Peripheral B Lymphocyte Serves as a Reservoir for the Persistently Covert Infection of Mandarin Fish Siniperca chuatsi Ranavirus
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Virus and Antibodies
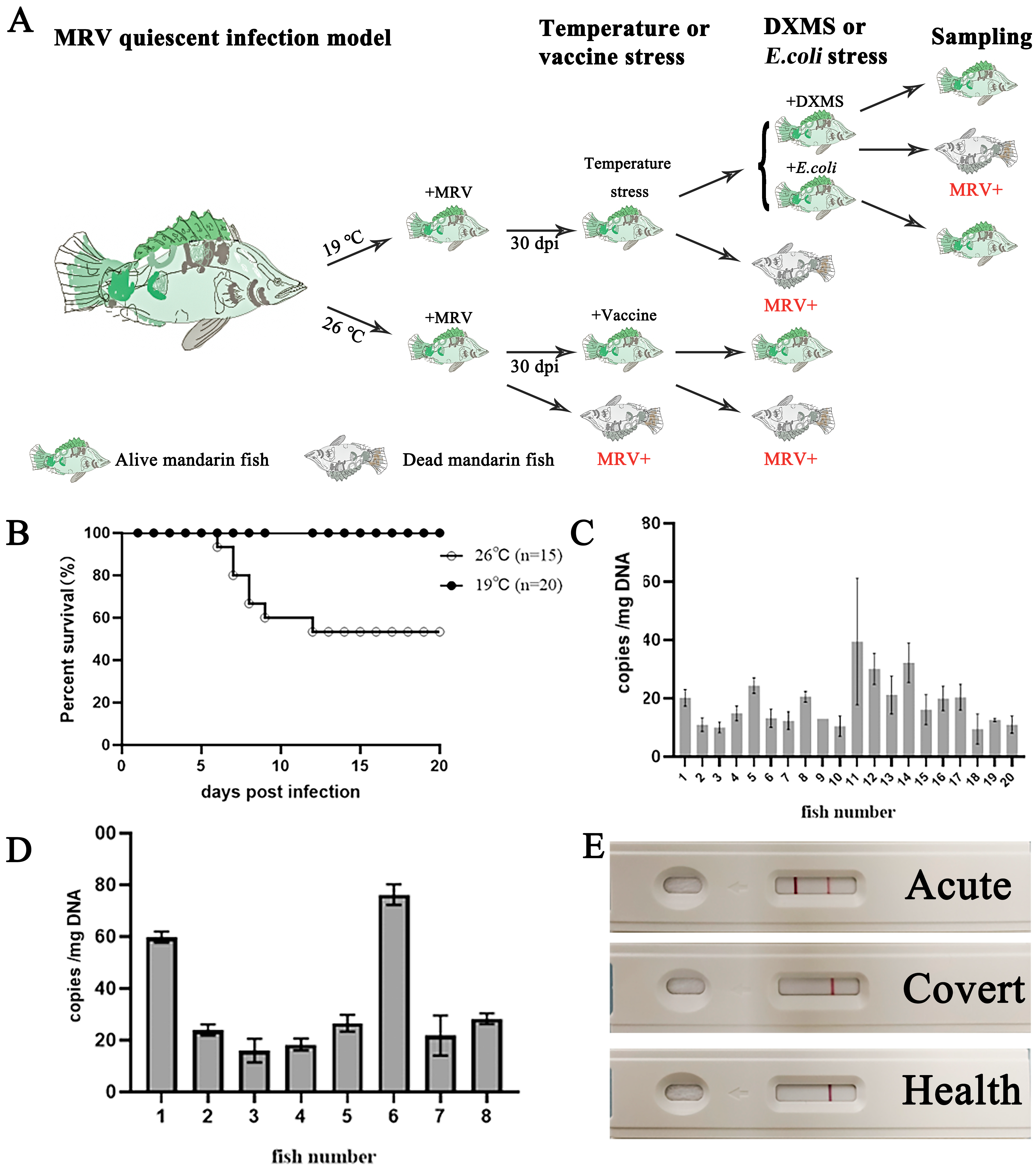
2.2. Microchip Label and Artificial Infection
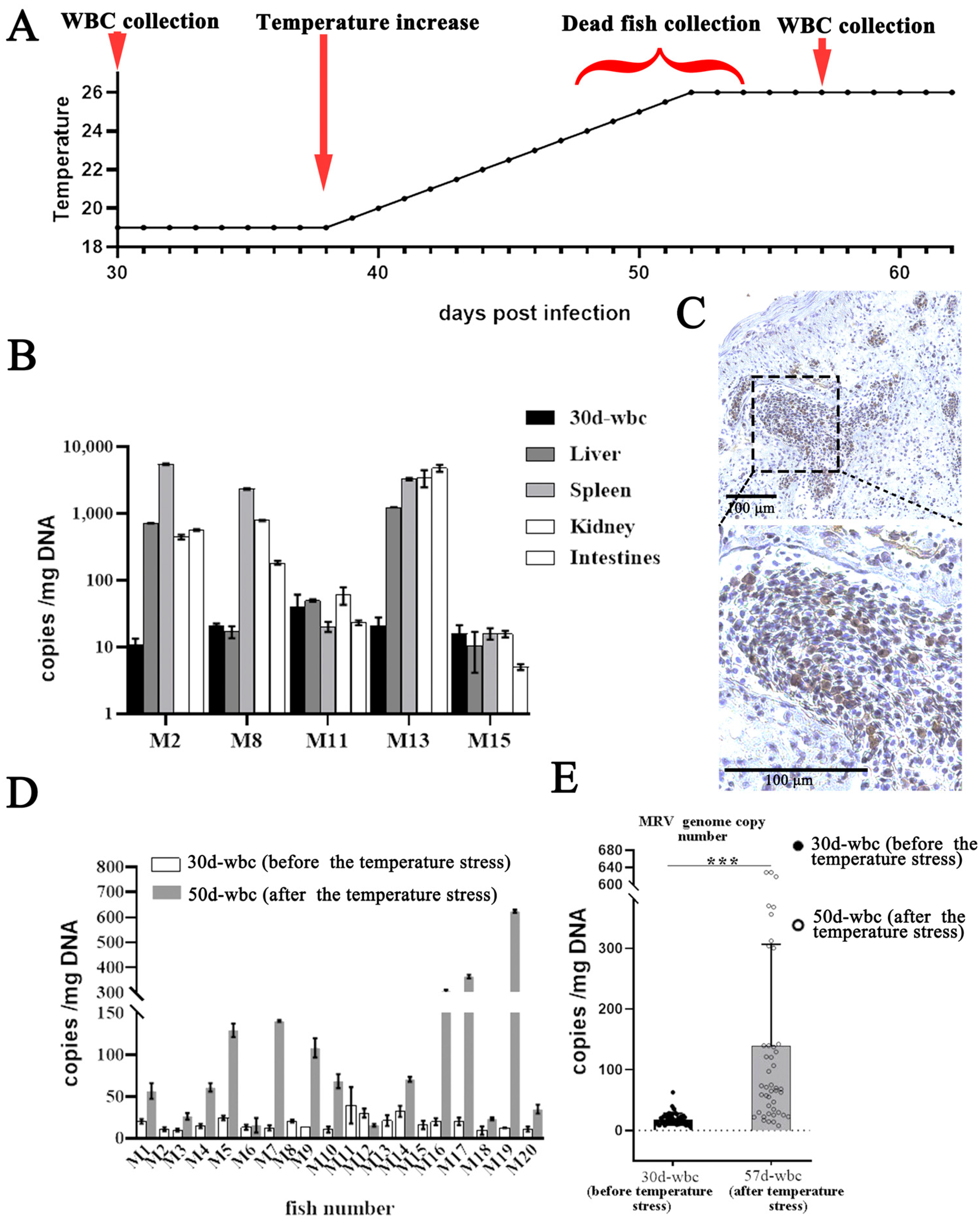
2.3. Raising Temperature Stress
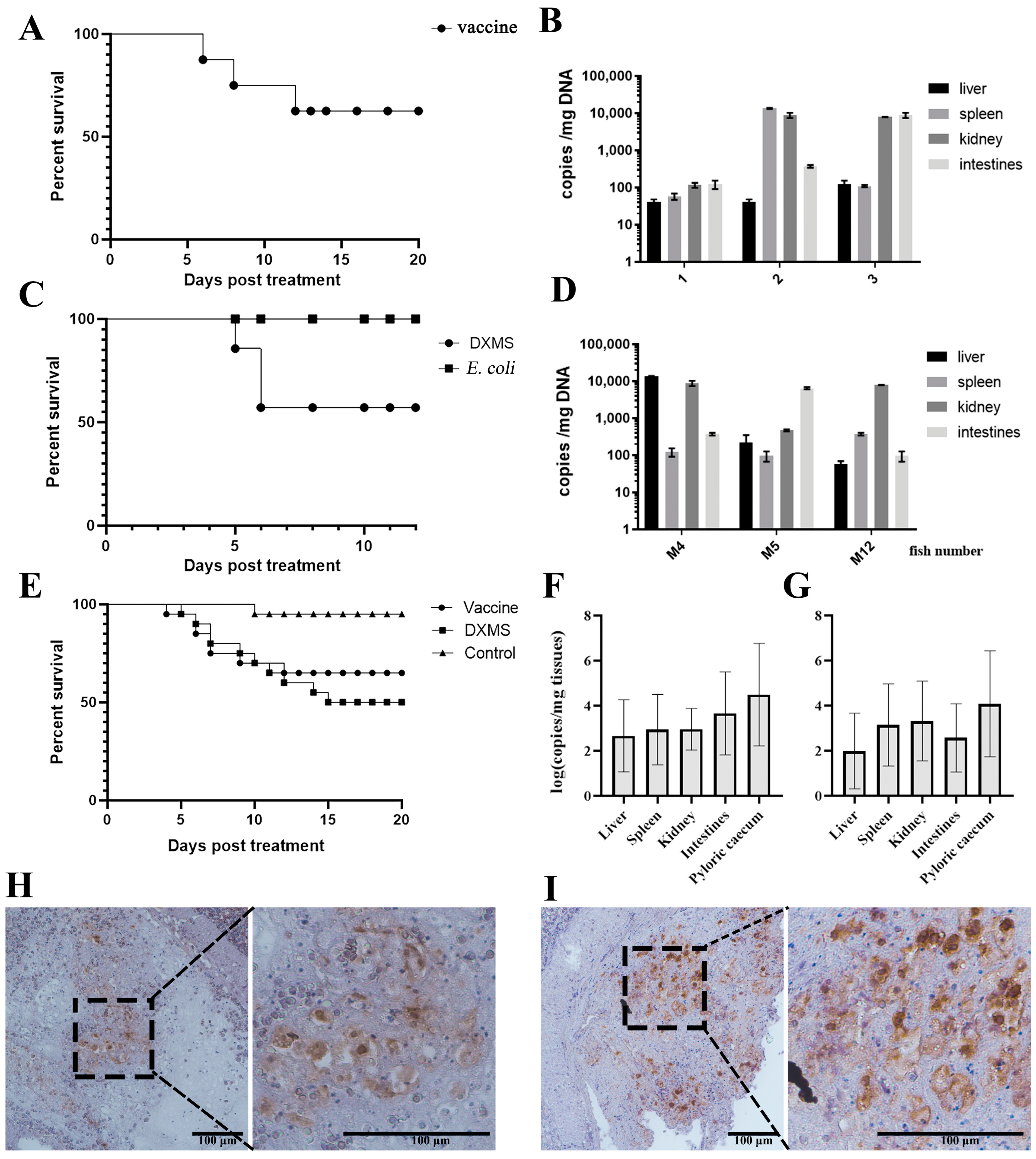
2.4. Vaccination, Dexamethasone (DXMS) Treatment and E. coil Stimulation
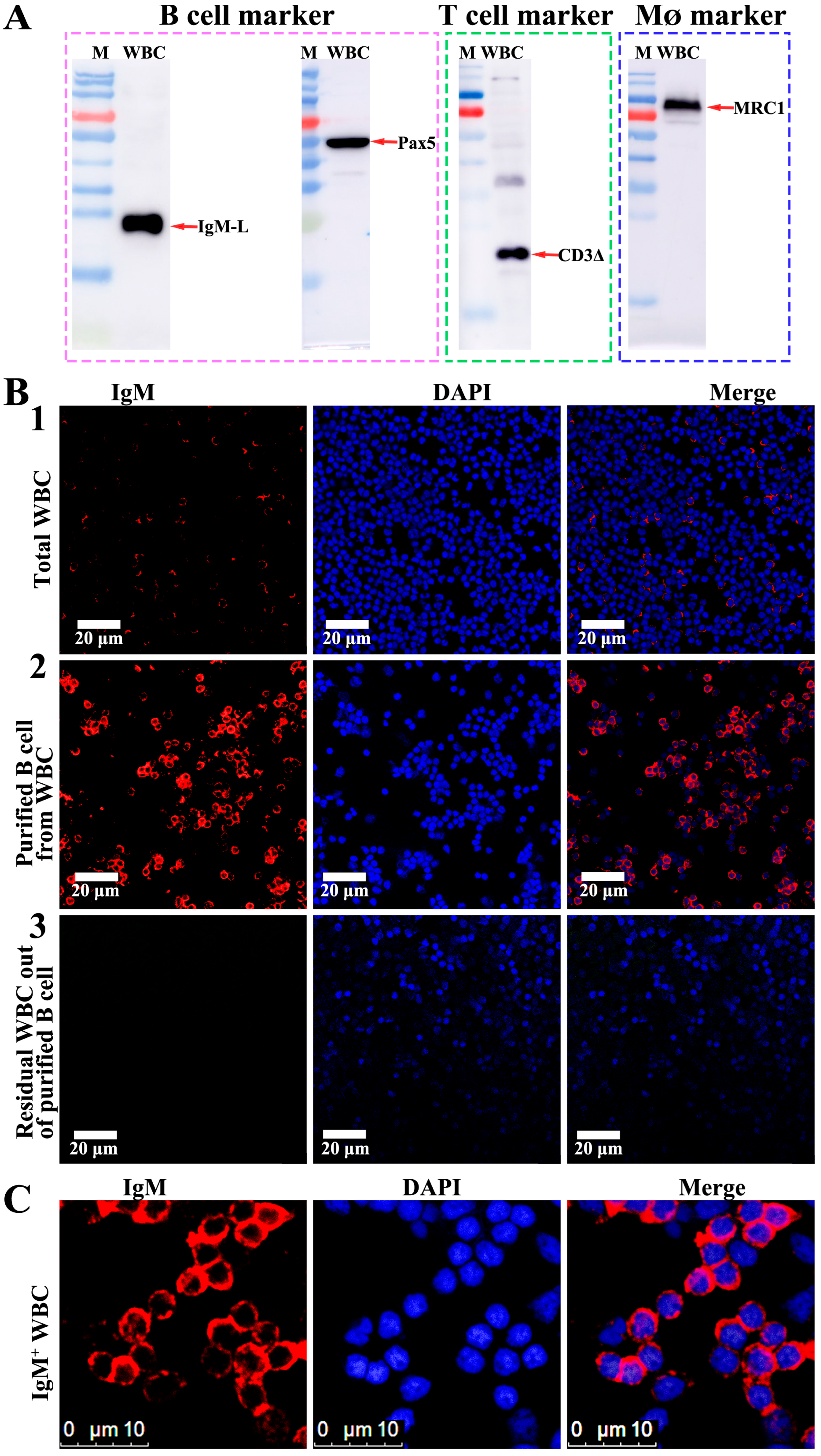
2.5. IgM+, CD3+ and MRC1+ WBC Isolation and Purification
2.6. Western Blotting
2.7. Confocal Microscopy Observation
2.8. Viral Load Measuement by qPCR
2.9. Quantitative Gene Expression
2.10. Immunohistochemistry Assay (IHC)
2.11. Statistical Analysis
3. Results
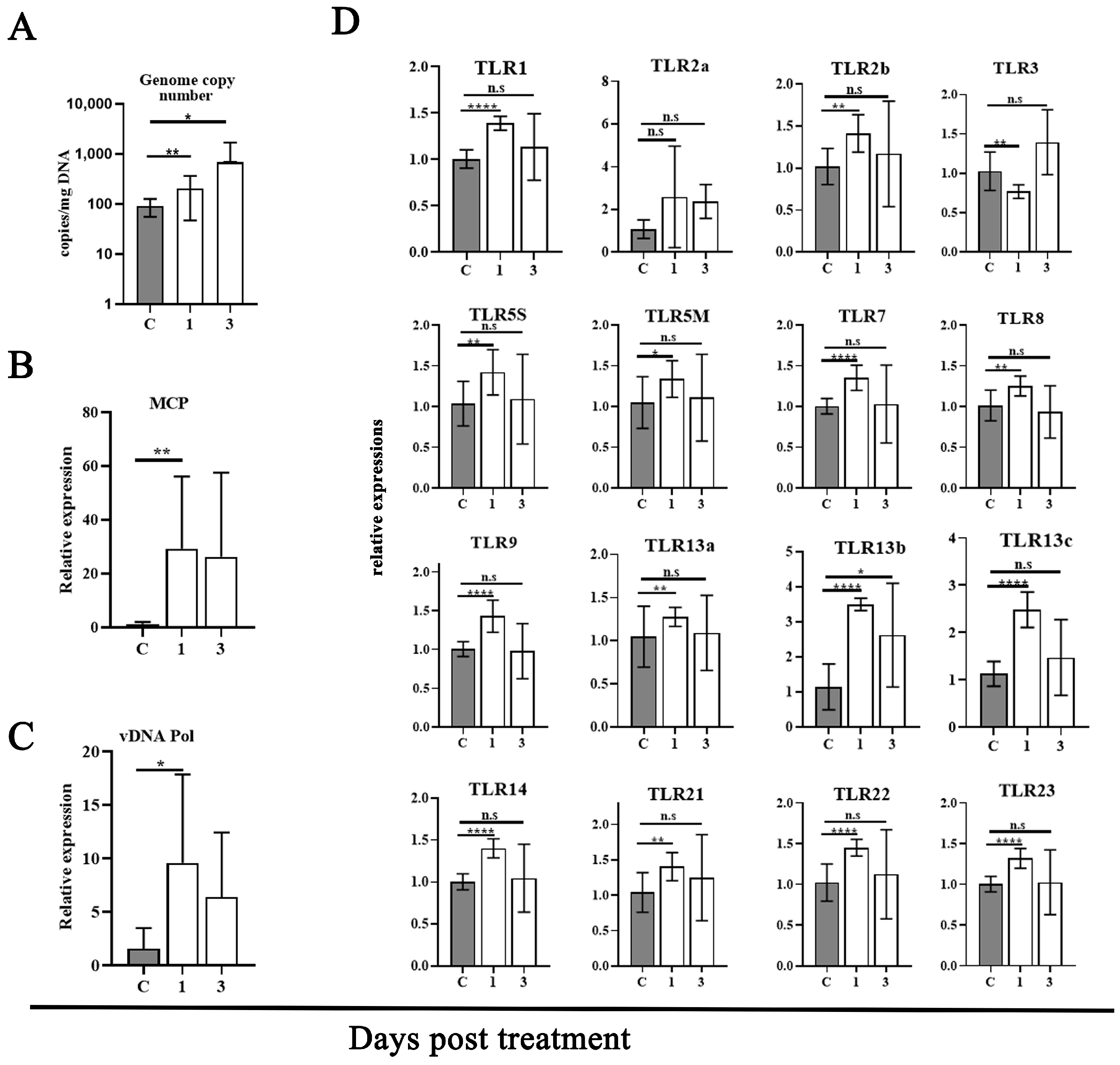
3.1. Transition of MRV from Acute Infection to Persistently Covert State
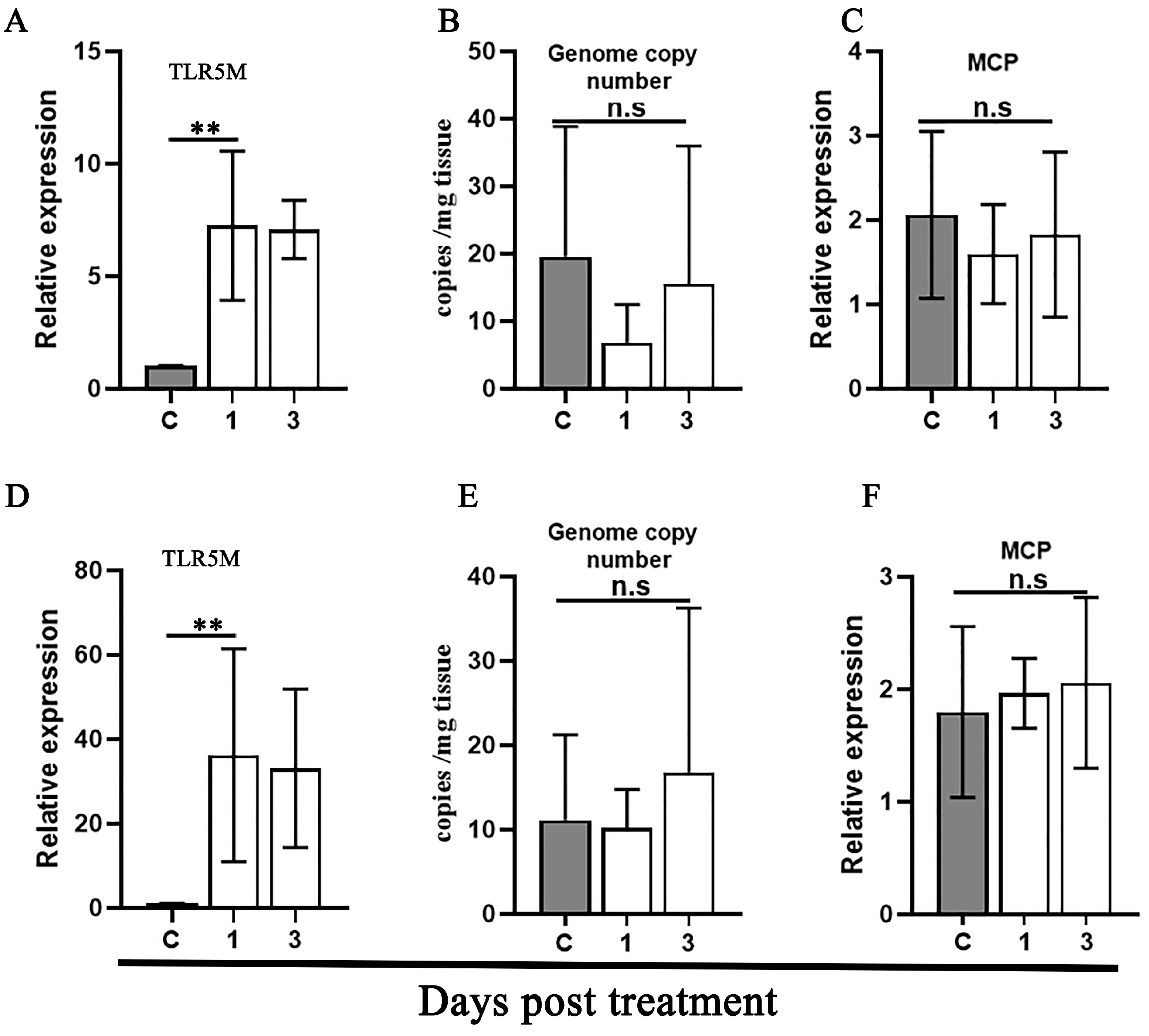
3.2. Reactivation of Covert MRV via Raising Temperature Stress
3.3. Reactivation of Covert MRV by Vaccination and DXMS Treatment
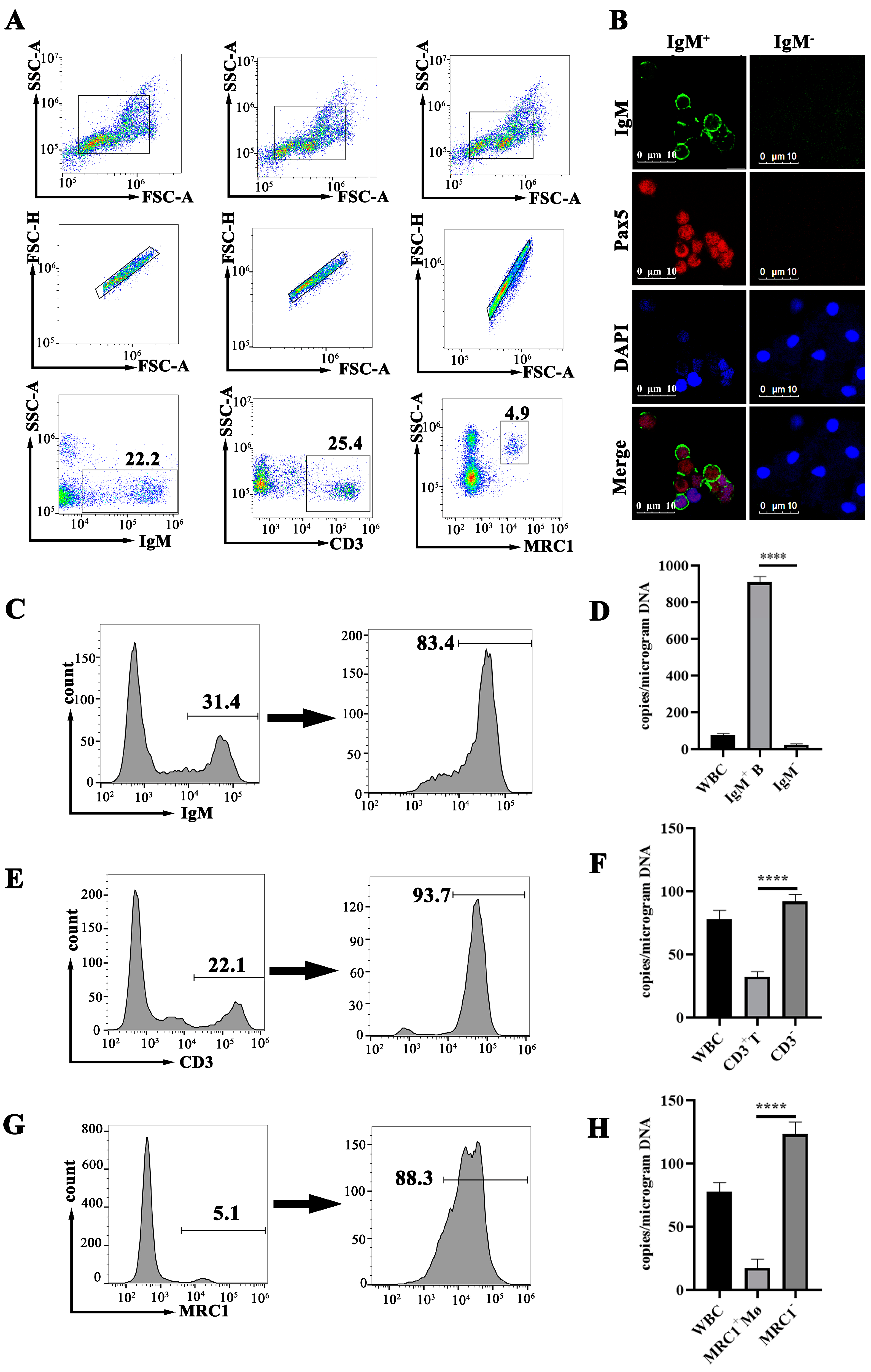
3.4. Purification of B Cells, T Cells and MØ
3.5. MRV Genome Assessment in B Cells, T Cells and MØ
3.6. Screening TLR Pathway Involved in the Reactivation of Covert MRV
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eaton, H.E.; Metcalf, J.; Penny, E.; Tcherepanov, V.; Upton, C.; Brunetti, C.R. Comparative genomic analysis of the family Iridoviridae: Re-annotating and defining the core set of iridovirus genes. Virol. J. 2007, 4, 11. [Google Scholar] [CrossRef]
- Chinchar, V.G.; Hick, P.; Ince, I.A.; Jancovich, J.K.; Marschang, R.; Qin, Q.; Subramaniam, K.; Waltzek, T.B.; Whittington, R.; Williams, T.; et al. ICTV Virus Taxonomy Profile: Iridoviridae. J. Gen. Virol. 2017, 98, 890–891. [Google Scholar] [CrossRef]
- Yu, X.D.; Ke, F.; Zhang, Q.Y.; Gui, J.F. Genome Characteristics of Two Ranavirus Isolates from Mandarin Fish and Largemouth Bass. Pathogens 2023, 12, 730. [Google Scholar] [CrossRef]
- Zhang, W.; Deng, H.; Fu, Y.; Fu, W.; Weng, S.; He, J.; Dong, C. Production and characterization of monoclonal antibodies against mandarinfish ranavirus and first identification of pyloric caecum as the major target tissue. J. Fish Dis. 2023, 46, 189–199. [Google Scholar] [CrossRef]
