuan, S.Y.; Yan, T.X.; Huang, L.B.; Hao, X.J.; Zhao, M.D.; Zhang, S.C.; Zhou, D.F.; Cheng, Z.Q. Cross-species pathogenicity of gyrovirus 3 in experimentally infected chickens and mice. Vet. Microbiol. 2021, 261, 109191. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhou, D.F.; Zhao, M.D.; Liu, Q.; Hao, X.J.; Yan, T.X.; Yuan, S.Y.; Zhang, S.C.; Cheng, Z.Q. Kinetic analysis of pathogenicity and tissue tropism of gyrovirus 3 in experimentally infected chickens. Vet. Res. 2021, 52, 120. [Google Scholar] [CrossRef]
- Cibulski, S.; Alves de Lima, D.; Fernandes Dos Santos, H.; Teixeira, T.F.; Tochetto, C.; Mayer, F.Q.; Roehe, P.M. A plate of viruses: Viral metagenomics of supermarket chicken, pork and beef from Brazil. Virology 2021, 552, 1–9. [Google Scholar] [CrossRef] [PubMed]


| Primer Name | Sequence (5′–3′) | Target Gene | Amplicon Size (bp) | Annealing Temp. (°C) |
|---|---|---|---|---|
| TTFeV1-81F TTFeV1-410R | TAGTCATGGAACTAGGAGCGC CTGAAATGTTGGGTGTAGTCTC | TATA Box-ORF2 gene | 330 bp (This study) | 55 |
| TTFeV2-837F TTFeV2-1451R | ATACCACCACCATCTAGCACAC CTTTTTATGAGCGGTTGGGGAG | ORF1-ORF2 genes | 615 bp (This study) | 57 |
| CAV 974F CAV 1328R | GTAGACGAGCTTTTAGGAAG GAGGGCAYGTTATTATCTAG | VP1 gene | 355 bp (This study) | 50 |
| AvGy2-1360F AvGy2-1969R | CTTGCAGGGGTGCCAATGGT GCTAGGAAATGACCAGGGTGC | VP1 gene | 610 bp (This study) | 51 |
| Gy3-1101Fn Gy3-1744Rn | ACCCCTATAACGCGATTAACCT TGGTATTGTGGTTTCATTAGCTGG | VP1 gene | 644 bp (This study) | 53 |
| Gy4-1259Fn Gy4-1938Rn | CTGAAACTTCTGCTTTTAGGGT CGTTTCACTCAATCCAGTAGCT | VP1 gene | 330 bp (This study) | 52 |
| Hfor Hrev | CAGGTGATGAATTTGCTACA CATTTGGATAAACTGGTGGT | VP2 gene | 630 bp [22] | 49 |
| Virus | Adults (n = 35) | Kittens (n = 56) | Total (n = 91) |
|---|---|---|---|
| FPV | 7/35 (20.00%) | 30/56 (53.57%) | 37/91 (40.65%) |
| TTFeV1 | 6/35 (17.14%) | 13/56 (23.21%) | 19/91 (20.88%) |
| CAV | 11/35 (31.43%) | 21/56 (37.50%) | 32/91 (35.16%) |
| AvGyV2 | 25/35 (71.43%) | 42/56 (75.00%) | 67/91 (73.63%) |
| GyV3 | 1/35 (3.86%) | 3/56 (5.36%) | 4/91 (4.40%) |
| GyV4 | 1/35 (3.86%) | 2/56 (3.57%) | 3/91 (3.30%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turan, T.; Işıdan, H.; Duran-Yelken, S.; Atasoy, M.O.; Özbek, R.; El Naggar, R.F.; Rohaim, M.A. Genetic and Statistical Study of Anelloviruses and Gyroviruses in Diarrheic Cats and Their Co-Occurrence Patterns. Viruses 2025, 17, 1413. https://doi.org/10.3390/v17111413
Turan T, Işıdan H, Duran-Yelken S, Atasoy MO, Özbek R, El Naggar RF, Rohaim MA. Genetic and Statistical Study of Anelloviruses and Gyroviruses in Diarrheic Cats and Their Co-Occurrence Patterns. Viruses. 2025; 17(11):1413. https://doi.org/10.3390/v17111413
Chicago/Turabian StyleTuran, Turhan, Hakan Işıdan, Selda Duran-Yelken, Mustafa Ozan Atasoy, Remziye Özbek, Rania F. El Naggar, and Mohammed A. Rohaim. 2025. "Genetic and Statistical Study of Anelloviruses and Gyroviruses in Diarrheic Cats and Their Co-Occurrence Patterns" Viruses 17, no. 11: 1413. https://doi.org/10.3390/v17111413
APA StyleTuran, T., Işıdan, H., Duran-Yelken, S., Atasoy, M. O., Özbek, R., El Naggar, R. F., & Rohaim, M. A. (2025). Genetic and Statistical Study of Anelloviruses and Gyroviruses in Diarrheic Cats and Their Co-Occurrence Patterns. Viruses, 17(11), 1413. https://doi.org/10.3390/v17111413








