Production and Characterization of Self-Assembled Virus-like Particles Comprising Capsid Proteins from Genotypes 3 and 4 Hepatitis E Virus (HEV) and Rabbit HEV Expressed in Escherichia coli
Abstract
1. Introduction
2. Materials and Methods
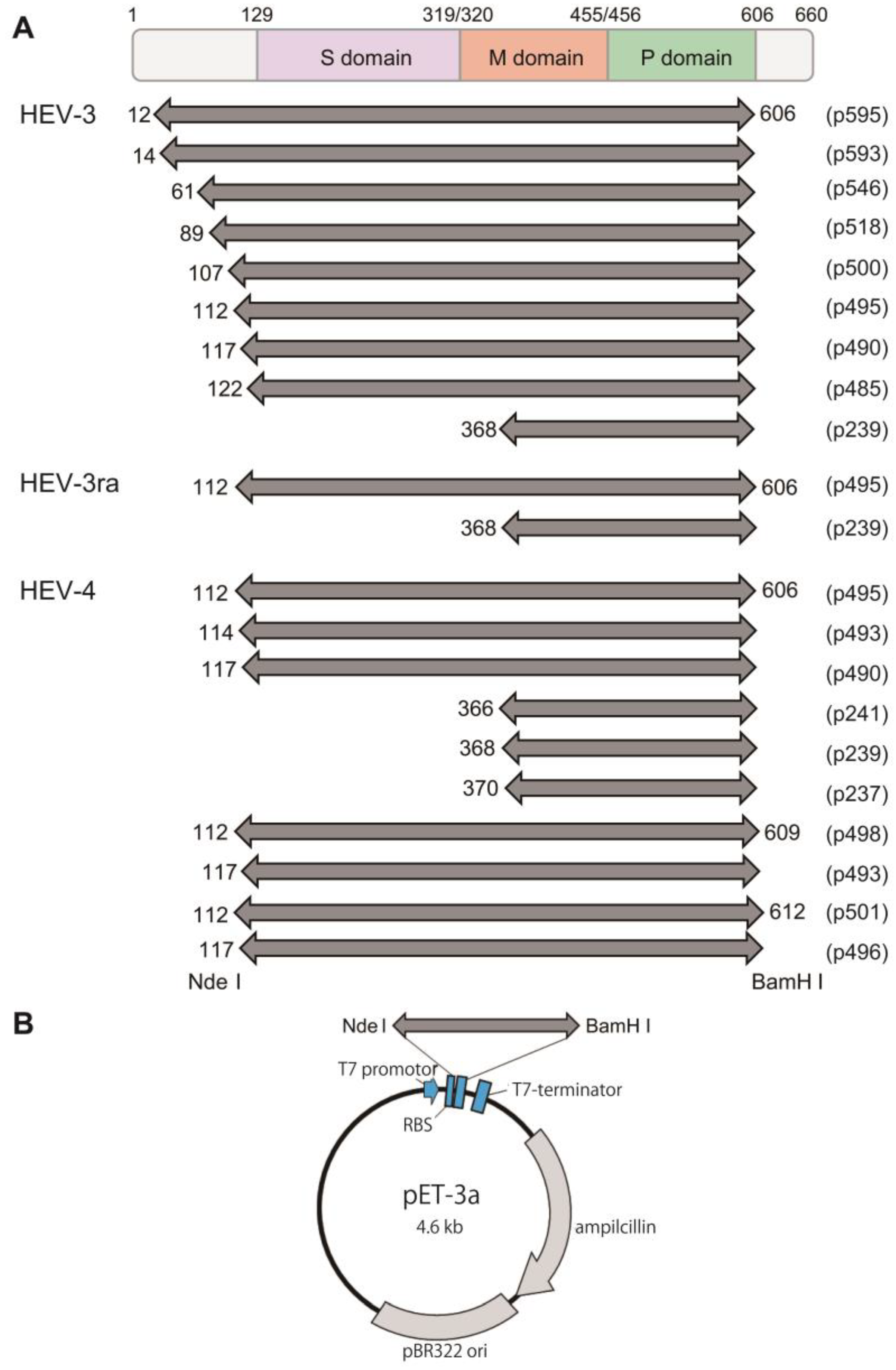
2.1. Plasmid Construction and Truncated HEV ORF2 Protein Expression
2.2. Purification of Recombinant HEV ORF2 Proteins
2.3. SDS-PAGE and Western Blot Analysis
2.4. Analysis of Purified HEV VLPs with the Bioanalyzer
2.5. Transmission Electron Microscopy (TEM)
2.6. Immunoelectron Microscopy (IEM)
2.7. Enzyme-Linked Immunosorbent Assays (ELISAs) for the Determination of Binding with Monoclonal Antibodies (MAbs) against ORF2 Protein of Human HEV (HEV-3 and HEV-4), Rabbit HEV (HEV-3ra), or Rat HEV (HEV-C1)
2.8. Immunization of HEV VLPs in Mice
3. Results
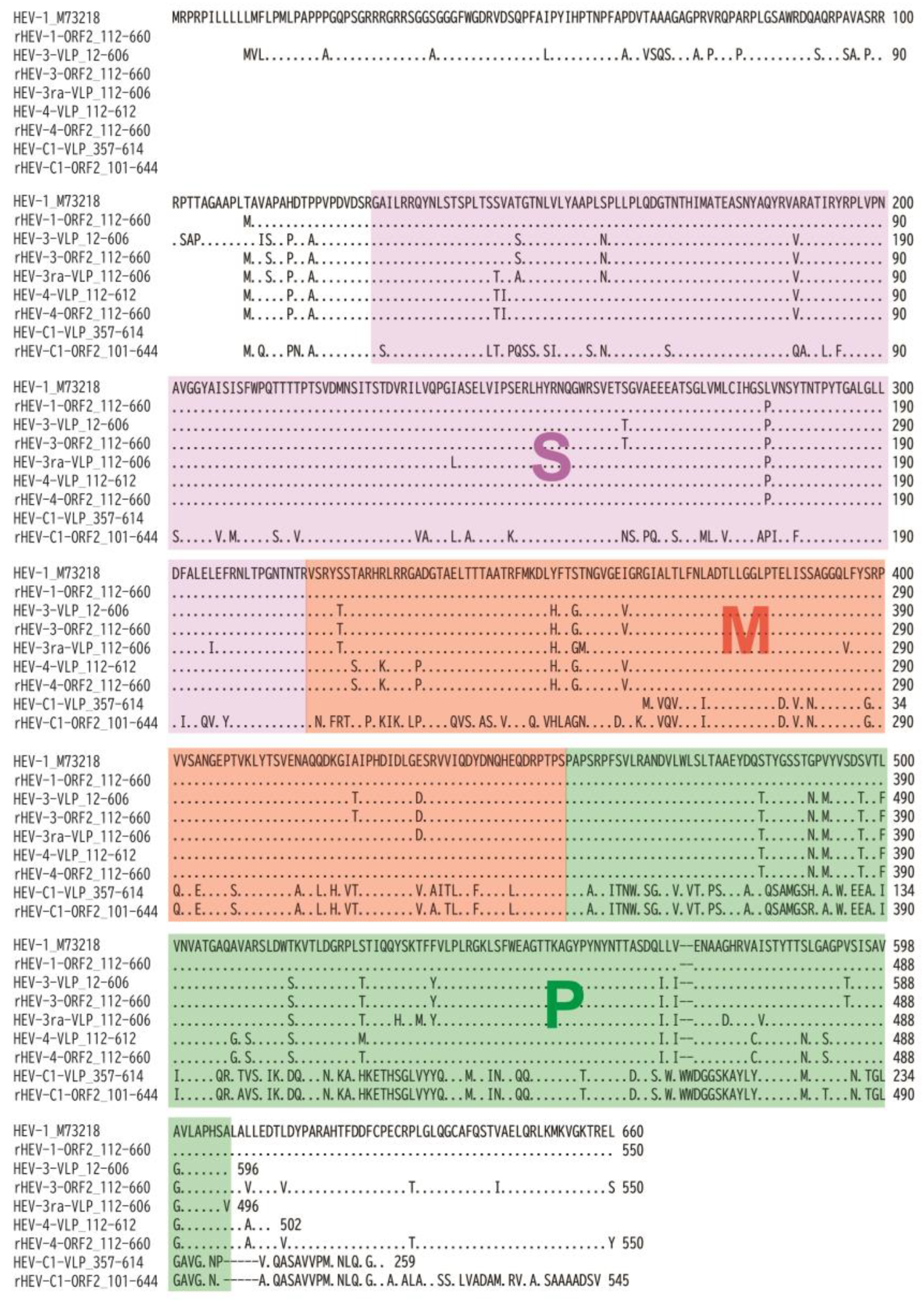
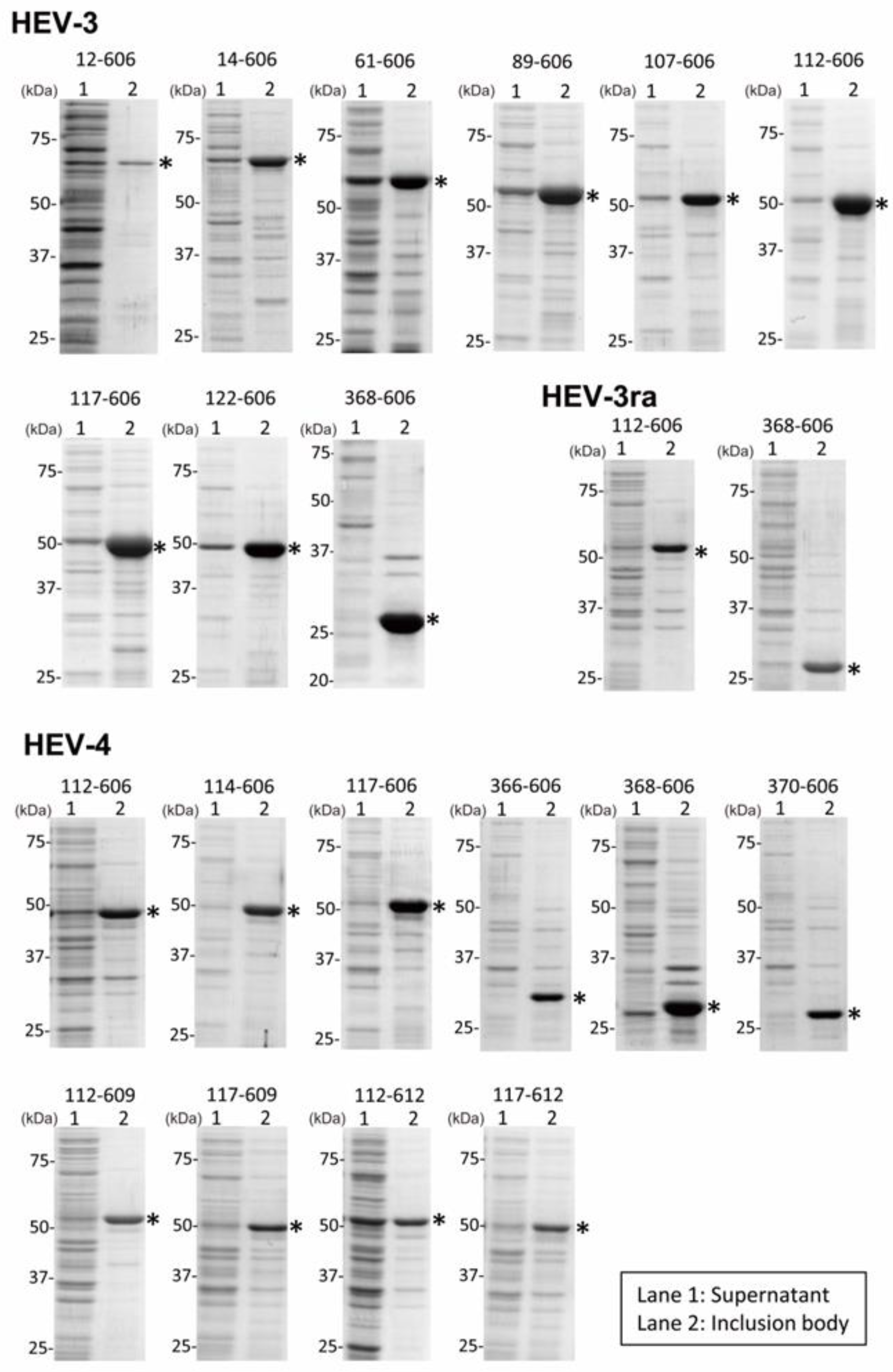
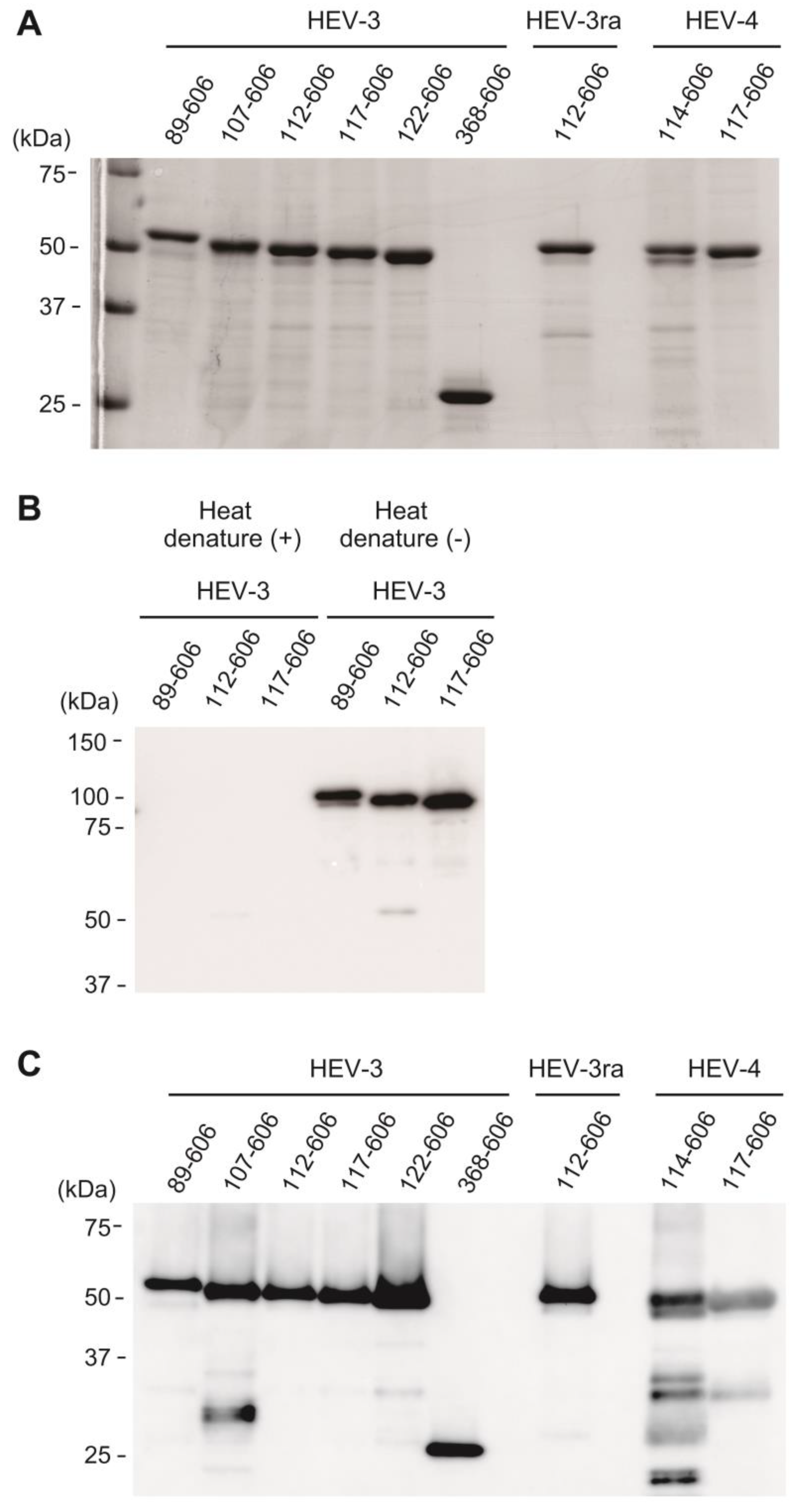
3.1. The Expression and Purification of Truncated HEV ORF2 Proteins
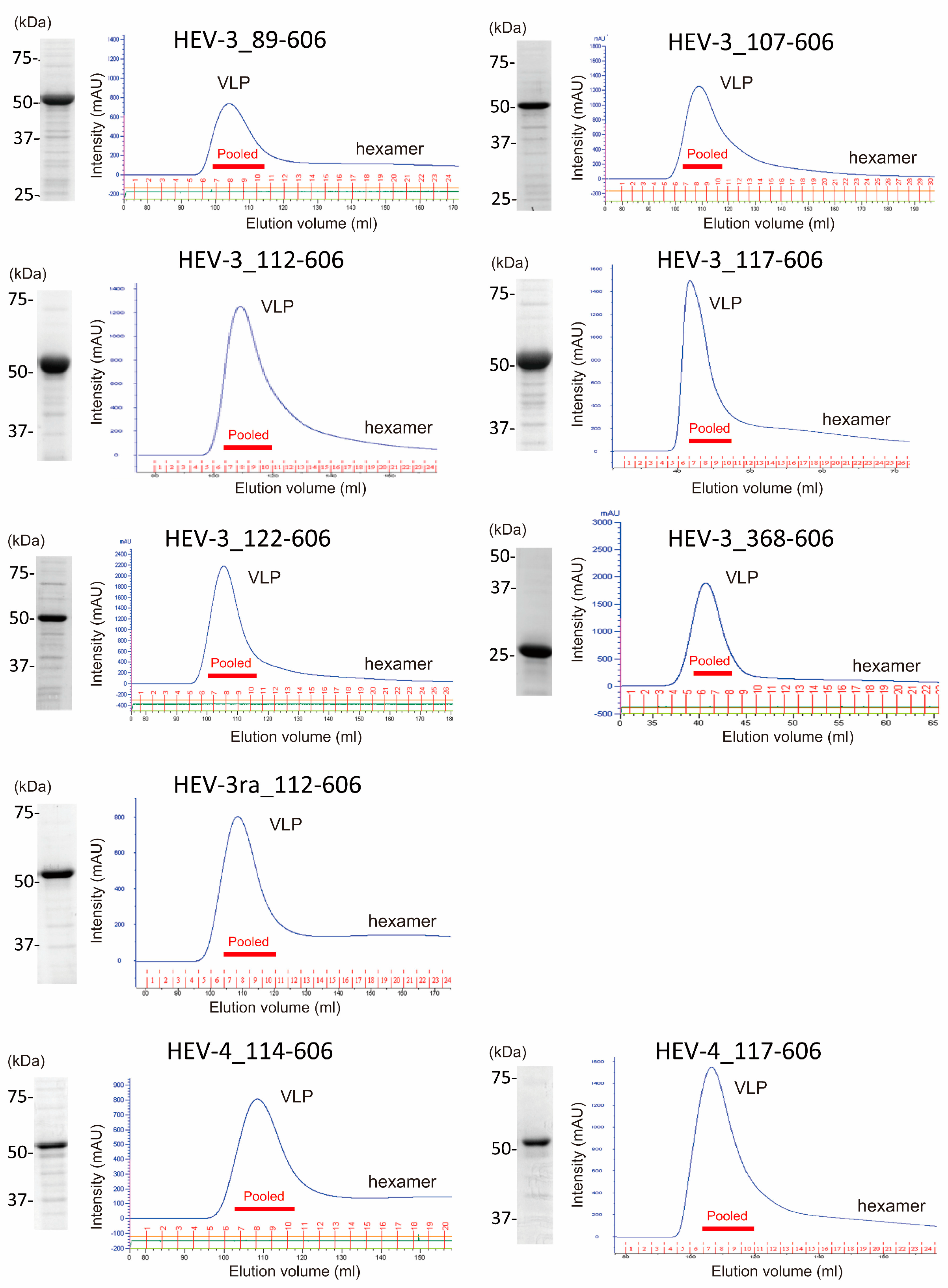
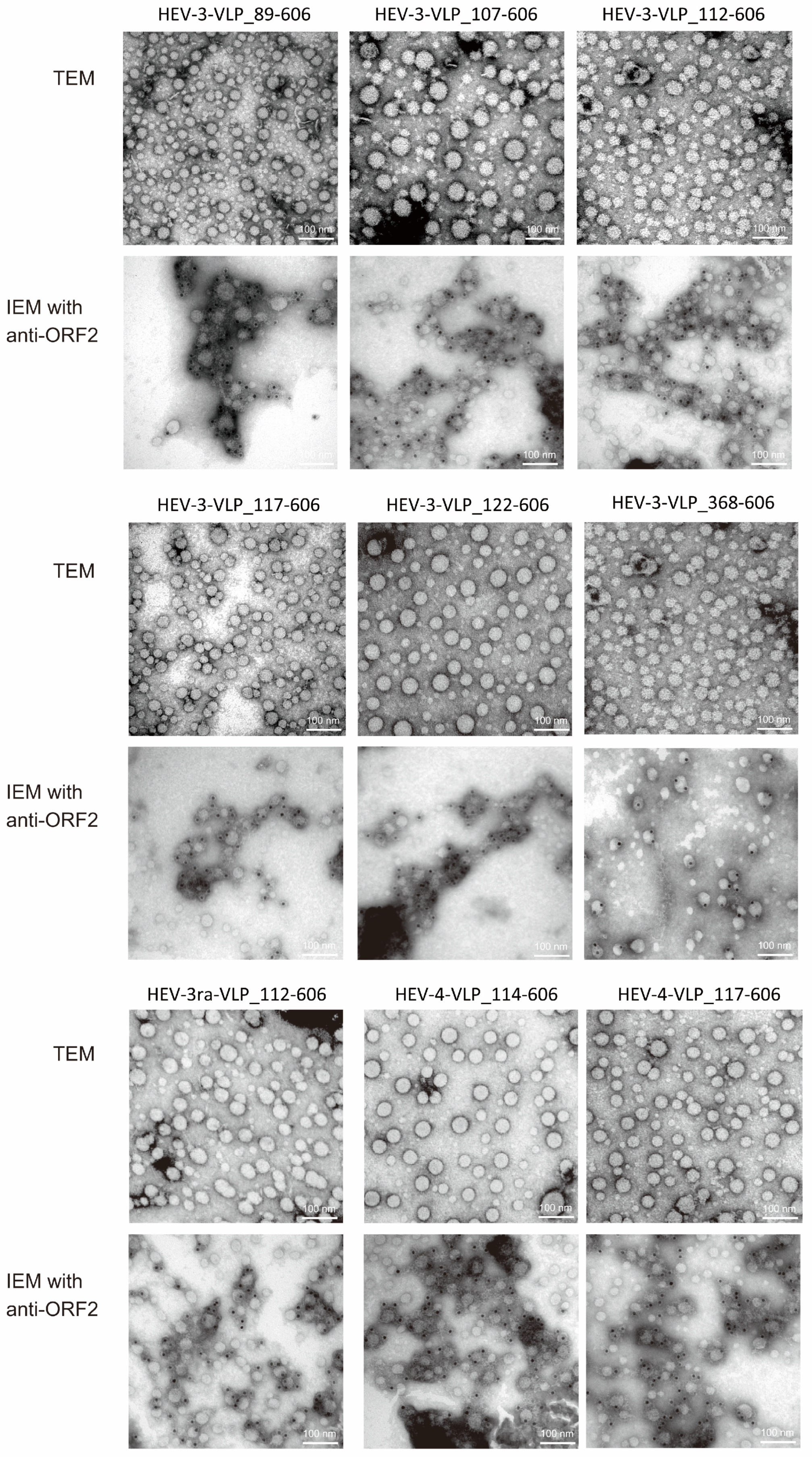
3.2. Characterization of the Purified VLPs of HEV-3, HEV-3ra, and HEV-4
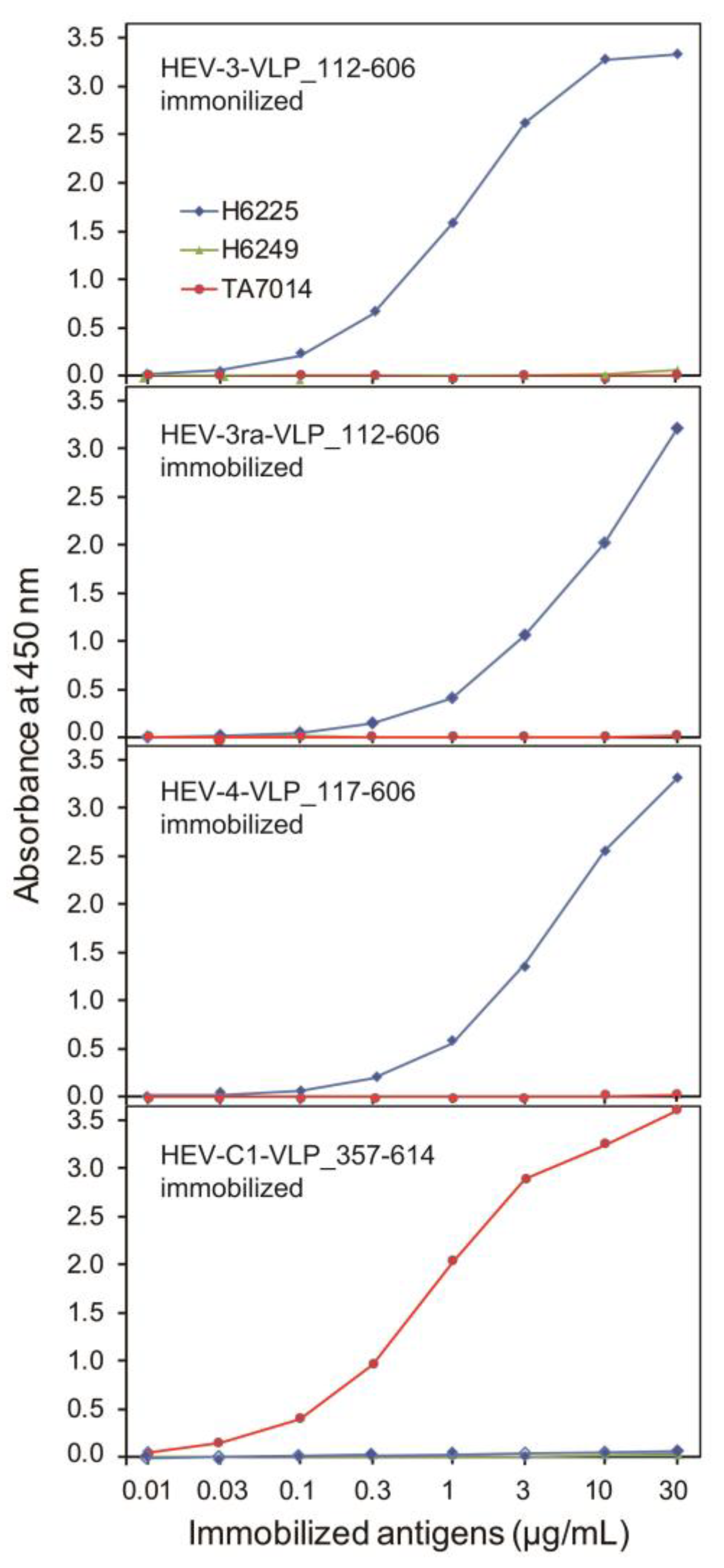
3.3. The Antigenicity of the Purified VLPs of HEV-3, HEV-3ra, and HEV-4
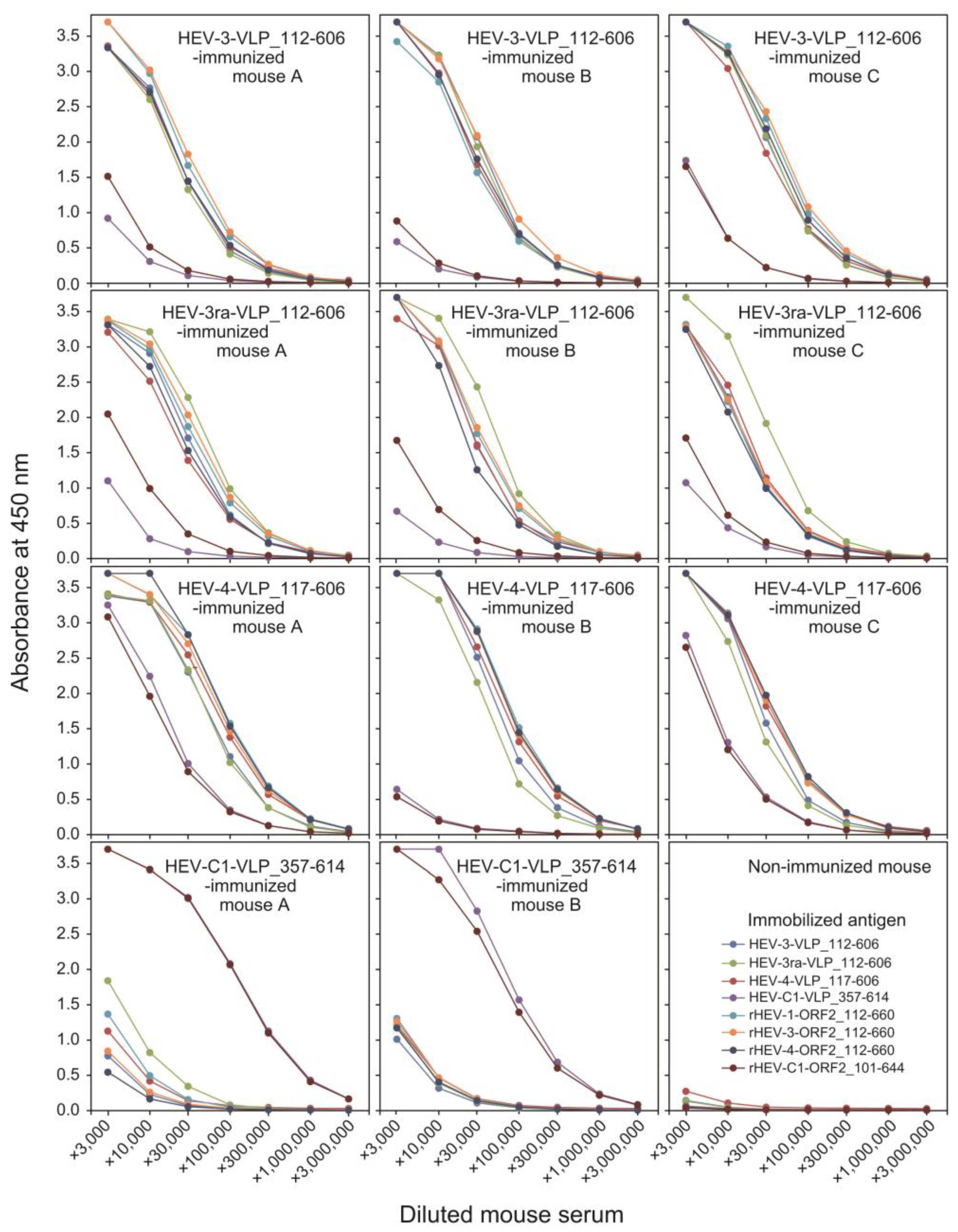
3.4. Immune Response of the Balb/cAJcl Mice to the Purified VLPs of HEV-3, HEV-3ra, and HEV-4
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Rein, D.B.; Stevens, G.A.; Theaker, J.; Wittenborn, J.S.; Wiersma, S.T. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology 2012, 55, 988–997. [Google Scholar] [CrossRef]
- Li, P.; Liu, J.; Li, Y.; Su, J.; Ma, Z.; Bramer, W.M.; Cao, W.; de Man, R.A.; Peppelenbosch, M.P.; Pan, Q. The global epidemiology of hepatitis E virus infection: A systematic review and meta-analysis. Liver Int. 2020, 40, 1516–1528. [Google Scholar] [CrossRef] [PubMed]
- Damiris, K.; Aghaie Meybodi, M.; Niazi, M.; Pyrsopoulos, N. Hepatitis E in immunocompromised individuals. World J. Hepatol. 2022, 14, 482–494. [Google Scholar] [CrossRef]
- Pischke, S.; Hartl, J.; Pas, S.D.; Lohse, A.W.; Jacobs, B.C.; Van der Eijk, A.A. Hepatitis E virus: Infection beyond the liver? J. Hepatol. 2017, 66, 1082–1095. [Google Scholar] [CrossRef] [PubMed]
- Berglov, A.; Hallager, S.; Weis, N. Hepatitis E during pregnancy: Maternal and foetal case-fatality rates and adverse outcomes-A systematic review. J. Viral Hepat. 2019, 26, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- Perez-Gracia, M.T.; Suay-Garcia, B.; Mateos-Lindemann, M.L. Hepatitis E and pregnancy: Current state. Rev. Med. Virol. 2017, 27, e1929. [Google Scholar] [CrossRef]
- Grange, Z.L.; Goldstein, T.; Johnson, C.K.; Anthony, S.; Gilardi, K.; Daszak, P.; Olival, K.J.; O’Rourke, T.; Murray, S.; Olson, S.H.; et al. Ranking the risk of animal-to-human spillover for newly discovered viruses. Proc. Natl. Acad. Sci. USA 2021, 118, e2002324118. [Google Scholar] [CrossRef]
- Nagashima, S.; Takahashi, M.; Kobayashi, T.; Tanggis; Nishizawa, T.; Nishiyama, T.; Primadharsini, P.P.; Okamoto, H. Characterization of the Quasi-Enveloped Hepatitis E Virus Particles Released by the Cellular Exosomal Pathway. J. Virol. 2017, 91, e00822-17. [Google Scholar] [CrossRef]
