Breaking Latent Infection: How ORF37/38-Deletion Mutants Offer New Hope against EHV-1 Neuropathogenicity
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus, Cell Lines, Primers, and Plasmids
2.2. Construction of EHV-1 ∆ORF38 and ∆ORF37/38 Mutants
2.3. Viral Growth Kinetics
2.4. Evaluation of Neuropathogenicity
2.5. Evaluation of the Latency of Reactivation
2.6. Evaluation of Immune Efficacy
2.7. Serological Test
2.8. Cell-Mediated Immune Responses
3. Results
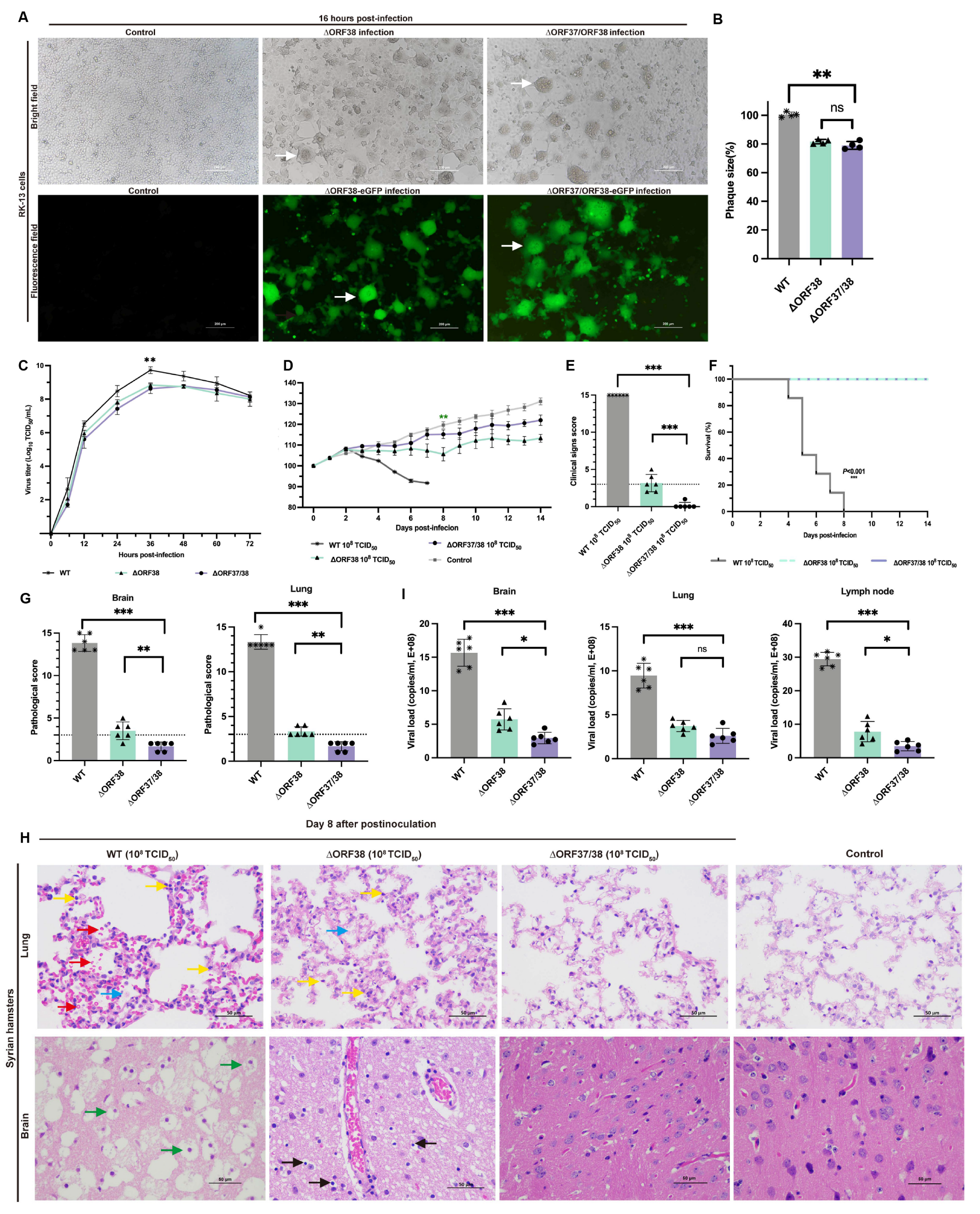
3.1. The ∆ORF37/38-Gene-Deletion Virus Does Not Induce Neurological Signs in Hamsters during Acute Infection
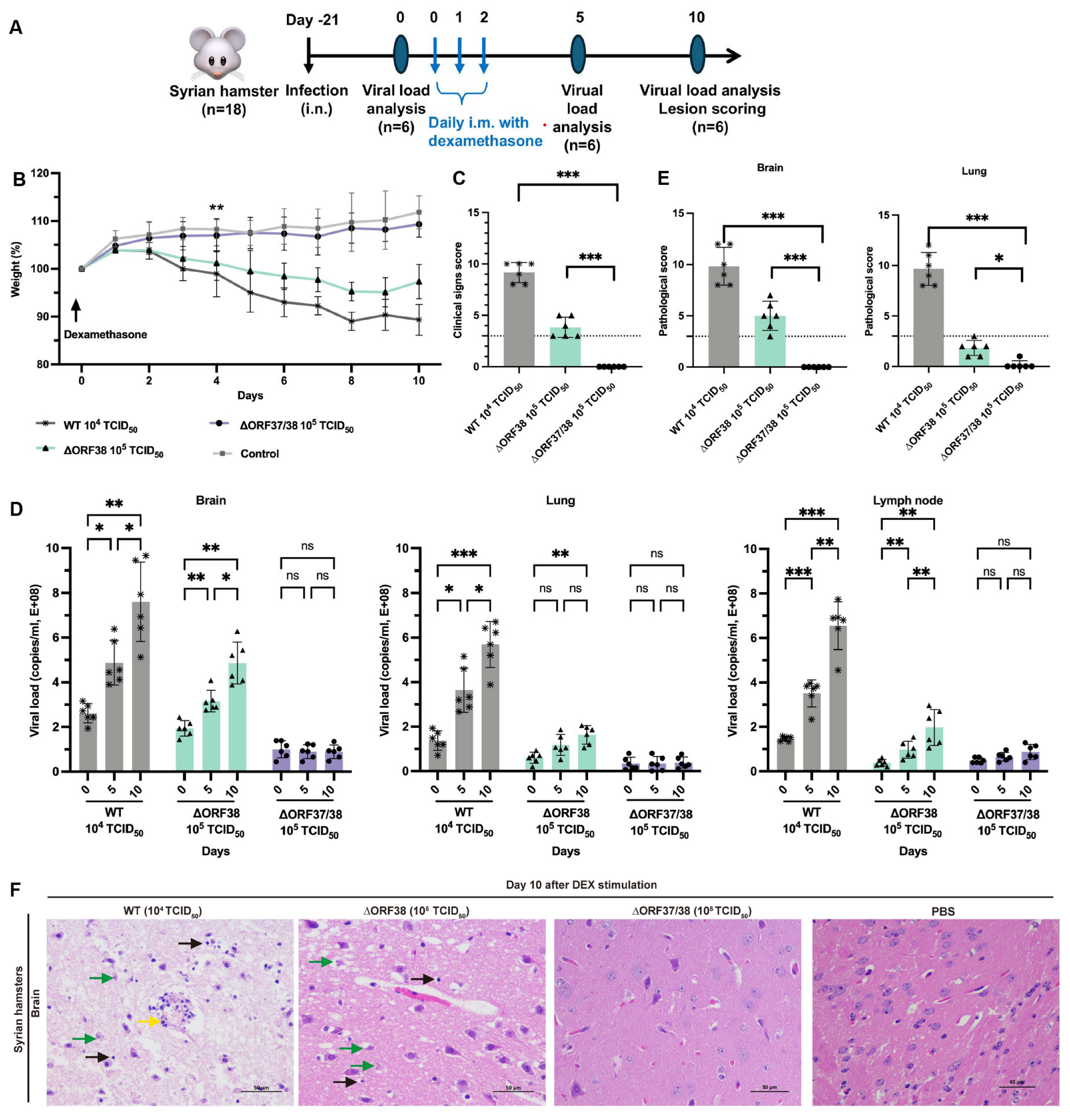
3.2. Inability of the ∆ORF37/38 Virus to Cause an Increase in Clinical Signs or Viral DNA Loads with Dexamethasone Stimulation
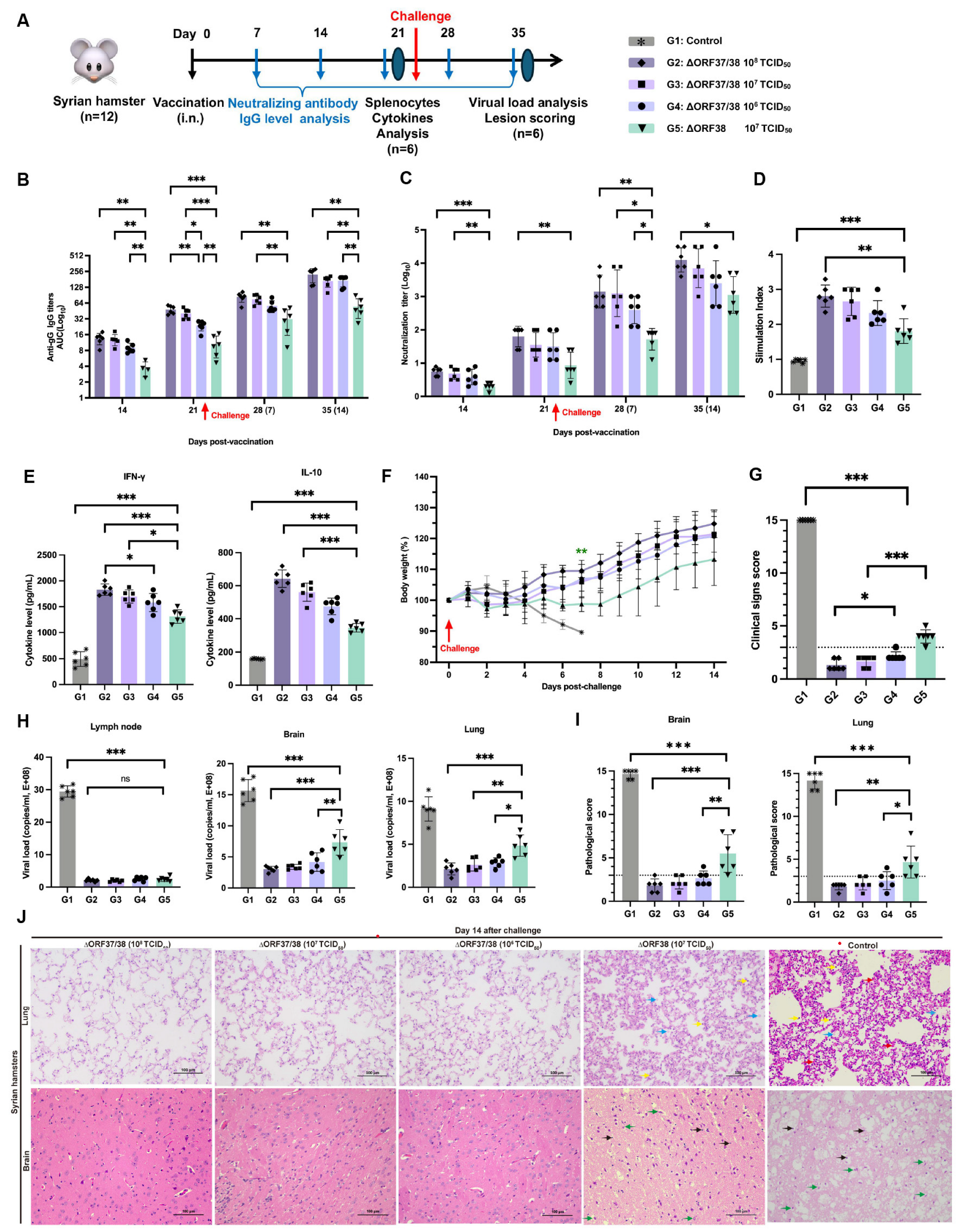
3.3. Intranasal Vaccination with the ∆ORF37/38 Mutant Effectively Protects Hamsters from Lethal Infections
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Afify, A.F.; Hassanien, R.T.; El Naggar, R.F.; Rohaim, M.A.; Munir, M. Unmasking the ongoing challenge of equid herpesvirus-1 (EHV-1): A comprehensive review. Microb. Pathog. 2024, 193, 106755. [Google Scholar] [CrossRef] [PubMed]
- Lunn, D.P.; Burgess, B.A.; Dorman, D.C.; Goehring, L.S.; Gross, P.; Osterrieder, K.; Pusterla, N.; Soboll-Hussey, G. Updated ACVIM consensus statement on equine herpesvirus-1. J. Vet. Intern. Med. 2024, 38, 1290–1299. [Google Scholar] [CrossRef] [PubMed]
- Van Galen, G.; Leblond, A.; Tritz, P.; Martinelle, L.; Pronost, S.; Saegerman, C. A retrospective study on equine herpesvirus type-1 associated myeloencephalopathy in France (2008–2011). Vet. Microbiol. 2015, 179, 304–309. [Google Scholar] [CrossRef]
- Mannini, A.; Ellero, N.; Urbani, L.; Balboni, A.; Imposimato, I.; Battilani, M.; Gialletti, R.; Freccero, F. Medical management and positive outcome after prolonged recumbency in a case of equine herpesvirus myeloencephalopathy. J. Equine Vet. Sci. 2024, 136, 105063. [Google Scholar] [CrossRef]
