Plant Compounds Inhibit the Growth of W12 Cervical Precancer Cells Containing Episomal or Integrant HPV DNA; Tanshinone IIA Synergizes with Curcumin in Cervical Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture: Cancer Cells
2.3. Cell Culture: W12 Cells
2.4. Proliferation Assay
2.5. Calculating the Combination Index
2.6. RNA Extraction and Quantitative Real-Time PCR
2.7. Gene Expression Analysis:
2.8. Docking Simulation:
2.9. Statistical Analysis
3. Results
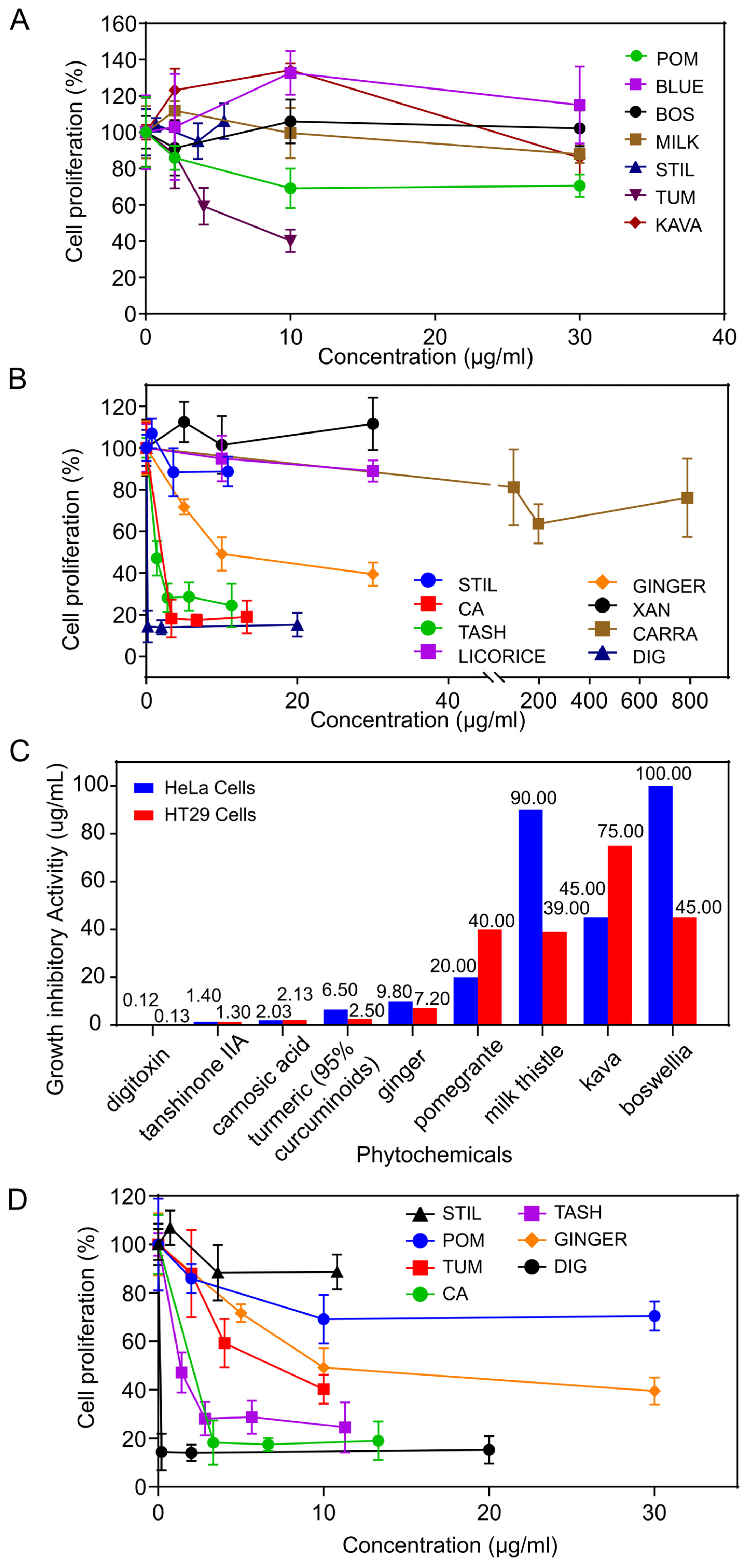
3.1. Growth Inhibitory Activity of Phytochemicals on Cervical Cancer Cells
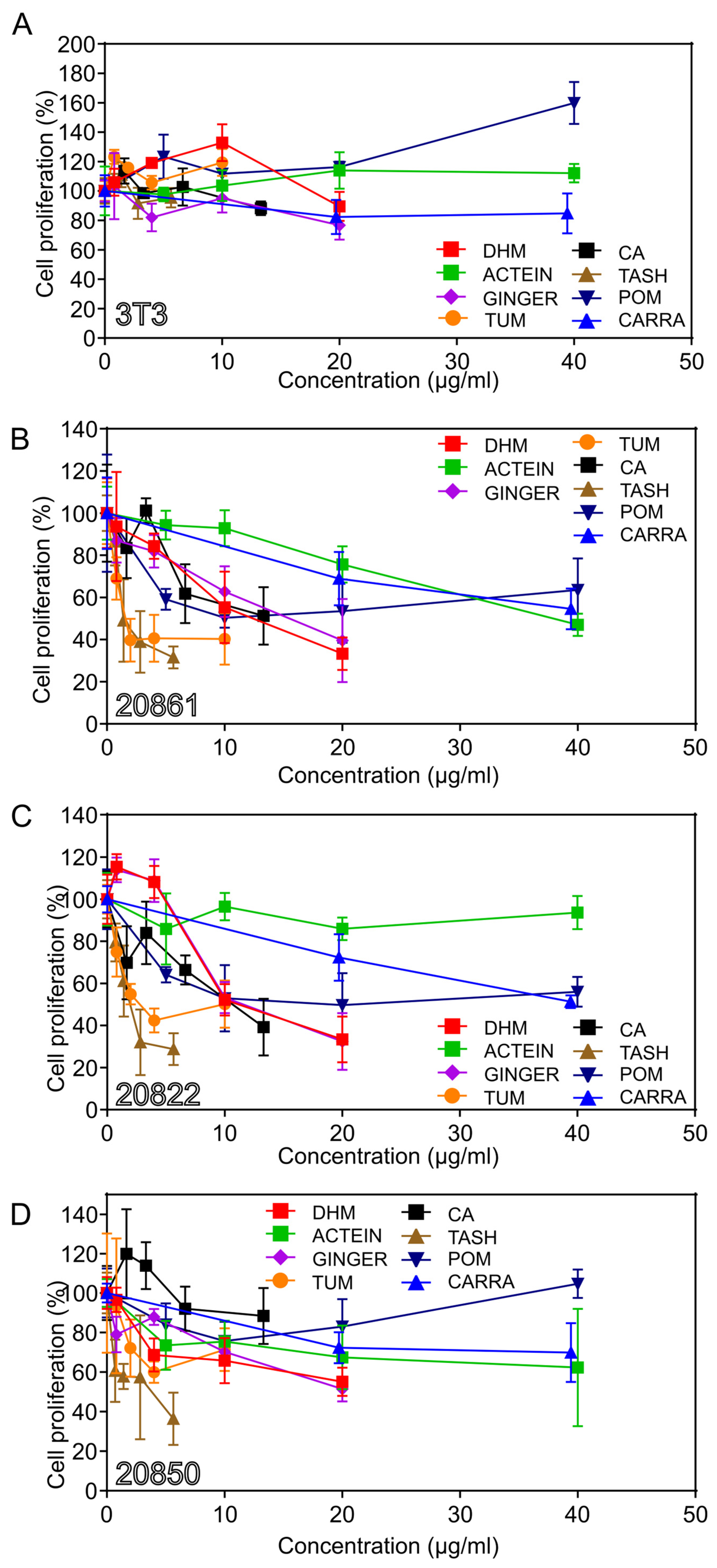
3.2. Growth Inhibitory Effects on W12 Cervical Cells
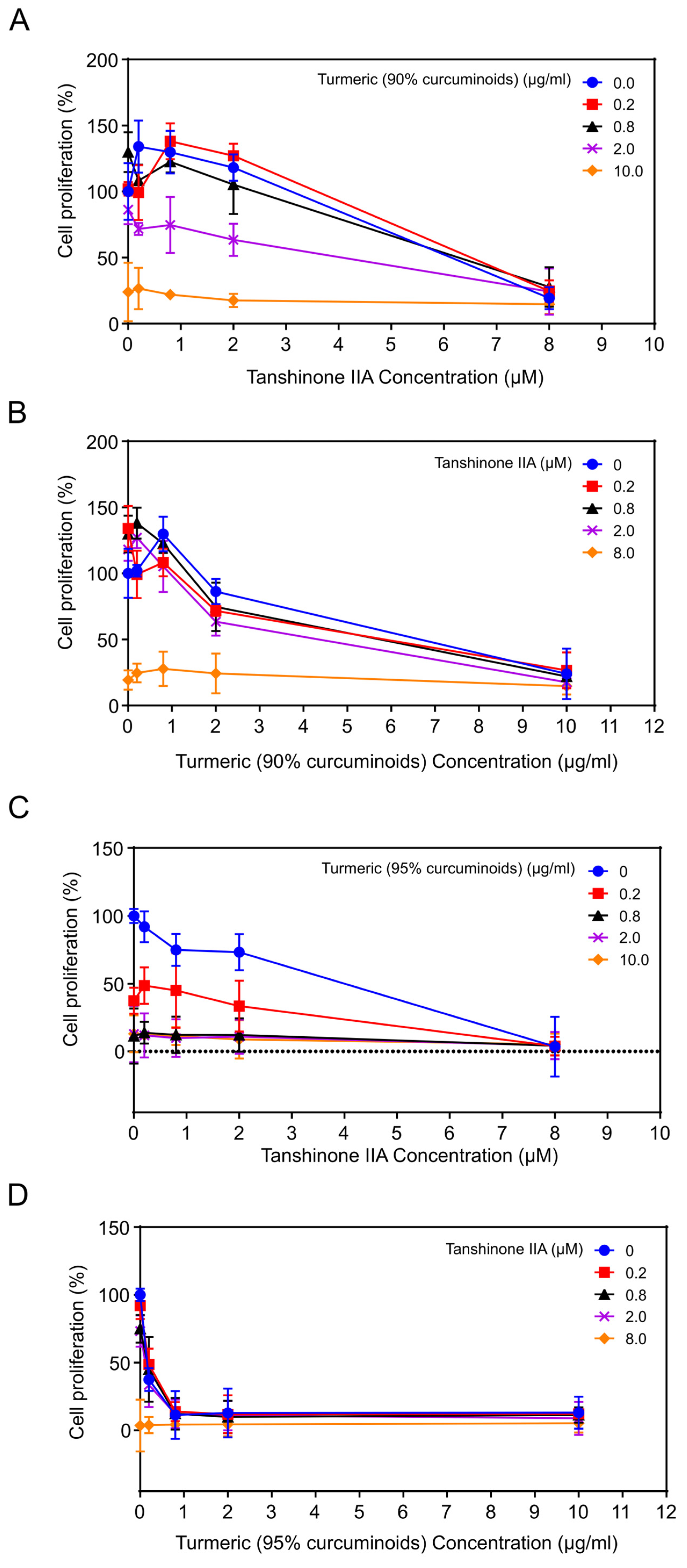
3.3. The Effects of Combinations of Phytochemicals
3.3.1. Combination of Tanshinone IIA Plus Turmeric (90% Curcuminoids)
3.3.2. Combination of Tanshinone IIA Plus Turmeric (95% Curcuminoids)
3.3.3. Combination of Turmeric (95% Curcuminoids) Plus Ginger or Carrageenan
3.4. The Effects of Tanshinone IIA on the Expression of p53 and HPV16 mRNAs
3.4.1. Effect of Tanshinone IIA on the Expression of p53
3.4.2. Effect of Tanshinone IIA on the Expression of HPV16 mRNAs
3.5. Gene Expression Analysis of the Effects of Curcumin