- Purdy, M.A.; Drexler, J.F.; Meng, X.J.; Norder, H.; Okamoto, H.; Van der Poel, W.H.M.; Reuter, G.; de Souza, W.M.; Ulrich, R.G.; Smith, D.B. ICTV Virus Taxonomy Profile: Hepeviridae 2022. J. Gen. Virol. 2022, 103, 001778. [Google Scholar] [CrossRef]
- Wang, B.; Meng, X.J. Structural and molecular biology of hepatitis E virus. Comput. Struct. Biotechnol. J. 2021, 19, 1907–1916. [Google Scholar] [CrossRef]
- Nimgaonkar, I.; Ding, Q.; Schwartz, R.E.; Ploss, A. Hepatitis E virus: Advances and challenges. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 96–110. [Google Scholar] [CrossRef]
- Nan, Y.; Zhang, Y.J. Molecular Biology and Infection of Hepatitis E Virus. Front. Microbiol. 2016, 7, 1419. [Google Scholar] [CrossRef]
- Graff, J.; Torian, U.; Nguyen, H.; Emerson, S.U. A bicistronic subgenomic mRNA encodes both the ORF2 and ORF3 proteins of hepatitis E virus. J. Virol. 2006, 80, 5919–5926. [Google Scholar] [CrossRef]
- Yin, X.; Ying, D.; Lhomme, S.; Tang, Z.; Walker, C.M.; Xia, N.; Zheng, Z.; Feng, Z. Origin, antigenicity, and function of a secreted form of ORF2 in hepatitis E virus infection. Proc. Natl. Acad. Sci. USA 2018, 115, 4773–4778. [Google Scholar] [CrossRef]
- Zhou, Y. Immunobiology and Host Response to HEV. Adv. Exp. Med. Biol. 2016, 948, 113–141. [Google Scholar] [PubMed]
- Glitscher, M.; Hildt, E. Hepatitis E virus egress and beyond—The manifold roles of the viral ORF3 protein. Cell Microbiol. 2021, 23, e13379. [Google Scholar] [CrossRef]
- Nagashima, S.; Jirintai, S.; Takahashi, M.; Kobayashi, T.; Tanggis; Nishizawa, T.; Kouki, T.; Yashiro, T.; Okamoto, H. Hepatitis E virus egress depends on the exosomal pathway, with secretory exosomes derived from multivesicular bodies. J. Gen. Virol. 2014, 95, 2166–2175. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.B.; Izopet, J.; Nicot, F.; Simmonds, P.; Jameel, S.; Meng, X.J.; Norder, H.; Okamoto, H.; van der Poel, W.H.M.; Reuter, G.; et al. Update: Proposed reference sequences for subtypes of hepatitis E virus (species Orthohepevirus A). J. Gen. Virol. 2020, 101, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Pavio, N.; Meng, X.J.; Renou, C. Zoonotic hepatitis E: Animal reservoirs and emerging risks. Vet. Res. 2010, 41, 46. [Google Scholar] [CrossRef]
- Wang, B.; Meng, X.J. Hepatitis E virus: Host tropism and zoonotic infection. Curr. Opin. Microbiol. 2020, 59, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Nishizawa, T.; Sato, H.; Sato, Y.; Jirintai; Nagashima, S.; Okamoto, H. Analysis of the full-length genome of a hepatitis E virus isolate obtained from a wild boar in Japan that is classifiable into a novel genotype. J. Gen. Virol. 2011, 92, 902–908. [Google Scholar]
- Takahashi, M.; Nishizawa, T.; Nagashima, S.; Jirintai, S.; Kawakami, M.; Sonoda, Y.; Suzuki, T.; Yamamoto, S.; Shigemoto, K.; Ashida, K.; et al. Molecular characterization of a novel hepatitis E virus (HEV) strain obtained from a wild boar in Japan that is highly divergent from the previously recognized HEV strains. Virus Res. 2014, 180, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Nishizawa, T.; Sato, Y.; Miyazaki, S.; Aikawa, T.; Ashida, K.; Tamaru, T.; Oguro, K.; Hayakawa, F.; Matsuoka, H.; et al. Prevalence and genotype/subtype distribution of hepatitis E virus (HEV) among wild boars in Japan: Identification of a genotype 5 HEV strain. Virus Res. 2020, 287, 198106. [Google Scholar] [CrossRef]
