Burden of Congenital CMV Infection: A Narrative Review and Implications for Public Health Interventions
Abstract
1. Introduction
2. Materials and Methods
3. Results
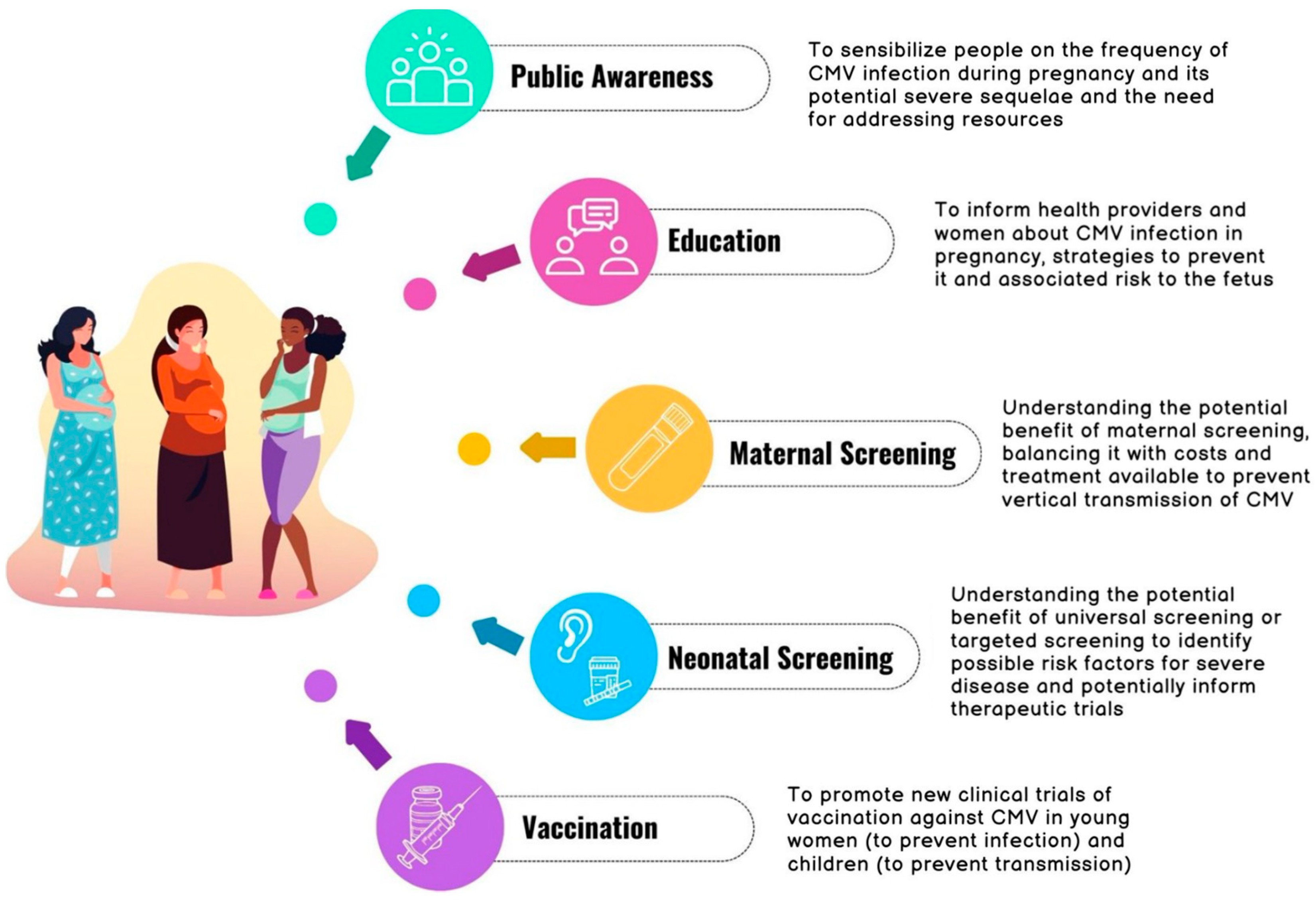
3.1. Awareness and Education in Pregnancy
3.2. Maternal Screening
3.3. Neonatal Screening
3.3.1. Universal Screening
| Author, Year, Ref, Country | Study Design and Time of Enrollment | Population | Method of Universal Screening | Results | Symptomatic Newborns at Birth (Other than Isolated HL) | Isolated HL Confirmed Cases | Treatment | Outcome | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Schlesinger, 2005 [49] Israel | Multicenter prospective study From May 98 to August 99 | Live-born infants | PCR on urine | 14 diagnosed/2000 screened for CMV | 2 symptomatic (microcephaly, hepatitis) | no HL found | n.a. | n.a. | This study did not identify any isolated SNHL, no information about follow-up for LO-SNHL was provided. |
| Lorenzoni, 2013 [48] Italy | Monocenter prospective From January 2012 to July 2013 | Premature newborns (<37 gw) and SGA term infants (weight <3rd percentile) | PCR on urine | 12 (10 preterms, 2 SGA)/383 screened/504 premature or SGA | 1 preterm (lissencephaly) | 2 | n.a. | n.a. | Increased incidence of cCMV and isolated SHL (17%) in this populations. |
| Barkai, 2014 [50] Israel | Single-center prospective study From May 2011 to May 2012 | Live-born infants | PCR on saliva confirmed by urine | 48 cCMV/9845 screened for CMV/10,137 live-born infants | 0 | 1 | 4 infants | 1 LO-SNHL at 3 months of age | Incidence of neonatal hearing loss: 2%. The infant diagnosed with HL passed the OAE screening and was confirmed on ABR. |
| Fowler, 2017 [47] USA | Multicenter prospective From March 2007 to March 2012 | Live-born infants | PCR on saliva or DBS | 443 cCMV identified out of 100,332 tested | n.a. | 35 confirmed | n.a. | n.a. | Incidence of neonatal hearing loss: 8%. The lack of CMV confirmation on urine may give some FP patients. 15 cCMV cases with confirmed SNHL passed the OAE screening. |
| Dar, 2017 [51] India | Multicenter, prospective study From December 2010 to May 2012 | Live-born infants | PCR on saliva | 20 diagnosed/1720 screened | 1 | 2 | n.a. | n.a. | Incidence of neonatal hearing loss: 10%. 1 out of 2 neonates with cCMV and SNHL passed the initial HS. The lack of CMV confirmation on urine may give some FP patients. |
| Yamamoto, 2020 [52] Brazil | Multicenter, prospective study From September 2013 to April 2017 | Live-born infants | PCR on saliva, confirmed on urine | 68 diagnosed/11,900 tested | 4 | 4 | 7 | Neonatal SNHL: between the 4 isolated SNHL, 1 progressed and 1 was stable at 18–48 months at follow-up For the 4 symptomatic babies, all had SNHL, one progressive and 3 stable at follow-up. For the other 49 cases, no late-onset HL was detected at a median 36-month follow-up | Incidence of neonatal hearing loss: 5.8%. 1 neonate with cCMV and SNHL passed the initial HS. Targeted screening would have missed 12.5% of infants with SNHL. |
| Yamada, 2020 [53] Japan | Multicenter prospective study From November 2009 to March 2018 | Live-born infants | PCR on urine | 56 diagnosed/11,736 tested for CMV | 19 | 4 | n.a. | Between the 4 isolated SNHL, 2 normal development and 2 mild sequele. | The incidence of isolated SNHL in this population is 7.1%. |
| Blazquez-Gamero, 2020 [54] Spain | Prospective, monocenter From February 2017 to February 2018 | Live-born infants | PCR on saliva, confirmed by urine | 15 positive out of 3190 tested | 2 | 0 | n.a. | No infants (13 available at follow-up) developed SNH at 25 months | The incidence of isolated SNHL in this population is 0%. |
| Letamendia-Richard, 2022 [55] France | Monocenter, retrospective From single unit, 2016–2020 | Live-born infants | PCR on saliva at birth, confirmed by urine | 63 confirmed infections/15,341 tested/15,649 live-born infants | 8 infants small for gestational age, no one with HL | 1 | n.a. | n.a. | The child with isolated SNHL had hepatomegaly at prenatal US and his mother had known seroconversion, so it would have been diagnosed without intervention. |
| Chiereghin, 2022 [56] Italy | Multicenter prospective study From February 2019 to July 2020 | Live-born infants | PCR on saliva confirmed by urine | 21 confirmed cCMV/3151 screened for CMV | 1 case with severe CNS disease and HL | 1 | 2 (6 months) | 1 asymptomatic infant developed LO-SNHL at 5 months of age | Incidence of neonatal hearing loss: 4.7%. No information regarding hearing screening test. |