3.6. Effect of Curcumin on the Activity of the Na+/K+-ATPase
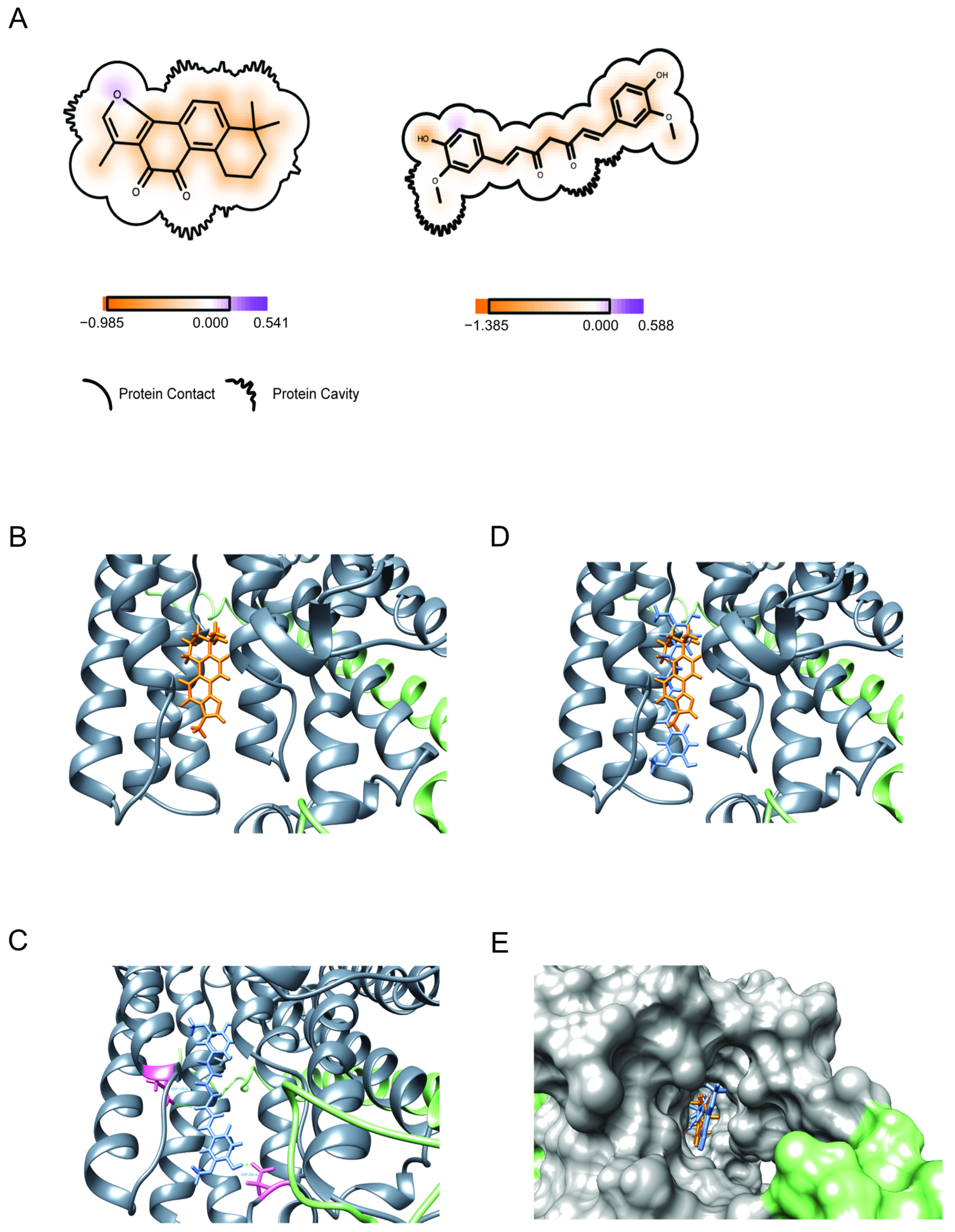
3.7. Binding (Molecular Docking) of Drugs to Na+/K+-ATPase
3.7.1. Binding of Tanshinone IIA
3.7.2. Binding of Curcumin
3.7.3. Binding of the Combination of Tanshinone IIA and Curcumin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [PubMed]
- WHO. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer (accessed on 4 December 2023).
- Graham, S.V. The human papillomavirus replication cycle, and its links to cancer progression: A comprehensive review. Clin. Sci. 2017, 131, 2201–2221. [Google Scholar]
- Alazawi, W.; Pett, M.; Arch, B.; Scott, L.; Freeman, T.; Stanley, M.A.; Coleman, N. Changes in cervical keratinocyte gene expression associated with integration of human papillomavirus 16. Cancer Res. 2002, 62, 6959–6965. [Google Scholar] [PubMed]
- Teymouri, M.; Pirro, M.; Johnston, T.P.; Sahebkar, A. Curcumin as a multifaceted compound against human papilloma virus infection and cervical cancers: A review of chemistry, cellular, molecular, and preclinical features. Biofactors 2017, 43, 331–346. [Google Scholar] [CrossRef]