- Primadharsini, P.P.; Takahashi, M.; Nishizawa, T.; Sato, Y.; Nagashima, S.; Murata, K.; Okamoto, H. The Full-Genome Analysis and Generation of an Infectious cDNA Clone of a Genotype 6 Hepatitis E Virus Variant Obtained from a Japanese Wild Boar: In Vitro Cultivation in Human Cell Lines. Viruses 2024, 16, 842. [Google Scholar] [CrossRef]
- Woo, P.C.; Lau, S.K.; Teng, J.L.; Tsang, A.K.; Joseph, M.; Wong, E.Y.; Tang, Y.; Sivakumar, S.; Xie, J.; Bai, R.; et al. New hepatitis E virus genotype in camels, the Middle East. Emerg. Infect. Dis. 2014, 20, 1044–1048. [Google Scholar] [CrossRef]
- Woo, P.C.; Lau, S.K.; Teng, J.L.; Cao, K.Y.; Wernery, U.; Schountz, T.; Chiu, T.H.; Tsang, A.K.; Wong, P.C.; Wong, E.Y.; et al. New Hepatitis E Virus Genotype in Bactrian Camels, Xinjiang, China, 2013. Emerg. Infect. Dis. 2016, 22, 2219–2221. [Google Scholar] [CrossRef]
- Nishizawa, T.; Takahashi, M.; Tsatsralt-Od, B.; Nyamdavaa, K.; Dulmaa, N.; Osorjin, B.; Tseren-Ochir, E.O.; Sharav, T.; Bayasgalan, C.; Sukhbaatar, B.; et al. Identification and a full genome analysis of novel camel hepatitis E virus strains obtained from Bactrian camels in Mongolia. Virus Res. 2021, 299, 198355. [Google Scholar] [CrossRef]
- Hara, Y.; Terada, Y.; Yonemitsu, K.; Shimoda, H.; Noguchi, K.; Suzuki, K.; Maeda, K. High prevalence of hepatitis E virus in wild boar (Sus scrofa) in Yamaguchi Prefecture, Japan. J. Wildl. Dis. 2014, 50, 378–383. [Google Scholar] [CrossRef]
- Nakano, T.; Takahashi, K.; Arai, M.; Okano, H.; Kato, H.; Ayada, M.; Okamoto, H.; Mishiro, S. Identification of European-type hepatitis E virus subtype 3e isolates in Japanese wild boars: Molecular tracing of HEV from swine to wild boars. Infect. Genet. Evol. 2013, 18, 287–298. [Google Scholar] [CrossRef]
- Okamoto, H. Genetic variability and evolution of hepatitis E virus. Virus Res. 2007, 127, 216–228. [Google Scholar] [CrossRef]
- Sato, Y.; Sato, H.; Naka, K.; Furuya, S.; Tsukiji, H.; Kitagawa, K.; Sonoda, Y.; Usui, T.; Sakamoto, H.; Yoshino, S.; et al. A nationwide survey of hepatitis E virus (HEV) infection in wild boars in Japan: Identification of boar HEV strains of genotypes 3 and 4 and unrecognized genotypes. Arch. Virol. 2011, 156, 1345–1358. [Google Scholar] [CrossRef]
- Wang, L.; Liu, L.; Wang, L. An overview: Rabbit hepatitis E virus (HEV) and rabbit providing an animal model for HEV study. Rev. Med. Virol. 2018, 28, e1961. [Google Scholar] [CrossRef] [PubMed]
- Jirintai, S.; Jinshan; Tanggis; Manglai, D.; Mulyanto; Takahashi, M.; Nagashima, S.; Kobayashi, T.; Nishizawa, T.; Okamoto, H. Molecular analysis of hepatitis E virus from farm rabbits in Inner Mongolia, China and its successful propagation in A549 and PLC/PRF/5 cells. Virus Res. 2012, 170, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Abravanel, F.; Lhomme, S.; El Costa, H.; Schvartz, B.; Peron, J.M.; Kamar, N.; Izopet, J. Rabbit Hepatitis E Virus Infections in Humans, France. Emerg. Infect. Dis. 2017, 23, 1191–1193. [Google Scholar] [CrossRef]