- Chinchar, V.G.; Waltzek, T.B. Ranaviruses: Not just for frogs. PLoS Pathog. 2014, 10, e1003850. [Google Scholar] [CrossRef]
- Whittington, R.J.; Becker, J.A.; Dennis, M.M. Iridovirus infections in finfish—Critical review with emphasis on ranaviruses. J. Fish Dis. 2010, 33, 95–122. [Google Scholar] [CrossRef]
- Gray, M.J.; Miller, D.L.; Hoverman, J.T. Ecology and pathology of amphibian ranaviruses. Dis. Aquat. Org. 2009, 87, 243–266. [Google Scholar] [CrossRef]
- Ma, H.; Peng, C.; Su, Y.; Feng, J.; Guo, Z. Isolation of a Ranavirus-type grouper iridovirus in mainland China and comparison of its pathogenicity with that of a Megalocytivirus-type grouper iridovirus. Aquaculture 2016, 463, 145–151. [Google Scholar] [CrossRef]
- Grizzle, J.M.; Altinok, I.; Fraser, W.A.; Francis-Floyd, R. First isolation of largemouth bass virus. Dis. Aquat. Org. 2002, 50, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.C.; Li, S.J.; Xie, J.; Bai, J.J.; Chen, K.C.; Ma, D.M.; Jiang, X.Y.; Lao, H.H.; Yu, L.Y. Characterization of a ranavirus isolated from cultured largemouth bass (Micropterus salmoides) in China. Aquaculture 2011, 312, 198–204. [Google Scholar] [CrossRef]
- George, M.R.; John, K.R.; Mansoor, M.M.; Saravanakumar, R.; Sundar, P.; Pradeep, V. Isolation and characterization of a ranavirus from koi, Cyprinus carpio L., experiencing mass mortalities in India. J. Fish Dis. 2015, 38, 389–403. [Google Scholar] [CrossRef]
- Kayansamruaj, P.; Rangsichol, A.; Dong, H.T.; Rodkhum, C.; Maita, M.; Katagiri, T.; Pirarat, N. Outbreaks of ulcerative disease associated with ranavirus infection in barcoo grunter, Scortum barcoo (McCulloch & Waite). J. Fish Dis. 2017, 40, 1341–1350. [Google Scholar]
- Hedrick, R.P.; McDowell, T.S. Properties of iridoviruses from ornamental fish. Vet. Res. 1995, 26, 423–427. [Google Scholar]
- Zhang, W.F.; Duan, C.; Zhang, H.T.; Weng, S.P.; He, J.G.; Dong, C.F. Widespread outbreaks of the emerging mandarinfish ranavirus (MRV) both in natural and ISKNV-FKC vaccinated mandarinfish Siniperca chuatsi in Guangdong, South China, 2017. Aquaculture 2020, 520, 734989. [Google Scholar] [CrossRef]
