A Possible Protective Effect of IgA Against Severe Acute Respiratory Syndrome Corona Virus 2 (SARS-CoV-2) in Bronchoalveolar Lavage in COVID-19 Patients Admitted to Intensive Care Unit
Abstract
1. Introduction
2. Material and Methods
2.1. Study Population
2.2. IgA and IgG from BAL and Serum of Patients
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Montague, B.T.; Wipperman, M.F.; Chio, E.; Crow, R.; Hooper, A.T.; O’Brien, M.P.; Simões, E.A.F. Elevated serum IgA following vaccination against SARS-CoV-2 in a cohort of high-risk first responders. Sci. Rep. 2022, 12, 14932. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gupta, S.L.; Tyagi, R.; Dhar, A.; Oswal, N.; Khandelwal, A.; Jaiswal, R.K. Children’s SARS-CoV-2 Infection and Their Vaccination. Vaccines 2023, 11, 418. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bohländer, F. A new hope? Possibilities of therapeutic IgA antibodies in the treatment of inflammatory lung diseases. Front. Immunol. 2023, 14, 1127339. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pantaleo, G.; Correia, B.; Fenwick, C.; Joo, V.S.; Perez, L. Antibodies to combat viral infections: Development strategies and progress. Nat. Rev. Drug Discov. 2022, 21, 676–696. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sterlin, D.; Mathian, A.; Miyara, M.; Mohr, A.; Anna, F.; Claër, L.; Quentric, P.; Fadlallah, J.; Devilliers, H.; Ghillani, P.; et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci. Transl. Med. 2021, 13, eabd2223. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Esmat, K.; Jamil, B.; Kheder, R.K.; Kombe, A.J.; Zeng, W.; Ma, H.; Jin, T. Immunoglobulin A response to SARS-CoV-2 infection and immunity. Heliyon 2024, 10, e24031. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Izikson, R.; Brune, D.; Bolduc, J.S.; Bourron, P.; Fournier, M.; Moore, T.M.; Pandey, A.; Perez, L.; Sater, N.; Shrestha, A.; et al. Safety and immunogenicity of a high-dose quadrivalent influenza vaccine administered concomitantly with a third dose of the mRNA-1273 SARS-CoV-2 vaccine in adults aged ≥65 years: A phase 2, randomized, open-label study. Lancet Respir. Med. 2022, 10, 392–402. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, M.H.; Kim, H.J.; Chang, J. Superior immune responses induced by intranasal immunization with recombinant adenovirus-based vaccine expressing full-length Spike protein of Middle East respiratory syndrome coronavirus. PLoS ONE. 2019, 14, e0220196. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Norton, N.J.; Ings, D.P.; Fifield, K.E.; Barnes, D.A.; Barnable, K.A.; Harnum, D.O.A.; Holder, K.A.; Russell, R.S.; Grant, M.D. Characteristics of Vaccine- and Infection-Induced Systemic IgA Anti-SARS-CoV-2 Spike Responses. Vaccines 2023, 11, 1462. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Magen, E.; Merzon, E.; Green, I.; Golan-Cohen, A.; Vinker, S.; Israel, A. Selective IgA deficiency and COVID-19. J. Allergy Clin. Immunol. Pract. 2023, 11, 1936–1938. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Çölkesen, F.; Kandemir, B.; Arslan, Ş.; Çölkesen, F.; Yıldız, E.; Korkmaz, C.; Vatansev, H.; Evcen, R.; Aykan, F.S.; Kılınç, M.; et al. Relationship between Selective IgA Deficiency and COVID-19 Prognosis. Jpn. J. Infect. Dis. 2022, 75, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.J.; Siracusano, G.; Cottignies-Calamarte, A.; Tudor, D.; Real, F.; Zhu, A.; Pastori, C.; Capron, C.; Rosenberg, A.R.; Temperton, N.; et al. Persistent but dysfunctional mucosal SARS-CoV-2-specific IgA and low lung IL-1β associate with COVID-19 fatal outcome: A cross-sectional analysis. Front. Immunol. 2022, 13, 842468. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gasser, R.M.; Cloutier, M.; Prévost, J.; Fink, C.; Ducas, É.; Ding, S.; Dussault, N.; Landry, P.; Tremblay, T.; Laforce-Lavoie, A.; et al. Major role of IgM in the neutralizing activity of convalescent plasma against SARS-CoV-2. Cell Rep. 2021, 34, 108790. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Verkerke, H.; Saeedi, B.J.; Boyer, D.; Allen, J.W.; Owens, J.; Shin, S.; Horwath, M.; Patel, K.; Paul, A.; Wu, S.C.; et al. Are We Forgetting About IgA? A Re-examination of Coronavirus Disease 2019 Convalescent Plasma. Transfusion 2021, 61, 1740–1748. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M. Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Delclaux, C.; Roupie, E.; Blot, F.; Brochard, L.; Lemaire, F.; Brun-Buisson, C. Lower respiratory tract colonization and infection during severe acute respiratory distress syndrome: Incidence and diagnosis. Am. J. Respir. Crit. Care Med. 1997, 156 Pt 1, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Gededzha, M.P.; Mampeule, N.; Jugwanth, S.; Zwane, N.; David, A.; Burgers, W.A.; Blackburn, J.M.; Grove, J.S.; George, J.A.; Sanne, I.; et al. Performance of the EUROIMMUN Anti-SARS-CoV-2 ELISA Assay for detection of IgA and IgG antibodies in South Africa. PLoS ONE 2021, 16, e0252317. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tyagi, R.; Basu, S.; Dhar, A.; Gupta, S.; Gupta, S.L.; Jaiswal, R.K. Role of Immunoglobulin A in COVID-19 and Influenza Infections. Vaccines 2023, 11, 1647. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Z.; Lorenzi, J.C.C.; Muecksch, F.; Finkin, S.; Viant, C.; Gaebler, C.; Cipolla, M.; Hoffmann, H.H.; Oliveira, T.Y.; Oren, D.A.; et al. Enhanced SARS-CoV-2 neutralization by dimeric IgA. Sci Transl Med. 2021, 13, eabf1555. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bemark, M.; Angeletti, D. Know your enemy or find your friend?—Induction of IgA at mucosal surfaces. Immunol. Rev. 2021, 303, 83–102. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Sousa-Pereira, P.; Woof, J.M. IgA: Structure, Function, and Developability. Antibodies 2019, 8, 57. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bakema, J.E.; van Egmond, M. Immunoglobulin A: A next generation of therapeutic antibodies? MAbs 2011, 3, 352–361. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Woof, J.M.; Russell, M.W. Structure and function relationships in IgA. Mucosal Immunol. 2011, 4, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Padoan, A.; Sciacovelli, L.; Basso, D.; Negrini, D.; Zuin, S.; Cosma, C.; Faggian, D.; Matricardi, P.; Plebani, M. IgA-Ab response to spike glycoprotein of SARS-CoV-2 in patients with COVID-19: A longitudinal study. Clin. Chim. Acta. 2020, 507, 164–166. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brandtzaeg, P. Secretory IgA: Designed for Anti-Microbial Defense. Immunol. Front. 2013, 4, 222. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bomsel, M.; Tudor, D.; Drillet, A.S.; Alfsen, A.; Ganor, Y.; Roger, M.G.; Mouz, N.; Amacker, M.; Chalifour, A.; Diomede, L. Immunization with HIV-1 gp41 subunit virosomes induces mucosal antibodies protecting nonhuman primates against vaginal SHIV challenges. Immunity 2011, 34, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Khamassi, M.; Xu, L.; Rey, J.; Duchemin, M.; Bouceba, T.; Tuffery, P.; Tudor, D.; Bomsel, M. The CH1α domain of mucosal gp41 IgA contributes to antibody specificity and antiviral functions in HIV-1 highly exposed Sero-Negative individuals. PLoS Pathog. 