- Pusterla, N.; Barnum, S.; Miller, J.; Varnell, S.; Dallap-Schaer, B.; Aceto, H.; Simeone, A. Investigation of an EHV-1 Outbreak in the United States Caused by a New H752 Genotype. Pathogens 2021, 10, 747. [Google Scholar] [CrossRef]
- Giessler, K.S.; Goehring, L.S.; Jacob, S.I.; Davis, A.; Esser, M.M.; Lee, Y.; Zarski, L.M.; Weber, P.S.D.; Hussey, G.S. Impact of the host immune response on the development of equine herpesvirus myeloencephalopathy in horses. J. Gen. Virol. 2024, 105, 001987. [Google Scholar] [CrossRef]
- Hue, E.S.; Richard, E.A.; Fortier, C.I.; Fortier, G.D.; Paillot, R.; Raue, R.; Pronost, S.L. Equine PBMC Cytokines Profile after In Vitro α- and γ-EHV Infection: Efficacy of a Parapoxvirus Ovis Based-Immunomodulator Treatment. Vaccines. 2017, 5, 28. [Google Scholar] [CrossRef]
- Pusterla, N.; Dorman, D.C.; Burgess, B.A.; Goehring, L.; Gross, M.; Osterrieder, K.; Soboll Hussey, G.; Lunn, D.P. Viremia and nasal shedding for the diagnosis of equine herpesvirus-1 infection in domesticated horses. J. Vet. Intern. Med. 2024, 38, 1765–1791. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, C.L.; Wimer, C.L.; Perkins, G.; Babasyan, S.; Freer, H.; Watts, C.; Rollins, A.; Osterrieder, N.; Wagner, B. Deletion of the ORF2 gene of the neuropathogenic equine herpesvirus type 1 strain Ab4 reduces virulence while maintaining strong immunogenicity. BMC Vet. Res. 2018, 14, 245. [Google Scholar] [CrossRef]
- Rickabaugh, T.M.; Brown, H.J.; Martinez-Guzman, D.; Wu, T.T.; Tong, L.; Yu, F.; Cole, S.; Sun, R. Generation of a latency-deficient gammaherpesvirus that is protective against secondary infection. J. Virol. 2004, 78, 9215–9223. [Google Scholar] [CrossRef]
- Brar, G.; Farhat, N.A.; Sukhina, A.; Lam, A.K.; Kim, Y.H.; Hsu, T.; Tong, L.; Lin, W.W.; Ware, C.F.; Blackman, M.A.; et al. Deletion of immune evasion genes provides an effective vaccine design for tumor-associated herpesviruses. NPJ Vaccines 2020, 5, 102. [Google Scholar] [CrossRef] [PubMed]
- Bryant, N.A.; Wilkie, G.S.; Russell, C.A.; Compston, L.; Grafham, D.; Clissold, L.; McLay, K.; Medcalf, L.; Newton, R.; Davison, A.J.; et al. Genetic diversity of equine herpesvirus 1 isolated from neurological, abortigenic and respiratory disease outbreaks. Transbound. Emerg. Dis. 2018, 65, 817–832. [Google Scholar] [CrossRef] [PubMed]
