Prevalence of Respiratory Viral Infections in Deceased Persons during the COVID-19 Pandemic Season 2021–2022: A Population-Based Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Epidemiological and Virological Surveillance of Resporatory-Viral-Infection-Related Deaths
2.3. Post-Mortem Recruitment and Testing
2.4. Other Sources of Information and Variables
2.5. Statistical Analysis
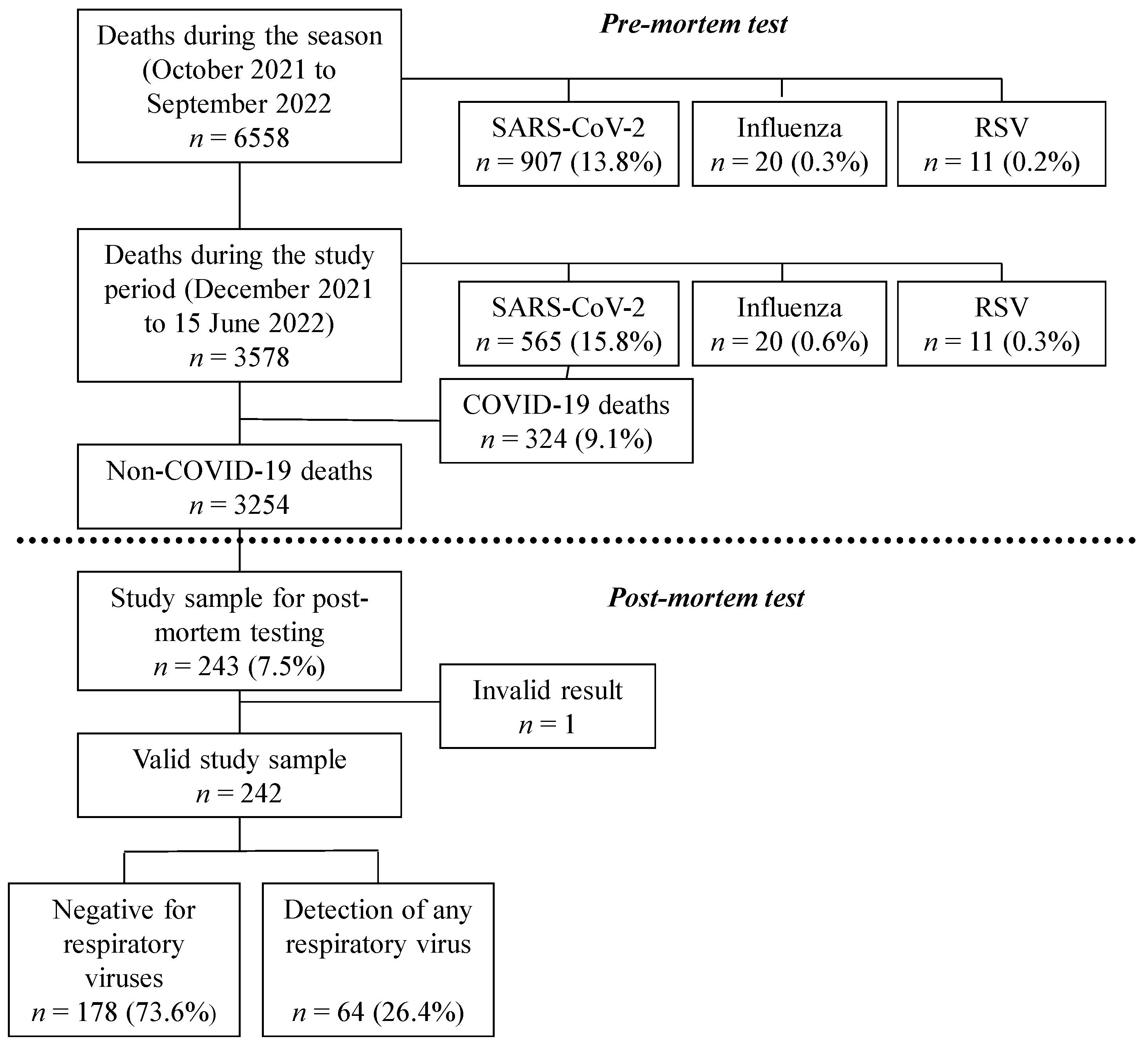
3. Results
3.1. Description of the Study Population
3.2. Prevalence of Respiratory Virus Infection from Post-Mortem Tests
3.3. Influenza and SARS-CoV-2 Characterisation
3.4. Co-Infections with More Than One Respiratory Virus
3.5. Prevalence Estimates of Respiratory Viral Infections in Deceased Persons of the Population
3.6. Characteristics of Deceased Persons with a Respitatory Viral Infection
3.7. Comparison of the Results of Clinical and Post-Mortem Detections of Respiratory Viral Infections
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, W.W.; Shay, D.K.; Weintraub, E.; Brammer, L.; Cox, N.; Anderson, L.J.; Fukuda, K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003, 289, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.L.; Chaves, S.S.; Demont, C.; Viboud, C. Mortality Associated with Influenza and Respiratory Syncytial Virus in the US, 1999–2018. JAMA Netw. Open 2022, 5, e220527. [Google Scholar] [CrossRef] [PubMed]
- Ackerson, B.; Tseng, H.F.; Sy, L.S.; Solano, Z.; Slezak, J.; Luo, Y.; Fischetti, C.A.; Shinde, V. Severe Morbidity and Mortality Associated with Respiratory Syncytial Virus Versus Influenza Infection in Hospitalized Older Adults. Clin. Infect. Dis. 2019, 69, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Gilca, R.; Amini, R.; Douville-Fradet, M.; Charest, H.; Dubuque, J.; Boulianne, N.; Skowronski, D.M.; De Serres, G. Other respiratory viruses are important contributors to adult respiratory hospitalizations and mortality even during peak weeks of the influenza season. Open Forum. Infect. Dis. 2014, 1, ofu086. [Google Scholar] [CrossRef]