- Izopet, J.; Dubois, M.; Bertagnoli, S.; Lhomme, S.; Marchandeau, S.; Boucher, S.; Kamar, N.; Abravanel, F.; Guerin, J.L. Hepatitis E virus strains in rabbits and evidence of a closely related strain in humans, france. Emerg. Infect. Dis. 2012, 18, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Andonov, A.; Robbins, M.; Borlang, J.; Cao, J.; Hatchette, T.; Stueck, A.; Deschambault, Y.; Murnaghan, K.; Varga, J.; Johnston, L. Rat Hepatitis E Virus Linked to Severe Acute Hepatitis in an Immunocompetent Patient. J. Infect. Dis. 2019, 220, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, S.; Yip, C.C.Y.; Wu, S.; Cai, J.; Zhang, A.J.; Leung, K.H.; Chung, T.W.H.; Chan, J.F.W.; Chan, W.M.; Teng, J.L.L.; et al. Rat Hepatitis E Virus as Cause of Persistent Hepatitis after Liver Transplant. Emerg. Infect. Dis. 2018, 24, 2241–2250. [Google Scholar] [CrossRef]
- Sridhar, S.; Yip, C.C.; Wu, S.; Chew, N.F.; Leung, K.H.; Chan, J.F.; Zhao, P.S.; Chan, W.M.; Poon, R.W.; Tsoi, H.W.; et al. Transmission of Rat Hepatitis E Virus Infection to Humans in Hong Kong: A Clinical and Epidemiological Analysis. Hepatology 2021, 73, 10–22. [Google Scholar] [CrossRef]
- Caballero-Gomez, J.; Pereira, S.; Rivero-Calle, I.; Perez, A.B.; Viciana, I.; Casares-Jimenez, M.; Rios-Munoz, L.; Rivero-Juarez, A.; Aguilera, A.; Rivero, A. Acute Hepatitis in Children Due to Rat Hepatitis E Virus. J. Pediatr. 2024, 273, 114125. [Google Scholar] [CrossRef]
- Johne, R.; Heckel, G.; Plenge-Bönig, A.; Kindler, E.; Maresch, C.; Reetz, J.; Schielke, A.; Ulrich, R.G. Novel hepatitis E virus genotype in Norway rats, Germany. Emerg. Infect. Dis. 2010, 16, 1452–1455. [Google Scholar] [CrossRef]
- Mulyanto; Wibawa, I.D.; Suparyatmo, J.B.; Amirudin, R.; Ohnishi, H.; Takahashi, M.; Nishizawa, T.; Okamoto, H. The complete genomes of subgenotype IA hepatitis A virus strains from four different islands in Indonesia form a phylogenetic cluster. Arch. Virol. 2014, 159, 935–945. [Google Scholar] [CrossRef]
- Purcell, R.H.; Engle, R.E.; Rood, M.P.; Kabrane-Lazizi, Y.; Nguyen, H.T.; Govindarajan, S.; St Claire, M.; Emerson, S.U. Hepatitis E virus in rats, Los Angeles, California, USA. Emerg. Infect. Dis. 2011, 17, 2216–2222. [Google Scholar] [CrossRef] [PubMed]
- Schofield, D.J.; Glamann, J.; Emerson, S.U.; Purcell, R.H. Identification by phage display and characterization of two neutralizing chimpanzee monoclonal antibodies to the hepatitis E virus capsid protein. J. Virol. 2000, 74, 5548–5555. [Google Scholar] [CrossRef] [PubMed]
- Mazalovska, M.; Kouokam, J.C. Progress in the Production of Virus-Like Particles for Vaccination against Hepatitis E Virus. Viruses 2020, 12, 826. [Google Scholar] [CrossRef]
- Hadj Hassine, I.; Ben M’hadheb, M.; Almalki, M.A.; Gharbi, J. Virus-like particles as powerful vaccination strategy against human viruses. Rev. Med. Virol. 2024, 34, e2498. [Google Scholar] [CrossRef]
- Cao, Y.F.; Tao, H.; Hu, Y.M.; Shi, C.B.; Wu, X.; Liang, Q.; Chi, C.P.; Li, L.; Liang, Z.L.; Meng, J.H.; et al. A phase 1 randomized open-label clinical study to evaluate the safety and tolerability of a novel recombinant hepatitis E vaccine. Vaccine 2017, 35, 5073–5080. [Google Scholar] [CrossRef] [PubMed]
- Li, T.C.; Takeda, N.; Miyamura, T.; Matsuura, Y.; Wang, J.C.; Engvall, H.; Hammar, L.; Xing, L.; Cheng, R.H. Essential elements of the capsid protein for self-assembly into empty virus-like particles of hepatitis E virus. J. Virol. 2005, 79, 12999–13006. [Google Scholar] [CrossRef] [PubMed]
- Li, S.W.; Zhao, Q.; Wu, T.; Chen, S.; Zhang, J.; Xia, N.S. The development of a recombinant hepatitis E vaccine HEV 239. Hum. Vaccin. Immunother. 2015, 11, 908–914. [Google Scholar] [CrossRef]
