The Feline calicivirus Leader of the Capsid (LC) Protein Contains a Putative Transmembrane Domain, Binds to the Cytoplasmic Membrane, and Exogenously Permeates Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Prediction and Validation of the Tertiary Structure of the WT and C40A Mutant LC Proteins
2.2. Prediction of the Transmembrane Domain of the WT and C40A Mutant LC Proteins
2.3. Cell Culture
2.4. Plasmid Purification and Transfection
2.5. Recombinant Protein Expression and Purification
2.6. In Vitro Exogenous Interaction of the LC Protein with the Plasmatic Membrane of CrFK Cells
2.7. Cell Permeation Assays
2.8. SDS-PAGE Western Blot Analysis
3. Results
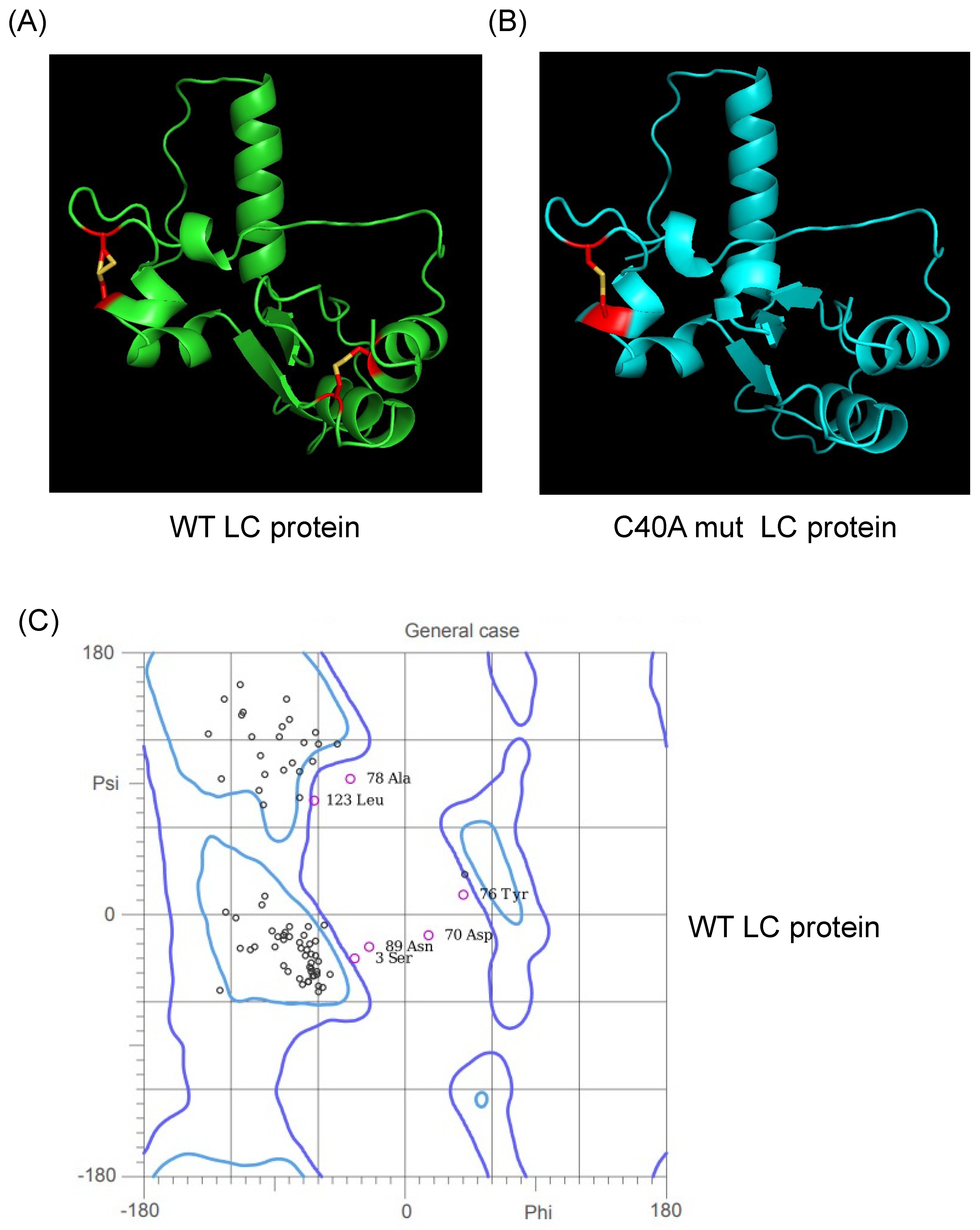
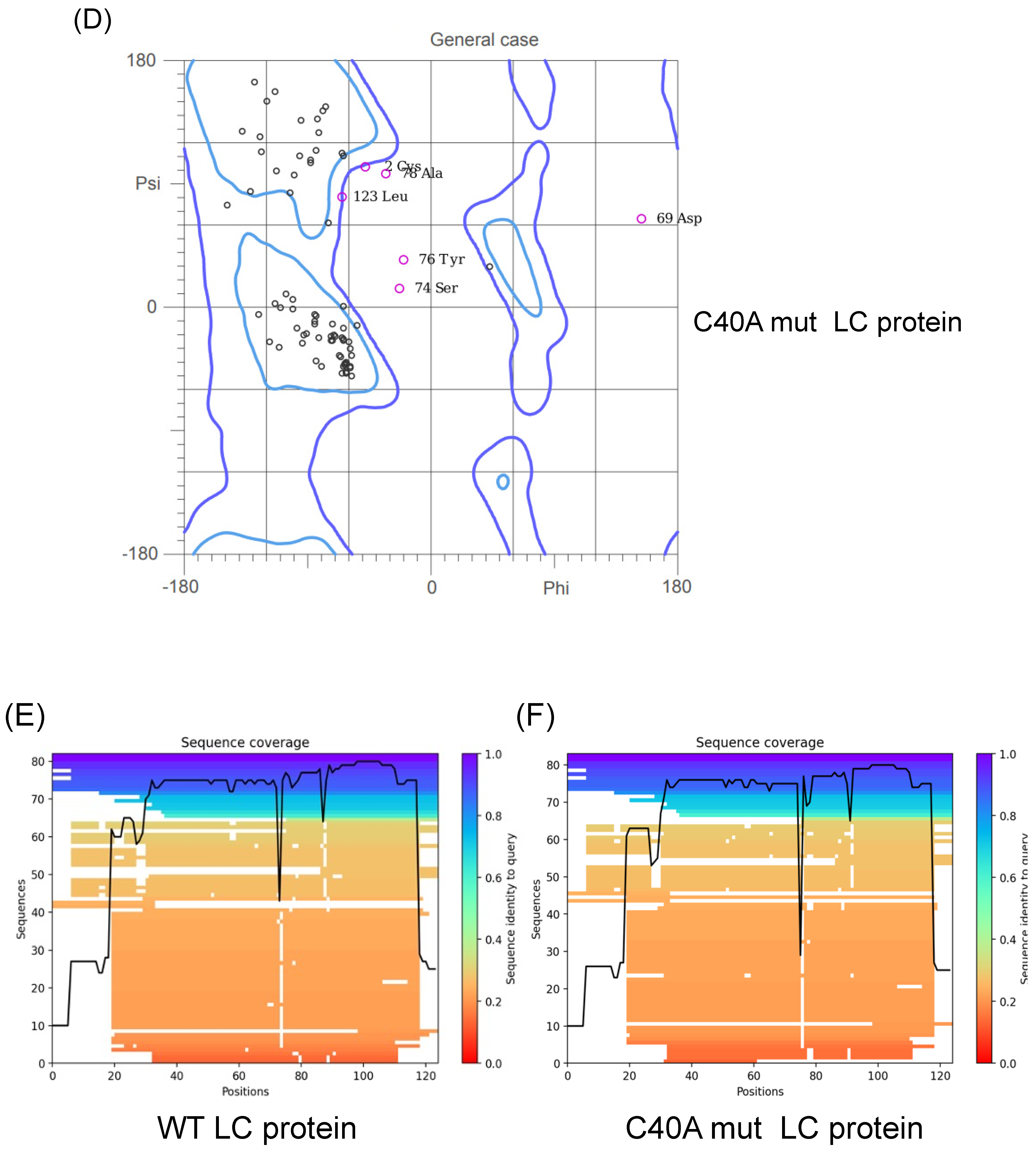
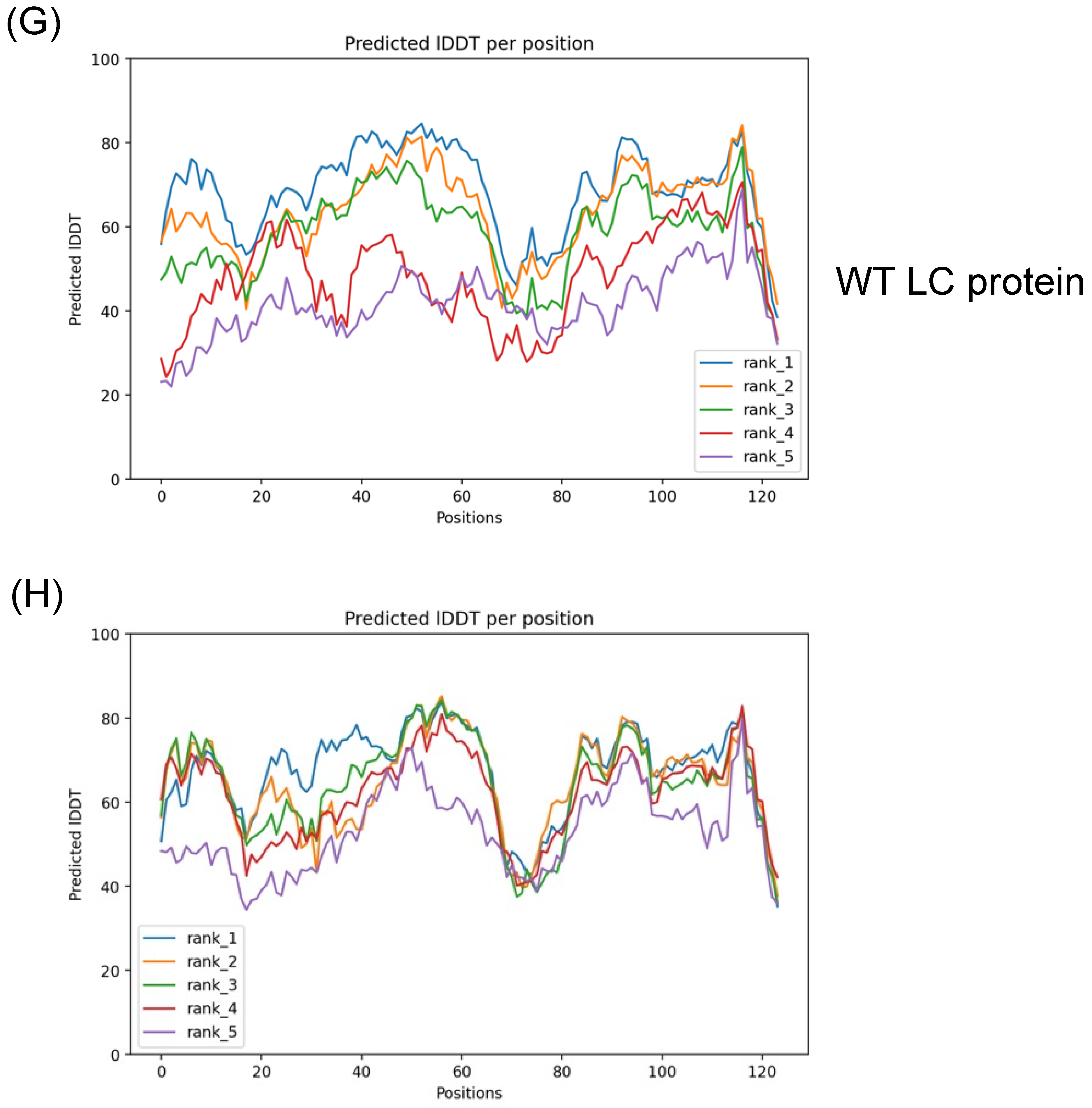
3.1. Validation of the Predicted Tertiary Structure of the Wild Type and the C40A Mutant LC Proteins from the FCV
3.2. Putative Wild-Type and C40A Mutant LC Protein Tertiary Structures Possess Intramolecular Disulfide Bonds in Accordance with Experimental Data
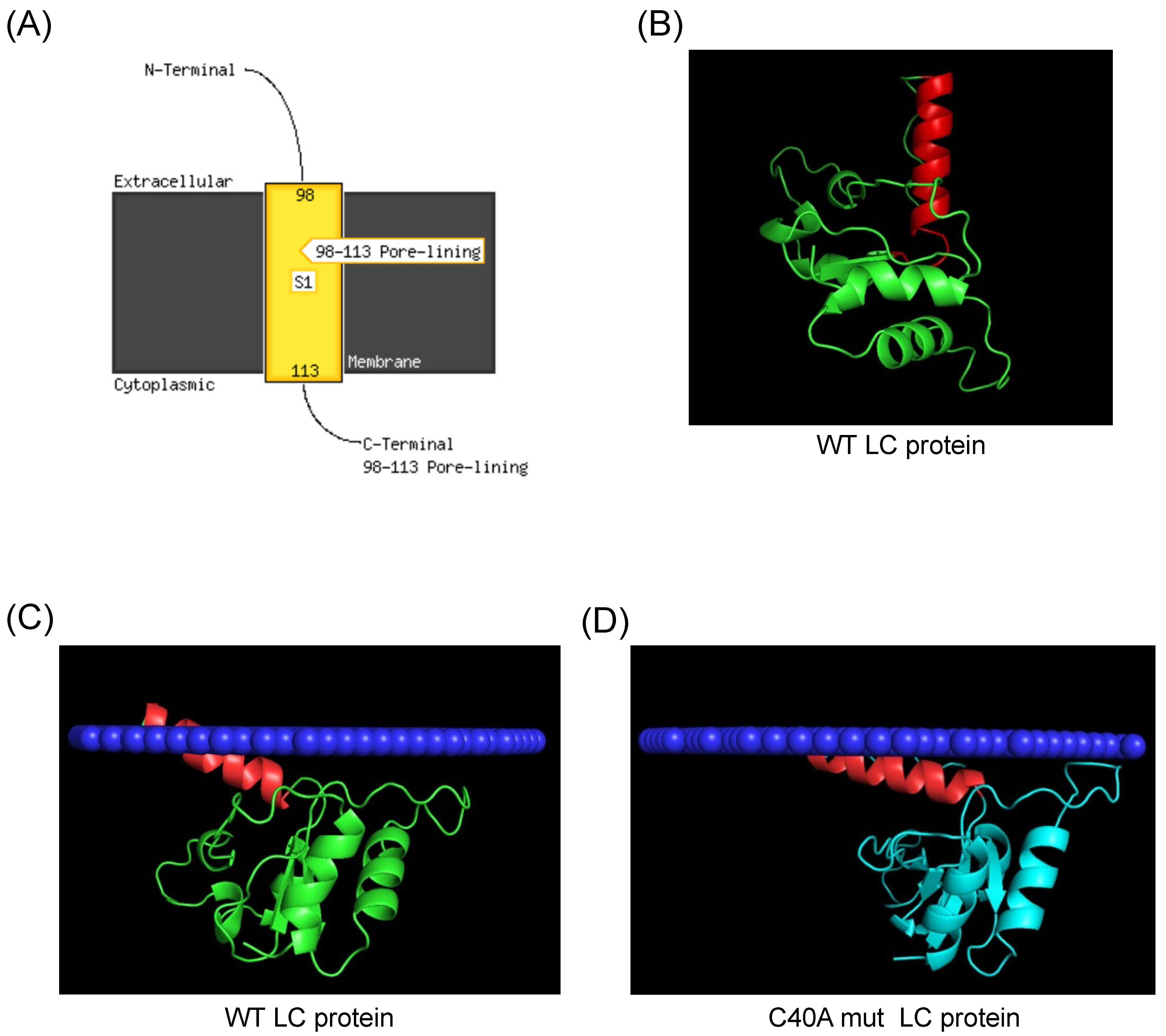
3.3. The FCV LC Protein Predicted Structure Lacks a Defensin γ-Core Signature in Its CR-I But Has a Putative C-Terminal Transmembrane Domain