- Pan, T.L.; Hung, Y.C.; Wang, P.W.; Chen, S.T.; Hsu, T.K.; Sintupisut, N.; Cheng, C.; Lyu, P. Functional proteomic and structural insights into molecular targets related to the growth inhibitory effect of tanshinone IIA on HeLa cells. Proteomics 2010, 10, 914–929. [Google Scholar] [CrossRef]
- Pan, T.L.; Wang, P.W.; Hung, Y.C.; Huang, C.H.; Rau, K.M. Proteomic analysis reveals tanshinone IIA enhances apoptosis of advanced cervix carcinoma CaSki cells through mitochondria intrinsic and endoplasmic reticulum stress pathways. Proteomics 2013, 13, 3411–3423. [Google Scholar] [CrossRef]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Gupta, R.C. Tanshinone IIA inhibits viral oncogene expression leading to apoptosis and inhibition of cervical cancer. Cancer Lett. 2015, 356 Pt B, 536–546. [Google Scholar] [CrossRef]
- Li, L.W.; Na, C.; Tian, S.Y.; Chen, J.; Ma, R.; Gao, Y.; Lou, G. Ellagic acid induces HeLa cell apoptosis via regulating signal transducer and activator of transcription 3 signaling. Exp. Ther. Med. 2018, 16, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Lu, J.L.; Liang, Y.R.; Li, Q.S. Suppressive effects of EGCG on cervical cancer. Molecules 2018, 23, 2334. [Google Scholar] [CrossRef]