- Khasawneh, A.I.; Himsawi, N.M.; Abu-Raideh, J.A.; Sammour, A.; Abu Safieh, H.; Obeidat, A.; Azab, M.; Tarifi, A.A.; Al Khawaldeh, A.; Al-Momani, H.; et al. Prevalence of SARS-COV-2 and other respiratory pathogens among a Jordanian subpopulation during Delta-to-Omicron transition: Winter 2021/2022. PLoS ONE 2023, 18, e0283804. [Google Scholar] [CrossRef] [PubMed]
- van Asten, L.; van den Wijngaard, C.; van Pelt, W.; van de Kassteele, J.; Meijer, A.; van der Hoek, W.; Kretzschmar, M.; Koopmans, M. Mortality attributable to 9 common infections: Significant effect of influenza A, respiratory syncytial virus, influenza B, norovirus, and parainfluenza in elderly persons. J. Infect. Dis. 2012, 206, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Almeida, T.; Guimarães, J.T.; Rebelo, S. Epidemiological Changes in Respiratory Viral Infections in Children: The Influence of the COVID-19 Pandemic. Viruses 2023, 15, 1880. [Google Scholar] [CrossRef] [PubMed]
- Kurskaya, O.G.; Prokopyeva, E.A.; Sobolev, I.A.; Solomatina, M.V.; Saroyan, T.A.; Dubovitskiy, N.A.; Derko, A.A.; Nokhova, A.R.; Anoshina, A.V.; Leonova, N.V.; et al. Changes in the Etiology of Acute Respiratory Infections among Children in Novosibirsk, Russia, between 2019 and 2022: The Impact of the SARS-CoV-2 Virus. Viruses 2023, 15, 934. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Implications of the Spread of the SARS-CoV-2 B.1.1.529 Variant of Concern (Omicron) for the EU/EEA—First Update. 2 December 2021; ECDC: Stockholm, Sweden, 2021; Available online: https://www.ecdc.europa.eu/sites/default/files/documents/threat-assessment-covid-19-emergence-sars-cov-2-variant-omicron-december-2021.pdf (accessed on 25 March 2024).