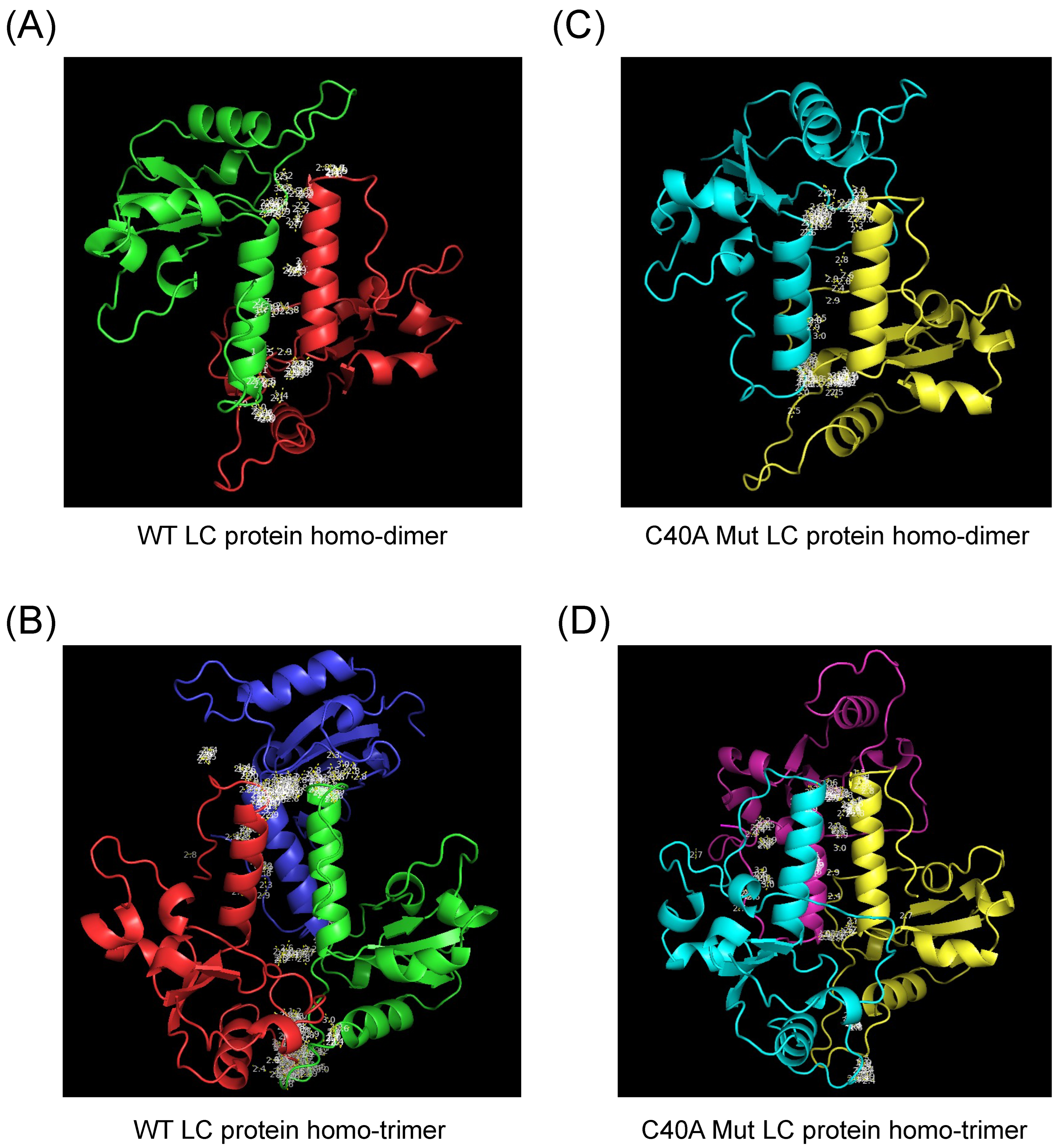
3.4. Modeling of FCV Wild Type and C40A Mutant LC Protein Oligomeric Forms by AlphaFold 2
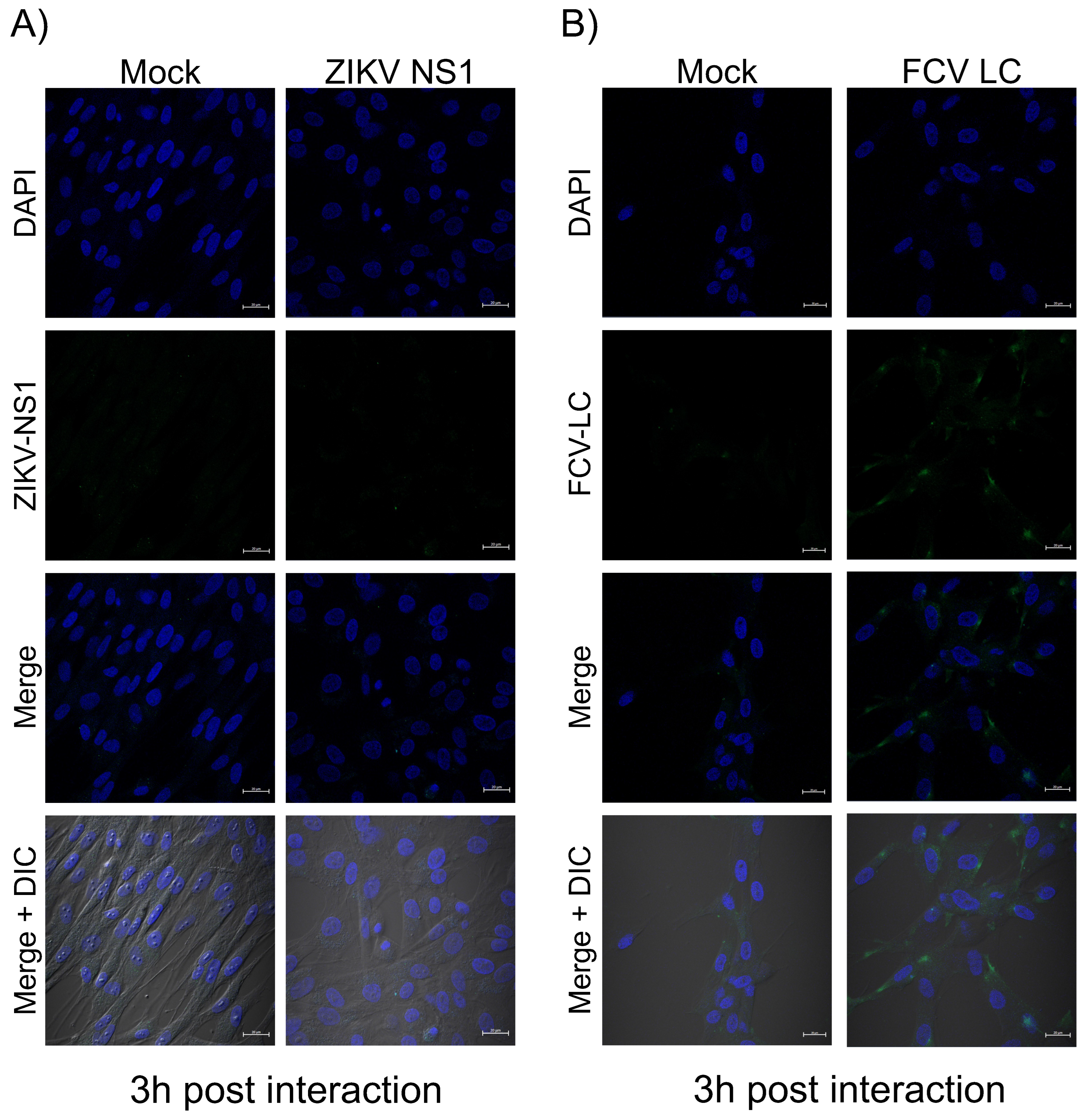
3.5. The Purified Histag-LC Protein from FCV Associates with the CrFK Cells’ Cytoplasmic Membrane through Exogenous Interaction
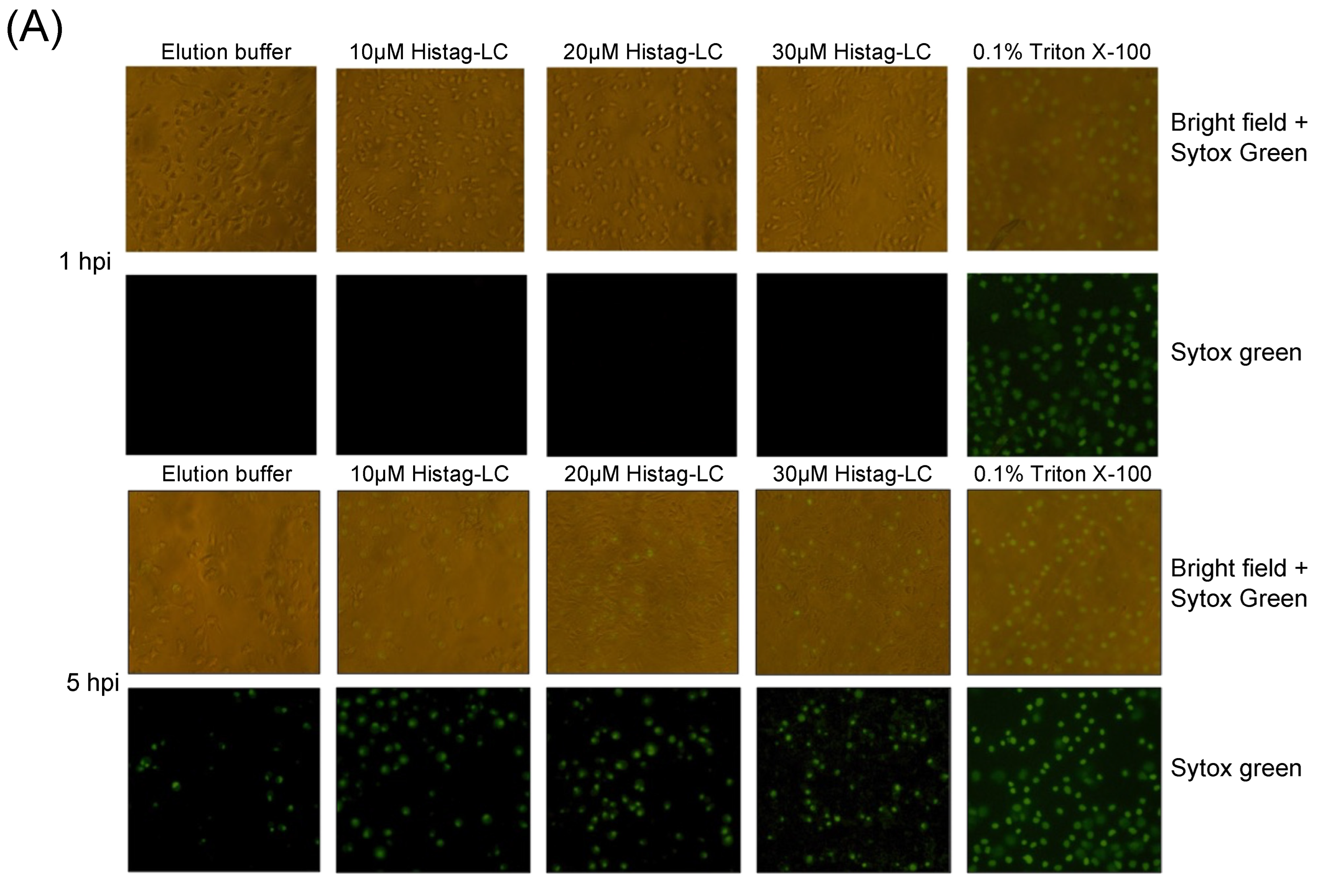
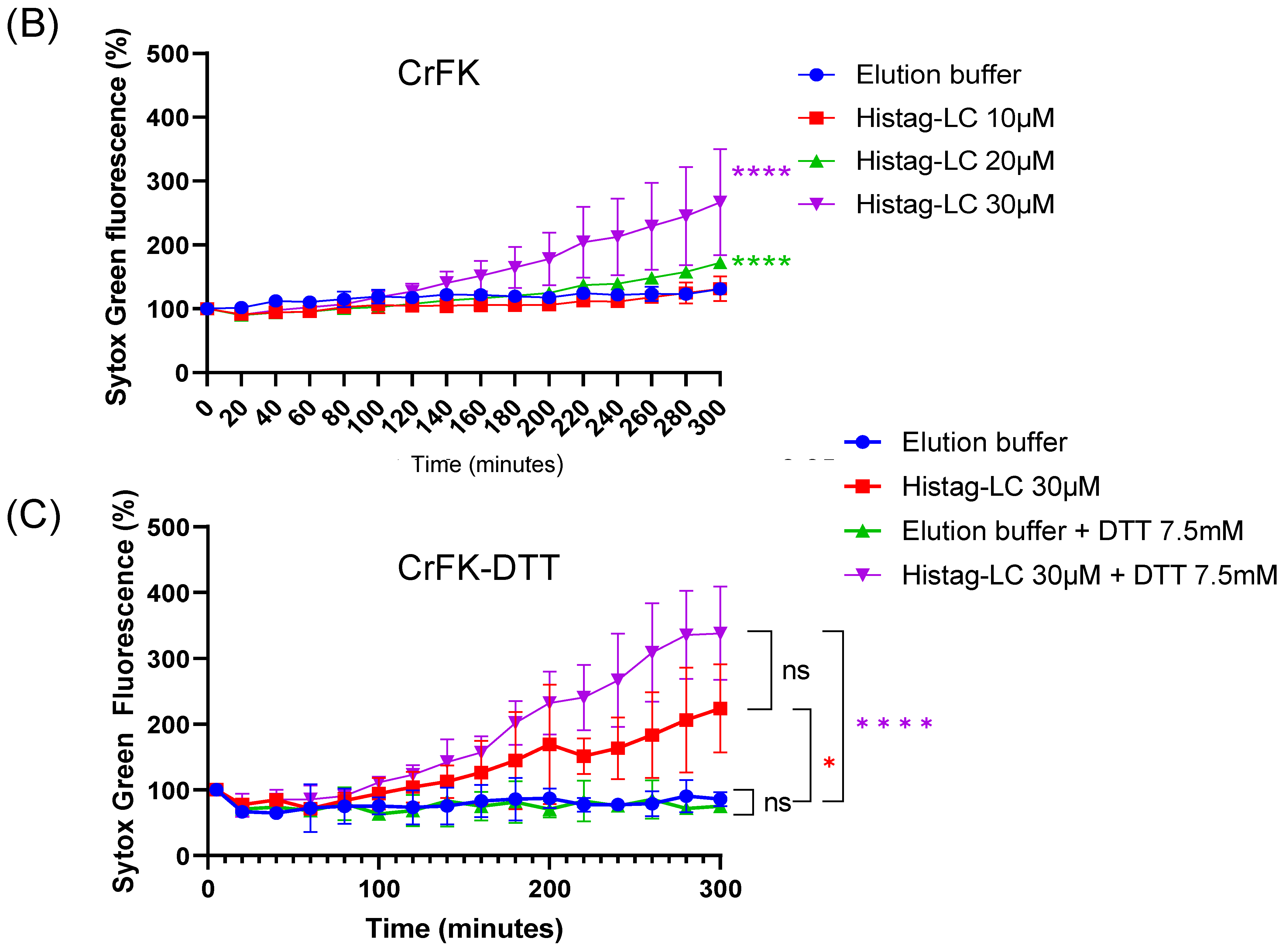
3.6. The Purified Histag-LC Protein from FCV Permeates CrFK Cells’ Cytoplasmic Membrane through Exogenous Interaction and in a Disulfide Bond-Independent Formation
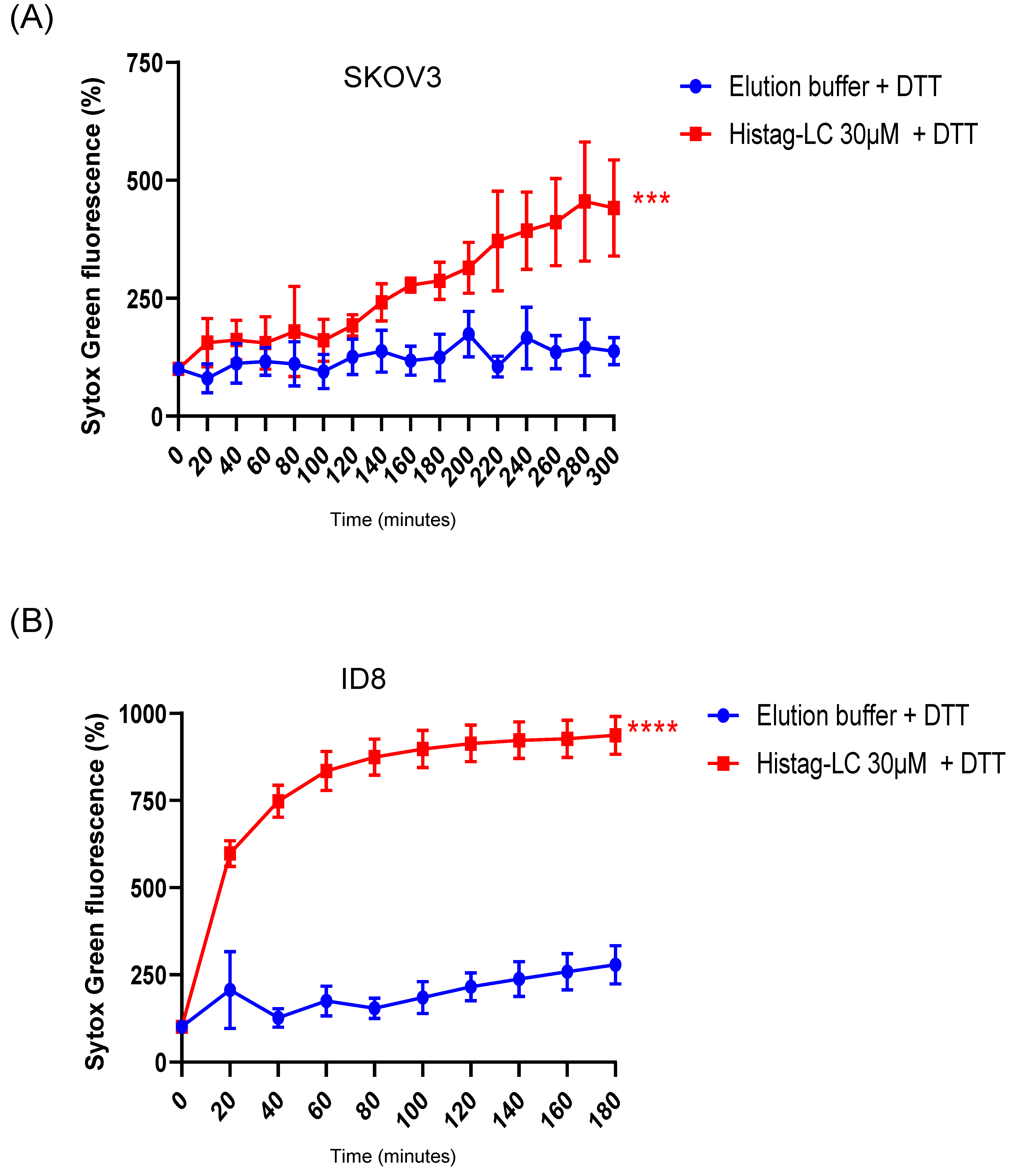
3.7. The Purified Histag-LC Protein from FCV Permeates the Cytoplasmic Membrane of the Human SKOV3 and the Murine ID8 Ovarian Cancer Cells through an Exogenous Interaction
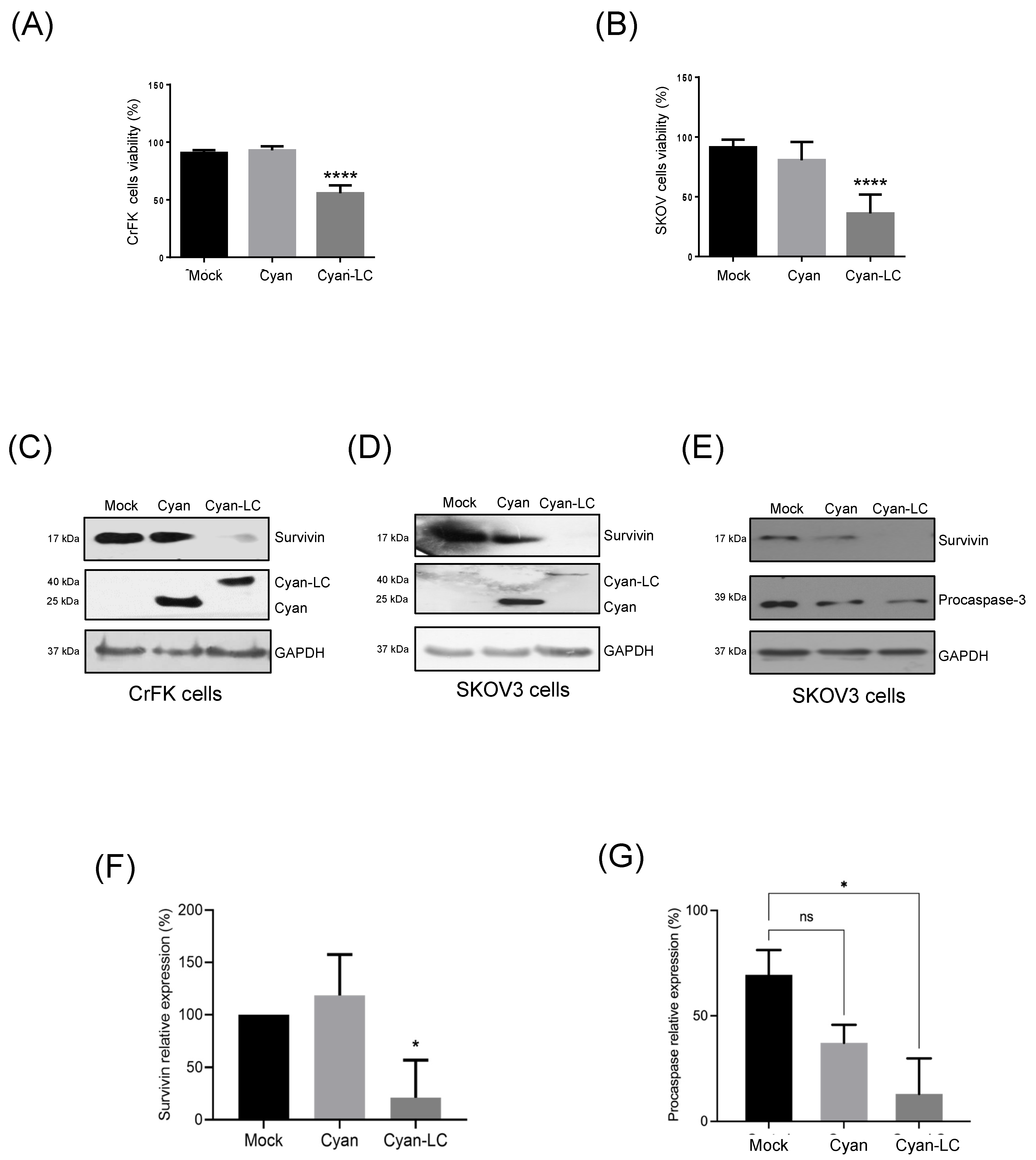
3.8. The FCV LC Protein Expression in a Virus-Free System Induces Apoptosis in SKOV3 Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peñaflor-Téllez, C.E.M.-R.Y.; Escolano, A.L.G. The Caliciviridae family. Rezaei, N., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 192–206. [Google Scholar]