- Dong, C.; Wang, Z.; Weng, S.; He, J. Occurrence of a lethal ranavirus in hybrid mandarin (Siniperca scherzeri × Siniperca chuatsi) in Guangdong, South China. Vet. Microbiol. 2017, 203, 28–33. [Google Scholar] [CrossRef]
- He, J.G.; Deng, M.; Weng, S.P.; Li, Z.; Zhou, S.Y.; Long, Q.X.; Wang, X.Z.; Chan, S.M. Complete genome analysis of the mandarin fish infectious spleen and kidney necrosis iridovirus. Virology 2001, 291, 126–139. [Google Scholar] [CrossRef]
- Dong, C.; Xiong, X.; Luo, Y.; Weng, S.; Wang, Q.; He, J. Efficacy of a formalin-killed cell vaccine against infectious spleen and kidney necrosis virus (ISKNV) and immunoproteomic analysis of its major immunogenic proteins. Vet. Microbiol. 2013, 162, 419–428. [Google Scholar] [CrossRef]
- Fu, Y.; Li, Y.; Zhang, W.; Fu, W.; Li, W.; Zhu, Z.; Weng, S.; He, J.; Dong, C. Effectively protecting Asian seabass Lates calcarifer from ISKNV-I, ISKNV-II, RSIV-II and SDDV by an inactivated ISKNV-I and SDDV bivalent vaccine. Aquaculture 2023, 566, 739218. [Google Scholar] [CrossRef]
- Fu, W.; Li, Y.; Fu, Y.; Zhang, W.; Luo, P.; Sun, Q.; Yu, F.; Weng, S.; Li, W.; He, J.; et al. The Inactivated ISKNV-I Vaccine Confers Highly Effective Cross-Protection against Epidemic RSIV-I and RSIV-II from Cultured Spotted Sea Bass Lateolabrax maculatus. Microbiol. Spectr. 2023, 11, e0449522. [Google Scholar] [CrossRef]
- Matsuyama, T.; Minami, T.; Fukuda, Y.; Sano, N.; Sakai, T.; Takano, T.; Nakayasu, C. Passive immunization against red sea bream iridoviral disease in five marine fish species. Fish. Pathol. 2016, 51, 32–35. [Google Scholar] [CrossRef][Green Version]
- Nakajima, K.; Maeno, Y.; Honda, A.; Yokoyama, K.; Tooriyama, T.; Manabe, S. Effectiveness of a vaccine against red sea bream iridoviral disease in a field trial test. Dis. Aquat. Org. 1999, 36, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.Y.; Fu, J.J.; Pan, W.Q.; Zhan, Z.P.; Weng, S.P.; Guo, C.J.; He, J.G. Low-temperature immunization attenuates the residual virulence of orf074r gene-deleted infectious spleen and kidney necrosis virus: A candidate immersion vaccine. J. Virol. 2023, 97, e01289-23. [Google Scholar] [CrossRef] [PubMed]