3.3.2. Targeted Screening
| Author, Year, Ref, Country | Study Design and Time of Enrollment | Patient | Method of Targeted Screening | Results | Symptomatic Newborns at Birth (Other than Isolated HL) | Isolated HL Confirmed Cases | Treatment | Outcomes of cCMV Cases | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Stehel, 2008 [60] Texas (USA) | Monocenter, retrospective From September 1999 to August 2004 | Patients failing HS, mother infected with HIV, clinical or lab signs suggestive | PCR on urine | 24 confirmed/483 screened/572 failing HS. | 9 | 8 | n.a. | n.a. | The inclusion criteria for screening were not stringent. It was hard to predict if the screening would be different to normal clinical practice. |
| Williams, 2014 [61] UK | Multicenter prospective From August 2010 to October 2012 | Infants < 22 days old failing NHS. Known cCMV excluded | PCR on urine or saliva | 6 diagnosed/407 screened/411 recruited after failing NHS | n.a. | 3 | n.a. | n.a. | Clinical data and outcome missing. |
| Kawada, 2015 [62] Japan | Prospective study From January 2011 to December 2013 | Infants failing NHS | PCR on saliva or urine | 6 confirmed out fo 127 failing NHS | 0 | 6 | valgancyclovir for 6 weeks | only 1 out of 6 improved at 1-year follow-up | Valganciclovir did not show to significantly improved hearing function. |
| Roth, 2017 [63] Israel | Single-center retrospective study From 2014 to 2015 | Infants failing NHS | PCR on saliva confirmed by urine | 4 confirmed cCMV/180 tested for CMV/200 failing NHS | 2 | 3 | n.a. | n.a. | Targeted screening identified 1 child (out of 200 failing NHS) who needed treatment. Outcomes missing. |
| Diener, 2017 [58] Utah (USA) | Retrospective multicenter From 2013 to 2015 | Live-born infants failing NHS. Infants with suggestive symptoms were excluded | PCR on saliva, confirmed on urine | 14 diagnosed/314 screened for CMV/509 failing HS | 0 | 6 | n.a. | n.a. | No information on follow-up and outcome. |
| Rawlinson, 2018 [59] Australia | Monocenter, retrospective study From October 2009 to Oct 2016 | Infants failing HS and formal audiological testing (ABR) | PCR on saliva up to 2011, after 2011, positivity on saliva was confirmed on urine | 19 diagnosed/323 screened/502 infants with confirmed HL | 4 | 15 | 6 out of 19 (only 4 started within the first month of life) | n.a. | No clinical outcome, no follow-up. Symptomatic infants were not excluded from the study (4 out of 19 confirmed) and were reasonably diagnosed without this intervention. |
| Beswick, 2019 [64] Australia | Multicenter, retrospective From August 2014 to April 2016 | Neonates failing NHS (twice OAE) | PCR on saliva, confirmed by urine and blood | 3 diagnosed out of 234 screened/347 failing NHS | 0 | 2 | 1, valganciclovir | n.a. | Intervention allowed diagnosis and treatment of one otherwise asymptomatic infant. No clinical outcome provided. |
| Pellegrinelli, 2019 [65] Italy | Observational single-center study From 2014 to 2018 | Infants failing NHS (AOE) | PCR on DBS | 5 DBS tested positive/82 DBS screened/89 failing NHS | n.a. | 5 | n.a. | n.a. | DBS method may have missed some CMV diagnoses. |
| Ronner, 2021 [57] Massachusetts (USA) | Monocenter, retrospective chart review, From 2013 to 2020 (screening from 2015). Targeted screening was implemented in 2015 for 2 nurseries, from 2016 to all nurseries | Infants failing NHS | Primary PCR on saliva | 8 confirmed/528 tested for CMV/891 failing NHS | n.a. | 6 | valganciclovir | hearing stable in 3, progressed in 2, improved in 1. | Hearing function improved in 1 patient out of 6 diagnosed and treated for isolated SNHL. Not specified if symptomatic infants were excluded from the study. |
| Khi Chung, 2022 [66] Netherlands | National, prospective observational From 2012 to 2016 | Infants failing NHS (three rounds: two OAEs, one ABR) | PCR on DBS | 54 confirmed/1374 DBS screened/1381 infants failing NHS | n.a. | n.a. (48 infants had confirmed HL, but other concurrent symptoms were not excluded or specified in the study) | n.a | n.a. | Symptomatic children were not excluded and granular data about clinical scenario were not provided. |