- Stefanelli, P.; Trentini, F.; Petrone, D.; Mammone, A.; Ambrosio, L.; Manica, M.; Guzzetta, G.; d’Andrea, V.; Marziano, V.; Zardini, A.; et al. Genomic SARS–CoV–2 National Surveillance Working Group; Italian Integrated Surveillance of COVID–19 Study Group; Italian Integrated Surveillance of COVID-19 Study Group. Tracking the progressive spread of the SARS-CoV-2 Omicron variant in Italy, December 2021 to January 2022. Eurosurveillance 2022, 27, 2200125. [Google Scholar] [CrossRef]
- Casado, I.; García Cenoz, M.; Egüés, N.; Burgui, C.; Martínez-Baz, I.; Castilla, J. COVID-19 infections, hospitalizations, and mortality in Navarre (Spain) between February 2020 and September 2022. An. del Sist. Sanit. Navar. 2023, 46, e1044. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Seasonal influenza 2021–2022. In ECDC Annual Epidemiological Report for 2021; ECDC: Stockholm, Sweden, 2022; Available online: https://www.ecdc.europa.eu/sites/default/files/documents/seasonal-influenza-annual-epidemiological-report-2020-2021.pdf (accessed on 25 March 2024).
- Milano, G.; Capitani, E.; Camarri, A.; Bova, G.; Capecchi, P.L.; Lazzeri, G.; Lipari, D.; Montomoli, E.; Manini, I. Surveillance of Influenza and Other Airborne Transmission Viruses during the 2021/2022 Season in Hospitalized Subjects in Tuscany, Italy. Vaccines 2023, 11, 776. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Baz, I.; Trobajo-Sanmartín, C.; Miqueleiz, A.; Casado, I.; Navascués, A.; Burgui, C.; Ezpeleta, C.; Castilla, J.; Guevara, M.; Working Group for the Study of COVID-19 in Navarra. Risk reduction of hospitalisation and severe disease in vaccinated COVID-19 cases during the SARS-CoV-2 variant Omicron BA.1-predominant period, Navarre, Spain, January to March 2022. Eurosurveillance 2023, 28, 2200337. [Google Scholar] [CrossRef] [PubMed]
- Trobajo-Sanmartín, C.; Miqueleiz, A.; Guevara, M.; Fernández-Huerta, M.; Burgui, C.; Casado, I.; Baigorria, F.; Navascués, A.; Ezpeleta, C.; Castilla, J. Comparison of the Risk of Hospitalization and Severe Disease Among Co-circulating Severe Acute Respiratory Syndrome Coronavirus 2 Variants. J. Infect. Dis. 2023, 227, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Boletín Informativo de Salud Pública. Temporada de Gripe 2021–2022 en Navarra. Instituto de Salud Pública y Laboral de Navarra. Número 121, Septiembre 2022. Available online: http://www.navarra.es/NR/rdonlyres/AECCD760-AB2A-4841-818A-FA53478FD6DC/482512/BOL12125.pdf (accessed on 25 March 2024).