- Van de Walle, G.R.; Goupil, R.; Wishon, C.; Damiani, A.; Perkins, G.A.; Osterrieder, N. A single-nucleotide polymorphism in a herpesvirus DNA polymerase is sufficient to cause lethal neurological disease. J. Infect. Dis. 2009, 200, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Sutton, G.; Thieulent, C.; Fortier, C.; Hue, E.S.; Marcillaud-Pitel, C.; Pléau, A.; Deslis, A.; Guitton, E.; Paillot, R.; Pronost, S. Identification of a New Equid Herpesvirus 1 DNA Polymerase (ORF30) Genotype with the Isolation of a C2254/H752 Strain in French Horses Showing no Major Impact on the Strain Behaviour. Viruses 2020, 12, 1160. [Google Scholar] [CrossRef]
- Hu, Y.; Jia, Q.; Liu, J.; Sun, W.; Bao, Z.; Che, C.; Wu, G.; Fan, B.; Jarhen; Ran, D. Molecular characteristics and pathogenicity of an equid alphaherpesvirus 1 strain isolated in China. Virus Genes 2022, 58, 284–293. [Google Scholar] [CrossRef]
- Carvalho, R.F.; Spilki, F.R.; Cunha, E.M.; Stocco, R.C.; Arns, C.W. Molecular data of UL24 homolog gene (ORF37) from Brazilian isolates of equine herpesvirus type 1. Res. Vet. Sci. 2012, 93, 494–497. [Google Scholar] [CrossRef]
- Kasem, S.; Yu, M.H.; Yamada, S.; Kodaira, A.; Matsumura, T.; Tsujimura, K.; Madbouly, H.; Yamaguchi, T.; Ohya, K.; Fukushi, H. The ORF37 (UL24) is a neuropathogenicity determinant of equine herpesvirus 1 (EHV-1) in the mouse encephalitis model. Virology 2010, 400, 259–270. [Google Scholar] [CrossRef]
- Ruan, P.; Wang, M.; Cheng, A.; Zhao, X.; Yang, Q.; Wu, Y.; Zhang, S.; Tian, B.; Huang, J.; Ou, X. Mechanism of herpesvirus UL24 protein regulating viral immune escape and virulence. Front. Microbiol. 2023, 14, 1268429. [Google Scholar] [CrossRef]
- Slater, J.D.; Gibson, J.S.; Field, H.J. Pathogenicity of a thymidine kinase-deficient mutant of equine herpesvirus 1 in mice and specific pathogen-free foals. J. Gen. Virol. 1993, 74, 819–828. [Google Scholar] [CrossRef]