- Poiesz, B.J.; Poiesz, M.J.; Choi, D. The human T-cell lymphoma/leukemia viruses. Cancer Investig. 2003, 21, 253–277. [Google Scholar] [CrossRef] [PubMed]
- Moar, P.; Premeaux, T.A.; Atkins, A.; Ndhlovu, L.C. The latent HIV reservoir: Current advances in genetic sequencing approaches. mBio 2023, 14, e01344-23. [Google Scholar] [CrossRef]
- Kukhanova, M.K.; Korovina, A.N.; Kochetkov, S.N. Human herpes simplex virus: Life cycle and development of inhibitors. Biochemistry 2014, 79, 1635–1652. [Google Scholar] [CrossRef] [PubMed]
- Eide, K.E.; Miller-Morgan, T.; Heidel, J.R.; Kent, M.L.; Bildfell, R.J.; LaPatra, S.; Watson, G.; Jin, L. Investigation of Koi Herpesvirus Latency in Koi. J. Virol. 2011, 85, 4954–4962. [Google Scholar] [CrossRef]
- Weidner-Glunde, M.; Kruminis-Kaszkiel, E.; Savanagouder, M. Herpesviral Latency-Common Themes. Pathogens 2020, 9, 125. [Google Scholar] [CrossRef]
- Croen, K.D. Latency of the human herpesviruses. Annu. Rev. Med. 1991, 42, 61–67. [Google Scholar] [CrossRef]
- Baichwal, V.R.; Sugden, B. Latency comes of age for herpesviruses. Cell 1988, 52, 787–789. [Google Scholar] [CrossRef]
- Hyatt, A.D.; Williamson, M.; Coupar, B.E.; Middleton, D.; Hengstberger, S.G.; Gould, A.R.; Selleck, P.; Wise, T.G.; Kattenbelt, J.; Cunningham, A.A.; et al. First identification of a ranavirus from green pythons (Chondropython viridis). J. Wildl. Dis. 2002, 38, 239–252. [Google Scholar] [CrossRef]
- Johnson, A.J.; Pessier, A.P.; Wellehan, J.F.; Childress, A.; Norton, T.M.; Stedman, N.L.; Bloom, D.C.; Belzer, W.; Titus, V.R.; Wagner, R.; et al. Ranavirus infection of free-ranging and captive box turtles and tortoises in the United States. J. Wildl. Dis. 2008, 44, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Maclaine, A.; Forzan, M.J.; Mashkour, N.; Scott, J.; Ariel, E. Pathogenesis of Bohle Iridovirus (Genus Ranavirus) in Experimentally Infected Juvenile Eastern Water Dragons (Intellagama lesueurii lesueurii). Vet. Pathol. 2019, 56, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Morales, H.D.; Abramowitz, L.; Gertz, J.; Sowa, J.; Vogel, A.; Robert, J. Innate immune responses and permissiveness to ranavirus infection of peritoneal leukocytes in the frog Xenopus laevis. J. Virol. 2010, 84, 4912–4922. [Google Scholar] [CrossRef]