- Li, T.C.; Yamakawa, Y.; Suzuki, K.; Tatsumi, M.; Razak, M.A.; Uchida, T.; Takeda, N.; Miyamura, T. Expression and self-assembly of empty virus-like particles of hepatitis E virus. J. Virol. 1997, 71, 7207–7213. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, M.; Pan, H.; Lin, Z.; Wang, K.; Weng, Z.; Zhu, Y.; Xin, L.; Zhang, J.; Li, S.; et al. Robust manufacturing and comprehensive characterization of recombinant hepatitis E virus-like particles in Hecolin((R)). Vaccine 2014, 32, 4039–4050. [Google Scholar] [CrossRef]
- Zheng, M.; Jiang, J.; Zhang, X.; Wang, N.; Wang, K.; Li, Q.; Li, T.; Lin, Q.; Wang, Y.; Yu, H.; et al. Characterization of capsid protein (p495) of hepatitis E virus expressed in Escherichia coli and assembling into particles in vitro. Vaccine 2018, 36, 2104–2111. [Google Scholar] [CrossRef]
- Kobayashi, T.; Takahashi, M.; Ohta, S.; Nagashima, S.; Primadharsini, P.P.; Mulyanto; Kunita, S.; Murata, K.; Okamoto, H. Production of capsid proteins of rat hepatitis E virus in Escherichia coli and characterization of self-assembled virus-like particles. Virus Res. 2021, 302, 198483. [Google Scholar] [CrossRef] [PubMed]
- Abravanel, F.; Lhomme, S. Hecolin vaccine: Long-term efficacy against HEV for a three-dose regimen. Lancet 2024, 403, 782–783. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Takahashi, M.; Kusano, E.; Okamoto, H. Development and evaluation of an efficient cell-culture system for Hepatitis E virus. J. Gen. Virol. 2007, 88, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Takahashi, M.; Hoshino, Y.; Takahashi, H.; Ichiyama, K.; Tanaka, T.; Okamoto, H. Construction of an infectious cDNA clone of hepatitis E virus strain JE03-1760F that can propagate efficiently in cultured cells. J. Gen. Virol. 2009, 90, 457–462. [Google Scholar] [CrossRef]
- Yamashita, T.; Mori, Y.; Miyazaki, N.; Cheng, R.H.; Yoshimura, M.; Unno, H.; Shima, R.; Moriishi, K.; Tsukihara, T.; Li, T.C.; et al. Biological and immunological characteristics of hepatitis E virus-like particles based on the crystal structure. Proc. Natl. Acad. Sci. USA 2009, 106, 12986–12991. [Google Scholar] [CrossRef]
- Tanaka, T.; Takahashi, M.; Takahashi, H.; Ichiyama, K.; Hoshino, Y.; Nagashima, S.; Mizuo, H.; Okamoto, H. Development and Characterization of a Genotype 4 Hepatitis E Virus Cell Culture System Using a HE-JF5/15F Strain Recovered from a Fulminant Hepatitis Patient. J. Clin. Microbiol. 2009, 47, 1906–1910. [Google Scholar] [CrossRef]
- Takahashi, M.; Hoshino, Y.; Tanaka, T.; Takahashi, H.; Nishizawa, T.; Okamoto, H. Production of monoclonal antibodies against hepatitis E virus capsid protein and evaluation of their neutralizing activity in a cell culture system. Arch. Virol. 2008, 153, 657–666. [Google Scholar] [CrossRef]
- Nishiyama, T.; Umezawa, K.; Yamada, K.; Takahashi, M.; Kunita, S.; Mulyanto; Kii, I.; Okamoto, H. The Capsid (ORF2) Protein of Hepatitis E Virus in Feces Is C-Terminally Truncated. Pathogens 2022, 11, 24. [Google Scholar] [CrossRef]