- Sosnovtsev, S.V.; Sosnovtseva, S.A.; Green, K.Y. Cleavage of the feline calicivirus capsid precursor is mediated by a virus-encoded proteinase. J. Virol. 1998, 72, 3051–3059. Available online: http://www.ncbi.nlm.nih.gov/pubmed/9525628 (accessed on 23 June 2024). [CrossRef]
- Abente, E.J.; Sosnovtsev, S.V.; Sandoval-Jaime, C.; Parra, G.I.; Bok, K.; Green, K.Y. The Feline Calicivirus Leader of the Capsid Protein Is Associated with Cytopathic Effect. J. Virol. 2013, 87, 3003–3017. [Google Scholar] [CrossRef]
- Barrera-Vazquez, O.S.; Cancio-Lonches, C.; Hernandez-Gonzalez, O.; Chavez-Munguia, B.; Villegas-Sepulveda, N.; Gutierrez-Escolano, A.L. The feline calicivirus leader of the capsid protein causes survivin and XIAP downregulation and apoptosis. Virology 2019, 527, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Penaflor-Tellez, Y.; Chavez-Munguia, B.; Lagunes-Guillen, A.; Salazar-Villatoro, L.; Gutierrez-Escolano, A.L. The Feline Calicivirus Leader of the Capsid Protein Has the Functional Characteristics of a Viroporin. Viruses 2022, 14, 635. [Google Scholar] [CrossRef]