- Martínez-Baz, I.; Casado, I.; Miqueleiz, A.; Navascués, A.; Pozo, F.; Trobajo-Sanmartín, C.; Albéniz, E.; Elía, F.; Burgui, C.; Fernández-Huerta, M.; et al. Effectiveness of influenza vaccination in preventing influenza in primary care, Navarre, Spain, 2021/22. Eurosurveillance 2022, 27, 2200488. [Google Scholar] [CrossRef] [PubMed]
- Navascués, A.; Casado, I.; Pérez-García, A.; Aguinaga, A.; Martínez-Baz, I.; Floristán, Y.; Ezpeleta, C.; Castilla, J. Detection of Respiratory Viruses in Deceased Persons, Spain, 2017. Emerg. Infect. Dis. 2018, 24, 1331–1334. [Google Scholar] [CrossRef]
- Castilla, J.; Moreno-Iribas, C.; Ibero Esparza, C.; Martínez-Baz, I.; Trobajo-Sanmartín, C.; Ezpeleta, C.; Guevara, M. First wave of the COVID-19 pandemic in Navarre, Spain, February-June 2020. An. Sist. Sanit. Navar. 2022, 31, e0954. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Rybak, A.; Levy, C.; Angoulvant, F.; Auvrignon, A.; Gembara, P.; Danis, K.; Vaux, S.; Levy-Bruhl, D.; van der Werf, S.; Béchet, S.; et al. Association of Nonpharmaceutical Interventions During the COVID-19 Pandemic With Invasive Pneumococcal Disease, Pneumococcal Carriage, and Respiratory Viral Infections Among Children in France. JAMA Netw. Open 2022, 5, e2218959. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Baz, I.; Miqueleiz, A.; Egüés, N.; Casado, I.; Burgui, C.; Echeverría, A.; Navascués, A.; Fernández-Huerta, M.; García Cenoz, M.; Trobajo-Sanmartín, C.; et al. Effect of COVID-19 vaccination on the SARS-CoV-2 transmission among social and household close contacts: A cohort study. J. Infect. Public Health 2023, 16, 410–417. [Google Scholar] [CrossRef]
- Fratty, I.S.; Reznik-Balter, S.; Nemet, I.; Atari, N.; Kliker, L.; Sherbany, H.; Keller, N.; Stein, M.; Mendelson, E.; Mandelboim, M. Outbreak of Influenza and Other Respiratory Viruses in Hospitalized Patients Alongside the SARS-CoV-2 Pandemic. Front. Microbiol. 2022, 13, 902476. [Google Scholar] [CrossRef]
- Agca, H.; Akalin, H.; Saglik, I.; Hacimustafaoglu, M.; Celebi, S.; Ener, B. Changing epidemiology of influenza and other respiratory viruses in the first year of COVID-19 pandemic. J. Infect. Public Health 2021, 14, 1186–1190. [Google Scholar] [CrossRef]
- Cillóniz, C.; Pericàs, J.M.; Rojas, J.R.; Torres, A. Severe Infections Due to Respiratory Viruses. Semin. Respir. Crit. Care Med. 2022, 43, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wu, T.; Zeng, Q.; Chen, Y.; Liu, Y.; Wu, D. Epidemiology of rhinovirus under the COVID-19 pandemic in Guangzhou, China, 2020. Immun. Inflamm. Dis. 2022, 10, e632. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Walsh, E.E.; Hayden, F.G. Rhinovirus and coronavirus infection-associated hospitalizations among older adults. J. Infect. Dis. 2002, 185, 1338–1341. [Google Scholar] [CrossRef] [PubMed]
- Fowlkes, A.; Giorgi, A.; Erdman, D.; Temte, J.; Goodin, K.; Di Lonardo, S.; Sun, Y.; Martin, K.; Feist, M.; Linz, R.; et al. Viruses associated with acute respiratory infections and influenza-like illness among outpatients from the Influenza Incidence Surveillance Project, 2010–2011. J. Infect. Dis. 2014, 209, 1715–1725. [Google Scholar] [CrossRef] [PubMed]
- Castilla, J.; Guevara, M.; Miqueleiz, A.; Baigorria, F.; Ibero-Esparza, C.; Navascués, A.; Trobajo-Sanmartín, C.; Martínez-Baz, I.; Casado, I.; Burgui, C.; et al. Risk Factors of Infection, Hospitalization and Death from SARS-CoV-2: A Population-Based Cohort Study. J. Clin. Med. 2021, 10, 2608. [Google Scholar] [CrossRef]
- Pyöriä, L.; Pratas, D.; Toppinen, M.; Hedman, K.; Sajantila, A.; Perdomo, M. Unmasking the tissue-resident eukaryotic DNA virome in humans. Nucleic Acids Res. 2023, 51, 3223–3239. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.P.; Ponomarenko, A. Roles for Pathogen Interference in Influenza Vaccination, with Implications to Vaccine Effectiveness (VE) and Attribution of Influenza Deaths. Infect. Dis. Rep. 2022, 14, 710–758. [Google Scholar] [CrossRef] [PubMed]
- Kissling, E.; Pozo, F.; Martínez-Baz, I.; Buda, S.; Vilcu, A.M.; Domegan, L.; Mazagatos, C.; Dijkstra, F.; Latorre-Margalef, N.; Kurečić Filipović, S.; et al. Influenza vaccine effectiveness against influenza A subtypes in Europe: Results from the 2021–2022 I-MOVE primary care multicentre study. Influ. Other Respir. Viruses 2023, 17, e13069. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control (ECDC). Influenza Virus Characterisation. Summary Europe, March 2022; ECDC: Stockholm, Sweden, 2022; Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Influenza-characterisation-report-march-2022.pdf (accessed on 25 March 2024).