- Kobayashi, T.; Takahashi, M.; Tanggis; Mulyanto; Jirintai, S.; Nagashima, S.; Nishizawa, T.; Okamoto, H. Characterization and epitope mapping of monoclonal antibodies raised against rat hepatitis E virus capsid protein: An evaluation of their neutralizing activity in a cell culture system. J. Virol. Methods 2016, 233, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Mizuo, H.; Suzuki, K.; Takikawa, Y.; Sugai, Y.; Tokita, H.; Akahane, Y.; Itoh, K.; Gotanda, Y.; Takahashi, M.; Nishizawa, T.; et al. Polyphyletic strains of hepatitis E virus are responsible for sporadic cases of acute hepatitis in Japan. J. Clin. Microbiol. 2002, 40, 3209–3218. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tang, X.; Seetharaman, J.; Yang, C.; Gu, Y.; Zhang, J.; Du, H.; Shih, J.W.; Hew, C.L.; Sivaraman, J.; et al. Dimerization of hepatitis E virus capsid protein E2s domain is essential for virus-host interaction. PLoS. Pathog. 2009, 5, e1000537. [Google Scholar] [CrossRef]
- Zhang, M.; Emerson, S.U.; Nguyen, H.; Engle, R.E.; Govindarajan, S.; Gerin, J.L.; Purcell, R.H. Immunogenicity and protective efficacy of a vaccine prepared from 53 kDa truncated hepatitis E virus capsid protein expressed in insect cells. Vaccine 2001, 20, 853–857. [Google Scholar] [CrossRef]
- Liu, Z.; Behloul, N.; Baha, S.; Wei, W.; Tao, W.; Zhang, T.; Li, W.; Shi, R.; Meng, J. Role of the C-terminal cysteines in virus-like particle formation and oligomerization of the hepatitis E virus ORF2 truncated proteins. Virology 2020, 544, 1–11. [Google Scholar] [CrossRef]
- Li, Y.; Huang, X.; Zhang, Z.; Li, S.; Zhang, J.; Xia, N.; Zhao, Q. Prophylactic Hepatitis E Vaccines: Antigenic Analysis and Serological Evaluation. Viruses 2020, 12, 109. [Google Scholar] [CrossRef]
- Vormittag, P.; Klamp, T.; Hubbuch, J. Ensembles of Hydrophobicity Scales as Potent Classifiers for Chimeric Virus-Like Particle Solubility—An Amino Acid Sequence-Based Machine Learning Approach. Front. Bioeng. Biotechnol. 2020, 8, 395. [Google Scholar] [CrossRef] [PubMed]
- Thrane, S.; Janitzek, C.M.; Matondo, S.; Resende, M.; Gustavsson, T.; de Jongh, W.A.; Clemmensen, S.; Roeffen, W.; van de Vegte-Bolmer, M.; van Gemert, G.J.; et al. Bacterial superglue enables easy development of efficient virus-like particle based vaccines. J. Nanobiotechnol. 2016, 14, 30. [Google Scholar] [CrossRef]
- Li, T.C.; Yoshimatsu, K.; Yasuda, S.P.; Arikawa, J.; Koma, T.; Kataoka, M.; Ami, Y.; Suzaki, Y.; Mai le, T.Q.; Hoa, N.T.; et al. Characterization of self-assembled virus-like particles of rat hepatitis E virus generated by recombinant baculoviruses. J. Gen. Virol. 2011, 92, 2830–2837. [Google Scholar] [CrossRef]
- Li, T.C.; Yang, T.; Yoshizaki, S.; Ami, Y.; Suzaki, Y.; Ishii, K.; Haga, K.; Nakamura, T.; Ochiai, S.; Takaji, W.; et al. Construction and characterization of an infectious cDNA clone of rat hepatitis E virus. J. Gen. Virol. 2015, 96, 1320–1327. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Kataoka, M.; Ami, Y.; Suzaki, Y.; Kishida, N.; Shirakura, M.; Imai, M.; Asanuma, H.; Takeda, N.; Wakita, T.; et al. Characterization of self-assembled virus-like particles of ferret hepatitis E virus generated by recombinant baculoviruses. J. Gen. Virol. 2013, 94, 2647–2656. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Kataoka, M.; Liu, Z.; Takeda, N.; Wakita, T.; Li, T.C. Characterization of self-assembled virus-like particles of dromedary camel hepatitis e virus generated by recombinant baculoviruses. Virus Res. 2015, 210, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Kataoka, M.; Ami, Y.; Suzaki, Y.; Takeda, N.; Muramatsu, M.; Li, T.C. Immunogenicity and Antigenicity of Rabbit Hepatitis E Virus-Like Particles Produced by Recombinant Baculoviruses. Viruses 2021, 13, 1573. [Google Scholar] [CrossRef] [PubMed]