- Huang, C.Y.; Yao, H.W.; Wang, L.C.; Shen, F.H.; Hsu, S.M.; Chen, S.H. Thymidine Kinase-Negative Herpes Simplex Virus 1 Can Efficiently Establish Persistent Infection in Neural Tissues of Nude Mice. J. Virol. 2017, 91, e01979-16. [Google Scholar] [CrossRef]
- Xie, Y.; Wu, L.; Wang, M.; Cheng, A.; Yang, Q.; Wu, Y.; Jia, R.; Zhu, D.; Zhao, X.; Chen, S.; et al. Alpha-Herpesvirus Thymidine Kinase Genes Mediate Viral Virulence and Are Potential Therapeutic Targets. Front. Microbiol. 2019, 10, 941. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.F.; Cheng, X.; Su, B.Q.; Duan, L.F.; Wang, C.R.; Niu, X.R.; Jia, R.; Zhu, D.; Zhao, X.; Chen, S.; et al. CRISPR/Cas9-based generation of a recombinant double-reporter pseudorabies virus and its characterization in vitro and in vivo. Vet. Res. 2021, 52, 95. [Google Scholar] [CrossRef]
- Hu, R.M.; Zhou, Q.; Song, W.B.; Sun, E.C.; Zhang, M.M.; He, Q.G.; Chen, H.C.; Wu, B.; Liu, Z.F. Novel pseudorabies virus variant with defects in T.K.; gE and gI protects growing pigs against lethal challenge. Vaccine 2015, 33, 5733–5740. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.; Zhu, L.; Xu, L.; Yang, Y.; Zhang, Y.; Liu, Z.; Xu, T.; Wen, J.; Deng, L.; Zhou, Y.; et al. The Construction and Immunogenicity Analyses of a Recombinant Pseudorabies Virus with Senecavirus A VP2 Protein Coexpression. Microbiol. Spectr. 2023, 28, e0522922. [Google Scholar] [CrossRef]
- Mesquita, L.P.; Arévalo, A.F.; Zanatto, D.A.; Miyashiro, S.I.; Cunha, E.M.S.; de Souza, M.D.C.C.; Villalobos, E.M.C.; Mori, C.M.C.; Maiorka, P.C.; Mori, E. Equine herpesvirus type 1 induces both neurological and respiratory disease in Syrian hamsters. Vet. Microbiol. 2017, 203, 117–124. [Google Scholar] [CrossRef]
- Cardiff, R.D.; Miller, C.H.; Munn, R.J. Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harb. Protoc. 2014, 2014, 655–658. [Google Scholar] [CrossRef]
- Gibson-Corley, K.N.; Olivier, A.K.; Meyerholz, D.K. Principles for valid histopathologic scoring in research. Vet. Pathol. 2013, 50, 1007–1015. [Google Scholar] [CrossRef]