| Fourgeaud, 2022 [67] France | Multicenter, prospective study From 2014 to 2017 | Newborns failing NHS (twice OAE in 3 centers, twice ABR in 2 centers) | PCR on saliva Confirmatory test on saliva and blood | 2 confirmed/231 screened for CMV/236 failing NHS | n.a. | n.a. (2 cases of HL but no information on other symptoms) | valganciclovir | n.a. | No granular data about clinical scenario of confirmed cases. Not specified if symptomatic infants were excluded from the study. |
| Webb, 2022 [68] Australia | Prospective, multicenter From June 2019 to March 2020 | Infants failing NHS | PCR on saliva, confirmed on urine and plasma | 1 positive out of 96 tested | 0 | 1 | valganciclovir started at 32 days of life for 6 months | n.a. | Good feasibility and acceptability. |
| Zhang, 2023 [69] Japan | Single-center observational prospective study From October 2018 to October 2021 | Newborns with suggestive perinatal conditions, including failing NHS (twice ABR) | PCR on urine | 1 positive out of 12 failing NHS, 1 positive screened because of abnormal CNS findings, 1 positive screened for suspected maternal infection during pregnancy | n.a. | 1 | 2 treated with valgancyclovir | n.a. | No clinical outcome. |
3.4. Vaccinations
| Vaccine | Author, Year or Trial ID Number | Study Design | Population | Outcome | Enrolment Time | Results |
|---|---|---|---|---|---|---|
| bB-MF59: MF59 adjuvated recombinant CMV envelope glicoproteinB subunit | Pass, 2009 [74] | Phase 2, placebo-controlled, randomized, double-blind trial. | Post-partum, seronegative women, aged 14–40 years and healthy. | Effectiveness in preventing CMV infection during a 42-month period | August 1999 to April 2006 | 464 subject enrolled. Vaccine recipients were more likely to remain uninfected than placebo recipients (p = 0.02). |
| bB-MF59: MF59 adjuvated recombinant CMV envelope glicoproteinB subunit | Bernstein, 2017 [75] | Phase 2, multicenter, randomized, double-blind, controlled study. | Healthy adolescent females. | Effectiveness in preventing CMV infection, immunogenicity, safety. | June 2006–June 2013 | 402 subjects enrolled. CMV infection occurred without significant differences between vaccinated and control individuals. |
| V160: whole-virus vaccine that is derived from the live-attenuated AD169 strain | Das, 2023 [76] | Phase 2b, multicenter, randomized, double-blind, placebo-controlled study. | Healthy, CMV-seronegative, non-pregnant, 16–35-year-old women of childbearing potential with exposure to children aged 5 years or younger. | Efficacy of three doses of V160 in reducing the incidence of primary CMV infection during the follow-up period starting 30 days after the last dose of vaccine; vaccine safety. | April 2018–August 2019 | 2220 enrolled. The vaccine efficacy for the V160 three-dose group was 44.6% (95% CI −15.2 to 74.8) at the final testing of the primary efficacy hypothesis, a result corresponding to failure to demonstrate the primary efficacy hypothesis. The study was terminated due to futility. |
| mRNA-1647 | NCT04232280 [78] | Phase 2, randomized, observer-blind, placebo-controlled, dose-finding trial. Part 1: to inform the selection of the middle dose level for further development. Part 2: to further evaluate the safety and immunogenicity of the middle dose level of mRNA-1647 vaccine or placebo. | Healthy participants seropositive or seronegative, males or females, 18 to 40 years of age. | Safety, immunogenicity (NAb titers) | September 2020–April 2023 | 315 subjects enrolled. No results reported. |
| mRNA-1647 | Ongoing trial NCT05085366 [79] | Phase 3, randomized, observer-blind, placebo-controlled study. | Participants aged ≥20 years, has or anticipates having direct exposure within 7 months after the planned first dose (in the home, socially, or occupationally) to at least 1 child ≤5 years of age. Enrollment estimated 6900 subjects. | Efficacy (seroconversion from a negative to a positive result) in females and in all participants. Safety. | October 2021–April 2024 | Enrolled 7454 patients, no results reported. |
4. Final Considerations and Future Prospectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dollard, S.C.; Grosse, S.D.; Ross, D.S. New Estimates of the Prevalence of Neurological and Sensory Sequelae and Mortality Associated with Congenital Cytomegalovirus Infection. Rev. Med. Virol. 2007, 17, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Faure-bardon, V.; Magny, J.; Parodi, M.; Couderc, S.; Garcia, P.; Maillotte, A.; Benard, M. Sequelae of Congenital Cytomegalovirus Following Maternal Primary Infections Are Limited to Those Acquired in the First Trimester of Pregnancy. Clin. Infect. Dis. 2019, 69, 1526–1532. [Google Scholar] [CrossRef] [PubMed]