2020, 16, e1009103. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Butler, S.E.; Crowley, A.R.; Natarajan, H.; Xu, S.; Weiner, J.A.; Bobak, C.A.; Mattox, D.E.; Lee, J.; Wieland-Alter, W.; Connor, R.I.; et al. Distinct Features and Functions of Systemic and Mucosal Humoral Immunity Among SARS-CoV-2 Convalescent Individuals. Front. Immunol. 2021, 11, 618685. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, H.Q.; Sun, B.Q.; Fang, Z.F.; Zhao, J.C.; Liu, X.Y.; Li, Y.M.; Sun, X.Z.; Liang, H.F.; Zhong, B.; Huang, Z.F.; et al. Distinct features of SARS-CoV-2-specific IgA response in COVID-19 patients. Eur. Respir. J. 2020, 56, 2001526. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zervou, F.N.; Louie, P.; Stachel, A.; Zacharioudakis, I.M.; Ortiz-Mendez, Y.; Thomas, K.; Aguero-Rosenfeld, M.E. SARS-CoV-2 antibodies: IgA correlates with severity of disease in early COVID-19 infection. J. Med. Virol. 2021, 93, 5409–5415. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ejemel, M.; Li, Q.; Hou, S.; Schiller, Z.A.; Tree, J.A.; Wallace, A.; Amcheslavsky, A.; Kurt, Y.; Buttigieg, K.R.; Elmore, M.J.; et al. A cross-reactive human IgA monoclonal antibody blocks SARS-CoV-2 spike-ACE2 interaction. Nat Commun. 2020, 11, 4198. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Göritzer, K.; Groppelli, E.; Grünwald-Gruber, C.; Figl, R.; Ni, F.; Hu, H.; Li, Y.; Liu, Y.; Hu, Q.; Puligedda, R.D.; et al. Recombinant neutralizing secretory IgA antibodies for preventing mucosal acquisition and transmission of SARS-CoV-2. Mol. Ther. 2024, 32, 689–703. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| 57 Patients | Anti SARS-CoV-2-IgA | Survivors | Not Survived | |
|---|---|---|---|---|
| Positive in BAL | ||||
| Mean age | 70.04 ± 9.43 years | |||
| Caucasian | All | |||
| Males | 41 | 31 | 15 | 16 |
| Females | 16 | 9 | 4 | 5 |
| Comorbidities | ||||
| Diabetes | 7 | 14 | ||
| Arterial hypertension | 16 | 15 | ||
| Obesity | 9 | 7 | ||
| Cardiovascular diseases | 7 | 6 | ||
| COPD | 2 | 3 | ||
| Allergy | 2 | 3 | ||