- Peñaflor-Téllez, Y.; de la Madrid, J.G.; Monge-Celestino, E.I.; Pérez-Ibáñez, C.; Miguel-Rodríguez, C.E.; Gutiérrez-Escolano, A.L. The Leader of the Capsid protein from Feline calicivirus must be palmitoylated and form oligomers through disulfide bonds for efficient viral replication. bioRxiv 2024. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Chakraborty, S.; Ahmad, M.; Kumar, V.; Tailor, P.B.; Biswal, B.K. Identification of probable inhibitors for the DNA polymerase of the Monkeypox virus through the virtual screening approach. Int. J. Biol. Macromol. 2023, 229, 515–528. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Williams, C.J.; Headd, J.J.; Moriarty, N.W.; Prisant, M.G.; Videau, L.L.; Deis, L.N.; Verma, V.; Keedy, D.A.; Hintze, B.J.; Chen, V.B.; et al. MolProbity: More and better reference data for improved all-atom structure validation. Protein Sci. 2018, 27, 293–315. [Google Scholar] [CrossRef] [PubMed]
- Lomize, A.L.; Todd, S.C.; Pogozheva, I.D. Spatial arrangement of proteins in planar and curved membranes by PPM 3.0. Protein Sci. 2022, 31, 209–220. [Google Scholar] [CrossRef]
- Ceroni, A.; Passerini, A.; Vullo, A.; Frasconi, P. DISULFIND: A disulfide bonding state and cysteine connectivity prediction server. Nucleic Acids Res. 2006, 4, W177–W181. [Google Scholar] [CrossRef] [PubMed]