- Klein, A.; Langenwalder, F.; Heinrich, F.; Meißner, K.; Schröder, A.S.; Püschel, K.; Ondruschka, B.; Lütgehetmann, M.; Heinemann, A. SARS-CoV 2-Zufallsentdeckungen bei Hamburger Todesfällen: Ein epidemiologisches Monitoring während des dynamischen Infektionsgeschehens im Frühjahr 2020 [SARS-CoV 2 incidental findings among Hamburg deaths: An epidemiological monitoring during the dynamic infection event in spring 2020]. Rechtsmedizin 2021, 31, 427–433. [Google Scholar] [CrossRef]
- Instituto de Salud Carlos III. MoMo. Monitorización de la Mortalidad Diaria Portodas las Causas y Atribuible a Temperatura. Situación a 28 de Diciembre de 2022. Available online: https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/MoMo/Documents/InformesMoMo2022/MoMo_Situaci%c3%b3n%20a%2028%20de%20diciembre%20de%202022_CNE.pdf (accessed on 25 March 2024).
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef]
- Lin, D.; Liu, L.; Zhang, M.; Hu, Y.; Yang, Q.; Guo, J.; Guo, Y.; Dai, Y.; Xu, Y.; Cai, Y.; et al. Co-infections of SARS-CoV-2 with multiple common respiratory pathogens in infected patients. Sci. China Life Sci. 2020, 63, 606–609. [Google Scholar] [CrossRef] [PubMed]
- Plenzig, S.; Bojkova, D.; Held, H.; Berger, A.; Holz, F.; Cinatl, J.; Gradhand, E.; Kettner, M.; Pfeiffer, A.; Verhoff, M.A.; et al. Infectivity of deceased COVID-19 patients. Int. J. Leg. Med. 2021, 135, 2055–2060. [Google Scholar] [CrossRef] [PubMed]
- Speers, D.J.; Moss, D.M.; Minney-Smith, C.; Levy, A.; Smith, D.W. Influenza and respiratory syncytial virus are the major respiratory viruses detected from prospective testing of pediatric and adult coronial autopsies. Influ. Other Respir. Viruses 2013, 7, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Brañas, P.; Muñoz-Gallego, I.; Espartosa, E.; Moral, N.; Abellán, G.; Folgueira, L. Dynamics of respiratory viruses other than SARS-CoV-2 during the COVID-19 pandemic in Madrid, Spain. Influ. Other Respir. Viruses 2023, 17, e13199. [Google Scholar] [CrossRef] [PubMed]
- Baker, T.; Schandl, C.; Presnell, S.E.; Madory, J.; Nolte, F.S.; Batalis, N. Use of an Automated Nested Multiplex Respiratory Pathogen PCR Panel Postmortem in the Pediatric Forensic Setting. J. Forensic. Sci. 2017, 62, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.; Jones, R. The use of coroner’s autopsy reports to validate the use of targeted swabbing rather than tissue collection for rapid confirmation of virological causes of sudden death in the community. J. Clin. Virol. 2015, 63, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Gilsenan-Reed, C.; Higgins, G.; Langlois, N. Determining a sampling regime for PCR detection of respiratory tract viral infection at coronial post-mortem examinations. Forensic. Sci. Med. Pathol. 2020, 16, 457–462. [Google Scholar] [CrossRef]
- World Health Organization. Strategy Considerations for Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and Other Respiratory Viruses in the WHO European Region during Autumn and Winter 2022/23: Protecting the Vulnerable with Agility, Efficiency, and Trust; WHO Regional Office for Europe: Copenhagen, Denmark, 2022. Available online: https://apps.who.int/iris/handle/10665/360408 (accessed on 25 March 2024).