- Grayfer, L.; Robert, J. Colony-Stimulating Factor-l-Responsive Macrophage Precursors Reside in the Amphibian (Xenopus laevis) Bone Marrow rather than the Hematopoietic Subcapsular Liver. J. Innate Immun. 2013, 5, 531–542. [Google Scholar] [CrossRef]
- Hoverman, J.T.; Gray, M.J.; Haislip, N.A.; Miller, D.L. Phylogeny, life history, and ecology contribute to differences in amphibian susceptibility to ranaviruses. EcoHealth 2011, 8, 301–319. [Google Scholar] [CrossRef]
- Robert, J.; Grayfer, L.; Edholm, E.S.; Ward, B.; De Jesus Andino, F. Inflammation-induced reactivation of the ranavirus Frog Virus 3 in asymptomatic Xenopus laevis. PLoS ONE 2014, 9, e112904. [Google Scholar] [CrossRef] [PubMed]
- Robert, J.; Abramowitz, L.; Gantress, J.; Morales, H.D. Xenopus laevis: A possible vector of Ranavirus infection? J. Wildl. Dis. 2007, 43, 645–652. [Google Scholar] [CrossRef]
- Samanta, M.; Yim, J.; De Jesus Andino, F.; Paiola, M.; Robert, J. TLR5-Mediated Reactivation of Quiescent Ranavirus FV3 in Xenopus Peritoneal Macrophages. J. Virol. 2021, 95, e00215-21. [Google Scholar] [CrossRef]
- Dong, C.; Weng, S.; Shi, X.; Xu, X.; Shi, N.; He, J. Development of a mandarin fish Siniperca chuatsi fry cell line suitable for the study of infectious spleen and kidney necrosis virus (ISKNV). Virus Res. 2008, 135, 273–281. [Google Scholar] [CrossRef]
- Wang, F.; Tian-long, L.; Hou-jun, P.; Shu-qin, W.; Jin-xian, Y.; Qiao-ping, L. Production and characterization of monoclonal antibodies against Siniperca chuatsi Ig. J. Fish. China 2006, 30, 285–288, (In Chinese with English abstract). [Google Scholar]
- Dong, Y.; Weng, S.; He, J.; Dong, C. Field trial tests of FKC vaccines against RSIV genotype Megalocytivirus in cage-cultured mandarin fish (Siniperca chuatsi) in an inland reservoir. Fish Shellfish Immunol. 2013, 35, 1598–1603. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.L.; Chen, S.N.; Huo, H.J.; Nie, P. Identification and expression analysis of sixteen Toll-like receptor genes, TLR1, TLR2a, TLR2b, TLR3, TLR5M, TLR5S, TLR7-9, TLR13a-c, TLR14, TLR21-23 in mandarin fish Siniperca chuatsi. Dev. Comp. Immunol. 2021, 121, 104100. [Google Scholar] [CrossRef]