- Yount, N.Y.; Yeaman, M.R. Multidimensional signatures in antimicrobial peptides. Proc. Natl. Acad. Sci. USA 2004, 101, 7363–7368. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Melnik, L.I.; Komin, A.; Wiedman, G.; Fuselier, T.; Morris, C.F.; Starr, C.G.; Searson, P.C.; Gallaher, W.R.; Hristova, K.; et al. Ebola Virus Delta Peptide Is a Viroporin. J. Virol. 2017, 91, 16. [Google Scholar] [CrossRef] [PubMed]
- Hoover, D.M.; Chertov, O.; Lubkowski, J. The structure of human beta-defensin-1: New insights into structural properties of beta-defensins. J. Biol. Chem. 2001, 276, 39021–39026. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.B.; Perminov, A.; Bekele, S.; Kedziora, G.; Farajollahi, S.; Varaljay, V.; Hinkle, K.; Molinero, V.; Meister, K.; Hung, C.; et al. AlphaFold2 models indicate that protein sequence determines both structure and dynamics. Sci. Rep. 2022, 12, 10696. [Google Scholar] [CrossRef] [PubMed]
- Alcala, A.C.; Maravillas, J.L.; Meza, D.; Ramirez, O.T.; Ludert, J.E.; Palomares, L.A. Dengue Virus NS1 Uses Scavenger Receptor B1 as a Cell Receptor in Cultured Cells. J. Virol. 2022, 96, e0166421. [Google Scholar] [CrossRef] [PubMed]