- Ip, D.K.; Lau, L.L.; Leung, N.H.; Fang, V.J.; Chan, K.H.; Chu, D.K.; Leung, G.M.; Peiris, J.S.; Uyeki, T.M.; Cowling, B.J. Viral Shedding and Transmission Potential of Asymptomatic and Paucisymptomatic Influenza Virus Infections in the Community. Clin. Infect. Dis. 2017, 64, 736–742. [Google Scholar] [CrossRef]
| Cases among Deceased Persons Tested, n (n = 242) | Proportion of All Positive Samples % (n = 64) | Cases among Deceased Persons Tested, % (n = 242) | Estimated Proportion of All Deaths in the Population, % | |
|---|---|---|---|---|
| SARS-CoV-2 | 27 | 42.2 | 11.2 | 19.2 |
| Rhinovirus | 14 | 21.9 | 5.8 | 5.8 |
| Coronavirus 229E or OC43 | 9 | 14.1 | 3.7 | 3.7 |
| Metapneumovirus | 6 | 9.4 | 2.5 | 2.5 |
| Respiratory syncytial virus | 4 | 6.3 | 1.7 | 1.7 |
| Parainfluenza virus | 4 | 6.3 | 1.7 | 1.7 |
| Influenza | 3 | 4.7 | 1.2 | 1.2 |
| Adenovirus | 2 | 3.1 | 0.8 | 0.8 |
| Bocavirus | 1 | 1.6 | 0.4 | 0.4 |
| All non-SARS-CoV-2 viruses a | 40 | 62.5 | 16.5 | 16.5 |
| Any respiratory virus b | 64 | 100 | 26.4 | 34.4 |
| Total with Valid Result N (%) | Any Respiratory Virus n (% of Testers) | p Value | SARS-CoV-2 n (% of Testers) | p Value | Other Respiratory Virus n (% of Testers) | p Value | |
|---|---|---|---|---|---|---|---|
| Total | 242 (100) | 64 (26.4) | 27 (11.2) | 40 (16.5) | |||
| Sex | 0.830 | 0.874 | 0.773 | ||||
| Male | 122 (50.4) | 33 (27.0) | 14 (11.5) | 21 (17.2) | |||
| Female | 120 (49.6) | 31 (25.8) | 13 (10.8) | 19 (15.8) | |||
| Age, years | 0.048 | 0.184 | 0.242 | ||||
| <70 | 42 (17.4) | 8 (19.0) | 2 (4.8) | 6 (14.3) | |||
| 70–79 | 41 (16.9) | 8 (19.5) | 4 (9.8) | 4 (9.8) | |||
| 80–89 | 76 (31.4) | 17 (22.4) | 7 (9.2) | 11 (14.5) | |||
| ≥90 | 83 (34.3) | 31 (37.3) | 14 (16.9) | 19 (22.9) | |||
| Month | 0.033 | 0.015 | 0.055 | ||||
| December | 57 (23.6) | 16 (28.1) | 2 (3.5) | 14 (24.6) | |||
| January | 28 (11.6) | 7 (25.0) | 4 (14.3) | 3 (10.7) | |||
| February | 35 (14.5) | 8 (22.9) | 7 (20.0) | 2 (5.7) | |||
| March | 52 (21.5) | 7 (13.5) | 1 (1.9) | 6 (11.5) | |||
| April | 37 (15.3) | 17 (44.7) | 8 (21.6) | 10 (26.3) | |||
| May | 24 (9.9) | 8 (33.3) | 4 (16.7) | 5 (20.8) | |||
| June | 9 (3.7) | 1 (11.1) | 1 (11.1) | 0 (0.0) | |||
| Respiratory symptoms | 0.467 | 0.093 | 0.115 | ||||
| Yes | 109 (45.0) | 32 (29.4) | 9 (8.3) | 24 (22.0) | |||
| No | 75 (31.0) | 16 (21.3) | 7 (9.3) | 9 (12.0) | |||