- He, J.G.; Zeng, K.; Weng, S.P.; Chan, S.M. Experimental transmission, pathogenicity and physical–chemical properties of infectious spleen and kidney necrosis virus (ISKNV). Aquaculture 2002, 204, 11–24. [Google Scholar] [CrossRef]
- Wei, C.; Kakazu, T.; Chuah, Q.Y.; Tanaka, M.; Kato, G.; Sano, M. Reactivation of cyprinid herpesvirus 2 (CyHV-2) in asymptomatic surviving goldfish Carassius auratus (L.) under immunosuppression. Fish Shellfish Immunol. 2020, 103, 302–309. [Google Scholar] [CrossRef] [PubMed]
- El-mayet, F.S.; Jones, C. Specificity protein 1 (Sp1) and glucocorticoid receptor (GR) stimulate bovine alphaherpesvirus 1 (BoHV-1) replication and cooperatively transactivate the immediate early transcription unit 1 promoter. J. Virol. 2024, 98, e01436-23. [Google Scholar] [CrossRef]
- Li, L.T.; Liu, J.; Luo, M.; Liu, J.S.; Zhang, M.M.; Zhang, W.J.; Chen, H.C.; Liu, Z.F. Establishment of pseudorabies virus latency and reactivation model in mice dorsal root ganglia culture. J. Gen. Virol. 2023, 104, 001921. [Google Scholar] [CrossRef]
- Mutoloki, S.; Alexandersen, S.; Evensen, Ø. Sequential study of antigen persistence and concomitant inflammatory reactions relative to side-effects and growth of Atlantic salmon (Salmo salar L.) following intraperitoneal injection with oil-adjuvanted vaccines. Fish Shellfish Immunol. 2004, 16, 633–644. [Google Scholar] [CrossRef]
- Gjessing, M.C.; Falk, K.; Weli, S.C.; Koppang, E.O.; Kvellestad, A. A sequential study of incomplete Freund’s adjuvant-induced peritonitis in Atlantic cod. Fish Shellfish Immunol. 2012, 32, 141–150. [Google Scholar] [CrossRef]
- Kang, H.-R.; Han, J.h.; Yee, C.N.; Ryu, S.; Park, J.-Y.; Chung, W.-C.; Song, Y.-J.; Chen, S.-T.; Brikey, W.J.; Ting, J.P.-Y.; et al. Dynamic bidirectional regulation of NLRC3 and gammaherpesviruses during viral latency in B lymphocytes. J. Med. Virol. 2024, 9, e29504. [Google Scholar] [CrossRef]