- Gindes, L.; Teperberg-Oikawa, M.; Sherman, D.; Pardo, J.; Rahav, G. Congenital Cytomegalovirus Infection Following Primary Maternal Infection in the Third Trimester. BJOG Int. J. Obstet. Gynaecol. 2008, 115, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Enders, G.; Daiminger, A.; Bäder, U.; Exler, S.; Enders, M. Intrauterine Transmission and Clinical Outcome of 248 Pregnancies with Primary Cytomegalovirus Infection in Relation to Gestational Age. J. Clin. Virol. 2011, 52, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Riga, M.; Korres, G.; Chouridis, P.; Naxakis, S.; Danielides, V. Congenital Cytomegalovirus Infection Inducing Non-Congenital Sensorineural Hearing Loss during Childhood; a Systematic Review. Int. J. Pediatr. Otorhinolaryngol. 2018, 115, 156–164. [Google Scholar] [CrossRef]
- Nigro, G.; Scholz, H.; Bartmann, U. Ganciclovir Therapy for Symptomatic Congenital Cytomegalovirus Infection in Infants: A Two-Regimen Experience. J. Pediatr. 1994, 124, 318–322. [Google Scholar] [CrossRef]
- Kimberlin, D.W.; Jester, P.M.; Sánchez, P.J.; Ahmed, A.; Arav-Boger, R.; Michaels, M.G.; Ashouri, N.; Englund, J.A.; Estrada, B.; Jacobs, R.F.; et al. Valganciclovir for Symptomatic Congenital Cytomegalovirus Disease. N. Engl. J. Med. 2015, 372, 933–943. [Google Scholar] [CrossRef]
- Whitley, R.J.; Cloud, G.; Gruber, W.; Storch, G.A.; Demmler, G.J.; Jacobs, R.F.; Dankner, W.; Spector, S.A.; Starr, S.; Pass, R.F.; et al. Ganciclovir Treatment of Symptomatic Congenital Cytomegalovirus Infection: Results of a Phase II Study. National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. J. Infect. Dis. 1997, 175, 1080–1086. [Google Scholar] [CrossRef]
- Oliver, S.E.; Cloud, G.A.; Sánchez, P.J.; Demmler, G.J.; Dankner, W.; Shelton, M.; Jacobs, R.F.; Vaudry, W.; Pass, R.F.; Soong, S.J.; et al. Neurodevelopmental Outcomes Following Ganciclovir Therapy in Symptomatic Congenital Cytomegalovirus Infections Involving the Central Nervous System. J. Clin. Virol. 2009, 46, 22–26. [Google Scholar] [CrossRef]
- Rawlinson, W.D.; Boppana, S.B.; Fowler, K.B.; Kimberlin, D.W.; Lazzarotto, T.; Alain, S.; Daly, K.; Doutré, S.; Gibson, L.; Giles, M.L.; et al. Congenital Cytomegalovirus Infection in Pregnancy and the Neonate: Consensus Recommendations for Prevention, Diagnosis, and Therapy. Lancet Infect. Dis. 2017, 17, e177–e188. [Google Scholar] [CrossRef]
- Luck, S.E.; Wieringa, J.W.; Blázquez-Gamero, D.; Henneke, P.; Schuster, K.; Butler, K.; Capretti, M.G.; Cilleruelo, M.J.; Curtis, N.; Garofoli, F.; et al. Congenital Cytomegalovirus a European Expert Consensus Statement on Diagnosis and Management. Pediatr. Infect. Dis. J. 2017, 36, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Dorfman, L.; Amir, J.; Attias, J.; Bilavsky, E. Treatment of Congenital Cytomegalovirus beyond the Neonatal Period: An Observational Study. Eur. J. Pediatr. 2020, 179, 807–812. [Google Scholar] [CrossRef]
- Amir, J.; Attias, J.; Pardo, J. Treatment of Late-Onset Hearing Loss in Infants with Congenital Cytomegalovirus Infection. Clin. Pediatr. 2014, 53, 444–448. [Google Scholar] [CrossRef]
- Randomized Controlled Trial of Valganciclovir for Cytomegalovirus Infected Hearing Impaired Infants—Full Text View—ClinicalTrials.Gov. Available online: https://classic.clinicaltrials.gov/ct2/show/study/NCT03107871 (accessed on 27 November 2023).
- Study Details|Valganciclovir Therapy in Infants and Children With Congenital CMV Infection and Hearing Loss|ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/study/NCT01649869 (accessed on 16 November 2023).
- Chatzakis, C.; Shahar-Nissan, K.; Faure-Bardon, V.; Picone, O.; Hadar, E.; Amir, J.; Egloff, C.; Vivanti, A.; Sotiriadis, A.; Leruez-Ville, M.; et al. The Effect of Valacyclovir on Secondary Prevention of Congenital Cytomegalovirus Infection, Following Primary Maternal Infection Acquired Periconceptionally or in the First Trimester of Pregnancy. An Individual Patient Data Meta-Analysis. Am. J. Obstet. Gynecol. 2023, 230, 109–117.e2. [Google Scholar] [CrossRef] [PubMed]
- Nigro, G.; Muselli, M. Prevention of Congenital Cytomegalovirus Infection: Review and Case Series of Valaciclovir versus Hyperimmune Globulin Therapy. Viruses 2023, 15, 1376. [Google Scholar] [CrossRef]
- MNDOH Public Health Interventions (Population-Based). Minn. Dep. Health 2019, 2, 453–468.