| Unknow | 58 (24.0) | 16 (27.6) | 11 (19.0) | 7 (12.1) | |||
| Hospitalized ≤ 30 days before death | 0.114 | 0.454 | 0.165 | ||||
| Yes | 117 (48.3) | 34 (30.7) | 13 (12.6) | 22 (19.7) | |||
| No | 125 (51.7) | 30 (24.0) | 14 (119.6) | 18 (13.0) | |||
| Pre-mortem diagnosis ≤ 30 days before death | <0.001 | <0.001 | 0.909 | ||||
| Yes | 13 (5.4) | 11 (84.6) | 9 (69.2) | 2 (15.4) | |||
| No | 229 (94.6) | 53 (23.1) | 18 (7.9) | 38 (16.6) | |||
| Influenza vaccination | 0.424 | 0.415 | 0.091 | ||||
| Unvaccinated | 70 (28.9) | 21(30.0) | 6 (8.6) | 16 (22.9) | |||
| Vaccinated | 172 (71.1) | 43 (25.0) | 21 (12.2) | 24 (14.0) | |||
| COVID-19 vaccination | 0.013 | 0.523 | 0.022 | ||||
| Unvaccinated | 10 (4.1) | 6 (60.0) | 1 (10.0) | 6 (60.0) | |||
| Vaccinated without booster dose | 36 (14.9) | 13 (36.1) | 6 (16.7) | 8 (22.2) | |||
| Vaccinated and booster dose | 196 (81.0) | 45 (23.0) | 20 (10.2) | 26 (13.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trobajo-Sanmartín, C.; Navascués, A.; Fernández-Huerta, M.; Martínez-Baz, I.; Casado, I.; Ezpeleta, C.; Castilla, J. Prevalence of Respiratory Viral Infections in Deceased Persons during the COVID-19 Pandemic Season 2021–2022: A Population-Based Observational Study. Viruses 2024, 16, 533. https://doi.org/10.3390/v16040533
Trobajo-Sanmartín C, Navascués A, Fernández-Huerta M, Martínez-Baz I, Casado I, Ezpeleta C, Castilla J. Prevalence of Respiratory Viral Infections in Deceased Persons during the COVID-19 Pandemic Season 2021–2022: A Population-Based Observational Study. Viruses. 2024; 16(4):533. https://doi.org/10.3390/v16040533
Chicago/Turabian StyleTrobajo-Sanmartín, Camino, Ana Navascués, Miguel Fernández-Huerta, Iván Martínez-Baz, Itziar Casado, Carmen Ezpeleta, and Jesús Castilla. 2024. "Prevalence of Respiratory Viral Infections in Deceased Persons during the COVID-19 Pandemic Season 2021–2022: A Population-Based Observational Study" Viruses 16, no. 4: 533. https://doi.org/10.3390/v16040533
APA StyleTrobajo-Sanmartín, C., Navascués, A., Fernández-Huerta, M., Martínez-Baz, I., Casado, I., Ezpeleta, C., & Castilla, J. (2024). Prevalence of Respiratory Viral Infections in Deceased Persons during the COVID-19 Pandemic Season 2021–2022: A Population-Based Observational Study. Viruses, 16(4), 533. https://doi.org/10.3390/v16040533