- Reed, A.N.; Izume, S.; Dolan, B.P.; LaPatra, S.; Kent, M.; Dong, J.; Jin, L. Identification of B cells as a major site for cyprinid herpesvirus 3 latency. J. Virol. 2014, 88, 9297–9309. [Google Scholar] [CrossRef]
- Stahl, P.D. The mannose receptor and other macrophage lectins. Curr. Opin. Immunol. 1992, 4, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Peng, C.; Yang, M.; Huang, F.; Duan, X.; Wang, S.; Cheng, H.; Yang, H.; Zhao, H.; Qin, Q. Single-cell RNA-seq landscape midbrain cell responses to red spotted grouper nervous necrosis virus infection. PLoS Pathog. 2021, 17, e1009665. [Google Scholar] [CrossRef]
- Ernst, D.N.; Shih, C.C. CD3 complex. J. Biol. Regul. Homeost. Agents 2000, 14, 226–229. [Google Scholar] [PubMed]
- Adams, B.; Dörfler, P.; Aguzzi, A.; Kozmik, Z.; Urbánek, P.; Maurer-Fogy, I.; Busslinger, M. Pax-5 encodes the transcription factor BSAP and is expressed in B lymphocytes, the developing CNS, and adult testis. Genes. Dev. 1992, 6, 1589–1607. [Google Scholar] [CrossRef] [PubMed]
- Zwollo, P. Dissecting teleost B cell differentiation using transcription factors. Dev. Comp. Immunol. 2011, 35, 898–905. [Google Scholar] [CrossRef]
- Cobaleda, C.; Schebesta, A.; Delogu, A.; Busslinger, M. Pax5: The guardian of B cell identity and function. Nat. Immunol. 2007, 8, 463–470. [Google Scholar] [CrossRef]
- Ohtani, M.; Miyadai, T.; Hiroishi, S. Identification of genes encoding critical factors regulating B-cell terminal differentiation in torafugu (Takifugu rubripes). Comp. Biochem. Physiol. Part D Genom. Proteom. 2006, 1, 109–114. [Google Scholar] [CrossRef]
- Pfeffer, P.L.; Gerster, T.; Lun, K.; Brand, M.; Busslinger, M. Characterization of three novel members of the zebrafish Pax2/5/8 family: Dependency of Pax5 and Pax8 expression on the Pax2.1 (noi) function. Development 1998, 125, 3063–3074. [Google Scholar] [CrossRef]
- Zwollo, P.; Haines, A.; Rosato, P.; Gumulak-Smith, J. Molecular and cellular analysis of B-cell populations in the rainbow trout using Pax5 and immunoglobulin markers. Dev. Comp. Immunol. 2008, 32, 1482–1496. [Google Scholar] [CrossRef]
- Chinchar, V.G.; Waltzek, T.B.; Subramaniam, K. Ranaviruses and other members of the family Iridoviridae: Their place in the virosphere. Virology 2017, 511, 259–271. [Google Scholar] [CrossRef]