- Demmler-Harrison, G.J. Congenital Cytomegalovirus: Public Health Action towards Awareness, Prevention, and Treatment. J. Clin. Virol. 2009, 46 (Suppl. S4), S1–S5. [Google Scholar] [CrossRef] [PubMed]
- Revello, M.G.; Tibaldi, C.; Masuelli, G.; Frisina, V.; Sacchi, A.; Furione, M.; Arossa, A.; Spinillo, A.; Klersy, C.; Ceccarelli, M.; et al. Prevention of Primary Cytomegalovirus Infection in Pregnancy. eBioMedicine 2015, 2, 1205–1210. [Google Scholar] [CrossRef]
- Adler, S.P.; Finney, J.W.; Manganello, A.M.; Best, A.M. Prevention of Child-to-Mother Transmission of Cytomegalovirus among Pregnant Women. J. Pediatr. 2004, 145, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Vauloup-Fellous, C.; Picone, O.; Cordier, A.G.; Parent-du-Châtelet, I.; Senat, M.V.; Frydman, R.; Grangeot-Keros, L. Does Hygiene Counseling Have an Impact on the Rate of CMV Primary Infection during Pregnancy? Results of a 3-Year Prospective Study in a French Hospital. J. Clin. Virol. 2009, 46 (Suppl. S4), S49–S53. [Google Scholar] [CrossRef]
- Knowledge and Practices of Obstetricians and Gynecologists Regarding Cytomegalovirus Infection During Pregnancy—United States. 2007. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5703a2.htm (accessed on 25 November 2023).
- Barber, V.; Calvert, A.; Vandrevala, T.; Star, C.; Khalil, A.; Griffiths, P.; Heath, P.T.; Jones, C.E. Prevention of Acquisition of Cytomegalovirus Infection in Pregnancy through Hygiene-Based Behavioral Interventions: A Systematic Review and Gap Analysis. Pediatr. Infect. Dis. J. 2020, 39, 949–954. [Google Scholar] [CrossRef] [PubMed]
- NICE Guideline, Antenatal Care. 2021. Available online: www.nice.org.uk/guidance/ng201 (accessed on 27 November 2023).
- Resources for Pregnant Women and Parents|CDC. Available online: https://www.cdc.gov/cmv/resources/pregnant-women-parents.html (accessed on 27 November 2023).
- CMV Action—What Is CMV? Available online: https://cmvaction.org.uk/ (accessed on 27 November 2023).
- Périllaud-Dubois, C.; Belhadi, D.; Laouénan, C.; Mandelbrot, L.; Picone, O.; Vauloup-Fellous, C. Current Practices of Management of Maternal and Congenital Cytomegalovirus Infection during Pregnancy after a Maternal Primary Infection Occurring in First Trimester of Pregnancy: Systematic Review. PLOS ONE 2021, 16, e0261011. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Tripathi, T.; Holmes, N.E.; Hui, L. Serological Screening for Cytomegalovirus during Pregnancy: A Systematic Review of Clinical Practice Guidelines and Consensus Statements. Prenat. Diagn. 2023, 43, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Leruez-Ville, M.; Chatzakis, C.; Lilleri, D.; Blazquez-Gamero, D.; Alarcon, A.; Bourgon, N.; Foulon, I.; Fourgeaud, J.; Gonce, A.; Jones, C.E.; et al. Consensus Recommendation for Prenatal, Neonatal and Postnatal Management of Congenital Cytomegalovirus Infection from the European Congenital Infection Initiative (ECCI). Lancet Reg. Health-Eur. 2024, 40, 100892. [Google Scholar] [CrossRef] [PubMed]
- Stagno, S.; Pass, R.F.; Dworsky, M.E.; Henderson, R.E.; Moore, E.G.; Walton, P.D.; Alford, C.A. Congenital Cytomegalovirus Infection. N. Engl. J. Med. 1982, 306, 945–949. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, X.; Bialek, S.; Cannon, M.J. Attribution of Congenital Cytomegalovirus Infection to Primary versus Non-Primary Maternal Infection. Clin. Infect. Dis. 2011, 52, 11–14. [Google Scholar] [CrossRef]
- Davis, N.L.; King, C.C.; Kourtis, A.P. Cytomegalovirus Infection in Pregnancy. Birth Defects Res. 2017, 109, 336–346. [Google Scholar] [CrossRef]
- Yamamoto, A.Y.; Mussi-Pinhata, M.M.; Isaac, M.D.L.; Amaral, F.R.; Carvalheiro, C.G.; Aragon, D.C.; Da Silva Manfredi, A.K.; Boppana, S.B.; Britt, W.J. Congenital Cytomegalovirus Infection as a Cause of Sensorineural Hearing Loss in a Highly Immune Population. Pediatr. Infect. Dis. J. 2011, 30, 1043–1046. [Google Scholar] [CrossRef]
- Revello, M.G.; Lazzarotto, T.; Guerra, B.; Spinillo, A.; Ferrazzi, E.; Kustermann, A.; Guaschino, S.; Vergani, P.; Todros, T.; Frusca, T.; et al. A Randomized Trial of Hyperimmune Globulin to Prevent Congenital Cytomegalovirus. N. Engl. J. Med. 2014, 370, 1316–1326. [Google Scholar] [CrossRef]
- Hughes, B.L.; Clifton, R.G.; Rouse, D.J.; Saade, G.R.; Dinsmoor, M.J.; Reddy, U.M.; Pass, R.; Allard, D.; Mallett, G.; Fette, L.M.; et al. A Trial of Hyperimmune Globulin to Prevent Congenital Cytomegalovirus Infection. N. Engl. J. Med. 2021, 385, 436–444. [Google Scholar] [CrossRef]