- Szlasa, W.; Zendran, I.; Zalesinska, A.; Tarek, M.; Kulbacka, J. Lipid composition of the cancer cell membrane. J. Bioenerg. Biomembr. 2020, 52, 321–342. [Google Scholar] [CrossRef]
- Choromanska, A.; Chwiłkowska, A.; Kulbacka, J.; Baczyńska, D.; Rembiałkowska, N.; Szewczyk, A.; Michel, O.; Gajewska-Naryniecka, A.; Przystupski, D.; Saczko, J. Modifications of Plasma Membrane Organization in Cancer Cells for Targeted Therapy. Molecules 2021, 26, 7. [Google Scholar] [CrossRef]
- Kiraz, Y.; Adan, A.; Yandim, M.K.; Baran, Y. Major apoptotic mechanisms and genes involved in apoptosis. Tumour Biol. 2016, 37, 8471–8486. [Google Scholar] [CrossRef]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 2. [Google Scholar] [CrossRef]
- Guindalini, R.S.C.; Machado, M.C.M.; Garicochea, B. Monitoring survivin expression in cancer: Implications for prognosis and therapy. Mol. Diagn. Ther. 2013, 17, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Smertina, E.; Carroll, A.J.; Boileau, J.; Emmott, E.; Jenckel, M.; Vohra, H.; Rolland, V.; Hands, P.; Hayashi, J.; Neave, M.J.; et al. Lagovirus Non-structural Protein p23: A Putative Viroporin That Interacts With Heat Shock Proteins and Uses a Disulfide Bond for Dimerization. Front. Microbiol. 2022, 13, 923256. [Google Scholar] [CrossRef]