- Mukherjee, S.; Debata, P.R.; Hussaini, R.; Chatterjee, K.; Baidoo, J.N.E.; Sampat, S.; Szerszen, A.; Navarra, J.P.; Fata, J.; Severinova, E.; et al. Unique synergistic formulation of curcumin, epicatechin gallate and resveratrol, tricurin, suppresses HPV E6, eliminates HPV+ cancer cells, and inhibits tumor progression. Oncotarget 2017, 8, 60904–60916. [Google Scholar] [CrossRef] [PubMed]
- Einbond, L.S.; Zhou, J.; Wu, H.-a.; Mbazor, E.; Guiyun, S.; Balick, M.; DeVoti, J.A.; Redenti, S.; Castellanos, M.R. A novel cancer preventative botanical mixture, TriCurin, inhibits viral transcripts and the growth of W12 cervical cells harboring extrachromosomal or integrated HPV16 DNA. Br. J. Cancer 2021, 24, 901–913. [Google Scholar] [CrossRef]
- Kalantari, M.; Lee, D.; Calleja-Macias, I.E.; Lambert, P.F.; Bernard, H.U. Effects of cellular differentiation, chromosomal integration and 5-aza-2′-deoxycytidine treatment on human papillomavirus-16 DNA methylation in cultured cell lines. Virology 2008, 374, 292–303. [Google Scholar] [CrossRef] [PubMed]
- ATCC. Maintaining High Standards in Cell Culture. 2010. Available online: https://www.cedarlanelabs.com/contents/files?filePath=Suppliers/Links/CellBiologyStandards.pdf (accessed on 8 August 2019).