| Renal failure/cancer | 1 | 1 | ||
| Asthma | 1 | |||
| Liver diseases | 1 | |||
| Bacterial infection | 1 |
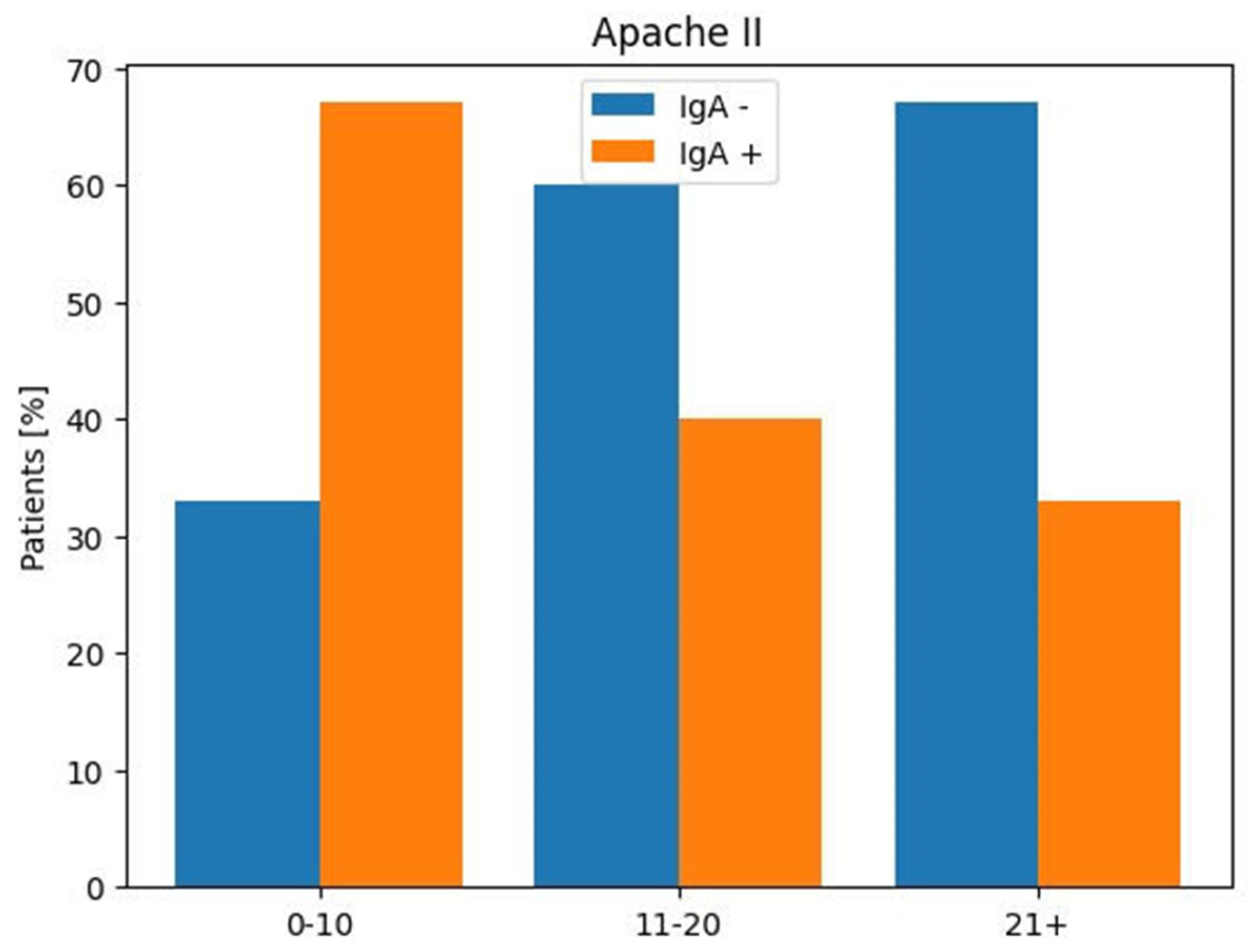
| APACHE II | Anti-SARS-CoV-2 IgA | Anti-SARS-CoV-2 IgA | |
| Score | Positive | Negative | |
| Patients | |||
| No (%) | No (%) | No (%) | |
| 0–10 | 14 (24%) | 8 (67%) | 4 (33%) |
| 11–20 | 38 (67%) | 10 (40%) | 15 (60%) |
| ≥21 | 5 (9%) | 1 (33%) | 2 (67%) |
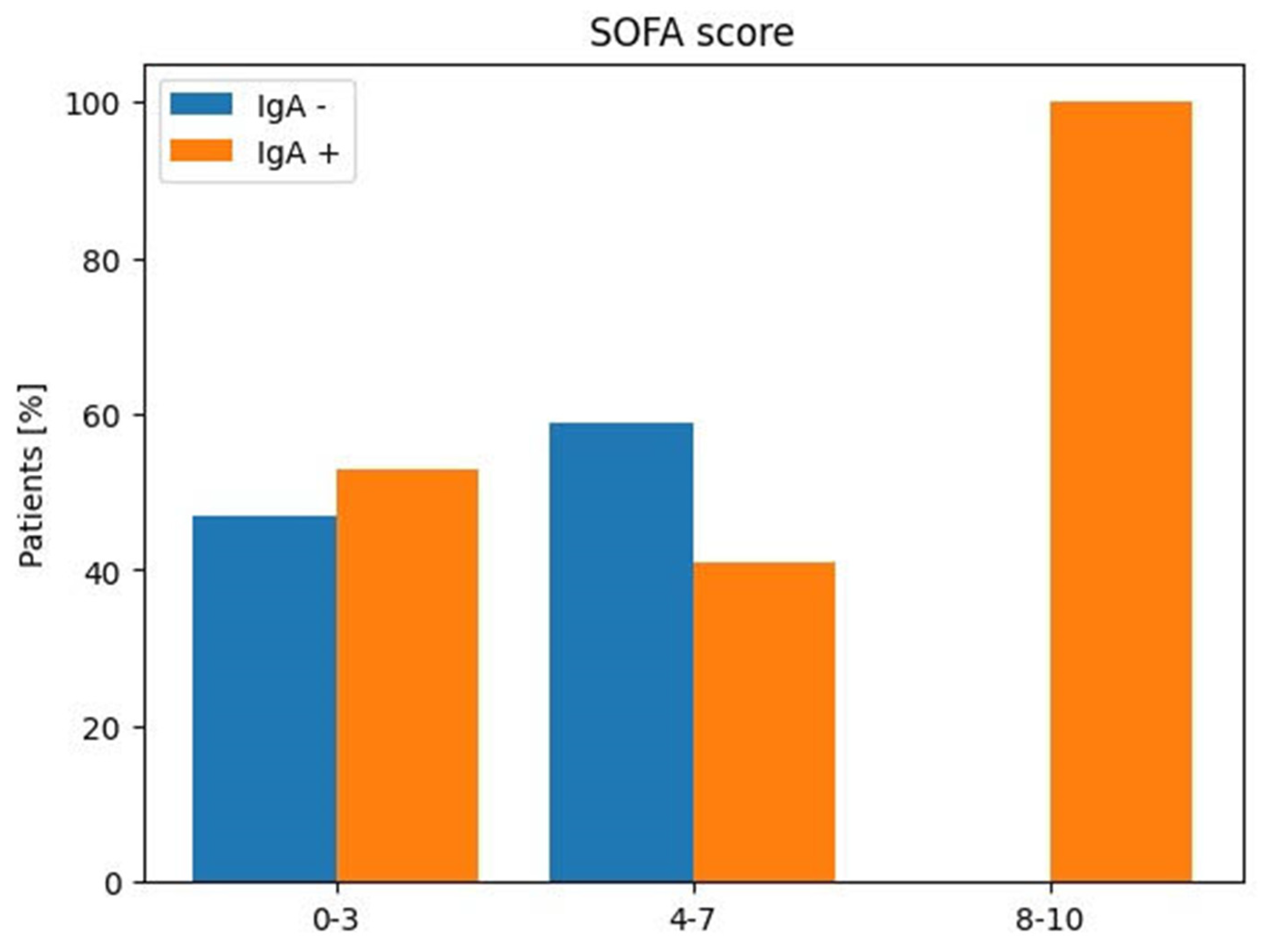
| SOFA | Anti-SARS-CoV-2 IgA | Anti-SARS-CoV-2 IgA | |
| Score | Positive | Negative | |
| Patients | |||
| No (%) | No (%) | No (%) | |
| 0–3 | 26 (46%) | 9 (53%) | 8 (47%) |
| 4–7 | 29 (51%) | 9 (41%) | 13 (59%) |
| 8–10 | 2 (3%) | 1 (100%) | 0 (0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Stefano, M.; Mirabella, L.; Cotoia, A.; Faleo, G.; Rauseo, M.; Rizzo, A.C.; Fiore, J.R.; Cinnella, G.; Serviddio, G. A Possible Protective Effect of IgA Against Severe Acute Respiratory Syndrome Corona Virus 2 (SARS-CoV-2) in Bronchoalveolar Lavage in COVID-19 Patients Admitted to Intensive Care Unit. Viruses 2024, 16, 1851. https://doi.org/10.3390/v16121851
Di Stefano M, Mirabella L, Cotoia A, Faleo G, Rauseo M, Rizzo AC, Fiore JR, Cinnella G, Serviddio G. A Possible Protective Effect of IgA Against Severe Acute Respiratory Syndrome Corona Virus 2 (SARS-CoV-2) in Bronchoalveolar Lavage in COVID-19 Patients Admitted to Intensive Care Unit. Viruses. 2024; 16(12):1851. https://doi.org/10.3390/v16121851
Chicago/Turabian StyleDi Stefano, Mariantonietta, Lucia Mirabella, Antonella Cotoia, Giuseppina Faleo, Michela Rauseo, Anna Chiara Rizzo, Josè Ramon Fiore, Gilda Cinnella, and Gaetano Serviddio. 2024. "A Possible Protective Effect of IgA Against Severe Acute Respiratory Syndrome Corona Virus 2 (SARS-CoV-2) in Bronchoalveolar Lavage in COVID-19 Patients Admitted to Intensive Care Unit" Viruses 16, no. 12: 1851. https://doi.org/10.3390/v16121851
APA StyleDi Stefano, M., Mirabella, L., Cotoia, A., Faleo, G., Rauseo, M., Rizzo, A. C., Fiore, J. R., Cinnella, G., & Serviddio, G. (2024). A Possible Protective Effect of IgA Against Severe Acute Respiratory Syndrome Corona Virus 2 (SARS-CoV-2) in Bronchoalveolar Lavage in COVID-19 Patients Admitted to Intensive Care Unit. Viruses, 16(12), 1851. https://doi.org/10.3390/v16121851