- Emerson, S.U.; Clemente-Casares, P.; Moiduddin, N.; Arankalle, V.A.; Torian, U.; Purcell, R.H. Putative neutralization epitopes and broad cross-genotype neutralization of Hepatitis E virus confirmed by a quantitative cell-culture assay. J. Gen. Virol. 2006, 87, 697–704. [Google Scholar] [CrossRef]
- Engle, R.E.; Yu, C.; Emerson, S.U.; Meng, X.J.; Purcell, R.H. Hepatitis E virus (HEV) capsid antigens derived from viruses of human and swine origin are equally efficient for detecting anti-HEV by enzyme immunoassay. J. Clin. Microbiol. 2002, 40, 4576–4580. [Google Scholar] [CrossRef]
- Li, T.C.; Kataoka, M.; Takahashi, K.; Yoshizaki, S.; Kato, T.; Ishii, K.; Takeda, N.; Mishiro, S.; Wakita, T. Generation of hepatitis E virus-like particles of two new genotypes G5 and G6 and comparison of antigenic properties with those of known genotypes. Vet. Microbiol. 2015, 178, 150–157. [Google Scholar] [CrossRef]
- Mulyanto; Suparyatmo, J.B.; Andayani, I.; Khalid; Takahashi, M.; Ohnishi, H.; Jirintai, S.; Nagashima, S.; Nishizawa, T.; Okamoto, H. Marked genomic heterogeneity of rat hepatitis E virus strains in Indonesia demonstrated on a full-length genome analysis. Virus Res. 2014, 179, 102–112. [Google Scholar] [CrossRef]
- Sridhar, S.; Situ, J.; Cai, J.P.; Yip, C.C.; Wu, S.; Zhang, A.J.; Wen, L.; Chew, N.F.; Chan, W.M.; Poon, R.W.; et al. Multimodal investigation of rat hepatitis E virus antigenicity: Implications for infection, diagnostics, and vaccine efficacy. J. Hepatol. 2021, 74, 1315–1324. [Google Scholar] [CrossRef]
- Zhang, W.; Ami, Y.; Suzaki, Y.; Doan, Y.H.; Muramatsu, M.; Li, T.C. Mongolia Gerbils Are Broadly Susceptible to Hepatitis E Virus. Viruses 2022, 14, 1125. [Google Scholar] [CrossRef]
- Xu, L.D.; Zhang, F.; Chen, C.; Peng, L.; Luo, W.T.; Chen, R.; Xu, P.; Huang, Y.W. Revisiting the Mongolian Gerbil Model for Hepatitis E Virus by Reverse Genetics. Microbiol. Spectr. 2022, 10, e0219321. [Google Scholar] [CrossRef]
| Primer Name | Sequence (5′–3′) | Note |
|---|---|---|
| hHEV_G3 Nde-ORF2 aa12 Fw | CATATG GTGCTTCTGCCTATGCTGCC | Nde I site (underlined) |
| hHEV_G3 Nde-ORF2 aa14 Fw | CATATG CTGCCTATGCTGCCCGCGCC | Nde I site (underlined) |
| hHEV_G3 Nde-ORF2 aa61 Fw | CATATG CCCTTTGCCGCCGATGTCG | Nde I site (underlined) |
| hHEV_G3 Nde-ORF2 aa89 Fw | CATATG GACCAGTCCCAGCGCCCCTC | Nde I site (underlined) |
| hHEV_G3 Nde-ORF2 aa107 Fw | CATATG GCTGCGCCGTTGACTGCTATC | Nde I site (underlined) |
| hHEV_G3 Nde-ORF2 aa112 Fw | CATATG GCTATCTCACCAGCCCCTGAC | Nde I site (underlined) |
| hHEV_G3 Nde-ORF2 aa117 Fw | CATATG CCTGACACAGCCCCTGTACC | Nde I site (underlined) |
| hHEV_G3 Nde-ORF2 aa122 Fw | CATATG GTACCTGATGTTGATTCACG | Nde I site (underlined) |
| hHEV_G3 Nde-ORF2 aa368 Fw | CATATG ATTGCTCTGACACTGTTCAAC | Nde I site (underlined) |
| rbHEV_G3 Nde-ORF2 aa112 Fw | CATATG GCTGTTTCACCTGCACCTG | Nde I site (underlined) |
| rbHEV_G3 Nde-ORF2 aa368 Fw | CATATG ATAGCCCTGACGCTGTTTAAC | Nde I site (underlined) |
| hHEV_G4 Nde-ORF2 aa112 Fw | CATATG GCTGTGGCCCCGGCCCCCGA | Nde I site (underlined) |
| hHEV_G4 Nde-ORF2 aa114 Fw | CATATG GCCCCGGCCCCCGATACTGC | Nde I site (underlined) |
| hHEV_G4 Nde-ORF2 aa117 Fw | CATATG CCCGATACTGCTCCTGTTCC | Nde I site (underlined) |
| hHEV_G4 Nde-ORF2 aa366 Fw | CATATG GTCGGTCGTGGTATAGCGC | Nde I site (underlined) |
| hHEV_G4 Nde-ORF2 aa368 Fw | CATATG CGTGGTATAGCGCTAACTCTG | Nde I site (underlined) |