- Ogutu, F.O.; Mu, T.H.; Sun, H.; Zhang, M. Ultrasonic Modified Sweet Potato Pectin Induces Apoptosis like Cell Death in Colon Cancer (HT-29) Cell Line. Nutr. Cancer 2018, 70, 136–145. [Google Scholar] [CrossRef]
- Buck, C.B.; Thompson, C.D.; Roberts, J.N.; Müller, M.; Lowy, D.R.; Schiller, J.T. Carrageenan is a potent inhibitor of papillomavirus infection. PLoS Pathog. 2006, 2, e69. [Google Scholar] [CrossRef]
- Gopal, J.; Muthu, M.; Chun, S. One-step ultrasonication mobilized solvent-free extraction/synthesis of nanocurcumin from turmeric. RSC Adv. 2015, 60, 48391–48398. [Google Scholar] [CrossRef]
- Einbond, L.S.; Su, T.; Wu, H.A.; Friedman, R.; Wang, X.; Ramirez, A.; Kronenberg, F.; Weinstein, I.B. The growth inhibitory effect of actein on human breast cancer cells is associated with activation of stress response pathways. Int. J. Cancer 2007, 121, 2073–2083. [Google Scholar] [CrossRef] [PubMed]
- Einbond, L.S.; Shimizu, M.; Nuntanakorn, P.; Seter, C.; Cheng, R.; Jiang, B.; Kronenberg, F.; Kennelly, E.J.; Weinstein, I.B. Actein and a fraction of black cohosh potentiate antiproliferative effects of chemotherapy agents on human breast cancer cells. Planta Med. 2006, 72, 1200–1206. [Google Scholar] [CrossRef] [PubMed]
- Einbond, L.S. Synergistic Combinations of Digoxin. The New York Botanical Garden: New York, NY, USA, 2025; manuscript in preparation. [Google Scholar]
- Cheng, Q.; Chen, J. Mechanism of p53 stabilization by ATM after DNA damage. Cell Cycle 2010, 9, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Einbond, L.S.; Wu, H.-A.; Kashiwazaki, R.; He, K.; Roller, M.; Su, T.; Wang, X.; Goldsberry, S. Carnosic acid inhibits the growth of ER-negative human breast cancer cells and synergizes with curcumin. Fitoterapia 2012, 83, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wu, S.; Burdette, J.E.; Cheng, X.; Kinghorn, A.D. Structural Insights into the Interactions of Digoxin and Na+/K+-ATPase and Other Targets for the Inhibition of Cancer Cell Proliferation. Molecules 2021, 26, 3672. [Google Scholar] [CrossRef]
- Pereira, D.G.; Salgado, M.A.R.; Rocha, S.C.; Santos, H.L.; Villar, J.A.F.P.; Contreras, R.G.; Fontes, C.F.; Barbosa, L.A.; Cortes, V.F. Involvement of Src Signaling in the Synergistic Effect Between Cisplatin and Digoxin on Cancer Cell Viability. J. Cell Biochem. 2018, 19, 3352–3362. [Google Scholar] [CrossRef] [PubMed]
- Elansary, H.O.; Mahmoud, E.A. Egyptian herbal tea infusions’ antioxidants and their antiproliferative and cytotoxic activities against cancer cells. Nat. Prod. Res. 2015, 29, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.S.; Chang, C.H.; Chou, Y.R.; Yeh, M.Y.; Au, M.K.; Lu, H.F.; Chu, Y.L.; Chou, H.M.; Chou, H.C.; Shih, Y.L.; et al. Curcumin causes DNA damage and affects associated protein expression in HeLa human cervical cancer cells. Oncol. Rep. 2016, 36, 2207–2215. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, J.G.; Qu, J.; Zhang, Q.; Prasad, C.; Wei, Z.J. Assessment of anti-cancerous potential of 6-gingerol (Tongling White Ginger) and its synergy with drugs on human cervical adenocarcinoma cells. Food Chem. Toxicol. 2017, 109 Pt 2, 910–922. [Google Scholar] [CrossRef]
- Li, R.; Chen, X.G.; Jia, K.; Liu, Z.P.; Peng, H.Y. A systematic determination of polyphenols constituents and cytotoxic ability in fruit parts of pomegranates derived from five Chinese cultivars. Springerplus 2016, 5, 914. [Google Scholar] [CrossRef]