- Visentin, S.; Manara, R.; Milanese, L.; Da Roit, A.; Forner, G.; Salviato, E.; Citton, V.; Magno, F.M.; Orzan, E.; Morando, C.; et al. Early Primary Cytomegalovirus Infection in Pregnancy: Maternal Hyperimmunoglobulin Therapy Improves Outcomes among Infants at 1 Year of Age. Clin. Infect. Dis. 2012, 55, 497–503. [Google Scholar] [CrossRef]
- Adler, S.P. Primary Maternal Cytomegalovirus Infection during Pregnancy: Do We Have a Treatment Option? Clin. Infect. Dis. 2012, 55, 504–506. [Google Scholar] [CrossRef]
- Nigro, G.; Adler, S.P.; La Torre, R.; Best, A.M. Passive Immunization during Pregnancy for Congenital Cytomegalovirus Infection. N. Engl. J. Med. 2005, 353, 1350–1362. [Google Scholar] [CrossRef]
- Nigro, G.; Adler, S.P.; Lasorella, S.; Iapadre, G.; Maresca, M.; Mareri, A.; Di Paolantonio, C.; Catenaro, M.; Tambucci, R.; Mattei, I.; et al. High-Dose Cytomegalovirus (CMV) Hyperimmune Globulin and Maternal CMV DNAemia Independently Predict Infant Outcome in Pregnant Women with a Primary CMV Infection. Clin. Infect. Dis. 2020, 71, 1491–1498. [Google Scholar] [CrossRef]
- Shahar-Nissan, K.; Pardo, J.; Peled, O.; Krause, I.; Bilavsky, E.; Wiznitzer, A.; Hadar, E.; Amir, J. Valaciclovir to Prevent Vertical Transmission of Cytomegalovirus after Maternal Primary Infection during Pregnancy: A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2020, 396, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Faure-Bardon, V.; Fourgeaud, J.; Stirnemann, J.; Leruez-Ville, M.; Ville, Y. Secondary Prevention of Congenital Cytomegalovirus Infection with Valacyclovir Following Maternal Primary Infection in Early Pregnancy. Ultrasound Obstet. Gynecol. 2021, 58, 576–581. [Google Scholar] [CrossRef]
- Egloff, C.; Sibiude, J.; Vauloup-Fellous, C.; Benachi, A.; Bouthry, E.; Biquard, F.; Hawkins-Villarreal, A.; Houhou-Fidouh, N.; Mandelbrot, L.; Vivanti, A.J.; et al. New Data on Efficacy of Valacyclovir in Secondary Prevention of Maternal–Fetal Transmission of Cytomegalovirus. Ultrasound Obstet. Gynecol. 2023, 61, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.T.; Marschall, M.; Rawlinson, W.D. Investigational Antiviral Therapy Models for the Prevention and Treatment of Congenital Cytomegalovirus Infection during Pregnancy. Antimicrob. Agents Chemother. 2021, 65, e01627-20. [Google Scholar] [CrossRef]
- Wroblewska-Seniuk, K.E.; Dabrowski, P.; Szyfter, W.; Mazela, J. Universal Newborn Hearing Screening: Methods and Results, Obstacles, and Benefits. Pediatr. Res. 2017, 81, 415–422. [Google Scholar] [CrossRef]
- Kennedy, C.R.; McCann, D.C.; Campbell, M.J.; Law, C.M.; Mullee, M.; Petrou, S.; Watkin, P.; Worsfold, S.; Yuen, H.M.; Stevenson, J. Language Ability after Early Detection of Permanent Childhood Hearing Impairment. N. Engl. J. Med. 2006, 354, 2131–2141. [Google Scholar] [CrossRef] [PubMed]
- Fowler, K.B.; Mccollister, F.P.; Sabo, D.L.; Shoup, A.G.; Owen, K.E.; Woodruff, J.L.; Cox, E.; Mohamed, L.S.; Choo, D.I.; Boppana, S.B. A Targeted Approach for Congenital Cytomegalovirus Screening Within Newborn Hearing Screening. Pediatrics 2017, 139. [Google Scholar] [CrossRef]
- Lorenzoni, F.; Lunardi, S.; Liumbruno, A.; Ferri, G.; Madrigali, V.; Fiorentini, E.; Forli, F.; Berrettini, S.; Boldrini, A.; Ghirri, P. Neonatal Screening for Congenital Cytomegalovirus Infection in Preterm and Small for Gestational Age Infants. J. Matern. Neonatal Med. 2014, 27, 1589–1593. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, Y.; Reich, D.; Eidelman, A.I.; Schimmel, M.S.; Hassanin, J.; Miron, D. Congenital Cytomegalovirus Infection in Israel: Screening in Different Subpopulations. Isr. Med. Assoc. J. 2005, 7, 237–240. [Google Scholar] [PubMed]
- Barkai, G.; Ari-Even Roth, D.; Barzilai, A.; Tepperberg-Oikawa, M.; Mendelson, E.; Hildesheimer, M.; Kuint, J. Universal Neonatal Cytomegalovirus Screening Using Saliva—Report of Clinical Experience. J. Clin. Virol. 2014, 60, 361–366. [Google Scholar] [CrossRef]
- Dar, L.; Namdeo, D.; Kumar, P.; Thakar, A.; Kant, S.; Rai, S.; Singh, P.K.; Kabra, M.; Fowler, K.B.; Boppana, S.B. Congenital Cytomegalovirus Infection and Permanent Hearing Loss in Rural North Indian Children. Pediatr. Infect. Dis. J. 2017, 36, 670. [Google Scholar] [CrossRef]
- Yamamoto, A.Y.; Anastasio, A.R.T.; Massuda, E.T.; Isaac, M.L.; Manfredi, A.K.S.; Cavalcante, J.M.S.; Carnevale-Silva, A.; Fowler, K.B.; Boppana, S.B.; Britt, W.J.; et al. Contribution of Congenital Cytomegalovirus Infection to Permanent Hearing Loss in a Highly Seropositive Population: The Brazilian Cytomegalovirus Hearing and Maternal Secondary Infection Study. Clin. Infect. Dis. 2020, 70, 1379–1384. [Google Scholar] [CrossRef]
- Yamada, H.; Tanimura, K.; Fukushima, S.; Fujioka, K.; Deguchi, M.; Sasagawa, Y.; Tairaku, S.; Funakoshi, T.; Morioka, I. A Cohort Study of the Universal Neonatal Urine Screening for Congenital Cytomegalovirus Infection. J. Infect. Chemother. 2020, 26, 790–794. [Google Scholar] [CrossRef] [PubMed]