| hHEV_G4 Nde-ORF2 aa370 Fw | CATATG ATAGCGCTAACTCTGTTCAATC | Nde I site (underlined) |
| hHEV_G3 ORF2-Bam aa606 Rev | GGATCC CTA AGCCGAGTGCGGGGCTAGTAC | BamH I site (underlined) |
| rbHEV_G3 ORF2-Bam aa606 Rev | GGATCC CTA AACTGAGTGCGGAGCAAGC | BamH I site (underlined) |
| hHEV_G4 ORF2-Bam aa606 Rev | GGATCC CTA CGCAGAGTGAGGTGCGAGGAC | BamH I site (underlined) |
| hHEV_G4 ORF2-Bam aa609 Rev | GGATCC CTA AGCGGCCAGCGCAGAGTGAG | BamH I site (underlined) |
| hHEV_G4 ORF2-Bam aa612 Rev | GGATCC CTA GTCCTCTAAAGCGGCCAGCG | BamH I site (underlined) |
| rbHEV_G3 T6686C Fw | TACGGCTCGTCAACTAACCC | nt 6684-6703 a (mutated nt underlined) |
| rbHEV_G3 T6686C Rev | TGTAGTTTGATCATACTCTG | nt 6664-6683 a |
| Purified VLPs | Amount (mL) Recovered after Gel Filtration | Concentration (Mean ± SD, mg/mL) a | Molecular Weight (Mean ± SD, kDa) b | Yield (Mean ± SD, mg) | Purity (Mean ± SD, %) b |
|---|---|---|---|---|---|
| HEV-3-VLP_89-606 (p518) | 48 | 0.5 ± 0.1 | 63.4 ± 0.7 | 24.0 ± 6.8 | 89.5 ± 2.8 |
| HEV-3-VLP_107-606 (p500) | 48 | 1.4 ± 0.1 | 61.1 ± 0.4 | 66.0 ± 6.0 | 90.7 ± 4.1 |
| HEV-3-VLP_112-606 (p495) | 48 | 1.9 ± 0.3 | 61.0 ± 0.5 | 89.6 ± 15.4 | 95.3 ± 1.4 |
| HEV-3-VLP_117-606 (p490) | 48 | 1.3 ± 0.1 | 60.3 ± 0.6 | 64.0 ± 5.5 | 91.9 ± 2.3 |
| HEV-3-VLP_122-606 (p485) | 48 | 1.6 ± 0.4 | 58.4 ± 1.1 | 76.8 ± 17.3 | 95.0 ± 1.6 |
| HEV-3-VLP_368-606 (p239) | 12 | 1.4 ± 0.6 | 27.1 ± 0.5 | 16.2 ± 7.6 | 93.1 ± 0.1 |
| HEV-3ra-VLP_112-606 (p495) | 16 | 0.9 ± 0.2 | 62.7 ± 0.6 | 13.9 ± 3.7 | 94.9 ± 1.9 |
| HEV-4-VLP_114-606 (p493) | 48 | 0.8 ± 0.1 | 61.9 ± 0.7 | 39.2 ± 5.0 | 74.7 ± 5.8 |
| HEV-4-VLP_117-606 (p490) | 48 | 1.3 ± 0.4 | 60.7 ± 0.3 | 62.4 ± 20.4 | 90.1 ± 8.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, T.; Takahashi, M.; Ohta, S.; Hoshino, Y.; Yamada, K.; Jirintai, S.; Primadharsini, P.P.; Nagashima, S.; Murata, K.; Okamoto, H. Production and Characterization of Self-Assembled Virus-like Particles Comprising Capsid Proteins from Genotypes 3 and 4 Hepatitis E Virus (HEV) and Rabbit HEV Expressed in Escherichia coli. Viruses 2024, 16, 1400. https://doi.org/10.3390/v16091400
Kobayashi T, Takahashi M, Ohta S, Hoshino Y, Yamada K, Jirintai S, Primadharsini PP, Nagashima S, Murata K, Okamoto H. Production and Characterization of Self-Assembled Virus-like Particles Comprising Capsid Proteins from Genotypes 3 and 4 Hepatitis E Virus (HEV) and Rabbit HEV Expressed in Escherichia coli. Viruses. 2024; 16(9):1400. https://doi.org/10.3390/v16091400
Chicago/Turabian StyleKobayashi, Tominari, Masaharu Takahashi, Satoshi Ohta, Yu Hoshino, Kentaro Yamada, Suljid Jirintai, Putu Prathiwi Primadharsini, Shigeo Nagashima, Kazumoto Murata, and Hiroaki Okamoto. 2024. "Production and Characterization of Self-Assembled Virus-like Particles Comprising Capsid Proteins from Genotypes 3 and 4 Hepatitis E Virus (HEV) and Rabbit HEV Expressed in Escherichia coli" Viruses 16, no. 9: 1400. https://doi.org/10.3390/v16091400
APA StyleKobayashi, T., Takahashi, M., Ohta, S., Hoshino, Y., Yamada, K., Jirintai, S., Primadharsini, P. P., Nagashima, S., Murata, K., & Okamoto, H. (2024). Production and Characterization of Self-Assembled Virus-like Particles Comprising Capsid Proteins from Genotypes 3 and 4 Hepatitis E Virus (HEV) and Rabbit HEV Expressed in Escherichia coli. Viruses, 16(9), 1400. https://doi.org/10.3390/v16091400