- McDougall, G.J.; Ross, H.A.; Ikeji, M.; Stewart, D. Berry extracts exert different antiproliferative effects against cervical and colon cancer cells grown in vitro. J. Agric. Food Chem. 2008, 56, 3016–3023. [Google Scholar] [CrossRef] [PubMed]
- Levin, M. Molecular bioelectricity: How endogenous voltage potentials control cell behavior and instruct pattern regulation in vivo. Mol. Biol. Cell 2014, 25, 3835–3850. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Payne, S.L.; Ram, P.; Srinivasan, D.H.; Le, T.T.; Levin, M.; Oudin, M.J. Potassium channel-driven bioelectric signalling regulates metastasis in triple-negative breast cancer. EBioMedicine 2022, 75, 103767. [Google Scholar] [CrossRef] [PubMed]
- Lobikin, M.; Chernet, B.; Lobo, D.; Levin, M. Resting Potential, Oncogene-induced Tumorigenesis, and Metastasis: The Bioelectric Basis of Cancer in vivo. Phys. Biol. 2012, 9, 065002. [Google Scholar] [CrossRef] [PubMed]
- Debata, P.R.; Castellanos, M.R.; Fata, J.E.; Baggett, S.; Rajupet, S.; Szerszen, A.; Begum, S.; Mata, A.; Murty, V.V.; Opitz, L.M.; et al. A novel curcumin-based vaginal cream Vacurin selectively eliminates apposed human cervical cancer cells. Gynecol. Oncol. 2013, 29, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Archambault, J.; Melendy, T. Targeing human papillomavirus genome replication for antiviral drug discovery. Antivir. Ther. 2013, 18, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves-de-Albuquerque, C.F.; Silva, A.R.; da Silva, C.I.; Castro-Faria-Neto, H.C.; Burth, P. Na/K Pump and Beyond: Na/K-ATPase as a Modulator of Apoptosis and Autophagy. Molecules 2017, 22, 578. [Google Scholar] [CrossRef]
- Themistocleous, S.C.; Yiallouris, A.; Tsioutis, C.; Zaravinos, A.; Johnson, E.O.; Patrikios, I. Clinical significance of P-class pumps in cancer. Oncol. Lett. 2021, 22, 658. [Google Scholar] [CrossRef]
- da Silva, C.I.; Gonçalves-de-Albuquerque, C.F.; Tavares de Moraes, B.P.; Garcia, D.G.; Burth, P. Na/K-ATPase: Their role in cell adhesion and migration in cancer. Biochimie 2021, 185, 1–8. [Google Scholar] [CrossRef]
- Zhang, J.; Chang, J.; Beg, M.A.; Huang, W.; Zhao, Y.; Dai, W.; Wu, X.; Cui, W.; Pillai, S.S.; Lakhani, H.V.; et al. Na/K-ATPase suppresses LPS-induced pro-inflammatory signaling through Lyn. iScience 2022, 25, 104963. [Google Scholar] [CrossRef]
- Yang, K.; Li, Z.; Chen, Y.; Yin, F.; Ji, X.; Zhou, J.; Yin, W. Na, K-ATPase α1 cooperates with its endogenous ligand to reprogram immune microenvironment of lung carcinoma and promotes immune escape. Sci. Adv. 2023, 9, eade5393. [Google Scholar] [CrossRef]
- Bejček, J.; Spiwok, V.; Kmoníčková, E.; Rimpelová, S. Na+/K+-ATPase Revisited: On Its Mechanism of Action, Role in Cancer, and Activity Modulation. Molecules 2021, 26, 1905. [Google Scholar] [CrossRef] [PubMed]
- Gould, H.J.; Miller, P.R.; Edenfield, S.; Sherman, K.J.; Brady, C.K.; Paul, D. Emergency Use of Targeted Osmotic Lysis for the Treatment of a Patient with Aggressive Late-Stage Squamous Cell Carcinoma of the Cervix. Curr. Oncol. 2021, 28, 2115–2122. [Google Scholar] [CrossRef]