- Malik, P.; Bálint, A.; Dán, A.; Pálfi, V. Molecular characterisation of the ORF68 region of equine herpesvirus-1 strains isolated from aborted fetuses in Hungary between 1977 and 2008. Acta Vet. Hung. 2012, 60, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wu, G.; Jia, Q.; Zhang, B.; Sun, W.; Sa, R.; Zhang, S.; Cai, W.; Jarhen; Ran, D.; et al. Development of a live attenuated vaccine candidate for equid alphaherpesvirus 1 control: A step towards efficient protection. Front. Immunol. 2024, 15, 1408510. [Google Scholar] [CrossRef]
- Guo, J.; Li, Q.; Jones, C. The bovine herpesvirus 1 regulatory proteins, bICP4 and bICP22, are expressed during the escape from latency. J. Neurovirol. 2019, 25, 42–49. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, L.; Xu, L.; Li, F.; Deng, H.; Huang, Y.; Gu, S.; Sun, X.; Zhou, Y.; Xu, Z. The Construction and Immunogenicity Analyses of Recombinant Pseudorabies Virus With NADC30-Like Porcine Reproductive and Respiratory Syndrome Virus-Like Particles Co-expression. Front. Microbiol. 2022, 13, 846079. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.B.; Kim, H.; Jeong, J.H.; Jang, U.S.; Jang, Y.; Roh, S.; Jeon, H.; Kim, E.J.; Han, S.Y.; Maeng, J.Y.; et al. Mosaic RBD nanoparticles induce intergenus cross-reactive antibodies and protect against SARS-CoV-2 challenge. Proc. Natl. Acad. Sci. USA 2023, 120, e2208425120. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Meneses-Ruiz, D.M.; Aguilar-Diaz, H.; Bobes, R.J.; Sampieri, A.; Vaca, L.; Laclette, J.P.; Carrero, J.C. Protection against Amoebic Liver Abscess in Hamster by Intramuscular Immunization with an Autographa californica Baculovirus Driving the Expression of the Gal-Lectin LC3 Fragment. Biomed Res. Int. 2015, 2015, 760598. [Google Scholar] [CrossRef]
- Pusterla, N.; Barnum, S.; Young, A.; Mendonsa, E.; Lee, S.; Hankin, S.; Brittner, S.; Finno, C.J. Molecular monitoring of EHV-1 in silently infected performance horses through nasal and environ- mental sample testing. Pathogens 2022, 11, 720. [Google Scholar] [CrossRef]
- Vandenberghe, E.; Boshuizen, B.; Delesalle, C.J.G.; Goehring, L.S.; Groome, K.A.; van Maanen, K.; de Bruijn, C.M. New insights into the management of an EHV-1 (Equine hospital) outbreak. Viruses 2021, 13, 1429. [Google Scholar] [CrossRef]