- Blázquez-Gamero, D.; Soriano-Ramos, M.; Vicente, M.; Pallás-Alonso, C.R.; Pérez-Rivilla, A.; García-Álvarez, M.; Pinilla Martín, M.T.; Freire, X.; De Vergas, J.; De Aragón, A.M.; et al. Prevalence and Clinical Manifestations of Congenital Cytomegalovirus Infection in a Screening Program in Madrid (PICCSA Study). Pediatr. Infect. Dis. J. 2020, 39, 1050–1056. [Google Scholar] [CrossRef]
- Letamendia-Richard, E.; Périllaud-Dubois, C.; de La Guillonnière, L.; Thouard, I.; Cordier, A.G.; Roque-Afonso, A.M.; de Luca, D.; Benachi, A.; Vauloup-Fellous, C. Universal Newborn Screening for Congenital Cytomegalovirus Infection: Feasibility and Relevance in a French Type-III Maternity Cohort. BJOG 2022, 129, 291–299. [Google Scholar] [CrossRef]
- Chiereghin, A.; Pavia, C.; Turello, G.; Borgatti, E.C.; Baiesi Pillastrini, F.; Gabrielli, L.; Gibertoni, D.; Marsico, C.; De Paschale, M.; Manco, M.T.; et al. Universal Newborn Screening for Congenital Cytomegalovirus Infection – From Infant to Maternal Infection: A Prospective Multicenter Study. Front. Pediatr. 2022, 10, 1–11. [Google Scholar] [CrossRef]
- Ronner, E.A.; Glovsky, C.K.; Herrmann, B.S.; Woythaler, M.A.; Pasternack, M.S.; Cohen, M.S. Congenital Cytomegalovirus Targeted Screening Implementation and Outcomes: A Retrospective Chart Review. Otolaryngol. Neck Surg. 2022, 167, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Diener, M.L.; Zick, C.D.; McVicar, S.B.; Boettger, J.; Park, A.H. Outcomes from a Hearing-Targeted Cytomegalovirus Screening Program. Pediatrics 2017, 139. [Google Scholar] [CrossRef] [PubMed]
- Rawlinson, W.D.; Palasanthiran, P.; Hall, B.; Al Yazidi, L.; Cannon, M.J.; Cottier, C.; van Zuylen, W.J.; Wilkinson, M. Neonates with Congenital Cytomegalovirus and Hearing Loss Identified via the Universal Newborn Hearing Screening Program. J. Clin. Virol. 2018, 102, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Stehel, E.K.; Shoup, A.G.; Owen, K.E.; Jackson, G.L.; Sendelbach, D.M.; Boney, L.F.; Sánchez, P.J. Newborn Hearing Screening and Detection of Congenital Cytomegalovirus Infection. Pediatrics 2008, 121, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.J.; Kadambari, S.; Berrington, J.E.; Luck, S.; Atkinson, C.; Walter, S.; Embleton, N.D.; James, P.; Griffiths, P.; Davis, A.; et al. Feasibility and Acceptability of Targeted Screening for Congenital CMV-Related Hearing Loss. Arch. Dis. Child. Fetal Neonatal Ed. 2014, 99, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Kawada, J.; Torii, Y.; Kawano, Y.; Suzuki, M.; Kamiya, Y.; Kotani, T.; Kikkawa, F.; Kimura, H.; Ito, Y. Viral Load in Children with Congenital Cytomegalovirus Infection Identified on Newborn Hearing Screening. J. Clin. Virol. 2015, 65, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Roth, D.A.E.; Lubin, D.; Kuint, J.; Teperberg-Oikawa, M.; Mendelson, E.; Strauss, T.; Barkai, G. Contribution of Targeted Saliva Screening for Congenital CMV-Related Hearing Loss in Newborns Who Fail Hearing Screening. Arch. Dis. Child. Fetal Neonatal Ed. 2017, 102, F519–F524. [Google Scholar] [CrossRef] [PubMed]
- Beswick, R.; David, M.; Higashi, H.; Thomas, D.; Nourse, C.; Koh, G.; Koorts, P.; Jardine, L.A.; Clark, J.E. Integration of Congenital Cytomegalovirus Screening within a Newborn Hearing Screening Programme. J. Paediatr. Child Health 2019, 55, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Pellegrinelli, L.; Galli, C.; Primache, V.; Alde, M.; Fagnani, E.; Di Berardino, F.; Zanetti, D.; Pariani, E.; Ambrosetti, U.; Binda, S. Diagnosis of Congenital CMV Infection via DBS Samples Testing and Neonatal Hearing Screening: An Observational Study in Italy. BMC Infect. Dis. 2019, 19, 652. [Google Scholar] [CrossRef] [PubMed]
- Chung, P.K.; Schornagel, F.; Oudesluys-Murphy, A.M.; De Vries, L.S.; Soede, W.; Van Zwet, E.; Vossen, A. Targeted Screening for Congenital Cytomegalovirus Infection: Clinical, Audiological and Neuroimaging Findings. Arch. Dis. Child. Fetal Neonatal Ed. 2023, 108, F302–F308. [Google Scholar] [CrossRef]
- Fourgeaud, J.; Boithias, C.; Walter-Nicolet, E.; Kermorvant, E.; Couderc, S.; Parat, S.; Pol, C.; Mousset, C.; Bussières, L.; Guilleminot, T.; et al. Performance of Targeted Congenital Cytomegalovirus Screening in Newborns Failing Universal Hearing Screening: A Multicenter Study. J. Bone Jt. Surg. 2022, 41, 478–481. [Google Scholar] [CrossRef]