- Shang, Q.; Xu, H.; Huang, L. Tanshinone IIA: A Promising Natural Cardioprotective Agent. Evid.-Based Complement. Altern. Med. 2012, 2012, 716459. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, J.; Liu, X.; Wang, W.; Zhao, Y.; Sun, W. Tanshinone IIA protects cardiomyocytes and prevents heart failure after myocardial infarction by activating the AMPK/mTOR-dependent autophagy pathway. J. Cardiovasc. Pharmacol. 2020, 75, 123–135. [Google Scholar]
- Wen, P.-Y.; Li, J.; Lu, B.-L.; Liu, J.; Yang, F.-Z.; Zhou, J.; Li, W.-W. Tanshinone IIA increases levels of NeuN, protein disulfide isomerase, and Na+/K+-ATPase and decreases evidence of microglial activation after cerebral ischemic injury. Neuroreport 2016, 27, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kesharwani, R.K.; Misra, K.; Rizvi, S.I. The modulation of erythrocyte Na(+)/K(+)-ATPase activity by curcumin. J. Adv. Res. 2015, 6, 1023–1030. [Google Scholar] [CrossRef]
- Levin, M. Bioelectric signaling: Reprogrammable circuits underlying embryogenesis, regeneration, and cancer. Cell 2021, 184, 1971–1989. [Google Scholar] [CrossRef] [PubMed]
- Chernet, B.; Levin, M. Endogenous Voltage Potentials and the Microenvironment: Bioelectric Signals that Reveal, Induce and Normalize Cancer. J. Clin. Exp. Oncol. 2013, S1, S1-002. [Google Scholar]
- Chernet, B.T.; Adams, D.S.; Lobikin, M.; Levin, M. Use of genetically encoded, light-gated ion translocators to control tumorigenesis. Oncotarget 2016, 7, 19575–19588. [Google Scholar] [CrossRef]
- Tuszynski, J.; Tilli, T.M.; Levin, M. Ion Channel and Neurotransmitter Modulators as Electroceutical Approaches to the Control of Cancer. Curr. Pharm. Des. 2017, 23, 4827–4841. [Google Scholar] [CrossRef] [PubMed]
- Straub, E.; Dreer, M.; Fertey, J.; Iftner, T.; Stubenrauch, F. The viral E8^E2C repressor limits productive replication of human papillomavirus 16. .J Virol. 2014, 88, 937–947. [Google Scholar] [CrossRef]
- Egawa, N.; Wang, Q.; Griffin, H.M.; Murakami, I.; Jackson, D.; Mahmood, R.; Doorbar, J. HPV16 and 18 genome amplification show different E4-dependence, with 16E4 enhancing E1 nuclear accumulation and replicative efficiency via its cell cycle arrest and kinase activation functions. PLoS Pathog. 2017, 13, e1006282. [Google Scholar] [CrossRef]
- Al-Haj, L.; lackshear, P.J.; Khabar, K.S.A. Regulation of p21/CIP1/WAF-1 Mediated Cell-Cycle Arrest by RNase L and Tristetraprolin, and Involvement of AU-rich Elements. Nucleic Acids Res. 2012, 40, 7739–7752. [Google Scholar] [CrossRef]
- Ma, D.; Jiang, C.; Hu, X.; Li, Q.; Li, T.; Yang, Y.; Li, Q. Methylation Patterns of the IFN-γ Gene in Cervical Cancer Tissues. Sci. Rep 2014, 4, 6331. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.I.; Woodby, B.I.; Ulicny, J.; Raikhy, G.; Orr, A.W.; Songock WKBodily, J.M. Human Papillomavirus 16 E5 Inhibits Interferon Signaling and Supports Episomal Viral Maintenance. J. Virol. 2020, 94, e01582-19. [Google Scholar] [CrossRef]


| ||||
| cells | HeLa | HT29 | ||
| compound (µg/mL) | ||||
| tanshinone IIA | 1.33 (4.52) * | 1.27 (4.32) * | ||
| carnosic acid | 2.03 (6.11) * | 2.13 (6.41) * | ||
| digoxin | 0.12 (0.15) * | 0.13 (0.17) * | ||
| fraction (µg/mL) | ||||
| turmeric (95% curcuminoids) | 6.5 | 2.5 | ||
| ginger | 9.8 | 7.2 | ||
| pomegranate | 20 | 40 | ||
| milk thistle | 90 | 39 | ||
| kava | 45 | 75 | ||
| boswellia | >100 | 45 | ||
| ||||
| W12 clone | 20850 | 20822 | 20862 | 20861 |
| compound (µg/mL) | ||||