- Spann, K.; Barnum, S.; Pusterla, N. Investigation of the Systemic Antibody Response and Antigen Detection Following Intranasal Administration of Two Commercial Equine Herpesvirus-1 Vaccines to Adult Horses. J. Equine Vet. Sci. 2023, 122, 104229. [Google Scholar] [CrossRef]
- Tong, P.; Yang, E. Identification of neuropathogenic Varicellovirus equidalpha1 as a potential cause of respiratory disease outbreaks among horses in North Xinjiang; China; from 2021–2023. BMC Vet. Res. 2024, 20, 77. [Google Scholar] [CrossRef]
- Kong, Z.; Yin, H.; Wang, F.; Liu, Z.; Luan, X.; Sun, L.; Liu, W.; Shang, Y. Pseudorabies virus tegument protein UL13 recruits RNF5 to inhibit STING-mediated antiviral immunity. PLoS Pathog 2022, 18, e1010544. [Google Scholar] [CrossRef]
- He, T.; Wang, M.; Cheng, A.; Yang, Q.; Wu, Y.; Jia, R.; Chen, S.; Zhu, D.; Liu, M.; Zhao, X.; et al. Duck plague virus UL41 protein inhibits RIG-I/MDA5-mediated duck IFN-β production via mRNA degradation activity. Vet. Res. 2022, 53, 22. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, M.; Ye, C.; Ruan, K.; Xu, A.; Gao, F.; Tong, G.; Zheng, H. Inhibition of the DNA-sensing pathway by pseudorabies virus UL24 protein via degradation of interferon regulatory factor 7. Vet. Microbiol. 2021, 255, 109023. [Google Scholar] [CrossRef] [PubMed]
- Osterrieder, K.; Dorman, D.C.; Burgess, B.A.; Goehring, L.S.; Gross, P.; Neinast, C.; Pusterla, N.; Hussey, G.S.; Lunn, D.P. Vaccination for the prevention of equine herpesvirus-1 disease in domesticated horses: A systematic review and meta-analysis. J. Vet. Intern. Med. 2024, 38, 1858–1871. [Google Scholar] [CrossRef] [PubMed]
- Cornick, J.; Martens, J.; Martens, R.; Crandell, R.; McConnell, S.; Kit, S. Safety and efficacy of a thymidine kinase negative equine herpesvirus-1 vaccine in young horses. Can. J. Vet. Res. 1990, 54, 260–266. [Google Scholar] [PubMed]
- Widjojoatmodjo, M.N.; Boes, J.; van Bers, M.; van Remmerden, Y.; Roholl, P.J.; Luytjes, W. A highly attenuated recombinant human respiratory syncytial virus lacking the G protein induces long-lasting protection in cotton rats. Virol. J. 2010, 7, 114. [Google Scholar] [CrossRef]