- Stevens, T.J.; Mizuguchi, K.; Arkin, I.T. Distinct protein interfaces in transmembrane domains suggest an in vivo folding model. Protein Sci. 2004, 13, 3028–3037. [Google Scholar] [CrossRef] [PubMed]
- Mravic, M.; He, L.; Kratochvil, H.T.; Hu, H.; Nick, S.E.; Bai, W.; Edwards, A.; Jo, H.; Wu, Y.; Di Maio, D.; et al. De novo-designed transmembrane proteins bind and regulate a cytokine receptor. Nat. Chem. Biol. 2024, 20, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Luo, R.Y.; Yang, J.; Cheng, Y.X. Knockdown of survivin contributes to antitumor activity in cisplatin-resistant ovarian cancer cells. Mol. Med. Rep. 2013, 7, 425–430. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peñaflor-Téllez, Y.; Escobar-Almazan, J.A.; Pérez-Ibáñez, C.; Miguel-Rodríguez, C.E.; Gómez de la Madrid, J.; Monge-Celestino, E.I.; Talamás-Rohana, P.; Gutiérrez-Escolano, A.L. The Feline calicivirus Leader of the Capsid (LC) Protein Contains a Putative Transmembrane Domain, Binds to the Cytoplasmic Membrane, and Exogenously Permeates Cells. Viruses 2024, 16, 1319. https://doi.org/10.3390/v16081319