| tanshinone IIA | 2.3 (7.8) * | 2.0 (6.8) * | 3.1 (10.6)* | 1.4 (4.8) * |
| DHM | 24.7 (89.4) * | 10.2 (36.9) * | 12.8 (46.3) * | 12.4 (44.9) * |
| actein | 41.7 (61.6) * | 55.7 (82.3) * | 36.3 (53.6) * * | 37.9 (56.0) * |
| carnosic acid | 13.0 (39.1) * | 10.7 (32.1) * | 10.0 (30.0) * | 14.1 (42.4) * |
| fraction (µg/mL) | ||||
| turmeric (95% curcuminoids) | 4.7 | 2.8 | 2.0 | 1.6 |
| ginger | 13.7 | 11.6 | 21.6 | 15.5 |
| pomegranate | 25.4 ** | 20 | 28 ** | 10 |
| Gene Identifier | Gene Name | SYMBOL | Ratio | p-Value |
|---|---|---|---|---|
| NM_005445 | Structural maintenance of chromosomes 3 | SMC3 | −3.89 | 0.0136 |
| NM_001042749 | Stromal antigen 2 | STAG2 | −3.56 | 0.0463 |
| NM_005030 | Polo-like kinase 1 (Drosophila) | PLK1 | 2.01 | 0.0293 |
| NM_002093 | Glycogen synthase kinase 3 beta | GSK3B | 2.09 | 0.0006 |
| NM_001950 | E2F transcription factor 4, p107/p130-binding | E2F4 | 2.37 | 0.0042 |
| NM_005862 | Stromal antigen 1 | STAG1 | 2.37 | 0.0277 |
| NM_003443 | Zinc finger and BTB domain containing 17 | ZBTB17 | 2.48 | 0.0115 |
| NM_001254 | Cell division cycle 6 homolog (S. cerevisiae) | CDC6 | 2.68 | 0.0000421 |
| NM_004064 | Cyclin-dependent kinase inhibitor 1B (p27, Kip1) | CDKN1B | 2.77 | 0.0457 |
| NM_001951 | E2F transcription factor 5, p130-binding | E2F5 | 2.94 | 0.0374 |
| NM_004153 | Origin recognition complex, subunit 1-like (yeast) | ORC1L | 3.10 | 0.0007 |
| NM_005359 | SMAD family member 4 | SMAD4 | 3.49 | 0.0129 |
| NM_005983 | S-phase kinase-associated protein 2 (p45) | SKP2 | 5.03 | 0.0076 |
| NM_000051 | Ataxia telangiectasia mutated | ATM | 15.43 | 0.0188 |
| NM_016263 | Fizzy/cell division cycle 20 related 1 (Drosophila) | CDC20 | 19.4 | 0.0476 |
| NM_138292 | Ataxia telangiectasia mutated | ATM | 35.94 | 0.00000342 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Einbond, L.S.; Zhou, J.; Huang, K.; Castellanos, M.R.; Mbazor, E.; Balick, M.; Ma, H.; DeVoti, J.A.; Redenti, S.; Wu, H.-a. Plant Compounds Inhibit the Growth of W12 Cervical Precancer Cells Containing Episomal or Integrant HPV DNA; Tanshinone IIA Synergizes with Curcumin in Cervical Cancer Cells. Viruses 2025, 17, 55. https://doi.org/10.3390/v17010055
Einbond LS, Zhou J, Huang K, Castellanos MR, Mbazor E, Balick M, Ma H, DeVoti JA, Redenti S, Wu H-a. Plant Compounds Inhibit the Growth of W12 Cervical Precancer Cells Containing Episomal or Integrant HPV DNA; Tanshinone IIA Synergizes with Curcumin in Cervical Cancer Cells. Viruses. 2025; 17(1):55. https://doi.org/10.3390/v17010055
Chicago/Turabian StyleEinbond, Linda Saxe, Jing Zhou, Kunhui Huang, Mario R. Castellanos, Emeka Mbazor, Michael Balick, Hongbao Ma, James A. DeVoti, Stephen Redenti, and Hsan-au Wu. 2025. "Plant Compounds Inhibit the Growth of W12 Cervical Precancer Cells Containing Episomal or Integrant HPV DNA; Tanshinone IIA Synergizes with Curcumin in Cervical Cancer Cells" Viruses 17, no. 1: 55. https://doi.org/10.3390/v17010055
APA StyleEinbond, L. S., Zhou, J., Huang, K., Castellanos, M. R., Mbazor, E., Balick, M., Ma, H., DeVoti, J. A., Redenti, S., & Wu, H.-a. (2025). Plant Compounds Inhibit the Growth of W12 Cervical Precancer Cells Containing Episomal or Integrant HPV DNA; Tanshinone IIA Synergizes with Curcumin in Cervical Cancer Cells. Viruses, 17(1), 55. https://doi.org/10.3390/v17010055