- Sun, L.; Tang, Y.; Yan, K.; Zhang, H. Construction of a quadruple gene-deleted vaccine confers complete protective immunity against emerging PRV variant challenge in piglets. Virol. J. 2022, 19, 19. [Google Scholar] [CrossRef]
- Karron, R.A.; Luongo, C.; Thumar, B.; Loehr, K.M.; Englund, J.A.; Collins, P.L.; Buchholz, U.J. A gene deletion that up-regulates viral gene expression yields an attenuated RSV vaccine with improved antibody responses in children. Sci. Transl. Med. 2015, 7, 312ra175. [Google Scholar] [CrossRef]
- Chen, X.; Kong, N.; Xu, J.; Wang, J.; Zhang, M.; Ruan, K.; Li, L.; Zhang, Y.; Zheng, H.; Tong, W.; et al. Pseudorabies virus UL24 antagonizes OASL-mediated antiviral effect. Virus. Res. 2021, 295, 198276. [Google Scholar] [CrossRef]
- Zhu, H.; Zheng, C. The Race between Host Antiviral Innate Immunity and the Immune Evasion Strategies of Herpes Simplex Virus 1. Mircobiol. Mol. Biol. Rev. 2023, 87, e0010323. [Google Scholar] [CrossRef]
- Eady, N.A.E.; Holmes, C.; Schnabel, C.; Babasyan, S.; Wagner, B. Equine herpesvirus type 1 (EHV-1) replication at the upper respiratory entry site is inhibited by neutralizing EHV-1-specific IgG1 and IgG4/7 mucosal antibodies. J. Virol. 2024, 98, e0025024. [Google Scholar] [CrossRef]
- Stasi, D.; Wagner, B.; Barnum, S.; Pusterla, N. Comparison of antibody and antigen response to intranasal and intramuscular EHV-1 modified-live vaccination in healthy adult horses. J. Equine Vet. Sci. 2024, 133, 104992. [Google Scholar] [CrossRef]
- Abdoli, M.; Shafaati, M.; Ghamsari, L.K.; Abdoli, A. Intranasal administration of cold-adapted live-attenuated SARS-CoV-2 candidate vaccine confers protection against SARS-CoV-2. Virus. Res. 2022, 319, 198857. [Google Scholar] [CrossRef]