- Webb, E.; Gillespie, A.N.; Poulakis, Z.; Gartland, T.; Buttery, J.; Casalaz, D.; Daley, A.J.; Donath, S.; Gwee, A.; Jacobs, S.E.; et al. Feasibility and Acceptability of Targeted Salivary Cytomegalovirus Screening through Universal Newborn Hearing Screening. J. Paediatr. Child Health 2022, 58, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Egashira, T.; Egashira, M.; Ogiwara, S.; Tomino, H.; Shichijo, A.; Mizukami, T.; Ogata, T.; Moriuchi, H.; Takayanagi, T.; et al. Expanded Targeted Screening for Congenital Cytomegalovirus Infection. Congenit. Anom. 2023, 63, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Carr, W.; Reingold, A.; Hunter, P.; Lee, G.; Temte, J.; Campos-Outcalt, D.; Rubin, L.; O’Leary, S.; Savoy, M.; et al. Updated Framework for Development of Evidence-Based Recommendations by the Advisory Committee on Immunization Practices. MMWR. Morb. Mortal. Wkly. Rep. 2018, 67, 1271–1272. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, P.D. Burden of Disease Associated with Human Cytomegalovirus and Prospects for Elimination by Universal Immunisation. Lancet Infect. Dis. 2012, 12, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.A.; Wang, D.; Oualim, A.; Diamond, D.J.; Kotton, C.N.; Mossman, S.; Carfi, A.; Anderson, D.; Dormitzer, P.R. The Status of Vaccine Development Against the Human Cytomegalovirus. J. Infect. Dis. 2020, 221, S113–S122. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.R.; Bialek, S.R.; Boppana, S.B.; Griffiths, P.D.; Laughlin, C.A.; Ljungman, P.; Mocarski, E.S.; Pass, R.F.; Read, J.S.; Schleiss, M.R.; et al. Priorities for CMV Vaccine Development. Vaccine 2013, 32, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Pass, R.F.; Zhang, C.; Evans, A.; Simpson, T.; Andrews, W.; Huang, M.-L.; Corey, L.; Hill, J.; Davis, E.; Flanigan, C.; et al. Vaccine Prevention of Maternal Cytomegalovirus Infection. N. Engl. J. Med. 2009, 360, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.I.; Munoz, F.M.; Callahan, S.T.; Rupp, R.; Wootton, S.H.; Edwards, K.M.; Turley, C.B.; Stanberry, L.R.; Patel, S.M.; Mcneal, M.M.; et al. Safety and Efficacy of a Cytomegalovirus Glycoprotein B (GB) Vaccine in Adolescent Girls: A Randomized Clinical Trial. Vaccine 2016, 34, 313. [Google Scholar] [CrossRef]
- Das, R.; Blázquez-Gamero, D.; Bernstein, D.I.; Gantt, S.; Bautista, O.; Beck, K.; Conlon, A.; Rosenbloom, D.I.S.; Wang, D.; Ritter, M.; et al. Safety, Efficacy, and Immunogenicity of a Replication-Defective Human Cytomegalovirus Vaccine, V160, in Cytomegalovirus-Seronegative Women: A Double-Blind, Randomised, Placebo-Controlled, Phase 2b Trial. Lancet Infect. Dis. 2023, 23, 1383–1394. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Karthigeyan, K.P.; Herbek, S.; Valencia, S.M.; Jenks, J.A.; Webster, H.; Miller, I.G.; Connors, M.; Pollara, J.; Andy, C.; et al. Human Cytomegalovirus MRNA-1647 Vaccine Candidate Elicits Potent and Broad Neutralization and Higher Antibody-Dependent Cellular Cytotoxicity Responses Than the GB/MF59 Vaccine. J. Infect. Dis. 2024, 230, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Study Details|Dose-Finding Trial to Evaluate the Safety and Immunogenicity of Cytomegalovirus (CMV) Vaccine MRNA-1647 in Healthy Adults |ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/study/NCT04232280?term=NCT04232280&rank=1 (accessed on 27 November 2023).
- Study Details|A Study to Evaluate the Efficacy, Safety, and Immunogenicity of MRNA-1647 Cytomegalovirus (CMV) Vaccine in Healthy Participants 16 to 40 Years of Age |ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/study/NCT05085366?term=NCT05085366&rank=1 (accessed on 27 November 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liberati, C.; Sturniolo, G.; Brigadoi, G.; Cavinato, S.; Visentin, S.; Cosmi, E.; Donà, D.; Rampon, O. Burden of Congenital CMV Infection: A Narrative Review and Implications for Public Health Interventions. Viruses 2024, 16, 1311. https://doi.org/10.3390/v16081311
Liberati C, Sturniolo G, Brigadoi G, Cavinato S, Visentin S, Cosmi E, Donà D, Rampon O. Burden of Congenital CMV Infection: A Narrative Review and Implications for Public Health Interventions. Viruses. 2024; 16(8):1311. https://doi.org/10.3390/v16081311
Chicago/Turabian StyleLiberati, Cecilia, Giulia Sturniolo, Giulia Brigadoi, Silvia Cavinato, Silvia Visentin, Erich Cosmi, Daniele Donà, and Osvalda Rampon. 2024. "Burden of Congenital CMV Infection: A Narrative Review and Implications for Public Health Interventions" Viruses 16, no. 8: 1311. https://doi.org/10.3390/v16081311
APA StyleLiberati, C., Sturniolo, G., Brigadoi, G., Cavinato, S., Visentin, S., Cosmi, E., Donà, D., & Rampon, O. (2024). Burden of Congenital CMV Infection: A Narrative Review and Implications for Public Health Interventions. Viruses, 16(8), 1311. https://doi.org/10.3390/v16081311