- Song, W.J.; Qin, Q.W.; Qiu, J.; Huang, C.H.; Wang, F.; Hew, C.L. Functional genomics analysis of Singapore grouper iridovirus: Complete sequence determination and proteomic analysis. J. Virol. 2004, 78, 12576–12590. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; Oh, M.J.; Nishizawa, T. Potential for a live red seabream iridovirus (RSIV) vaccine in rock bream Oplegnathus fasciatus at a low rearing temperature. Vaccine 2014, 32, 363–368. [Google Scholar] [CrossRef] [PubMed]
| Genes | Primers | Sequences |
|---|---|---|
| MCP probe | Forward | 5′-CACGCCGCACTCTCGTT-3′ |
| Reverse | 5′-GCGTCCAGGAAAGCAGTGTT-3′ | |
| Probe | 5′-AACGAGATTCAGGCCCAG-3′ | |
| MCP | Forward | 5′-TCGCCACTTATGACAGCCTTGA-3′ |
| Reverse | 5′-CGGCACTGATGGCACTTGAC-3′ | |
| vDNA polymerase II | Forward | 5′-TCTGCGTTAGGGTGACTGGTTT-3′ |
| Reverse | 5′-CGGCACTGATGGCACTTGAC-3′ | |
| β-actin | Forward | 5′-AGAGGGAAATCGTGCGTG-3′ |
| Reverse | 5′-GAAGGAAGGCTGGAAGAGG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Gong, H.; Sun, Q.; Fu, Y.; Wu, X.; Deng, H.; Weng, S.; He, J.; Dong, C. Peripheral B Lymphocyte Serves as a Reservoir for the Persistently Covert Infection of Mandarin Fish Siniperca chuatsi Ranavirus. Viruses 2024, 16, 1895. https://doi.org/10.3390/v16121895
Zhang W, Gong H, Sun Q, Fu Y, Wu X, Deng H, Weng S, He J, Dong C. Peripheral B Lymphocyte Serves as a Reservoir for the Persistently Covert Infection of Mandarin Fish Siniperca chuatsi Ranavirus. Viruses. 2024; 16(12):1895. https://doi.org/10.3390/v16121895
Chicago/Turabian StyleZhang, Wenfeng, Hui Gong, Qianqian Sun, Yuting Fu, Xiaosi Wu, Hengwei Deng, Shaoping Weng, Jianguo He, and Chuanfu Dong. 2024. "Peripheral B Lymphocyte Serves as a Reservoir for the Persistently Covert Infection of Mandarin Fish Siniperca chuatsi Ranavirus" Viruses 16, no. 12: 1895. https://doi.org/10.3390/v16121895
APA StyleZhang, W., Gong, H., Sun, Q., Fu, Y., Wu, X., Deng, H., Weng, S., He, J., & Dong, C. (2024). Peripheral B Lymphocyte Serves as a Reservoir for the Persistently Covert Infection of Mandarin Fish Siniperca chuatsi Ranavirus. Viruses, 16(12), 1895. https://doi.org/10.3390/v16121895