Peñaflor-Téllez Y, Escobar-Almazan JA, Pérez-Ibáñez C, Miguel-Rodríguez CE, Gómez de la Madrid J, Monge-Celestino EI, Talamás-Rohana P, Gutiérrez-Escolano AL. The Feline calicivirus Leader of the Capsid (LC) Protein Contains a Putative Transmembrane Domain, Binds to the Cytoplasmic Membrane, and Exogenously Permeates Cells. Viruses. 2024; 16(8):1319. https://doi.org/10.3390/v16081319
Chicago/Turabian StylePeñaflor-Téllez, Yoatzin, Jesús Alejandro Escobar-Almazan, Carolina Pérez-Ibáñez, Carlos Emilio Miguel-Rodríguez, Jaury Gómez de la Madrid, Erick I. Monge-Celestino, Patricia Talamás-Rohana, and Ana Lorena Gutiérrez-Escolano. 2024. "The Feline calicivirus Leader of the Capsid (LC) Protein Contains a Putative Transmembrane Domain, Binds to the Cytoplasmic Membrane, and Exogenously Permeates Cells" Viruses 16, no. 8: 1319. https://doi.org/10.3390/v16081319
APA StylePeñaflor-Téllez, Y., Escobar-Almazan, J. A., Pérez-Ibáñez, C., Miguel-Rodríguez, C. E., Gómez de la Madrid, J., Monge-Celestino, E. I., Talamás-Rohana, P., & Gutiérrez-Escolano, A. L. (2024). The Feline calicivirus Leader of the Capsid (LC) Protein Contains a Putative Transmembrane Domain, Binds to the Cytoplasmic Membrane, and Exogenously Permeates Cells. Viruses, 16(8), 1319. https://doi.org/10.3390/v16081319