- Welch, H.M.; Bridges, C.G.; Lyon, A.M.; Griffiths, L.; Edington, N. Latent equid herpesviruses 1 and 4: Detection and distinction using the polymerase chain reaction and co-cultivation from lymphoid tissues. J. Gen. Virol. 1992, 73, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Goehring, L.; Dorman, D.C.; Osterrieder, K.; Burgess, B.A.; Dougherty, K.; Gross, P.; Neinast, C.; Pusterla, N.; Soboll-Hussey, G.; Lunn, D.P. Pharmacologic interventions for the treatment of equine herpesvirus-1 in domesticated horses: A systematic review. J. Vet. Intern. Med. 2024, 38, 1892–1905. [Google Scholar] [CrossRef] [PubMed]
- Dunowska, M. A review of equid herpesvirus 1 for the veterinary practitioner. Part B: Pathogenesis and epidemiology. New Zealand Vet. J. 2014, 62, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Oladunni, F.S.; Horohov, D.W.; Chambers, T.M. EHV-1: A Constant Threat to the Horse Industry. Front. Microbiol. 2019, 10, 2668. [Google Scholar] [CrossRef]
- Li, L.T.; Liu, J.; Luo, M.; Liu, J.S.; Zhang, M.M.; Zhang, W.J.; Chen, H.C.; Liu, Z.F. Establishment of pseudorabies virus latency and reactivation model in mice dorsal root ganglia culture. J. Gen. Virol. 2023, 104, 001921. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Zhang, S.-Y.; Sun, W.-C.; Feng, Y.-R.; Gong, H.-R.; Ran, D.-L.; Zhang, B.-Z.; Liu, J.-H. Breaking Latent Infection: How ORF37/38-Deletion Mutants Offer New Hope against EHV-1 Neuropathogenicity. Viruses 2024, 16, 1472. https://doi.org/10.3390/v16091472
Hu Y, Zhang S-Y, Sun W-C, Feng Y-R, Gong H-R, Ran D-L, Zhang B-Z, Liu J-H. Breaking Latent Infection: How ORF37/38-Deletion Mutants Offer New Hope against EHV-1 Neuropathogenicity. Viruses. 2024; 16(9):1472. https://doi.org/10.3390/v16091472
Chicago/Turabian StyleHu, Yue, Si-Yu Zhang, Wen-Cheng Sun, Ya-Ru Feng, Hua-Rui Gong, Duo-Liang Ran, Bao-Zhong Zhang, and Jian-Hua Liu. 2024. "Breaking Latent Infection: How ORF37/38-Deletion Mutants Offer New Hope against EHV-1 Neuropathogenicity" Viruses 16, no. 9: 1472. https://doi.org/10.3390/v16091472
APA StyleHu, Y., Zhang, S.-Y., Sun, W.-C., Feng, Y.-R., Gong, H.-R., Ran, D.-L., Zhang, B.-Z., & Liu, J.-H. (2024). Breaking Latent Infection: How ORF37/38-Deletion Mutants Offer New Hope against EHV-1 Neuropathogenicity. Viruses, 16(9), 1472. https://doi.org/10.3390/v16091472





